Abstract
Background
Little is known regarding the impact of diastolic function on cardiac output (CO) in patients with heart failure, particularly in patients with lower ejection fraction. This study aimed to evaluate the impact of end‐diastolic pressure–volume relationship (EDPVR) on CO and end‐diastolic pressure (EDP).
Methods and Results
We retrospectively analyzed 1840 consecutive patients who underwent heart catheterization. We divided patients into 8 groups according to ejection fraction (EF) (35–45%, 46–55%, 56–65%, and 66–75%) and EDP (>16 or ≤16 mm Hg). We estimated EDPVR from single measurements in the catheterization data set. Then, we replaced EDPVRs of high‐EDP groups with those of normal‐EDP groups and compared CO before and after EDPVR replacement. Normalized EDPVR significantly increased CO at EDP=10 mm Hg regardless of EF (EF 35–45%, from 4.5±1.6 to 4.9±1.0; EF 46–55%, 4.6±1.3 to 5.1±1.1; EF 56–65%, 4.9±1.5 to 5.2±1.0; EF 66–75%, 4.9±1.5 to 5.2±1.1). Changes in CO were similar across EF groups.
Conclusions
Diastolic function normalization was associated with higher CO irrespective of EF. Diastolic dysfunction plays an important role in determining CO irrespective of EF in heart failure patients.
Keywords: cardiac output, diastolic dysfunction, heart failure
Subject Categories: Heart Failure, Hemodynamics
Introduction
Diastolic dysfunction of the left ventricle is one of the major pathological factors in heart failure (HF). However, evaluation of diastolic dysfunction is insufficient because of methodological difficulty. Annually, 5.1 million people have HF,1 and the medical cost accounts for $31 billion dollars in the United States.2 The prevalence of HF with preserved ejection fraction (HFpEF), which accounts for more than 50% of HF cases, has been increasing.3 The prognosis of HF is devastating.3, 4 Diastolic dysfunction has been considered a major pathological factor of HFpEF, but because there has been increased focus on several other comorbid conditions that might contribute to HFpEF pathogenesis such as arterial stiffness,5 diabetes mellitus and anemia,6 elevated peripheral vessel resistance,7, 8 left atrial dysfunction,9 and baroreflex dysfunction,10, 11 the influence of diastolic dysfunction on pathophysiology in HFpEF patients is not evaluated precisely. Additionally, the impact of diastolic dysfunction on disease pathophysiology is unknown in not only patients with HFpEF but also those with HF with reduced ejection fraction (HFrEF). In HFrEF patients, the combination of systolic dysfunction and diastolic dysfunction makes it more difficult to evaluate the impact of diastolic dysfunction on the hemodynamic pathophysiology. The evaluation of diastolic function in clinical settings is practically limited and mainly depends on echocardiographic examination.12 Echocardiographic assessment of diastolic function is insufficient to determine the diastolic property of the left ventricle, because heart rate, fluid retention/depletion, and several conditions might change diastolic filling pattern on Doppler echocardiography or annular diastolic velocity pattern on tissue Doppler imaging. While end‐diastolic pressure‐volume relationship (EDPVR) represents the diastolic property of the left ventricle, measuring EDPVR is not practical in the clinical setting, since it requires perturbing preload or afterload during recording instantaneous left ventricular volume and its pressure. Previous studies demonstrated a method to estimate entire EDPVR from a single set of end‐diastolic pressure and volume,13, 14 and we used the method to evaluate diastolic function in this study. The purpose of this study was to assess the impact of diastolic dysfunction on left ventricular end‐diastolic pressure and cardiac output (CO) in HFrEF and HFpEF patients by evaluating entire EDPVR.
Methods
Study Design and Patient Selection
The ethics committee of Aso‐Iizuka Hospital approved all study protocols; the requirement for informed consent was waived because of the retrospective nature of the study. We retrospectively examined the impact of the diastolic property on hemodynamics in 2417 consecutive patients who underwent cardiac catheter examination on both sides of the heart from January 2003 to December 2013. We analyzed patient etiology, underlying diseases, and sex distribution. End‐systolic volume (ESV), end‐diastolic volume (EDV), and left ventricular ejection fraction (EF) were derived from left ventriculography. CO was calculated by multiplying (EDV−ESV) by heart rate (HR). We removed incomplete data and the 10 patients who had the largest or smallest systolic aortic pressure, left ventricular pressure, left ventricular EDV, or ESV as outliers. We removed patients with EF <35% or >75% due to the small number of samples. The final number of patients included in the data set was 1840. Then, we classified the patients into 4 groups according to EF: 35% to 45% (n=280), 46% to 55% (n=345), 56% to 65% (n=564), and 66% to 75% (n=651). Then, we divided each group into 2 subgroups according to end‐diastolic pressure (EDP): a high‐EDP group (EDP ≥16 mm Hg) and normal‐EDP group (EDP <16 mm Hg), as previously reported (Figure 1).15
Figure 1.
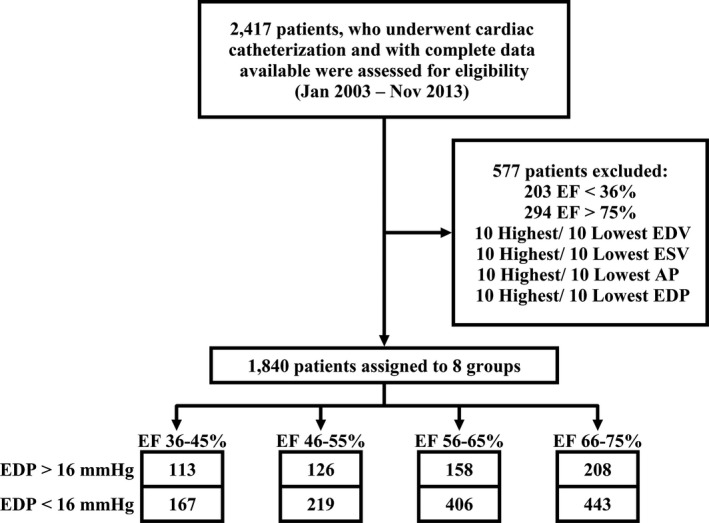
Patient disposition. Out of 2417 patients assessed for eligibility, a total of 1840 patients were assigned to 8 groups according to EF and EDP. AP indicates arterial pressure; EDP, end‐diastolic pressure; EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume.
Estimation of EDPVR From a Single Data Set of EDV and EDP
We estimated EDPVR by referring to previous articles employing a single set of EDV and EDP data.13, 14, 16 Briefly, EDPVR is considered generally invariant among subjects after normalization of volumes. EDPVR after normalizing LV volume axis was expressed by the following equation,
where EDVn is normalized EDV and An and Bn are coefficient values of 27.8 mm Hg and 2.76 (no units), respectively. Then we calculated EDV at EDP=0, 15, and 30 mm Hg (Vu, V15, and V30) based on previous reports as follows:
where Vm and Pm are the measured EDV and EDP. Then, coefficients of diastolic properties β and α were calculated according to the following equations:
Finally, we estimated each EDPVR as
where α and β represent coefficients of diastolic properties. We compared EDV at EDP=0, 15, and 30 mm Hg between the high‐EDP and normal‐EDP subgroups in each EF group (Figures 2 and 3).
Figure 2.
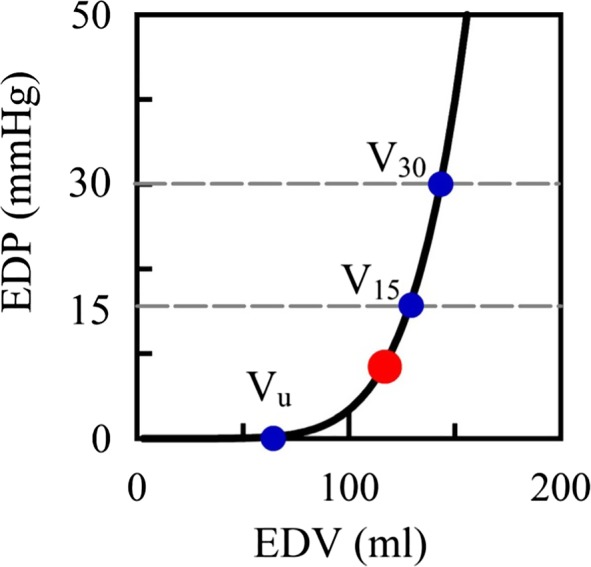
Estimated entire EDPVR and Vu, V15, and V30. Estimated entire EDPVR and end‐diastolic volumes (EDVs) at end‐diastolic pressure (EDP) of 0, 15, and 30 mm Hg (Vu, V15, and V30, respectively). Vu, V15, and V30 are plotted in blue dots. Measured (EDV, EDP) is plotted in red dot. EDPVR values superimposed. EDP=α EDV β where α and β represent coefficients of diastolic properties. EDPVR, end‐diastolic pressure–volume relationship.
Figure 3.
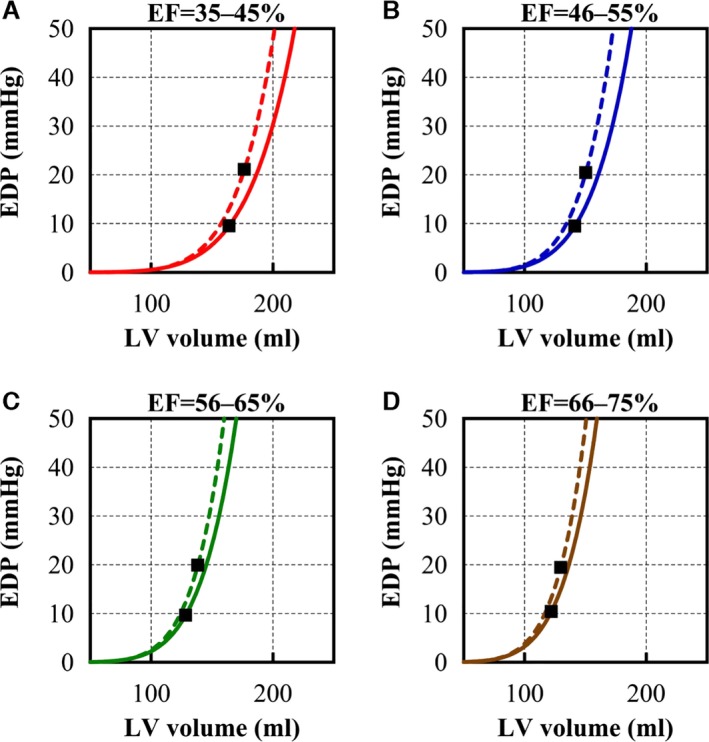
Calculated EDPVRs and single sets of (mean EDV, mean EDP). Calculated EDPVRs and single sets of (mean EDV, mean EDP) are plotted (squares) in (A through D). Solid lines indicate EDPVRs of normal EDP subgroups and dashed lines indicate EDPVRs of elevated EDP subgroups. In lower EF groups, EDVs are larger than that of higher EF groups. EDPVRs from elevated EDP subgroups are shifted upward compared to EDPVRs from normal EDP subgroups. EDP indicates end‐diastolic pressure; EDPVR, end‐diastolic pressure–volume relationship; EDV, end‐diastolic volume; EF, ejection fraction; LV, left ventricular.
Calculation of Cardiac Output
CO is theoretically determined by the following formula:
where Ees is left ventricular end‐systolic elastance, Ea is the effective arterial elastance, and V0 is the theoretical volume at zero pressure.17, 18 To simplify the calculation, we considered V0 as zero19 and used 0.9 times peak systolic arterial pressure20 as the arterial end‐systolic pressure, which is required to determine Ees and Ea. Thus, combining those 2 equations, CO is expressed as follows using α and β:
First, we calculated mean diastolic parameters (ie, α and β) of the normal‐EDP subgroup in each EF group and replaced the diastolic parameters of the high‐EDP subgroup with those of the normal diastolic group. Thus, we mathematically restored diastolic properties to clarify the impact on the hemodynamics. We then compared COs at EDP=10 mm Hg between before and after changing diastolic parameters in the high‐EDP group.
Statistical Analysis
Statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute, Inc., Cary, NC) and Microsoft Excel 2010 (Microsoft Corp., Redmond, WA). We compared hemodynamic variables of the 8 groups by using Kruskal–Wallis analysis of variance. We used the Wilcoxon rank–sum test to analyze data among the same EF groups. Categorical variables are expressed as numbers and percentages. The χ2 test was used for comparisons of categorical variables. Data are expressed as mean±SD in Table and mean±SE in Figure 4.
Table 1.
Characteristics of Study Population and Hemodynamic Variables
| EF=40% | EF=50% | EF=60% | EF=70% | P Valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| EDP elevation | − | + | − | + | − | + | − | + | |
| N | 167 | 113 | 219 | 126 | 406 | 158 | 443 | 208 | |
| Age, y | 69.0±11.4 | 69.6±11.9 | 69.8±10.9 | 68.9±13.3 | 66.8±12.9 | 68.6±12.1 | 68.4±11.6 | 68.3±10.3 | 0.10 |
| Female sex, n (%) | 65 (38.9) | 32 (28.3) | 62 (28.3) | 49 (38.9) | 137 (33.7) | 59 (37.3) | 158 (35.7) | 80 (38.5) | 0.17 |
| Body weight, kg | 55.2±11.7 | 57.7±11.1 | 56.8±11.4 | 58.5±12.1 | 58.1±11.6 | 59.9±12.5 | 58.5±11.5 | 60.3±11.8b | 0.0009 |
| Heart rate, bpm | 73±17 | 73±15 | 72±17 | 71±16 | 69±15 | 67±13 | 66±14 | 61±13b | <0.0001 |
| LV peak systolic pressure, mm Hg | 120±25 | 130±28b | 124±25 | 140±31b | 130±25 | 140±28b | 133±24 | 146±28b | <0.0001 |
| LV end‐diastolic pressure, mm Hg | 10±4 | 21±5b | 10±4 | 20±4b | 10±3 | 20±4b | 10±3 | 19±3b | <0.0001 |
| LV end‐systolic volume, mL | 97±36 | 106±39 | 70±22 | 75±24 | 50±17 | 55±16b | 36±11 | 39±13b | <0.0001 |
| LV end‐diastolic volume, mL | 164±58 | 177±61 | 140±43 | 150±46 | 128±41 | 138±38b | 122±35 | 130±37b | <0.0001 |
| Underlying diseases | |||||||||
| Hypertension, n (%) | 86 (51.5) | 53 (46.9) | 124 (56.6) | 72 (57.1) | 214 (52.7) | 88 (55.7) | 223 (50.3) | 94 (45.2) | 0.21 |
| Diabetes mellitus, n (%) | 49 (29.3) | 32 (28.3) | 47 (21.5) | 31 (24.6) | 83 (20.4) | 31 (19.6) | 76 (17.1) | 31 (14.9) | 0.004 |
| Etiology | |||||||||
| Ischemic heart disease, n (%) | 107 (64.1) | 93 (82.3) | 152 (69.4) | 102 (81.0) | 243 (59.9) | 111 (70.3) | 267 (60.3) | 128 (61.5) | <0.0001 |
| Mitral valve regurgitation, n (%) | 18 (10.8) | 9 (8.0) | 9 (4.1) | 5 (4.0) | 26 (6.4) | 7 (4.4) | 18 (4.1) | 9 (4.3) | 0.036 |
| Aortic valve regurgitation, n (%) | 7 (4.2) | 8 (7.1) | 3 (1.4) | 6 (4.8) | 24 (5.9) | 4 (2.5) | 18 (4.1) | 12 (5.8) | 0.16 |
| Aortic valve stenosis, n (%) | 2 (1.2) | 7 (6.2) | 7 (3.2) | 5 (4.0) | 6 (1.5) | 6 (3.8) | 19 (4.3) | 10 (4.8) | 0.089 |
| Dilated cardiomyopathy, n (%) | 15 (9.0) | 3 (2.7) | 4 (1.8) | 1 (0.8) | 4 (1.0) | 0 (0) | 0 (0) | 0 (0) | <0.0001 |
| Hypertrophic cardiomyopathy, n (%) | 1 (0.6) | 1 (0.9) | 3 (1.4) | 2 (1.6) | 8 (2.0) | 4 (2.5) | 5 (1.1) | 7 (3.4) | 0.43 |
| Calculated values | |||||||||
| α (10−11 mm Hg) | 1.2±5.9 | 13.5±82b | 1.4±3.1 | 17.6±59 | 2.8±8.6 | 12.8±56 | 2.5±5.9 | 18.8±69 | <0.0001 |
| β (unit less) | 5.8±0.14 | 5.9±0.53 | 5.8±0.14 | 6.0±0.50b | 5.8±0.13 | 6.0±0.44b | 5.8±0.12 | 6.0±0.41b | <0.0001 |
| Vu, mL | 89±31 | 83±29 | 77±23 | 72±22b | 69±22 | 66±18b | 66±19 | 63±19b | <0.0001 |
| V15, mL | 181±64 | 166±57 | 156±49 | 142±43b | 141±44 | 132±36b | 133±41 | 124±36b | <0.0001 |
| V30, mL | 204±72 | 187±64 | 176±56 | 160±49b | 158±50 | 148±41b | 149±46 | 140±41b | <0.0001 |
Continuous variables are expressed as mean±SD. Categorical variables are expressed as numbers and percentages. bpm indicates beats per minute; EDP, end‐diastolic pressure; EF, ejection fraction; LV, left ventricular; Vu, V15, and V30, end‐diastolic volumes at which end‐diastolic pressure is 0 (Vu), 15 (V15), and 30 (V30) mm Hg.
P values obtained from Kruskal–Wallis test for continuous variables and from the χ2 test for categorical variables.
P<0.05 vs normal‐EDP subgroup in same EF group using Wilcoxon rank‐sum test.
Figure 4.
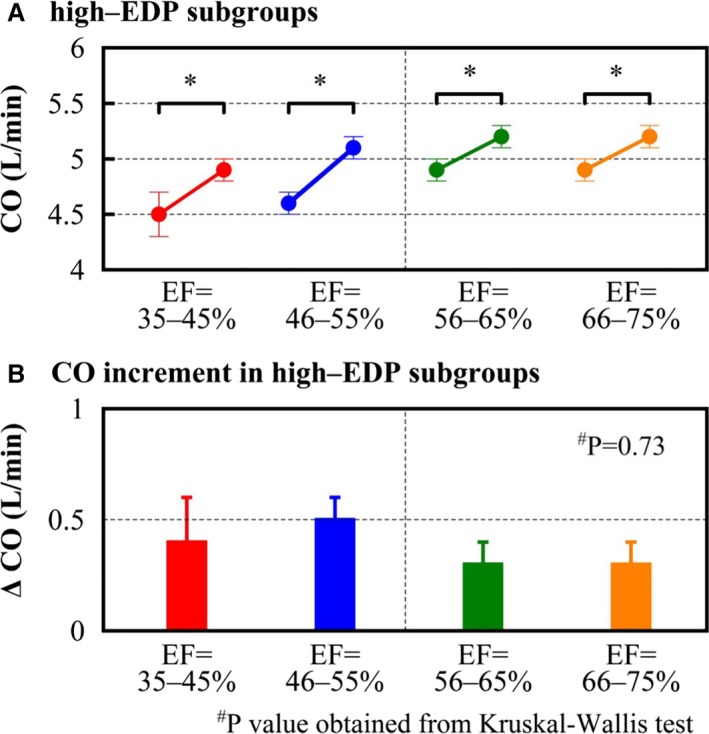
Cardiac output changes and cardiac output increment. In the high‐EDP subgroup, changing diastolic properties (α and β) to those of the normal‐EDP group significantly increased CO (A). There were no significant differences in the increment of CO among EF groups (B). CO indicates cardiac output; EDP, end‐diastolic pressure; EF, ejection fraction. * P<0.05 vs normal‐EDP subgroup of the same EF group using the paired t test. # P values obtained from Kruskal–Wallis test.
Results
Comparison of EDPVR Between High‐EDP and Normal‐EDP Subgroups
The characteristics of the study population and hemodynamic variables are detailed in Table. There was no significant difference in age and sex. Mean age was around 70 years old, and 28% to 38% of patients were female. The presence of hypertension was not significantly different among EF groups. The prevalence of ischemic heart disease was more than 60% in all EF groups. The percentages of patients with diabetes mellitus, mitral regurgitation, and diastolic cardiomyopathy were lower in higher EF groups. Percentage of patients with hypertrophic cardiomyopathy showed a reverse trend.
Higher EF groups tended to have higher left ventricular (LV) peak systolic pressure, lower HR, smaller LV ESV, and smaller LV EDV. Within the same EF groups, high‐EDP groups tended to have higher LV pressures and volumes. Estimated Vu, V15, and V30 were smaller in the high‐EDP subgroup than in normal‐EDP subgroup regardless of EF. The lower EF groups tended to have greater Vu, V15, and V30. Figure 3 shows mean EDV and EDP and the estimated EDPVR of each EF group. EDPVR shifted toward the right, which indicated enlarged LV volume as EF was reduced. Within the same EF groups, EDPVRs in high‐EDP groups (dashed line) were steeper than those in normal‐EDP groups (solid line). The calculated coefficients α and β values were higher in patients in the EDP‐elevated group than in patients in the normal‐EDP group.
Change in Cardiac Output Before and After Normalizing EDPVR
COs at EDP=10 mm Hg before and after changing diastolic properties are shown in Figure 4. Normalizing diastolic properties in high‐EDP groups significantly increased CO in all EF groups (4.5±1.6 to 4.9±1.0 L/min in EF 35–45% group, 4.6±1.3 to 5.1±1.1 L/min in EF 46–55% group, 4.9±1.5 to 5.2±1.0 L/min in EF 56–65% group, and 4.9±1.5 to 5.2±1.1 L/min in EF 66–75% group) (Figure 4A). The degrees of increase in CO were similar among all EF groups (Figure 4B).
Discussion
This study revealed that diastolic dysfunction plays a similar role in the pathophysiology of HF in patients with high EDP irrespective of EF. In addition, this study demonstrates that the mathematical estimation of EDPVR is feasible and may be an attractive tool for evaluating the contribution of diastolic function to hemodynamics.
Hemodynamic Characteristics and EDPVR According to EF and EDP Subsets
The present study demonstrated that CO was consistently preserved even in HF patients with lower EF. Borlaug et al21 also reported that CO was preserved even in HFpEF patients. Moreover, Schwartzenberg et al reported that CO was increased in HFpEF patients.22 These findings support our results and suggest that the mathematical CO estimation is adequate for clinical use. Meanwhile, within each EF group, EDPVRs of the high‐EDP subgroup were shifted left and steeper compared with the normal‐EDP subgroup (Figure 3). This report is the first to reveal how EDPVR differs according to EDP.
Change in Cardiac Output After EDPVR Normalization in the High‐EDP Group
Diastolic dysfunction, which is the key pathophysiological mechanism in HFpEF, is also considered a potential pathophysiological factor of HFrEF.23 In the present study, EDPVR normalization was significantly associated with higher CO regardless of EF in high‐EDP patients. Furthermore, there were no differences in the degree of change in CO among EF groups, suggesting that diastolic dysfunction exerts a similar hemodynamic effect regardless of EF. Although several previous articles have mentioned the importance of diastolic dysfunction in HFrEF,24, 25 this study is the first to demonstrate its quantitative impact on CO. This finding highlights the profound impact of diastolic dysfunction on hemodynamics in patients with HFrEF.
Previous studies26, 27 used the same single‐point EDPVR estimation method as we used in the current study. In those studies, α and β were defined as the diastolic curve fitting constant and stiffness constant, respectively. Those studies reported that the both α and β values were significantly different between HFpEF and HFrEF.26, 27 It is difficult to distinguish the effect of each specific coefficient on diastolic function. Therefore, we changed both coefficients in the current study to clarify the effect of diastolic properties.
In the guideline28 of management of HF, it is mentioned that abnormalities of systolic and diastolic dysfunction coexist. The current study using mathematical method revealed that diastolic dysfunction might prevail in HF pathophysiology regardless of EF. Especially in HF patients with low EF, physicians are apt to focus on their low systolic function. The current study might be helpful for physicians to become conscious of diastolic dysfunction in patients with HF independently of EF. Although specific therapies for diastolic dysfunction are lacking, the guideline28 recommends systolic and diastolic blood pressure control, correcting volume overload status, coronary revascularization for patients with coronary artery disease, and atrial fibrillation management. These recommendations also might be beneficial for HF patients with low EF.
The previous studies reported that diastolic function is impaired in the female29 and aged30 patients. Other studies showed that diabetes mellitus31 and hypertension32 were the major risk factors for HFpEF. In the current study, there were no significant difference between EDP elevation groups and normal EDP groups in age and sex. In same EF groups, we could not find an apparent trend that patients with hypertension or diabetes mellitus had impaired diastolic function. There was a trend that patients with ischemic heart disease had impaired diastolic function. The previous study33 showed ischemic heart disease is common in HFpEF. The current result supported myocardial ischemia reduced diastolic function. The previous study34 showed that myocardial ischemia slowed ventricular relaxation and reduced ventricular distensibility, although the current study could not clarify the mechanism. Because the numbers of patients with valvular heart disease or cardiomyopathy were small, we could not evaluate the impact of each pathological condition on diastolic function.
Limitations
First, we estimated EDPVR from a single point based on the previously reported mathematical model that hypothesized EDPVR as 1 simple nonlinear curve. This limited the accuracy of estimating true EDPVR. However, perturbation of volume load to measure EDPVR during catheterization is ethically unacceptable in HF patients. Therefore, estimating EDPVR from a single data set is considered the best method to determine EDPVR at this time. Second, LV volume was measured from left ventriculography, although the “gold standard” for LV volumetry is computed tomography or magnetic resonance imaging. However, left ventriculography is still widely performed and often used as a reference value to evaluate other volumetric methods.35, 36 It is reasonable to think that our results would not differ substantially with the use of computed tomography or magnetic resonance imaging to evaluate LV volume.
Conclusions
Although diastolic dysfunction is one of the major pathophysiological causes of HFpEF, our results revealed that diastolic dysfunction also plays a key role in the pathogenesis of HFrEF. The mathematical estimation of EDPVR can provide clinicians with detailed information about the role of diastolic dysfunction in individual patients and thus appears to be an attractive tool for practical applications.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e003389. DOI: 10.1161/JAHA.116.003389.)
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:28–292.24323793 [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 4. Zarrinkoub R, Wettermark B, Wändell P, Mejhert M, Szulkin R, Ljunggren G, Kahan T. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15:995–1002. [DOI] [PubMed] [Google Scholar]
- 5. Shibata S, Hastings JL, Prasad A, Fu Q, Bhella PS, Pacini E, Krainski F, Palmer MD, Zhang R, Levine BD. Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol. 2011;110:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction a community‐based study. Circ Heart Fail. 2012;5:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tartière‐Kesri L, Tartière J‐M, Logeart D, Beauvais F. Cohen Solal A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59:455–461. [DOI] [PubMed] [Google Scholar]
- 8. Ennezat PV, Maréchaux S, Six‐Carpentier M, Pinçon C, Sediri I, Delsart P, Gras M, Mounier‐Véhier C, Gautier C, Montaigne D, Jude B, Asseman P, Le Jemtel TH. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant. 2011;26:3908–3913. [DOI] [PubMed] [Google Scholar]
- 9. Fang F, Lee AP, Yu CM. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr Opin Cardiol. 2014;29:430–436. [DOI] [PubMed] [Google Scholar]
- 10. Hosokawa K, Ide T, Tobushi T, Sakamoto K, Onitsuka K, Sakamoto T, Fujino T, Saku K, Sunagawa K. Bionic baroreceptor corrects postural hypotension in rats with impaired baroreceptor. Circulation. 2012;126:1278–1285. [DOI] [PubMed] [Google Scholar]
- 11. Funakoshi K, Hosokawa K, Kishi T, Ide T, Sunagawa K. Striking volume intolerance is induced by mimicking arterial baroreflex failure in normal left ventricular function. J Card Fail. 2014;20:53–59. [DOI] [PubMed] [Google Scholar]
- 12. Penicka M, Vanderheyden M, Bartunek J. Diagnosis of heart failure with preserved ejection fraction: role of clinical Doppler echocardiography. Heart. 2014;100:68–76. [DOI] [PubMed] [Google Scholar]
- 13. Klotz S, Dickstein ML, Burkhoff D. A computational method of prediction of the end‐diastolic pressure‐volume relationship by single beat. Nat Protoc. 2007;2:2152–2158. [DOI] [PubMed] [Google Scholar]
- 14. Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, Kass DA, Burkhoff D. Single‐beat estimation of end‐diastolic pressure‐volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:403–412. [DOI] [PubMed] [Google Scholar]
- 15. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 16. Ten Brinke EA, Burkhoff D, Klautz RJ, Tschöpe C, Schalij MJ, Bax JJ, van der Wall EE, Dion RA, Steendijk P. Single‐beat estimation of the left ventricular end‐diastolic pressure‐volume relationship in patients with heart failure. Heart. 2010;96:213–219. [DOI] [PubMed] [Google Scholar]
- 17. Uemura K, Sugimachi M, Kawada T, Kamiya A, Jin Y, Kashihara K, Sunagawa K. A novel framework of circulatory equilibrium. Am J Physiol Heart Circ Physiol. 2004;286:H2376–H2385. [DOI] [PubMed] [Google Scholar]
- 18. Uemura K, Kawada T, Kamiya A, Aiba T, Hidaka I, Sunagawa K, Sugimachi M. Prediction of circulatory equilibrium in response to changes in stressed blood volume. Am J Physiol Heart Circ Physiol. 2005;289:H301–H307. [DOI] [PubMed] [Google Scholar]
- 19. Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, Fang JC, Sweitzer NK, Borlaug BA, Kass DA, St John Sutton M, Cappola TP. Ventricular‐arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single‐beat determination of left ventricular end‐systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. [DOI] [PubMed] [Google Scholar]
- 21. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–451. [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. Erratum in: Eur J Heart Fail. 2013;15:361–362. [DOI] [PubMed] [Google Scholar]
- 24. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 25. Dokainish H. Left ventricular diastolic function and dysfunction: central role of echocardiography. Glob Cardiol Sci Pract. 2015;2015:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam CSP, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular‐vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwarzl M, Ojeda F, Zeller T, Seiffert M, Becher PM, Munzel T, Wild PS, Blettner M, Lackner KJ, Pfeiffer N, Beutel ME, Blankenberg S. Westermann D. Risk factors for heart failure are associated with alterations of the LV end‐diastolic pressure‐volume relationship in non‐heart failure individuals: data from a large‐scale, population‐based cohort. Eur Heart J. 2016;37:1807–1814. [DOI] [PubMed] [Google Scholar]
- 28. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:240–327. [DOI] [PubMed] [Google Scholar]
- 29. den Ruijter H, Pasterkamp G, Rutten FH, Lam CSP, Chi C, Tan KH, van Zonneveld AJ, Spaanderman M, de Kleijn DPV. Heart failure with preserved ejection fraction in women: the Dutch Queen of Hearts program. Neth Heart J. 2015;23:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Riet EES, Hoes AW, Wagenaar KP, Limburg A, Landman MAJ, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18:242–252. [DOI] [PubMed] [Google Scholar]
- 31. Lindman BR, Dávila‐Román VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de Las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teo LYL, Chan LL, Lam CSP. Heart failure with preserved ejection fraction in hypertension. Curr Opin Cardiol. 2016;31:410–416. [DOI] [PubMed] [Google Scholar]
- 33. Samson R, Jaiswal A, Ennezat PV, Cassidy M, Le Jemtel TH. Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5:e002477 DOI: 10.1161/JAHA.115.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohara T, Little WC. Evolving focus on diastolic dysfunction in patients with coronary artery disease. Curr Opin Cardiol. 2010;25:613–621. [DOI] [PubMed] [Google Scholar]
- 35. Khorsand A, Gyöngyösi M, Graf S, Zamini S, Schuster E, Sochor H, Porenta G. Assessment of left ventricular volume and ejection fraction: comparison of QGS and MBGS analyses of ECG‐gated myocardial perfusion SPECT imaging. Nucl Med Commun. 2009;30:300–307. [DOI] [PubMed] [Google Scholar]
- 36. Iino M, Shiraishi H, Ichihashi K, Hoshina M, Saitoh M, Hirakubo Y, Morimoto Y, Momoi MY. Volume measurement of the left ventricle in children using real‐time three‐dimensional echocardiography: comparison with ventriculography. J Cardiol. 2007;49:221–229. [PubMed] [Google Scholar]


