Abstract
Background
Despite the epidemic rise in obesity, few studies have evaluated the effect of obesity on cost following cardiac surgery. We hypothesized that increasing body mass index (BMI) is associated with worse risk‐adjusted outcomes and higher cost.
Methods and Results
Medical records for 13 637 consecutive patients who underwent coronary artery bypass grafting (9702), aortic (1535) or mitral (837) valve surgery, and combined valve–coronary artery bypass grafting (1663) procedures were extracted from a regional Society of Thoracic Surgeons certified database. Patients were stratified by BMI: normal to overweight (BMI 18.5–30), obese (BMI 30–40), and morbidly obese (BMI >40). Differences in outcomes and cost were compared between BMI strata and also modeled as a continuous function of BMI with adjustment for preoperative risk using Society of Thoracic Surgeons predictive risk indices. Morbidly obese patients incurred nearly 60% greater observed mortality than normal weight patients. Moreover, morbidly obese patients had greater than 2‐fold increase in renal failure and 6.5‐fold increase in deep sternal wound infection. After risk adjustment, a significant association was found between BMI and mortality (P<0.001) and major morbidity (P<0.001). The risk‐adjusted odds ratio for mortality for morbidly obese patients was 1.57 (P=0.02) compared to normal patients. Importantly, risk‐adjusted total hospital cost increased with BMI, with 17.2% higher costs in morbidly obese patients.
Conclusions
Higher BMI is associated with increased mortality, major morbidity, and cost for hospital care. As such, BMI should be more strongly considered in risk assessment and resource allocation.
Keywords: complication, cost, obesity, surgery
Subject Categories: Cardiovascular Surgery, Obesity, Quality and Outcomes, Cost-Effectiveness
Introduction
Obesity is increasing in prevalence throughout the world, producing significant repercussions in cardiovascular disease morbidity, mortality, and healthcare costs.1, 2, 3, 4, 5 Consequently, obese patients frequently present for cardiac surgery, and surgery in morbidly obese patients has become common.6, 7 Few studies have evaluated the effect of obesity on outcomes and resource utilization following cardiac surgery. Some studies have found that obese patients have higher incidence of morbidity and mortality after coronary artery bypass grafting (CABG).8, 9 Conversely, other studies have concluded that obesity does not adversely affect morbidity or mortality of patients after CABG and a few studies found an inverse relationship between obesity and adverse outcomes, which is referred to as the “obesity paradox.”10, 11 Many prior studies were limited to single‐center data with limited sample sizes. Moreover, all prior studies evaluated outcome by categorization of patients based on body mass index (BMI) or weight strata, which may have affected results.12 Furthermore, the cost of obesity on the healthcare system is enormous. It is estimated that medical costs secondary to obesity‐related illness exceed $150 billion per year to Medicare alone.13 Although there are some data on resource utilization for surgery in obese patients, very few are specific to cardiac surgery. The relationship of obesity to resource utilization after cardiac surgery is important to define for appropriate resource allocation.
In this study, we evaluated the effect of BMI on risk‐adjusted morbidity, mortality, and resource utilization in a large multi‐institutional regional cohort. We hypothesized that increasing BMI is associated with worse risk‐adjusted outcomes and higher cost.
Methods
The Virginia Cardiac Surgery Quality Initiative (VCSQI) is a collaborative group of 18 different cardiac surgical centers within the Commonwealth of Virginia. Collectively, the VCSQI centers perform ≈99% of the Commonwealth's cardiac operations. Each center individually contributes patient data to the national Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database. The purpose of this study was 2‐fold: (1) to study the impact of BMI on outcomes of cardiac surgery and (2) to then determine the impact of BMI on postoperative complications, hospital resource utilization, and risk‐adjusted costs. This investigation was exempt from institutional review board review at each participating center because of its deidentified nature as a quality database and because of the absence of Health Insurance Portability and Accountability Act patient identifiers.
Patients and Data Acquisition
Deidentified patient records were obtained from the VCSQI database for the study period 2010–2012 for all patients (n=17 483) who underwent cardiac surgery. We excluded patients (n=5595) who did not have a STS predicted risk of operative mortality (PROM) to allow for risk‐adjusted comparison. STS PROM scores were available on all patients who underwent the following: isolated CABG, aortic valve replacement (AVR), mitral valve replacement (MVR), and combined CABG and valve replacement (CABG+AVR/MVR) as defined by the STS procedure‐type algorithm. Thus, patients who underwent tricuspid valve surgery, aortic surgery, mechanical circulatory support, or heart transplantation were excluded. Patient preoperative, operative, and postoperative variables were retrieved from the VCSQI database for each patient. STS predicted risk of morbidity or mortality and PROM were individually calculated. Standardized STS definitions for preoperative comorbidities such as hypertension, diabetes mellitus (DM), chronic lung disease, cerebrovascular accident, and congestive heart failure (CHF) were utilized.14 Left ventricular ejection fraction and pulmonary artery systolic pressure were extracted from the VCSQI database from patients with available hemodynamic data. Patients were then stratified by BMI, utilizing World Health Organization definitions of obesity: Underweight (BMI <18.5), Normal (BMI 18.5–24.5), Overweight (BMI 25–29.9), Obese (BMI 30–39.9), and Morbidly Obese (BMI ≥40).15
Cost Data and Acquisition
The VCSQI data registry combines standardized clinical data extracted from the STS data entry forms with hospital inpatient discharge financial data. Hospital inpatient data from Universal Billing (UB‐04) files are matched with each STS patient record. By the use of hospital‐specific cost‐to‐charge ratios, estimated hospital costs are determined with previously described methods.14, 16, 17 VCSQI maintains a 99% matching rate between STS patient records and billing data. All cost data are reported in U.S. dollars at the time of the surgery. As costs were not compared over time, costs were not adjusted for inflation.
Measured Outcomes
The primary outcomes were frequency of postoperative complications, length of stay, operative mortality, and total hospital cost. Standardized STS adult cardiac surgery database definitions for intraoperative and postoperative factors were used for this study. Operative mortality was defined as all patient deaths occurring during hospitalization as well as those within 30 days of the date of surgery despite discharge status. Ventilation time, intensive care unit hours, and hospital length of stay from surgery to discharge were measured. Standard STS definitions for postoperative events and complications were utilized, including cerebrovascular accident, renal failure (increase in serum creatinine level ≥4.0 with at least a 0.5 mg/dL rise, tripling of the most recent preoperative creatinine, or new requirement for dialysis), prolonged ventilation (>24 hours of mechanical ventilation), presence of any new‐onset atrial fibrillation, deep sternal wound infection (DSWI), and administration of intraoperative or postoperative blood products.14
Statistical Analysis
Descriptive statistics
All study outcomes, data analyses, regression coefficients, statistical modeling techniques, and methods were established a priori before data collection. Categorical variables are expressed as group percentages, while continuous variables are expressed as either mean±SD or median (25th, 75th percentile) depending upon overall variable distribution. Univariate comparisons included either Pearson's χ2 or Fisher's exact test for categorical variables and either independent‐sample single‐factor ANOVA for normally distributed data or the Wilcoxon rank sum test for non‐normally distributed data. Two‐sided P<0.05 defined statistically significant variable associations.
Risk‐adjusted regression models
Multilevel, mixed effects regression models were first used to estimate confounder‐adjusted associations between the probability of outcomes and patient BMI. Patient BMI was modeled as a continuous function using restricted cubic spline (RCS) smoothing transformations to account for both linear and nonlinear associations with outcomes rather than including categories of BMI (eg, normal, overweight, obese, or morbidly obese) in the regression analyses. RCS functions are beneficial because they utilize all data points to estimate the shape of the relationship between an exposure (eg, BMI) and an outcome (eg, operative mortality), whereas categories of BMI assume an identical relationship between the exposure and the outcome across the range of values included in each category. The use of RCS transformations, therefore, provides a more robust method to determine whether nonlinear relationships exist between a continuous variable and a dependent outcome. Use of RCS forces the tails of a function to be linear, which simplifies the representation. For RCS functions, continuous variables were analyzed using a total of 3 knots placed at standard 5th, 50th, and 95th percentiles to define the tails of each function. Individual hospital was entered as a random effect within each model to account for clustering of correlated events occurring within institutions. The predicted association between BMI and outcomes or cost was adjusted for the confounding effects of preoperative patient risk profile through inclusion of individual, calculated STS PROM scores for each patient. Inclusion of the STS PROM to adjust for preoperative patient risk utilizes a validated risk assessment score that is embraced and accepted by the STS and has been demonstrated to provide effective mortality and morbidity risk adjustment in cardiothoracic surgery literature.17, 18 In addition to STS PROM, age, sex, presence of hypertension, DM, renal failure, and heart failure were included as additional covariates. The risk‐adjusted RCS function for BMI versus the probability of patient outcomes was graphically represented in order to identify apparent threshold values (or inflection point) for patient BMI that correlated with an increase or decrease in the likelihood of each predicted outcome or cost. Regression model performance was assessed using the C statistic and the Nagelkerke Pseudo R 2 statistic, while the Hosmer‐Lemeshow test was utilized to assess goodness of fit. Each model demonstrated adequate performance characteristics with C statistics ranging from 0.78 to 0.86, Nagelkerke Pseudo R 2 statistics of 0.24 to 0.38, and Hosmer‐Lemeshow P>0.05. All statistical analyses were conducted using R statistical software, version 3.0.2 (http://www.R-project.org).
Results
Patients
A total of 17 483 patients underwent cardiac surgery in our regional cohort during the study period. Of these, 13 637 (78%) patients underwent operations that had an STS PROM score. Preoperative characteristics for each BMI strata are presented in Table 1. Obese (BMI 30–40) and morbidly obese (BMI >40) patients composed 40.8% (n=5586) of the cohort. Obesity was associated with younger age and increased comorbidities of DM and hypertension compared to normal weight patients. Morbidly obese patients consisted of more females (48% versus 27%; P<0.05), End Stage Renal Disease (5% versus 3%; P<0.05), CHF (27% versus 22%; P<0.05), and chronic lung disease (79% versus 24%; P<0.05) compared to normal weight patients. Despite the increased comorbidities, the median STS PROM was lower in obese (BMI 30–40) patients (1.1% versus 1.4%; P<0.05) and equivalent in morbidly obese (BMI >40) patients (1.4% versus 1.4%; P=0.91).
Table 1.
Preoperative Characteristics for BMI Strata
| Characteristic | Normal/Overweight (BMI 18.5–30) n=8051 | Obese (BMI 30–40) n=4797 | Morbidly Obese (BMI >40) n=789 |
|---|---|---|---|
| Age | 67±11 | 64±10* | 61±10* |
| Female sex | 2188 (27%) | 1429 (30) | 378 (48%)* |
| DM | 2420 (31%) | 1429 (51%)* | 378 (65%)* |
| Hypertension | 6302 (80%) | 4257 (89%)* | 745 (94%)* |
| ESRD | 230 (3%) | 124 (3%) | 38 (5%)* |
| CHF | 1762 (22%) | 1047 (22%) | 213 (27%)* |
| LV EF (%) | 55 (45, 60) | 55 (45, 60) | 55 (45, 60) |
| Chronic lung disease | 1723 (24%) | 1071 (22%) | 630 (79%) |
| PASP, mm Hg | 33 (27.42) | 36 (29, 45)* | 40 (32, 51)* |
| CVA | 1277 (17%) | 682 (14%)* | 106 (13%) |
| STS‐PROM | 1.4 (0.7, 3.1) | 1.1 (0.6, 2.3)* | 1.4 (0.7, 3.0) |
All continuous variables are presented as median and 25% and 75% percentiles. BMI indicates body mass index; CHF, congestive heart failure; CVA, history of cerebrovascular accident; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; ESRD, end‐stage renal disease; PASP, pulmonary artery systolic pressure; STS PROM, Society of Thoracic Surgery Predicted Risk of Mortality.
*P<0.05 compared to normal weight BMI Strata.
Operative Characteristics
Operative characteristics for each BMI strata are presented in Table 2. Operations in this cohort included isolated CABG (n=9702; 70.5%), AVR (n=1535; 11.2%) or MVR (n=837; 6.1%) valve surgery, or combined valve‐CABG (n=1663; 12.1%). In all strata, CABG was the most common operation, followed by AVR. There was no significant difference in median cardiopulmonary bypass or aortic cross‐clamp time between BMI strata. Obese and morbidly obese patients less commonly received intraoperative blood products (22% and 23%, respectively, versus 28%; P<0.05) compared to normal weight patients.
Table 2.
Operative Characteristics
| Characteristic | Normal/Overweight (BMI 18.5–30) n=8051 | Obese (BMI 30–40) n=4797 | Morbidly Obese (BMI >40) n=789 |
|---|---|---|---|
| CPB time, minutes | 97 (76 126) | 98 (77 126) | 97 (78 125) |
| Cross‐clamp time, minutes | 71 (54, 93) | 71 (55, 93) | 71 (54, 91) |
| Operation | |||
| CABG | 5534 (70%) | 3548 (74%) | 550 (70%) |
| AVR | 853 (11%) | 523 (11%) | 139 (18%) |
| MVR | 226 (3%) | 89 (2%) | 21 (3%) |
| CABG+AVR/MVR | 890 (11%) | 469 (10%) | 54 (7%) |
| Operative status | |||
| Elective | 3434 (44%) | 2170 (45%) | 358 (45%) |
| Urgent | 4155 (52%) | 2481 (52%) | 408 (52%) |
| Emergent | 258 (3%) | 141 (3%) | 22 (3%) |
| Received intraoperative blood products | 2243 (28%) | 1075 (22%)* | 182 (23%)* |
All continuous variables are presented as median and 25% and 75% percentiles. AVR indicates aortic valve replacement; BMI, body mass index; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; MVR, mitral valve repair or replacement.
*P<0.05 compared to normal.
Unadjusted Outcomes
Unadjusted operative outcomes for each BMI strata are presented in Table 3. There were no significant differences in rates of stroke, atrial fibrillation, or MI across BMI strata. Composite major morbidity was greater in obese (16.9%) and morbidly obese (22.2%) compared to normal weight patients (15%; P<0.05). Renal failure was more common in morbidly obese patients (6.8%) and obese (4.3%) compared to normal weight patients (3.1%; P<0.05). Prolonged ventilation was more common in morbidly obese (16.9%), and obese (12.2%) compared to normal weight patients (10.6%; P<0.05). DSWI was more common in morbidly obese (1.3%) and obese patients (0.4%) compared to normal weight patients (0.2%; P<0.05). Morbidly obese patients more frequently developed pneumonia (4.3% versus 3.0%; P<0.05) compared to normal weight patients. Morbidly obese patients had a longer intensive care unit (50 versus 46 hours; P<0.05) and hospital length of stay (6.4 versus 6.1 days; P<0.05). Operative mortality was greatest in morbidly obese patients (4.6%).
Table 3.
Outcomes by BMI Strata
| Outcome | Normal/Overweight (BMI 18.5–30) n=8051 | Obese (BMI 30–40) n=4797 | Morbidly Obese (BMI >40) n=789 |
|---|---|---|---|
| Stroke | 137 (1.7%) | 67 (1.8%) | 14 (1.8%) |
| Renal failure | 249 (3.1%) | 206 (4.3%)a | 54 (6.8%)a |
| w/Hemodialysis | 115 (1.5%) | 98 (2.0%)a | 28 (3.5%)a |
| DSWI | 16 (0.2%) | 19 (0.4%)a | 10 (1.3%) a |
| MI | 228 (2.9%) | 112 (2.3%) | 36 (4.6%) |
| AF | 1769 (22.3%) | 1090 (22.7%) | 193 (24.5%) |
| Prolonged ventilation | 843 (10.6%) | 584 (12.2%)a | 133 (16.9%)a |
| Pneumonia | 241 (3.0%) | 149 (3.1%) | 34 (4.3%)a |
| Composite major morbidity | 1197 (15%) | 809 (16.9%)a | 175 (22.2%)a |
| ICU LOS | 46 (25, 76) | 47 (25, 80) | 50 (27, 98)a |
| Hospital LOS in days | 6.1 (4, 8) | 6.4 (4, 8)a | 6.4 (5, 9)a |
| Mortality | 228 (2.9%) | 112 (2.3%) | 36 (4.6%)a |
| Total hospital cost ($) | 35 866 (28 341, 48 117) | 36 209 (27 846, 48 353) | 39 684a (30 378, 54 910) |
Composite major morbidity includes stroke, renal failure, DSWI, MI, AF, prolonged ventilation, pneumonia. All continuous variables are presented as median and 25% and 75% percentiles. AF indicates atrial fibrillation; BMI, body mass index; DSWI, deep sternal wound infection; ICU LOS, intensive care unit length of stay; MI, myocardial infarction.
P<0.05 compared to normal.
Risk‐Adjusted Outcomes
Risk‐adjusted morbidity and mortality across BMI utilizing the cubic spline modeling are demonstrated in Figures 1 and 2. The risk‐adjusted probability of major morbidity increased with increasing BMI in monotonic fashion, indicating that BMI is an independent risk factor for increased morbidity. Similarly, the probability of mortality overall increased with increasing BMI; however, the relationship was nonmonotonic, as mortality did not consistently increase with increasing BMI. There was a slight decrease in operative mortality probability in overweight patients, but operative mortality probability began to increase with BMI greater than 30. Morbidly obese patients’ risk‐adjusted odds ratio for mortality was 1.57 (P=0.02) compared to normal weight patients.
Figure 1.
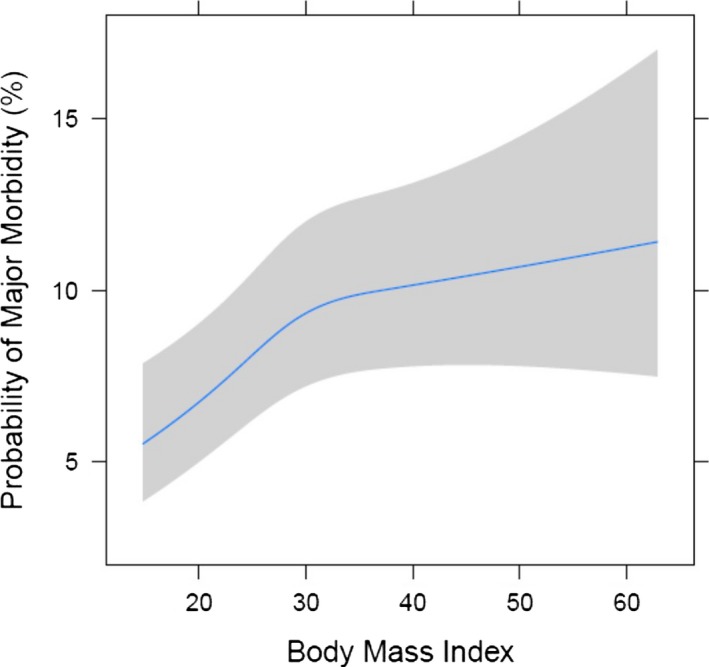
Risk‐adjusted major morbidity by BMI (body mass index).
Figure 2.
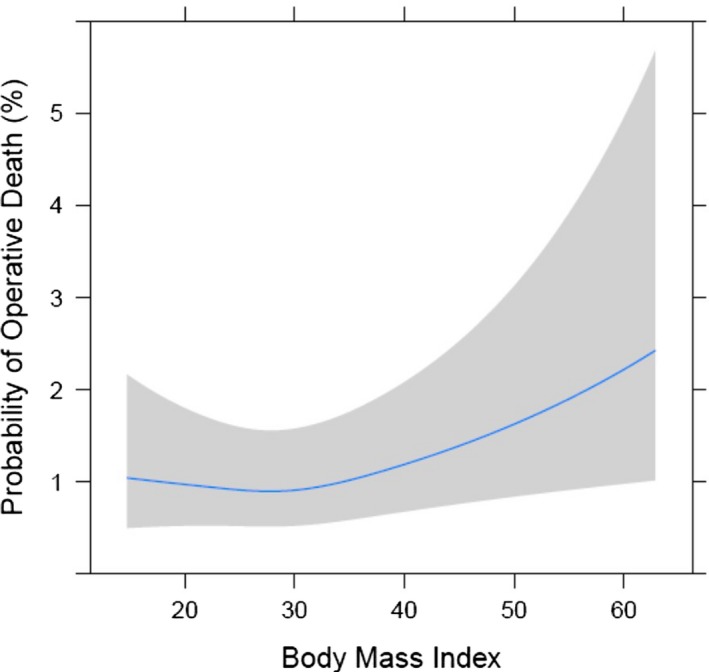
Risk‐adjusted mortality by BMI (body mass index).
Cost
Total hospital cost for each BMI strata are shown in Table 3. Morbidly obese patients had higher median total hospital costs compared to normal weight patients ($39 684 versus $35 866). The relationship of BMI to total hospital cost is shown in Figure 3. After risk adjustment, total hospital cost has an increasing relationship with BMI. By utilizing the slope of the relationship of BMI and cost, we observed a $426 increase in total cost of hospitalization for each unit increase in BMI.
Figure 3.
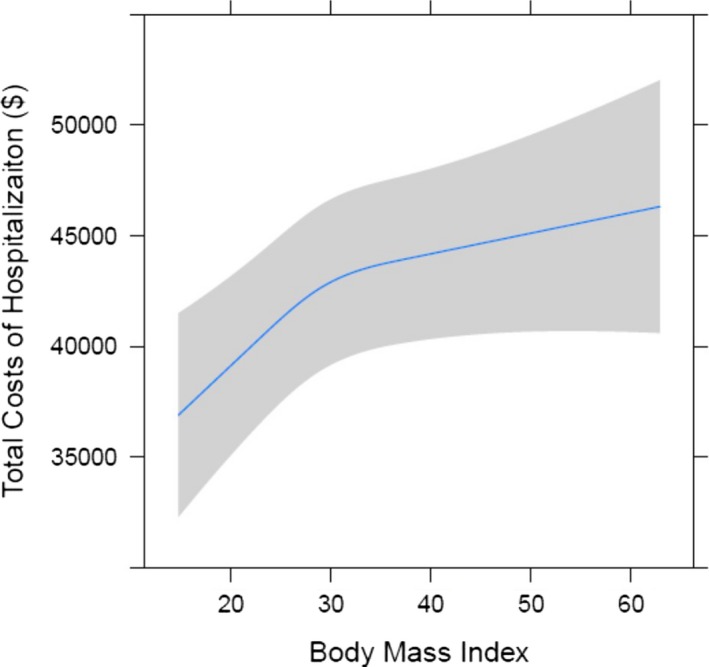
Total hospital cost by BMI (body mass index).
Discussion
Obese and morbidly obese patients frequently undergo cardiac surgery. In a contemporary multi‐institutional regional cohort of 13 637 patients, we found that after adjusting for preoperative risk, higher BMI adversely affects outcomes and resource utilization following cardiac surgery. Although higher BMI patients had more preoperative comorbidities, including DM, hypertension, and CHF, the STS PROM was lower in obese patients and equivalent in morbidly obese patients to normal weight patients in part due to their younger age. Obese and morbidly obese patients, however, incurred greater morbidity, including prolonged ventilation, pneumonia, DSWI, and renal failure. Consequently, higher BMI was associated with longer intensive care unit and hospital lengths of stay and higher hospital costs. After adjusting for risk, a linear relationship of BMI to major morbidity was observed (Figure 1). Although obese patients had similar observed mortality to normal weight patients, risk‐adjusted mortality increased with a BMI ≥30 (Figure 2). After risk adjustment, morbidly obese patients had 1.57 increased odds for mortality compared to normal weight patients. In addition, risk‐adjusted total hospital cost was 17.2% higher ($36 584 for BMI 20 versus $42 866 for BMI 40; P<0.05) in morbidly obese patients. After adjusting for risk, a significant association between BMI and total hospital cost was identified (Figure 3).
Few studies have evaluated outcomes and cost of cardiac surgery in the obese and morbidly obese patient population. In a study of the STS database from 1997 to 2000, Prabhakar and colleagues found that moderate obesity (BMI >35) and morbid obesity (BMI >40) had 1.21 and 1.58 odds of higher operative mortality after CABG.7 A number of studies, however, have demonstrated an “obesity paradox,” indicating a similar or lower mortality in obese patients compared to normal weight patients.10, 11, 19 The contradictory findings in many prior studies may be secondary to differences in patient cohorts, risk stratification, and arbitrary BMI categorization. In this study, we evaluate a large multi‐institutional cohort of patients and utilize the STS PROM to risk stratify patients. The STS PROM is the most widely utilized and validated model of risk stratification for cardiac surgery in the United States. BMI categorization may bias results as there are variations in categorization schemes and inherent statistical inferences may be invalid, as BMI is a continuous variable. Filardo and colleagues found that BMI categorizations significantly affected results in prior studies of CABG and AVR and suggest that modeling BMI as a continuous variable is a more appropriate statistical technique to evaluate the relationship of BMI on outcomes.12, 20 In this study, we utilized a cubic spline analysis to evaluate the association of BMI to risk‐adjusted outcomes and cost. Using these techniques, there is a clear association of increased operative mortality with increasing BMI, refuting the “obesity paradox” for operative outcomes.
Although this study is focused on operative outcomes, BMI clearly affects long‐term outcomes. In a large study of 1.46 million white adults in America, Berrington de Gonzalez and colleagues showed that obesity is associated with greater all‐cause mortality, with lowest mortality in people with normal BMI.5 Obesity is implicated in the pathogenesis of cardiovascular disease, including development of hypertension, CHF, DM, coronary artery disease, and peripheral vascular disease. In Framingham Heart Study participants, for every 1‐unit increase in BMI, the risk of development of CHF increased by 5%.21 Although obesity increases risk for development of cardiovascular disease, there are several studies that show that obese patients have a more favorable prognosis and improved survival compared with normal weight individuals.10, 19 A number of theories have been proposed to explain this finding, including cytokine and neuroendocrine profiles, renin–angiotensin responses, and differences in the pathogenesis of cardiovascular in obese versus nonobese patients.22 Thus, the “obesity paradox” may exist in longer‐term outcomes following cardiac surgery, such that overweight and obese patients may indeed have improved survival, but patients at the extreme ends of weight appear to have worse survival.
Our study shows that increased BMI significantly increases probability of major postoperative morbidity, specifically DSWI, acute renal failure, prolonged ventilation, and pneumonia. Mediastinitis after cardiac surgery is reported in 0.9 to 1.3% of patients, but carries high mortality.23, 24, 25, 26 Our study showed that morbidly obese patients had a nearly 6.5‐fold increase in DSWI rates compared to normal weight patients. Because they are at increased risk for sternal dehiscence, additional attention to sternal closure is warranted.27 In addition, the use of wound vacuum assisted closure to manage edema and incisional drainage may be helpful to reduce DSWI in morbidly obese patients.28, 29 Prior studies have also found an increased risk of acute kidney injury in obese patients following cardiac surgery.30, 31 The etiology of postoperative acute kidney injury in obesity is unclear and may be secondary to chronic inflammation, alterations of the renin–angiotensin–aldosterone system, and the increased incidence of comorbidities such as hypertension and DM.32, 33, 34 Our study also showed that morbidly obese patients have a 2.0‐fold increase in acute kidney injury compared with normal weight patients. Further understanding of the molecular basis to find the association is critical to design preventive strategies. We prefer that an aggressive hemodynamic optimization by volume expansion be done in obese and morbidly obese patients undergoing cardiac surgery.35 Obese patients are at increased risk of prolonged ventilation and pneumonia.36 Obese patients are considered more likely to have a low respiratory reserve with a ventilation/perfusion mismatch and decreased functional residual capacity. Impaired respiratory function could be attributable to slow release of anesthetic agents stored in fatty tissues into the bloodstream. Several perioperative strategies were considered to be effective in preventing pneumonia in obese patients undergoing cardiac surgery. Perioperative physiotherapy was found to decrease the prevalence of postoperative pneumonia in high‐risk patients and is strongly recommended by some studies.37, 38 Use of anesthetic agents that are more rapidly eliminated may reduce length of respiratory support duration and can prevent development of ventilator‐associated pneumonia in obese patients.39
In this study, obesity was a protective factor for bleeding, as obese patients were less likely to receive blood products. Similar findings have been reported in prior studies.40 Obese individuals have abundant mediastinal fat and large abdominal pressure, which may lead to increased intrathoracic pressure that compresses sites of minor bleeding. In addition, less hemodilution in obese patients may also contribute to lower risk of postoperative bleeding. Thus, obese patients have significantly lower risk of surgical reintervention because of bleeding than nonobese patients.
Prior studies have shown higher mortality, composite morbidity, and increased cost of hospitalization in underweight patients (BMI <18.5).11, 19 Reeves and colleagues found that in 4372 patients undergoing CABG, underweight patients have a 4 times greater risk of death or complication than normal weight patients.11 Similarly, Johnson and colleagues found a 1.35 times increased risk of death in the underweight population following cardiac surgery.19 Our study population had very few underweight patients (N=127; 0.9% of the total cohort), making statistical inference problematic. In this small cohort of underweight patients, we also observed increased morbidity (26.6% versus 15%; P<0.05), operative mortality (5.5% versus 2.9%; P<0.05), and cost ($44 344 versus $35 866; P<0.05). Thus, underweight patients, like obese patients, have increased operative morbidity and mortality.
To our knowledge, no prior study has evaluated the impact of BMI on cost of cardiac surgery. St Julien and colleagues studied the association between the BMI and cost of operation in 19 337 undergoing lobectomy for primary lung cancer.41 They found that increase in BMI is associated with increased total operating room time, regardless of institutional experience with obese patients. In general, obese patients tend to have a longer operation time and higher resource utilization. Hawn and colleagues found that in 1375 patients undergoing cholecystectomy, unilateral mastectomy, and colectomy, obese patients have a significantly longer operation time.42 Similarly, Kremers and colleagues found that obesity is associated with longer hospital stays and higher cost following orthopedic surgery.43 The effect of cost appeared to be independent of obesity‐related comorbid conditions and complications. Our study showed that there was an increase in total hospital cost and increased healthcare resource allocation necessary to treat morbidly obese patients who are candidates for cardiac surgery. Utilizing the cubic spline analysis, we found that there is a $426 increase in total cost of hospitalization for each unit increase in BMI. This additional cost may be accounted for by increased operative time, anesthesia costs, length of stay, and cost to care for pulmonary, renal, and DSWI complications. LaPar and colleagues found that after adjusting for preoperative risk, postoperative complications of pneumonia and DSWI increase total hospital costs by $50 025 and $56 003, respectively, following CABG.18
This study has several important limitations. The reported results are limited to a description of the observed associations between postoperative complications and hospital resource utilization and costs without assessing a direct cause‐and‐effect relationship. In addition, all analyses were limited to short‐term operative outcomes, and intermediate or long‐term follow‐up data were not available. In this study, we utilized the STS PROM and well‐known cardiovascular comorbidities for risk adjustment. The STS PROM is the most widely utilized and validated risk measure in cardiac surgery. The potential impact of unmeasured confounders not included in the STS PROM (such as pulmonary hypertension or liver dysfunction) could have affected model performance. Many of these confounders are not captured in the VCSQI or STS database, which may bias our results. In addition, this study included only patients who underwent operations with defined STS PROM scores and may not be fully descriptive of patients who undergo cardiac operations without STS risk scores (such as tricuspid valve or aortic surgery). Determination of cost remains a challenge in health care. We utilized individual hospital cost–charge ratios to estimate total in‐hospital costs for each patient and have found this methodology to be the best estimate of the costs associated with the delivery of cardiac surgical care in the Commonwealth of Virginia. Cost data were limited to total in‐hospital costs. Additional factors, such as rehabilitation cost and home health care, were not included in the cost analysis. Including these healthcare costs may have increased overall cost in the morbidly obese patient population. In addition, cost data were limited to total cost, so individual drivers of increased cost (ie, operative cost, intensive care unit care, etc) could not be evaluated.
Conclusions
This study found that higher BMI is associated with increased mortality, major morbidity, and cost for hospital care. As such, BMI should be more heavily considered in risk assessment. Prior studies that have identified an “obesity paradox” with better operative outcomes in obese patients may have been biased by sample size, lack of risk adjustment, and arbitrary BMI stratification. Additional measures to prevent DSWI, prolonged ventilation, and renal failure in the morbidly obese should be further evaluated. Finally, these data should be considered when advocating for greater reimbursement for cardiac surgery in the morbidly obese.
Disclosures
Ailawadi discloses consulting fees from Abbot Vascular, Mitralign, Edwards Lifesciences, and St. Jude Medical.
(J Am Heart Assoc. 2017;6:e003831. DOI: 10.1161/JAHA.116.003831.)
References
- 1. McLellan F. Obesity rising to alarming levels around the world. Lancet. 2002;359:1412. [DOI] [PubMed] [Google Scholar]
- 2. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard‐Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. [DOI] [PubMed] [Google Scholar]
- 3. Chen Z, Yang G, Offer A, Zhou M, Smith M, Peto R, Ge H, Yang L, Whitlock G. Body mass index and mortality in China: a 15‐year prospective study of 220 000 men. Int J Epidemiol. 2012;41:472–481. [DOI] [PubMed] [Google Scholar]
- 4. Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, He J, Gupta PC, Ramadas K, Tsugane S, Irie F, Tamakoshi A, Gao YT, Wang R, Shu XO, Tsuji I, Kuriyama S, Tanaka H, Satoh H, Chen CJ, Yuan JM, Yoo KY, Ahsan H, Pan WH, Gu D, Pednekar MS, Sauvaget C, Sasazuki S, Sairenchi T, Yang G, Xiang YB, Nagai M, Suzuki T, Nishino Y, You SL, Koh WP, Park SK, Chen Y, Shen CY, Thornquist M, Feng Z, Kang D, Boffetta P, Potter JD. Association between body‐mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton‐Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman‐Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch‐Jacquotte A, Willett WC, Thun MJ. Body‐mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alnasser SM, Huang W, Gore JM, Steg PG, Eagle KA, Anderson FA Jr, Fox KA, Gurfinkel E, Brieger D, Klein W, van de Werf F, Avezum A, Montalescot G, Gulba DC, Budaj A, Lopez‐Sendon J, Granger CB, Kennelly BM, Goldberg RJ, Fleming E, Goodman SG; Investigators G . Late consequences of acute coronary syndromes: global registry of acute coronary events (GRACE) follow‐up. Am J Med. 2015;128:766–775. [DOI] [PubMed] [Google Scholar]
- 7. Prabhakar G, Haan CK, Peterson ED, Coombs LP, Cruzzavala JL, Murray GF. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons’ database. Ann Thorac Surg. 2002;74:1125–1130; discussion 1130‐1131 [DOI] [PubMed] [Google Scholar]
- 8. Edwards FH, Carey JS, Grover FL, Bero JW, Hartz RS. Impact of gender on coronary bypass operative mortality. Ann Thorac Surg. 1998;66:125–131. [DOI] [PubMed] [Google Scholar]
- 9. Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Effects of obesity and small body size on operative and long‐term outcomes of coronary artery bypass surgery: a propensity‐matched analysis. Ann Thorac Surg. 2005;79:1976–1986. [DOI] [PubMed] [Google Scholar]
- 10. Stamou SC, Nussbaum M, Stiegel RM, Reames MK, Skipper ER, Robicsek F, Lobdell KW. Effect of body mass index on outcomes after cardiac surgery: is there an obesity paradox? Ann Thorac Surg. 2011;91:42–47. [DOI] [PubMed] [Google Scholar]
- 11. Reeves BC, Ascione R, Chamberlain MH, Angelini GD. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2003;42:668–676. [DOI] [PubMed] [Google Scholar]
- 12. Filardo G, Hamilton C, Hamman B, Ng HK, Grayburn P. Categorizing BMI may lead to biased results in studies investigating in‐hospital mortality after isolated CABG. J Clin Epidemiol. 2007;60:1132–1139. [DOI] [PubMed] [Google Scholar]
- 13. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer‐and service‐specific estimates. Health Aff. 2009;28:w822–w831. [DOI] [PubMed] [Google Scholar]
- 14. LaPar DJ, Crosby IK, Ailawadi G, Ad N, Choi E, Spiess BD, Rich JB, Kasirajan V, Fonner E Jr, Kron IL, Speir AM; Investigators for the Virginia Cardiac Surgery Quality I . Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:796–803; discussion 803‐804 [DOI] [PubMed] [Google Scholar]
- 15. Organization WH . WHO global database on body mass index (BMI). An interactive surveillance tool for monitoring nutrition transition. 2006.
- 16. Speir AM, Kasirajan V, Barnett SD, Fonner E Jr. Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in Virginia. Ann Thorac Surg. 2009;88:40–45; discussion 45‐46 [DOI] [PubMed] [Google Scholar]
- 17. Ghanta RK, Lapar DJ, Kern JA, Kron IL, Speir AM, Fonner E Jr, Quader M, Ailawadi G. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: a real‐world multi‐institutional analysis. J Thorac Cardiovasc Surg. 2015;149:1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaPar DJ, Crosby IK, Rich JB, Fonner E Jr, Kron IL, Ailawadi G, Speir AM; Investigators for Virginia Cardiac Surgery Quality I . A contemporary cost analysis of postoperative morbidity after coronary artery bypass grafting with and without concomitant aortic valve replacement to improve patient quality and cost‐effective care. Ann Thorac Surg. 2013;96:1621–1627. [DOI] [PubMed] [Google Scholar]
- 19. Johnson AP, Parlow JL, Whitehead M, Xu J, Rohland S, Milne B. Body mass index, outcomes, and mortality following cardiac surgery in Ontario, Canada. J Am Heart Assoc. 2015;4:e002140 DOI: 10.1161/JAHA.115.002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filardo G, Hamilton C, Hamman B, Grayburn P. Obesity and stroke after cardiac surgery: the impact of grouping body mass index. Ann Thorac Surg. 2007;84:720–722. [DOI] [PubMed] [Google Scholar]
- 21. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antunes PE, de Oliveira JF, Antunes MJ. Risk‐prediction for postoperative major morbidity in coronary surgery. Eur J Cardiothorac Surg. 2009;35:760–766; discussion 766‐767 [DOI] [PubMed] [Google Scholar]
- 24. Bitkover CY, Gardlund B. Mediastinitis after cardiovascular operations: a case‐control study of risk factors. Ann Thorac Surg. 1998;65:36–40. [DOI] [PubMed] [Google Scholar]
- 25. Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, Stewart RW, Golding LA, Taylor PC. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg. 1990;49:179–186; discussion 186‐177 [DOI] [PubMed] [Google Scholar]
- 26. Birkmeyer NJ, Charlesworth DC, Hernandez F, Leavitt BJ, Marrin CA, Morton JR, Olmstead EM, O'Connor GT. Obesity and risk of adverse outcomes associated with coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1998;97:1689–1694. [DOI] [PubMed] [Google Scholar]
- 27. Molina JE, Lew RS, Hyland KJ. Postoperative sternal dehiscence in obese patients: incidence and prevention. Ann Thorac Surg. 2004;78:912–917; discussion 912‐917 [DOI] [PubMed] [Google Scholar]
- 28. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov. 2009;16:140–146. [DOI] [PubMed] [Google Scholar]
- 29. Dohmen PM, Misfeld M, Borger MA, Mohr FW. Closed incision management with negative pressure wound therapy. Expert Rev Med Devices. 2014;11:395–402. [DOI] [PubMed] [Google Scholar]
- 30. Thourani VH, Keeling WB, Kilgo PD, Puskas JD, Lattouf OM, Chen EP, Guyton RA. The impact of body mass index on morbidity and short‐ and long‐term mortality in cardiac valvular surgery. J Thorac Cardiovasc Surg. 2011;142:1052–1061. [DOI] [PubMed] [Google Scholar]
- 31. O'Sullivan KE, Byrne JS, Hudson A, Murphy AM, Sadlier DM, Hurley JP. The effect of obesity on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:1622–1628. [DOI] [PubMed] [Google Scholar]
- 32. Billings FTt, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. 2012;23:1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhuo JL, Li XC. New insights and perspectives on intrarenal renin‐angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32:1551–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J. 2015;170:895–902. [DOI] [PubMed] [Google Scholar]
- 35. Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10:500–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuduvalli M, Grayson AD, Oo AY, Fabri BM, Rashid A. Risk of morbidity and in‐hospital mortality in obese patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg. 2002;22:787–793. [DOI] [PubMed] [Google Scholar]
- 37. Hulzebos EH, Helders PJ, Favie NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high‐risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296:1851–1857. [DOI] [PubMed] [Google Scholar]
- 38. Zarbock A, Mueller E, Netzer S, Gabriel A, Feindt P, Kindgen‐Milles D. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest. 2009;135:1252–1259. [DOI] [PubMed] [Google Scholar]
- 39. Krdzalic A, Kosjerina A, Jahic E, Rifatbegovic Z, Krdzalic G. Influence of remifentanil/propofol anesthesia on ventilator‐associated pneumonia occurrence after major cardiac surgery. Med Arch. 2013;67:407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim J, Hammar N, Jakobsson K, Luepker RV, McGovern PG, Ivert T. Obesity and the risk of early and late mortality after coronary artery bypass graft surgery. Am Heart J. 2003;146:555–560. [DOI] [PubMed] [Google Scholar]
- 41. St Julien JB, Aldrich MC, Sheng S, Deppen SA, Burfeind WR Jr, Putnam JB, Lambright ES, Nesbitt JC, Grogan EL. Obesity increases operating room time for lobectomy in the Society of Thoracic Surgeons database. Ann Thorac Surg. 2012;94:1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hawn MT, Bian J, Leeth RR, Ritchie G, Allen N, Bland KI, Vickers SM. Impact of obesity on resource utilization for general surgical procedures. Ann Surg. 2005;241:821–826; discussion 826‐828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kremers HM, Visscher SL, Kremers WK, Naessens JM, Lewallen DG. The effect of obesity on direct medical costs in total knee arthroplasty. J Bone Joint Surg Am. 2014;96:718–724. [DOI] [PubMed] [Google Scholar]


