Abstract
Background
Microvascular dysfunction is a marker of early vascular disease that predicts cardiovascular events. Whether metabolically healthy obese individuals have impaired microvascular function remains unclear. The aim of this study was to evaluate the relation of obesity phenotypes stratified by metabolic status to microvascular function.
Methods and Results
We meta‐analyzed aggregate data from 3 large cohorts (Brazilian Longitudinal Study of Adult Health, the Framingham Heart Study, and the Gutenberg Heart Study; n=16 830 participants, age range 19–90, 51.3% men). Regression slopes between cardiovascular risk factors and microvascular function, measured by peripheral arterial tonometry (PAT), were calculated. Individuals were classified as normal‐weight, overweight, or obese by body mass index (BMI) and stratified by healthy or unhealthy metabolic status based on metabolic syndrome using the ATP‐III criteria. Male sex, BMI, and metabolic risk factors were associated with higher baseline pulse amplitude and lower PAT ratio. There was stepwise impairment of vascular measures from normal weight to obesity in both metabolic status strata. Metabolically healthy obese individuals had more impaired vascular function than metabolically healthy normal‐weight individuals (baseline pulse amplitude 6.12±0.02 versus 5.61±0.01; PAT ratio 0.58±0.01 versus 0.76±0.01, all P<0.0001). Metabolically unhealthy obese individuals had more impaired vascular function than metabolically healthy obese individuals (baseline pulse amplitude 6.28±0.01 versus 6.12±0.02; PAT ratio 0.49±0.01 versus 0.58±0.01, all P<0.0001).
Conclusions
Metabolically healthy obese individuals have impaired microvascular function, though the degree of impairment is less marked than in metabolically unhealthy obese individuals. Our findings suggest that obesity is detrimental to vascular health irrespective of metabolic status.
Keywords: body mass index, cardiovascular risk factors, cohort studies, endothelial function, metabolic syndrome
Subject Categories: Vascular Disease, Obesity, Epidemiology, Risk Factors, Endothelium/Vascular Type/Nitric Oxide
Introduction
Obesity is an independent risk factor for all‐cause mortality in the general population, mostly due to cardiovascular disease.1, 2 According to the World Health Organization, the prevalence of obesity has increased more than 100% worldwide since 1980, and the number of obese individuals in 2014 has been estimated to be about 600 million, making obesity a healthcare public health problem worldwide.3 Previous works have shown that obese persons without metabolic disturbances, or metabolically healthy obese, account for around 10% to 51% of obese individuals.4 Whether metabolically healthy obesity is a benign condition in terms of cardiovascular risk is still a matter of controversy.5, 6
Endothelial dysfunction is characterized by decreased nitric oxide bioavailability, resulting in vascular inflammation, vasoconstriction, and thrombosis—a phenotype prone to cardiovascular events.7, 8 Dysfunctional endothelium is an early manifestation of vascular disease and may be mechanistically related to the greater risk of cardiovascular events in obese individuals.9 Microvascular function measured by peripheral arterial tonometry (PAT) in the digits has been established as a measure of endothelial function.10 An impaired PAT response correlates with a greater burden of cardiovascular risk factors11, 12, 13 and predicts cardiovascular events.14, 15 Previous community‐based cohorts of geographically diverse backgrounds have reported the strongest associations of impaired PAT responses to be with male sex and metabolic risk factors, such as obesity, diabetes mellitus, and dyslipidemia.11, 12, 13
By using meta‐analysis of aggregate data from 3 large cohort studies, we aimed to evaluate the relation of body mass index (BMI) to microvascular function, studying individuals stratified by their obesity status and metabolic phenotype. We hypothesized that metabolically healthy obesity has more impaired microvascular function than normal‐weight metabolically healthy individuals.
Subjects and Methods
Study Cohorts
Aggregate data from 3 community‐based cohorts of geographically diverse background that had studied microvascular endothelial function using PAT were included: The Longitudinal Study of Adult Health (ELSA‐Brasil), a study from Brazil, a middle‐income country with racial admixture; The Framingham Heart Study (FHS), a predominantly white American cohort; and The Gutenberg Study (GHS), a European cohort. A brief description of each participating cohort is provided below. More details can be found elsewhere.12, 16, 17, 18 Participants were excluded from the analysis if they had missing data on dependent variables or primary covariates. After applying the exclusion criteria, a total of 16 830 individuals were included across the 3 cohorts. The participating studies comply with the Declaration of Helsinki, were approved by an ethics committee at the individual institutions, and all study participants provided written informed consent.
Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil)
The ELSA‐Brasil is a multicenter cohort of 15 105 civil servants designed to investigate the determinants of cardiovascular diseases and diabetes mellitus.16 Eligibility criteria included active or retired employees of 5 universities and 1 research center, aged 35 to 74 years, who volunteered to participate. This study sample derived from the 3115 participants enrolled at the Minas Gerais Investigation Center, where PAT data acquisition began partway through the baseline visit (2008–2010). From the 1695 participants who were eligible for PAT examinations, 1535 participants had valid data and were included in this analysis.13 Reproducibility of PAT data in the ELSA‐Brasil cohort has been previously published.19
Framingham Heart Study (FHS)
This study sample comprised participants from the FHS Offspring cohort who underwent digital vascular function assessment at the eighth examination cycle (2005–2008, n=2395), and participants from the FHS Third Generation cohort with available PAT data that were acquired starting partway through the first examination cycle (2002–2005, n=1957).20 Therefore, the current analysis included 4325 FHS participants (age range 19–90 years). The design for the Offspring and the Third Generation cohort are described elsewhere.17, 18
Gutenberg Heart Study (GHS)
Between 2007 and 2012, GHS enrolled 15 010 individuals randomly selected from the general population of residents of the area Mainz and Mainz/Bingen. The age range was 35 to 74 years, and enrollment was performed stratified by age decade, sex, and residence (urban and rural). During a 5‐hour visit at the study center, interviews on cardiovascular risk factors, lifestyle, environmental conditions, and socioeconomic status were performed, fasting blood was drawn, and anthropometric and noninvasive cardiovascular measurements were conducted. Medication intake was recorded by self‐report. Because of capacity reasons, vascular function measurements could not be performed in all individuals, resulting in missing data. The final sample included in this analysis is 10 943 GHS participants for analyses not involving the metabolic phenotype for which the sample was further restricted to 10 763 individuals.12
Assessment of Digital Vascular Measures
Digital vascular measures were obtained with an automated device (EndoPAT2000, Itamar Medical, Caesarea, Israel) under comparable conditions in the 3 cohorts.11, 12, 13 PAT probes were placed on each index finger and the cuff was placed 2 cm above the cubital fossa in ELSA and GHS, and on the forearm in FHS. Baseline pulse amplitude was measured and arterial flow was interrupted on 1 side for 5 minutes by inflating the cuff to suprasystolic pressure. Then, the cuff was deflated to induce reactive hyperemia and PAT signal was recorded. The contralateral finger was used to control for systemic changes. Mean baseline pulse amplitude reflects basal peripheral vascular tone, density and compliance, and pulse pressure and was calculated by averaging the mean baseline amplitude values from the control and the occluded arm. PAT ratio is the ratio of postdeflation mean pulse amplitude 90 to 120 s after cuff release to the mean baseline pulse amplitude. This result is divided by the corresponding ratio from the control finger. Mean baseline pulse amplitude and PAT ratio were both transformed to their natural logarithm for analysis because of skewed distributions. As has been previously reported, quality control procedures were included in all 3 cohorts to exclude technically inadequate studies, including for incomplete cuff occlusion.11, 12, 13
Assessment and Definition of Covariates
The 3 cohorts had standardized measurements of anthropometry, blood pressures, heart rate, blood lipids, and glucose, as well as assessment of current smoking, prior cardiovascular disease, and medication use. BMI was calculated as weight in kilograms divided by height in meters squared. More details of the individual study protocols have been previously published.16, 17, 18 To have comparable data across the cohorts, we standardized the covariate definitions. As in the GHS, the period of fasting before assessing blood glucose was variable, diabetes mellitus was defined as individuals with self‐reported diabetes mellitus or treated with a hypoglycemic agent, or who had a fasting blood glucose ≥126 mg/dL if fasting more than 8 hours, or ≥200 mg/dL if fasting less than 8 hours. Hypertension was diagnosed if antihypertensive drugs were taken, or a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm was measured. Prevalent cardiovascular disease was defined as the self‐reported presence of myocardial infarction, revascularization, stroke, or heart failure for ELSA‐Brasil and GHS. For FHS, the presence of prevalent cardiovascular diseases cited above was adjudicated. For the analysis regarding the relation of BMI to PAT measures and the effect modification caused by metabolic phenotype, we classified participants as normal weight if BMI <25 kg/m2, as overweight if BMI was ≥25 and <30 kg/m2, and as obese if BMI ≥30 kg/m2. Underweight individuals (BMI <18.5 kg/m2) were included in the normal weight group given the small number (n=118). Metabolic phenotype was classified as healthy or unhealthy. A metabolically unhealthy phenotype was diagnosed in the presence of any 3 of the following traits, based on the ATP III criteria for metabolic syndrome21: abdominal obesity, defined as a waist circumference in men ≥102 cm (40 in) and in women ≥88 cm (35 in.); triglycerides ≥150 mg/dL (1.7 mmol/L); HDL cholesterol <40 mg/dL (1 mmol/L) in men and <50 mg/dL (1.3 mmol/L) in women; systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or hypertension treatment; and fasting plasma glucose ≥100 mg/dL (if fasting ≥6 hours) or drug treatment for elevated blood glucose. Otherwise, participants were classified as metabolically healthy. In GHS, if fasting <6 hours, participants could not be classified if glucose ≥100 mg/dL and none of the other criteria for metabolic syndrome were satisfied.
Statistical Analysis
Dichotomous variables are expressed as frequencies and continuous variables as mean± SD, unless otherwise noted. Triglycerides were transformed into natural logarithm values to account for their skewed distribution. For the comparison of clinical characteristics across the cohorts, F‐tests were used for continuous and χ2 tests for categorical variables. Heterogeneity in estimates between‐cohorts was evaluated using the Q‐test and I2 statistics.22 To combine estimates across the 3 cohorts, we used DerSimonian and Laird random effects meta‐analysis with inverse variance weighting.23 We combined estimates of the participants' clinical characteristics as well as the regression slopes for the strength of associations of digital vascular measures with age‐ and sex‐adjusted cardiovascular risk factors. To ensure comparable regression slopes, continuous predictors in all cohorts were standardized using pooled standard deviations from across the 3 cohorts.
We then stratified participants according to BMI categories (normal‐weight, overweight, and obese) and metabolic phenotype (healthy and unhealthy) and calculated the age‐ and sex‐adjusted mean baseline pulse amplitude and PAT ratio for each of the 6 subgroups. Using the metabolically healthy normal‐weight group as reference, we compared the weighted mean difference of the other 5 subgroups with a Wald test including Bonferroni adjustment for multiple comparisons. We tested for a linear trend across BMI categories for both metabolically healthy and metabolically unhealthy.
All analyses were performed using SAS version 9.3 (SAS Institute Inc). Two‐sided P<0.05 were considered statistically significant, unless stated otherwise.
Results
Participant Characteristics and Vascular Measures
Table 1 shows the characteristics of participants: the mean age was 53±6 years and 8665 participants (51.3%) were men. The overall mean BMI was 27.1±2.8 kg/m2 and was comparable across cohorts. The mean glucose was higher in ELSA‐Brasil, contributing to a higher prevalence of diabetes mellitus when compared to GHS and FHS. In FHS, there was a higher proportion of participants using lipid‐lowering treatment compared to GHS and ELSA‐Brasil, which may have contributed to the lower means for total/HDL cholesterol and triglycerides in FHS. Smoking was more prevalent in GHS, reflecting the higher prevalence of smoking in Germany than in Brazil or the United States. For the digital vascular function measures, ELSA‐Brasil had higher mean baseline pulse amplitude and lower PAT ratio that may reflect differences in participant characteristics, including the higher burden of metabolic risk factors.
Table 1.
Clinical Characteristics and Digital Vascular Measures of Participants According to Cohort and Overall
| Characteristica | GHS (n=10 943) | FHS (n=4352) | ELSA‐Brasil (n=1535) | Overall (n=16 830) |
|---|---|---|---|---|
| Age, y | 54±11 | 54±16 | 52±9 | 53±6 |
| Sex, male, n (%) | 5639 (52) | 2166 (49) | 860 (56) | 8665 (51) |
| Systolic blood pressure, mm Hg | 131±17 | 123±17 | 122±17 | 125±10 |
| Diastolic blood pressure, mm Hg | 82±9 | 74±10 | 78±11 | 78±6 |
| Heart rate, bpm | 69±11 | 62±10 | 69±10 | 67±6 |
| BMI, kg/m2 | 27.1±4.7 | 27.7±5.5 | 26.8±4.6 | 27.1±2.8 |
| Smoking, n (%) | 2130 (19) | 600 (14) | 184 (12) | 2914 (17) |
| Total/HDL cholesterol | 4.1±1.2 | 3.6±1.2 | 4.1±1.0 | 4.0±0.7 |
| triglycerides, ln(mg/dL)b | 4.7±0.5 | 4.6±0.5 | 4.8±0.5 | 4.7±0.3 |
| Glucose, mg/dL | 94±18 | 102±22 | 111±31 | 99±13 |
| Diabetes mellitus, n (%) | 766 (7) | 393 (9) | 197 (13) | 1356 (8) |
| Hypertension, n (%) | 5314 (49) | 1745 (40) | 576 (38) | 7635 (45) |
| Hypertension treatment, n (%) | 3091 (28) | 1361 (31) | 435 (28) | 4887 (29) |
| Lipid‐lowering treatment, n (%) | 1375 (13) | 1216 (28) | 197 (13) | 2788 (16) |
| Prevalent CVD, n (%) | 596 (5) | 405 (9) | 64 (4) | 1065 (6) |
| PAT ratio | 0.65±0.41 | 0.69±0.41 | 0.49±0.37 | 0.60±0.23 |
| Baseline pulse amplitude | 5.99±0.90 | 5.66±0.89 | 6.00±0.83 | 5.89±0.50 |
Combined estimate for continuous variables using inverse variance weighting. Continuous variables are given as mean±SD. BMI indicates body mass index; CVD, cardiovascular disease; ELSA‐Brasil, Brazilian Longitudinal Study of Adult Health; FHS, Framingham Heart Study; GHS, Gutenberg Heart Study; HDL, high‐density level; PAT, peripheral arterial tonometry.
P<0.005 for comparison of all characteristics between cohorts.
Triglycerides and PAT measures are natural logarithm transformed.
Meta‐Analysis of Clinical Correlates of Digital Vascular Measures
Figure 1 shows the combined estimates and 95% CI of the data pooled from the 3 cohorts regarding the clinical correlates of digital vascular measures. Male sex, prevalent cardiovascular disease, and metabolic risk factors (prevalent diabetes mellitus, and higher mean BMI, glucose, triglycerides, and total/HDL cholesterol) were significantly associated with higher mean baseline pulse amplitude and lower PAT ratio, both denoting more impaired vascular function. Advancing age and smoking were only significantly correlated to higher mean baseline pulse amplitude. Systolic and diastolic blood pressures were not correlated with PAT measures. Higher prevalence of hypertension and lipid‐lowering treatment were associated with more impaired PAT responses. There was substantial heterogeneity among the studies shown by statistically significant Q‐test results and I2 estimates that were mostly greater than 50%.
Figure 1.
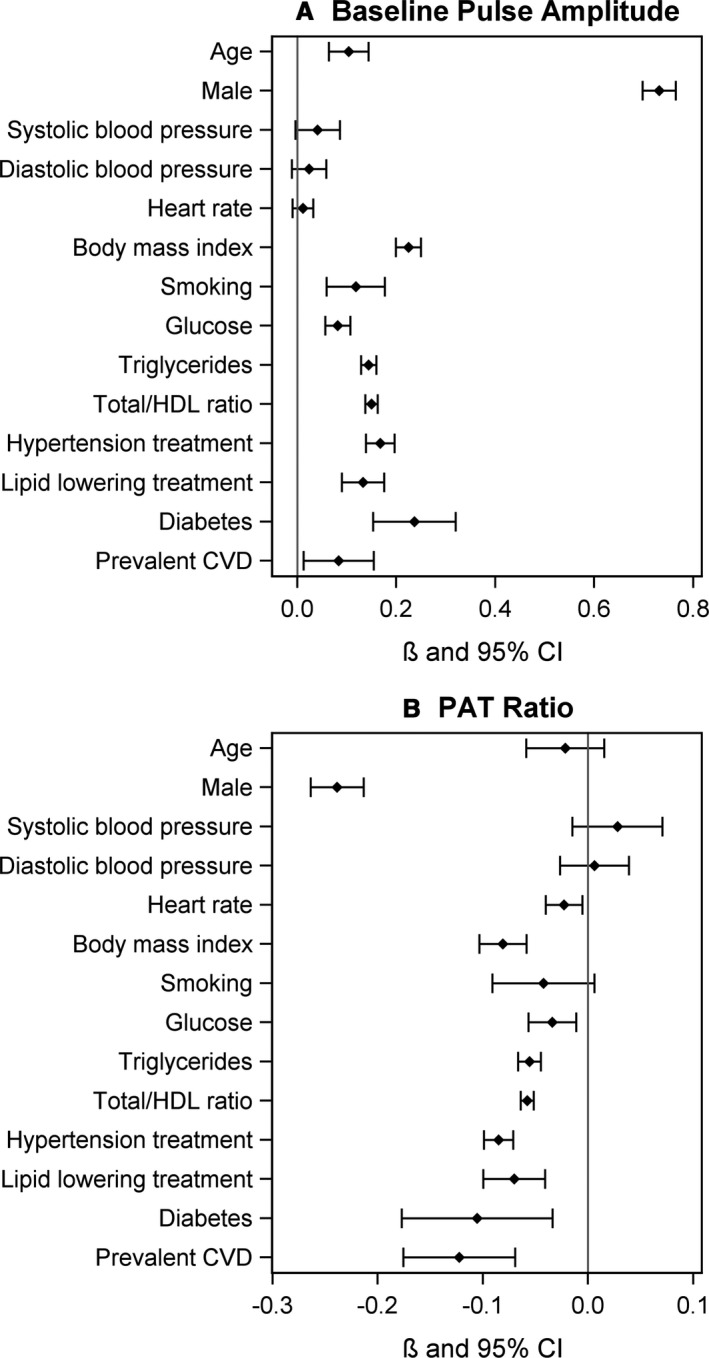
Forest plots of clinical correlates of digital vascular measures across the 3 cohorts. A, Baseline pulse amplitude. B, PAT ratio. Data were age‐ and sex‐adjusted. Per SD on continuous variables. Combined estimate using DerSimonian and Laird random effects model. Q test for heterogeneity. CVD indicates cardiovascular disease; HDL, high‐density cholesterol; PAT, peripheral arterial tonometry.
The values of the combined estimates and the heterogeneity statistics used in Figure 1 can be found in Tables S1 and S2. The relations of clinical correlates to digital vascular function measures in the individual cohorts are shown in Tables S3 and S4.
Relation of Obesity to Digital Vascular Function Stratified by Metabolic Status
Table 2 shows the number of participants according to BMI categories stratified by metabolic status. In the overall sample, 24% of the participants were classified as obese (n=4055) and 40% (n=6792) as overweight. Among the obese individuals, 33% were considered metabolically healthy (n=1348). In the metabolically healthy strata (n=11 233), 48% of the subjects were classified as normal‐weight, 40% overweight, and 12% obese; whereas in the metabolically unhealthy strata, 8% of the individuals were normal‐weight, 42% overweight, and 50% obese.
Table 2.
Number of Participants, Baseline Pulse Amplitude, and PAT Ratio According to BMI Categories Stratified by Metabolic Status, Per Cohort and Overall
| Cohort | Metabolically Healthy | Metabolically Unhealthy | ||||
|---|---|---|---|---|---|---|
| Normal | Overweight | Obese | Normal | Overweight | Obese | |
| Number of participants | ||||||
| ELSA‐Brasil | 472 | 261 | 67 | 126 | 351 | 253 |
| FHS | 1351 | 1064 | 395 | 116 | 668 | 811 |
| GHS | 3564 | 3173 | 886 | 222 | 1275 | 1643 |
| Overall | 5387 | 4498 | 1348 | 464 | 2294 | 2707 |
| Baseline pulse amplitude | ||||||
| ELSA‐Brasil | 5.74 (0.034) | 5.90 (0.046) | 6.17 (0.091) | 6.01 (0.066) | 6.15 (0.040) | 6.36 (0.046) |
| FHS | 5.33 (0.021) | 5.62 (0.022) | 5.70 (0.037) | 5.62 (0.068) | 5.84 (0.029) | 6.09 (0.026) |
| GHS | 5.70 (0.013) | 6.01 (0.013) | 6.24 (0.020) | 5.81 (0.022) | 6.13 (0.018) | 6.35 (0.017) |
| Overall | 5.61 (0.010) | 5.91 (0.011) | 6.12 (0.017) | 5.82 (0.020) | 6.06 (0.014) | 6.28 (0.014) |
| PAT ratio | ||||||
| ELSA‐Brasil | 0.55 (0.016) | 0.51 (0.022) | 0.44 (0.043) | 0.52 (0.031) | 0.45 (0.019) | 0.38 (0.022) |
| FHS | 0.82 (0.011) | 0.72 (0.012) | 0.69 (0.019) | 0.69 (0.035) | 0.61 (0.021) | 0.53 (0.013) |
| GHS | 0.77 (0.006) | 0.65 (0.006) | 0.56 (0.010) | 0.71 (0.011) | 0.59 (0.009) | 0.49 (0.008) |
| Overall | 0.76 (0.005) | 0.66 (0.005) | 0.58 (0.008) | 0.69 (0.010) | 0.57 (0.007) | 0.49 (0.007) |
Data are presented as mean and SE. Data are age‐ and sex‐adjusted. P value for linear trend across BMI categories (within each metabolic level) <0.0001 for both baseline pulse amplitude and PAT ratio in the overall sample. P values for comparison between metabolic levels for each BMI category (normal, overweight, obese) <0.0001 for both baseline pulse amplitude and PAT ratio in the overall sample. BMI indicates body mass index; ELSA‐Brasil, Brazilian Longitudinal Study of Adult Health; FHS, Framingham Heart Study; GHS, Gutenberg Heart Study; PAT, peripheral arterial tonometry.
As shown in Table 2, increasing obesity was associated with more impaired vascular function in both metabolically healthy and metabolically unhealthy participants. Furthermore, the metabolically unhealthy participants had greater impairment of vascular function at each level of obesity.
Figure 2 displays the greater degree of vascular abnormality associated with both greater obesity and with metabolically unhealthy status as compared to the normal‐weight metabolically healthy individuals.
Figure 2.
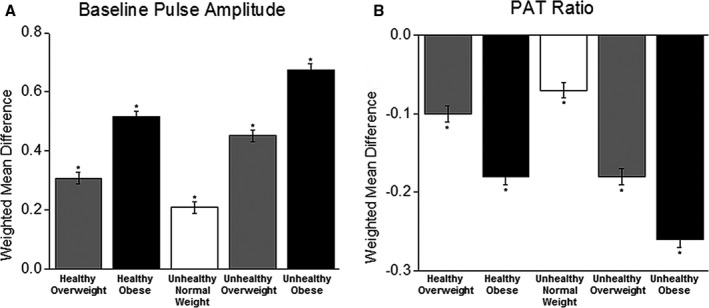
Meta‐analysis of digital vascular measures by metabolic and body mass index categories. A, Baseline pulse amplitude. B, PAT ratio. Data are shown as weighted mean difference compared with metabolically healthy normal‐weight person (reference). Data were age‐ and sex‐adjusted. *P<0.0001 compared to normal weight metabolically healthy. Baseline pulse amplitude and PAT ratio are unitless. PAT indicates peripheral arterial tonometry.
Discussion
In this large sample size study with data from 3 community‐based cohorts of geographically diverse background, we confirmed the associations of impaired PAT measures with obesity and metabolic risk factors. In obese individuals with a metabolically healthy profile, we observed impairment in microvascular function when compared to normal‐weight metabolically healthy subjects. Moreover, comparing individuals within the same BMI category, we found that vascular measures were more impaired in the presence of metabolic abnormalities. Taken together, our findings suggest that increased weight is detrimental to vascular health irrespective of metabolic status, although having metabolic abnormalities is associated with a further decrement in microvascular function.
Prior Studies With Microvascular Function and Cardiovascular Risk Factors
Vascular dysfunction is an early expression of vascular injury, the substrate for the most prevalent cardiovascular events, including myocardial infarction and stroke, and is able to predict cardiovascular outcomes.7 The relations of cardiovascular risk factors with PAT measures herein described are consistent with the findings of prior reports that impaired microvascular function is related to male sex, higher BMI, which is a marker of adiposity, and metabolic risk factors.11, 12, 13 Importantly, we confirmed in a large sample of geographically diverse individuals that blood pressure was not significantly correlated with PAT measures and advancing age was not correlated to PAT ratio. These findings confirm the associations of obesity and metabolic risk factors with small vessel dysfunction across multiple cohorts.12, 20
Obesity, Metabolic Status, and Vascular Function
Obesity is an independent risk factor for cardiovascular disease.1, 2 Recently, there has been considerable interest in a subgroup of obese and overweight subjects who have normal metabolic features, such as lipid profile, glucose tolerance, blood pressure, and waist circumference—the “metabolically healthy” phenotype.24, 25 Previous studies have shown that a metabolically healthy phenotype reduces the impact of excess weight on cardiovascular mortality.5, 6 However, a recent meta‐analysis found an increased risk of death in metabolically healthy overweight and obese when studies with more than 10 years of follow‐up were evaluated, suggesting that prolonged exposure to excess weight, even without metabolic dysfunction, is associated with heightened cardiovascular mortality.24
The relation of metabolically healthy obesity to vascular function has been previously evaluated in a community‐based setting in 1016 participants of a Swedish cohort of 70‐year‐old individuals.26 Studying the microvasculature by venous occlusion plethysmography and the conduit vessel function by flow‐mediated dilation using brachial artery ultrasound, they found impairment in vascular function measured by venous plethysmography, but not in flow‐mediated dilation, in metabolically healthy obese compared to normal‐weight.26 More recently, Schinzari et al evaluated the vasodilator response of metabolically healthy obese (n=34) and found that they have abnormal vascular reactivity when compared to lean subjects, although their endothelial dysfunction is less pronounced than in metabolically unhealthy obese subjects.27
Our results add information to the controversy of whether metabolically healthy obesity is benign in terms of cardiovascular disease. We found that, in a large sample of geographically diverse background, metabolically healthy obese had more impaired microvascular function than their normal‐weight counterparts, suggesting that there is not a benign pattern of increased weight for vascular health. We also found that in the 3 BMI categories (normal‐weight, overweight, and obese), the presence of metabolic disturbances caused further impairment in microvascular function. As vascular dysfunction is an early expression of atherosclerosis,7 its presence may reveal the effects of increased weight in the cardiovascular system at an early stage of vascular injury. Moreover, the vascular dysfunction that results from obesity may contribute to the development of metabolic disturbances.9
The mechanisms by which higher weight leads to vascular dysfunction are not completely elucidated. Higher resting flow in the finger with increasing weight may contribute to increased baseline pulsatility and reduced vasodilator responses. In addition, metabolic alterations including higher insulin levels may alter resting and dynamic tone. The adipose tissue, through histological and functional alterations, acts negatively on the cardiovascular system through the ectopic deposition of fat and by promoting insulin resistance and inflammation.28 Vascular dysfunction is linked to insulin resistance and a pro‐inflammatory state and our data suggest that excess weight has a negative influence on vascular function separately from the effect of metabolic risk factors.9, 29 Furthermore, improvement in vascular function has been described after weight loss, particularly in hyperinsulinemic obese individuals, suggesting that vascular dysfunction may be reversible when obese individuals lose weight.30 More research is needed to elucidate whether vascular dysfunction in obese individuals is only a marker of insulin resistance and inflammation, or if it is independently related to the higher cardiovascular risk of obese individuals, perhaps acting via other pathways that lead to cardiovascular events, such as an impaired coagulation or fibrinolysis.26
It is also important to emphasize 2 other findings of our study. First, even though studying the metabolically healthy obesity is important to better understand the complex interaction between the adipose tissue and the cardiovascular system, the metabolic abnormalities are considerably more prevalent in individuals with increased weight. Similarly to other studies, metabolically healthy obese accounted for one third of obese individuals,5, 26, 31 revealing that most obese persons have metabolic disturbances, and thus are in the subgroup of greater cardiovascular risk, emphasizing the burden of obesity. Secondly, PAT measures of overweight individuals are intermediate between normal‐weight and obese subjects in both metabolic strata, suggesting that the effect of excess weight on microvascular function has a dose–response gradient. Further studies are needed to evaluate whether this latter finding is clinically relevant, especially considering that 1.9 billion persons in the world were classified as overweight in 2014.3
Strengths and Limitations
The strengths of our study include the strategy of combining aggregate data from 3 large and geographically diverse community‐based cohorts with a wide age range, enabling us to study subgroups of participants that we were not powered to evaluate in each cohort separately. The strict and standardized protocols followed by the 3 cohorts for the assessment of vascular function and cardiovascular risk factors also must be acknowledged. Taken together, these aspects allowed us to provide more robust and generalizable findings.
Limitations of our study should also be considered. First, although the protocols for data collection were similar across cohorts, some methodological differences could have impacted the data comparability. To minimize these eventual differences, we made specific definitions for the present study. Regarding PAT examination, the different cuff position—forearm in FHS and upper arm in GHS and ELSA‐Brasil—was, initially, a cause for concern. Prior studies show that the cuff placed in the upper arm causes more vasodilation as a result of reactive hyperemia than in the forearm.32 However, as GHS and ELSA‐Brasil had lower mean PAT ratio than FHS, we believe that the cuff position did not impact the comparability of PAT vasodilator response. Secondly, we studied vascular function in the digits, which is only partly dependent on nitric oxide bioavailability,33 and the evaluation in different vascular beds may provide complementary information.12, 20 However, microvascular function measured by PAT has been shown to be more closely correlated to metabolic risk factors than conduit vessel function and has also been prospectively validated as a predictor of cardiovascular events.12, 14, 15 Third, other variables such as fat distribution and fat mass could add to our interpretation of the relation of adiposity to vascular function.34 Fourth, to address the heterogeneity found in our study for the association of most cardiovascular risk factors to PAT measures, we used the random effects model to meta‐analyze the data, seeking to incorporate heterogeneity into our combined estimates and their confidence intervals. We speculate that the high heterogeneity found could reflect differences among the populations studied, including ethnic composition. We further note that all the risk factors with significant associations with vascular function were consistent in the directionality of their associations across the 3 cohorts, making any distortions due to heterogeneity a matter of the degree of association rather than whether it is present. Lastly, due to the observational design of the cohorts, endothelium‐independent vasodilation was not evaluated and the use of medications could not be precluded. Since our analysis is cross‐sectional, it prevents inferences of causality or temporality.
Clinical Implications
From the clinical point of view, some aspects of our study should be highlighted. Our data corroborate that BMI and metabolic risk factors provide complementary information regarding markers of vascular health. The findings support further studies with prospective outcomes to evaluate the clinical utility of digital vascular function as a tool to refine cardiovascular risk prediction in obese individuals. Reversal of vascular dysfunction in obesity may be possible with both weight loss and treatments directed at metabolic risk factors. Furthermore, treatments targeted at the endothelium may protect the vasculature from the adverse consequences of obesity and metabolic dysfunction. It may be that vascular function assessment has potential to risk stratify obese individuals who may derive the greatest health benefits from weight reduction interventions. Lastly, our results support the recommendations of weight control as a population‐based preventive strategy for cardiovascular disease, since we found no benign pattern of excess weight for vascular health.
Conclusions
Our results suggest that obese individuals without metabolic abnormalities have more impaired microvascular function when compared to their normal‐weight counterparts. Although we emphasize that metabolic disturbances strengthen the association of excess weight to impaired vascular function, our data indicate that increased weight is detrimental to vascular health irrespective of metabolic status, challenging the concept of a healthy pattern of obesity.
Sources of Funding
The ELSA‐Brasil baseline study was supported by the Brazilian Ministries of Health and of Science and Technology, grant 01060278.00MG. This ancillary study was supported by FAPEMIG (Minas Gerais, Brazil). Santander Bank supported the collaboration between Boston University and Universidade Federal de Minas Gerais. Ribeiro and Barreto receive grants for established researchers from CNPq (Brazil). Ribeiro is also supported by “Pesquisador Mineiro” grant from FAPEMIG (Minas Gerais, Brazil). The Framingham Heart Study is funded by NIH/NHLBI contract HHSN268201500001I and N01‐HC 25195. The project was supported by NIH grants HL70100, HL076784, AG028321, HL080124, and the Donald W. Reynolds Foundation. Dr Hamburg is supported by NIH grants HL083781, HL102299, HL81587, HL115391; Dr Benjamin is supported by NIH grants 1R01HL128914, 2R01HL092577, 1P50HL12016. The Gutenberg Health Study is funded through the Government of Rheinland‐Pfalz (“Stiftung Rheinland Pfalz für Innovation,” contract number AZ 961‐386261/733), the research programs “Wissen schafft Zukunft” and “Center for Translational Vascular Biology (CTVB)” of the Johannes Gutenberg‐University of Mainz and its contract with Boehringer Ingelheim and PHILIPS Medical Systems including an unrestricted grant for the Gutenberg Health Study. Dr Wild is funded by the Federal Ministry of Education and Research (BMBF 01EO1503). Drs Münzel and Wild are PI of the German Center for Cardiovascular Research (DZHK). This work was further supported by research grants from the Brandenburg Ministry of Economics, Germany, and the European Regional Development Fund (EFRE/ERDF). The test kits for Copeptin, Ct‐pro‐endothelin‐1, MR‐proADM, and MR‐proANP were provided by B.R.A.H.M.S., Hennigsdorf, and for Nt‐proBNP by Roche Diagnostics, Mannheim at no cost. Dr Schnabel is supported by Deutsche Forschungsgemeinschaft (German Research Foundation) Emmy Noether Program SCHN 1149/3‐1 and Bundesministerium für Bildung und Forschung (BMBF, 01ZX1408A).
Disclosures
The PAT device and measurements were provided to FHS by Itamar Medical Ltd at no cost to the study. Dr Mitchell is owner of Cardiovascular Engineering, Inc, a company that designs and manufactures vascular stiffness measurement devices, and serves as a consultant to Novartis, Servier, Merck, and Philips Healthcare. The remaining authors have no disclosures.
Supporting information
Table S1. Meta‐Analysis of Clinical Correlates of Baseline Pulse Amplitude Adjusted on Age and Sex
Table S2. Meta‐Analysis of Clinical Correlates of PAT Ratio Adjusted on Age and Sex
Table S3. Relations of Baseline Pulse Amplitude and Cardiovascular Risk Factors According to Cohort
Table S4. Relations of PAT Ratio and Cardiovascular Risk Factors According to Cohort
Acknowledgments
The authors thank the staff and participants of Brazilian Longitudinal Study of Adult Health, Framingham Heart Study, and Gutenberg Heart Study for their important contributions.
(J Am Heart Assoc. 2017;6:e004199. DOI: 10.1161/JAHA.116.004199.)
References
- 1. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton‐Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman‐Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch‐Jacquotte A, Willett WC, Thun MJ. Body‐mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA. 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO) . Available at: www.who.int/mediacentre/factsheets/fs311/en/. Accessed November 15, 2015.
- 4. Rey‐Lopez JP, de Rezende LF, Pastor‐Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014;15:781–790. [DOI] [PubMed] [Google Scholar]
- 5. Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring). 2012;20:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, Rogers WJ, Reis SE. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–713. [DOI] [PubMed] [Google Scholar]
- 7. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. [DOI] [PubMed] [Google Scholar]
- 9. Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity‐associated insulin resistance and hypertension. Physiology (Bethesda). 2007;22:252–260. [DOI] [PubMed] [Google Scholar]
- 10. Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T. Noninvasive vascular function measurement in the community: cross‐sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4:371–380. [DOI] [PubMed] [Google Scholar]
- 13. Brant LC, Hamburg NM, Barreto SM, Benjamin EJ, Ribeiro AL. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil) cohort study. J Am Heart Assoc. 2014;3:e001279 doi: 10.1161/JAHA.114.001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 15. Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, Matsubara J, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H, Nagayoshi Y, Yamamuro M, Sakamoto K, Iwashita S, Jinnouchi H, Taguri M, Morita S, Matsui K, Kimura K, Umemura S, Ogawa H. Peripheral endothelial function and cardiovascular events in high‐risk patients. J Am Heart Assoc. 2013;2:e000426 doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aquino EM, Barreto SM, Bensenor IM, Carvalho MS, Chor D, Duncan BB, Lotufo PA, Mill JG, Molina Mdel C, Mota EL, Passos VM, Schmidt MI, Szklo M. Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil): objectives and design. Am J Epidemiol. 2012;175:315–324. [DOI] [PubMed] [Google Scholar]
- 17. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 18. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 19. Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens. 2013;31:1984–1990. [DOI] [PubMed] [Google Scholar]
- 20. Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham Heart Study. Hypertension. 2011;57:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 24. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta‐analysis. Ann Intern Med. 2013;159:758–769. [DOI] [PubMed] [Google Scholar]
- 25. Rey‐Lopez JP, de Rezende LF, de Sa TH, Stamatakis E. Is the metabolically healthy obesity phenotype an irrelevant artifact for public health? Am J Epidemiol. 2015;182:737–741. [DOI] [PubMed] [Google Scholar]
- 26. Lind L, Siegbahn A, Ingelsson E, Sundstrom J, Arnlov J. A detailed cardiovascular characterization of obesity without the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2011;31:e27–e34. [DOI] [PubMed] [Google Scholar]
- 27. Schinzari F, Iantorno M, Campia U, Mores N, Rovella V, Tesauro M, Di Daniele N, Cardillo C. Vasodilator responses and endothelin‐dependent vasoconstriction in metabolically healthy obesity and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2015;309:E787–E792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beleigoli A, Diniz Mde F. Two (or more) sides of a coin. Heart. 2014;100:1399–1401. [DOI] [PubMed] [Google Scholar]
- 29. Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bigornia SJ, Farb MG, Tiwari S, Karki S, Hamburg NM, Vita JA, Hess DT, Lavalley MP, Apovian CM, Gokce N. Insulin status and vascular responses to weight loss in obesity. J Am Coll Cardiol. 2013;62:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. [DOI] [PubMed] [Google Scholar]
- 32. Mannion TC, Vita JA, Keaney JF Jr, Benjamin EJ, Hunter L, Polak JF. Non‐invasive assessment of brachial artery endothelial vasomotor function: the effect of cuff position on level of discomfort and vasomotor responses. Vasc Med. 1998;3:263–267. [DOI] [PubMed] [Google Scholar]
- 33. Nohria A, Gerhard‐Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. [DOI] [PubMed] [Google Scholar]
- 34. Okorodudu DO, Jumean MF, Montori VM, Romero‐Corral A, Somers VK, Erwin PJ, Lopez‐Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta‐analysis. Int J Obes (Lond). 2010;34:791–799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Meta‐Analysis of Clinical Correlates of Baseline Pulse Amplitude Adjusted on Age and Sex
Table S2. Meta‐Analysis of Clinical Correlates of PAT Ratio Adjusted on Age and Sex
Table S3. Relations of Baseline Pulse Amplitude and Cardiovascular Risk Factors According to Cohort
Table S4. Relations of PAT Ratio and Cardiovascular Risk Factors According to Cohort


