Abstract
Background
Rheumatic heart disease (RHD) remains a leading cause of cardiovascular morbidity and mortality in children and young adults in disadvantaged populations. The emergence of echocardiographic screening provides the opportunity for early disease detection and intervention. Using our own multistate model of RHD progression derived from Australian RHD register data, we performed a cost–utility analysis of echocardiographic screening in indigenous Australian children, with the dual aims of informing policy decisions in Australia and providing a model that could be adapted in other countries.
Methods and Results
We simulated the outcomes of 2 screening strategies, assuming that RHD could be detected 1, 2, or 3 years earlier by screening. Outcomes included reductions in heart failure, surgery, mortality, disability‐adjusted life‐years, and corresponding costs. Only a strategy of screening all indigenous 5‐ to 12‐year‐olds in half of their communities in alternate years was found to be cost‐effective (incremental cost‐effectiveness ratio less than AU$50 000 per disability‐adjusted life‐year averted), assuming that RHD can be detected at least 2 years earlier by screening; however, this result was sensitive to a number of assumptions. Additional modeling of improved adherence to secondary prophylaxis alone resulted in dramatic reductions in heart failure, surgery, and death; these outcomes improved even further when combined with screening.
Conclusions
Echocardiographic screening for RHD is cost‐effective in our context, assuming that RHD can be detected ≥2 years earlier by screening. Our model can be adapted to any other setting but will require local data or acceptable assumptions for model parameters.
Keywords: cost‐effectiveness, echocardiography, pediatrics, rheumatic heart disease, screening
Subject Categories: Rheumatic Heart Disease, Cost-Effectiveness, Echocardiography, Pediatrics, Valvular Heart Disease
Introduction
Rheumatic heart disease (RHD) has long been a target of public health screening, and the World Health Organization (WHO) continues to recommend screening as a component of RHD control in high‐prevalence areas.1 Over the past decade, many groups around the world have undertaken population‐based screening for RHD using portable echocardiography, demonstrating its feasibility in different contexts.2 To date, the role of screening has primarily been to define disease burden, and enthusiasm for scaling up screening programs has been tempered by concerns about potential costs and the unclear significance of subclinical echocardiographic abnormalities.3, 4 The publication of the World Heart Federation (WHF) criteria for the echocardiographic diagnosis of RHD in 20125 has provided standardization and improved specificity, and the utility of these criteria in the screening context is now widely recognized by the cardiology community.2
In this light, RHD is moving closer to fulfilling the criteria for a disease suitable for screening.4 It has been accepted previously that in high‐prevalence regions, there is an obvious disease burden, there is treatment available (4‐week regimen of benzathine penicillin G [BPG]), and treatment at an early stage of disease improves outcome (BPG prevents recurrences of acute rheumatic fever (ARF), which is known to worsen RHD). There is also a “latent” stage that can be detected (by echocardiography using WHF criteria), and although conjecture remains about the natural history of WHF's “borderline” category,6 there is an evolving consensus that screen‐detected “definite RHD” represents true disease and is an indication for BPG prophylaxis.2
Economic evaluations provide important information about proposed public health interventions. Given that a systematic, large‐scale, echocardiographic screening program for RHD has not yet been instigated, mathematical modeling is required to evaluate the possible economic and health outcomes. Two previous groups have published cost–utility analyses of RHD screening in a hypothetical cohort of children.7, 8 Zachariah et al set their analysis in the Northern Territory (NT) of Australia, but there were a number of questionable assumptions about health care delivery in remote Australia and potential inaccuracies in the proposed costs. Both analyses were limited by the lack of an accepted model of RHD progression. To date, there has not been a comprehensive economic evaluation of RHD echocardiographic screening based on an accepted model of RHD progression and real‐life screening data.
We recently prepared a multistate model of RHD progression in indigenous Australians, based on serial clinical data from a contemporary cohort of 591 indigenous patients aged 5 to 24 years.9 Our analysis demonstrated a bleak prognosis for young people diagnosed with severe RHD and highlighted the need for earlier detection and treatment—an opportunity afforded by echocardiographic screening. We believe that our model can accurately predict the trajectory of RHD in our population, and in this study we use this model, together with data from our screening study in indigenous Australian children,10 to estimate the cost‐effectiveness of a proposed echocardiographic screening program compared with current practice. Our aims were to inform health policy in Australia, to identify the major drivers of cost for RHD screening programs, and to provide a model that could be relatively easily adapted to other settings, including low‐resource settings.
Methods
Population, Setting, and Current Practice
Geographic context
This economic evaluation is set in the NT of Australia, a vast area of 1.35 million square kilometers, with a population density of 0.2 person per square kilometer. The estimated indigenous population is 69 000 (representing nearly 30% of the NT population), of whom ≈80% live in rural or remote locations.11 Although most remote communities have a primary care clinic, the provision of health care to a small population dispersed over a large remote area poses challenges, including limited availability of general practitioners, minimal access to medical specialists, and high travel costs to access these services at a regional center.
The NT Department of Health identifies ≈80 remote communities in which it currently conducts health‐screening activities among children.12 The all‐age populations of the communities range between 100 and 3000, and >95% of residents are indigenous. Using 2010 data from the NT Department of Health gains planning, it is estimated that ≈10 000 indigenous children aged 5 to 14 years live in rural or remote communities of the NT.
Target population
The peak incidence of ARF is in school‐aged children (aged 5–14 years), and this group has been targeted in most international echocardiographic screening surveys to date. In our own echocardiographic screening study, the mean age of children detected with definite RHD (as defined by the WHF5) was 10.4 years (SD 2.5 years), and the peak prevalence was 23.5 per 1000 in 12‐year‐olds.10 Although some groups have identified a role for screening in older age groups,13 poor high school attendance would make this virtually impossible in our context. Furthermore, it is hoped that if there were an effective school screening program, children would be identified before late adolescence.
A recent audit of the NT RHD register confirmed that RHD incidence was nearly as high as ARF incidence in children aged 5 to 14 years (194 per 100 000),14 highlighting that RHD onset is also observed in childhood. Reviewing the data used for our own disease‐progression model (Figure S1), we determined that there was an average of 27.6 new cases of RHD per year in indigenous children aged 5 to 15 years in the NT (we included 15‐year‐olds for reasons outlined in Sensitivity and specificity of echo).
Current practice: clinical diagnosis of RHD
Children with RHD in the NT are currently identified in 2 ways. Most commonly, they present symptomatically with ARF (or occasionally RHD) at their local primary care clinic. Consistent with recommendations in the Australian ARF/RHD guidelines,15 most suspected cases (we estimate 90%, based on our experience) are transferred to one of 2 NT referral hospitals and have a full assessment including echocardiography to confirm acute carditis or chronic RHD. Alternatively, a cardiac murmur is opportunistically detected by auscultation during routine physical examination or school screening (which currently continues, although we have recently reported that this is not a useful approach16), and children are referred for an outpatient diagnostic echocardiogram.
Proposal: Echocardiographic Screening for RHD
In this study, we evaluate 2 echocardiographic screening strategies (Table 1) that are the product of consultation with local stakeholders and experts and that incorporated data and insights gained from undertaking our own screening study in the NT.17 The first (“Echo A”) is to visit all 80 remote NT communities on an annual basis and to screen only 8‐ and 12‐year‐olds (an estimated 2000 children per year). The second (“Echo B”) reduces annual travel by visiting half of the communities in alternate years and screening all children aged 5 to 12 years inclusive (an estimated 4000 children per year). We estimate that both strategies would require the staffing equivalent of 1 full‐time echocardiographer, 1 full‐time nurse, and a pediatric cardiologist for 4 hours per week.
Table 1.
Definitions Used in This Paper
| Activity/Hypothesis | Label | Description |
|---|---|---|
| Screening strategy | Echo A | Screen all indigenous children aged 8 and 12 years living in 80 rural/remote communities of the NT annually |
| Echo B | Screen all indigenous children aged 5–12 years in approximately half (40) of the rural/remote NT communities in alternate years | |
| Screening effectiveness hypothesis | Scenario 1 | Assumes that screening diagnoses RHD 1 year earlier than current practice |
| Scenario 2 | Assumes that screening diagnoses RHD 2 years earlier than current practice | |
| Scenario 3 | Assumes that screening diagnoses RHD 3 years earlier than current practice |
NT indicates Northern Territory; RHD, rheumatic heart disease.
Although we resourced Echo A and Echo B to screen the estimated number of age‐eligible children, we assumed baseline screening attendance of 75%, which approximates the average school attendance of indigenous children in the NT.18
We evaluated a number of alternative approaches based around these strategies, such as increasing the number of children screened (eg, including screening children aged 8, 10, and 12 years every year in every community). However, these alternatives did not alter our main conclusions, and we found the above 2 strategies were the most feasible.
In both strategies, a screening echocardiogram would be performed by a cardiac sonographer on a portable machine (Vivid i [GE Healthcare], or equivalent) in the community. A screen would be considered positive if there were structural and/or functional changes of the left‐sided heart valves that might meet the WHF criteria for RHD. (Although it is anticipated that a number of congenital anomalies would also be detected by screening,10 evaluating the costs and benefits of earlier detection of this group is beyond the scope of this analysis.)
Positive screens would be reviewed off‐site by the program's pediatric cardiologist, who would determine whether a cardiology consultation and a more detailed diagnostic echocardiogram were required. This follow‐up may be possible during routine cardiology outreach clinics to certain communities but more commonly would require the child to travel to a regional hospital. Because of uncertainty about the significance of borderline RHD, only children diagnosed with definite RHD would begin a 4‐week regimen of BPG prophylaxis.2
Sensitivity and specificity of echo
Estimating the sensitivity and specificity of echocardiographic screening for RHD is difficult, given that there is currently no alternative gold standard diagnostic test. There is an evolving consensus, however, that the 2012 WHF criteria5 should be used to make an echocardiographic diagnosis of RHD, and thus we assumed that diagnoses of definite RHD made on screening echocardiography using these criteria would be 100% sensitive. Because there was no way of knowing how many true positives would be missed by this approach, we varied the test sensitivity to 95% in the sensitivity analysis (Table 3, Figure 2).
Table 3.
Costs and Parameter Estimates for Sensitivity Analysis
| Parameter | Baseline | Minimum–Maximum | Distribution | Reference |
|---|---|---|---|---|
| Cost, RHD management | ||||
| Per‐episode costs | ||||
| ARF/RHD outpatient diagnosis | $1428 | $428–$4564 | Triangular | Table S1 |
| ARF/RHD hospital admission | $11 471 | $8661–$30 200 | Dirichleta | Table S2 |
| RHD surgery | $88 126 | $46 503–$138 749 | Triangular | Table S3 |
| Annual costs (outpatient management) | Table S4 | |||
| Inactive | $198 | $164–$231 | Triangular | |
| Mild RHD | $2567 | $1676–$4233 | Triangular | |
| Moderate RHD | $3267 | $1843–$6534 | Triangular | |
| Severe RHD | $4732 | $3368–$11 976 | Triangular | |
| Severe‐surgery RHD | $4732 | $3368–$13 809 | Triangular | |
| Cost—RHD screening | Table S5 | |||
| Annual costs | ||||
| Equipmentb | $37 045 | Not varied | ··· | |
| Admin and consumables | $5500 | $4500–$6500 | Triangular | |
| Staff salaries | $259 000 | $216 100–$297 000 | Triangular | |
| Travel (Echo A) | $221 270 | $156 524–$270 782 | Triangular | |
| Travel (Echo B) | $136 399 | $89 274–$206 115 | Triangular | |
| Per‐episode costs | ||||
| Cardiology follow‐up (per child) | $1260 | $260–$2324 | Triangular | |
| Other parameter estimates | ||||
| Discount factor | 5% | 3–7% | Uniform | PBAC22 |
| Incidence of RHD (cases per year) | 27.6 | 22.1–33.2 | Uniform | Figure S1 |
| Health state transition probabilities | Bootstrap | Cannon et al9 | ||
| Disability weights | Table 2 | |||
| Mild | 0.031 | 0.017–0.050 | Triangular | |
| Moderate | 0.037 | 0.021–0.058 | Triangular | |
| Severe (no surgery) | 0.186 | 0.128–0.261 | Triangular | |
| Severe after surgery | 0.070 | 0.044–0.102 | Triangular | |
| Screening parameters | ||||
| Screened, % | 75% | 50–100% | Uniform | Assumed |
| Sensitivity of echo | 100% | 95–100% | Uniform | Assumed |
| Diagnosed mild, % | 80% | 65–95% | Dirichletc | Assumed |
| Diagnosed moderate, % | 15% | 5–25% | Dirichletc | Assumed |
| Diagnosed severe, % | 5% | 0–10% | Dirichletc | Assumed |
| Cardiology follow‐up, % | 2.5% | 2–5% | Uniform | Assumed |
All costs are presented in Australian dollars at 2013 price levels. ARF indicates acute rheumatic fever; PBAC, Pharmaceutical Benefits Advisory Council; RHD, rheumatic heart disease.
A Dirichlet distribution was used to sample the proportion of mild (n=10), moderate (n=7), and severe (n=1) cases, based on severity of new cases in our screening study.10
Annuity in advance over 5 years calculated as the upfront cost ($182 720) minus discounted resale price of 10% purchase price.
A Dirichlet distribution was used to sample the proportion of cases admitted with Australian refined diagnosis‐related groups F69A (n=12), F69B (n=47), F75A (n=4), F75B (n=33), F75C (n=113), I66A (n=10), and I66B (n=140), derived from Royal Darwin Hospital admission data 2008–2013.
Figure 2.
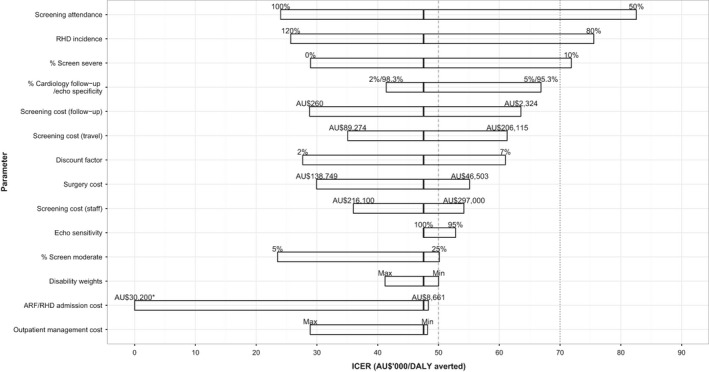
Tornado plot showing the effect of varying individual parameter estimates on the ICER of echocardiographic screening for RHD (Echo B, scenario 2). The solid line in each bar represents the baseline assumption. The dashed line represents an ICER threshold of AU$50 000 per DALY averted. The dotted line represents an ICER threshold of AU$70 000 (which approximates Australia's per capita gross domestic product26) per DALY averted. *At a maximum admission cost of AU$30 200, screening was cost‐saving (ICER less than AU$0 per DALY averted). ARF indicates acute rheumatic fever; DALY, disability‐adjusted life‐year; ICER, incremental cost‐effectiveness ratio; RHD, rheumatic heart disease.
Specificity is also difficult to estimate, and there have been concerns that echocardiographic screening yields a high number of false‐positive results.4 Our previous echo screening study had a high proportion of positive screens (14.4%) largely because screening began before the publication of the WHF criteria, and our criteria at that time were deliberately overinclusive to avoid missing cases in the context of a research study.17 Other groups have demonstrated much lower positive screen rates.19 We believe that with appropriate training of sonographers and consistent application of the WHF criteria, the proportion of positive screens—namely, those that have a possible abnormality requiring further review—could be reduced to ≈5%. We also estimated that detailed review of high‐quality images by a cardiologist could further reduce the number of children requiring clinical follow‐up (to confirm a diagnosis) to ≈2.5% (varied from 2% to 5% in the sensitivity analysis [Table 3, Figure 2]).20
Given test sensitivity of 100%, specificity can be derived by comparing the number of children with a negative screen with the total number screened minus the expected number of diagnoses (Figure S1). An inverse relationship exists between screening specificity and the proportion of cases requiring cardiology follow‐up. Using our baseline assumptions of 100% sensitivity and 75% screening attendance (Echo B), specificity was 95.3%, 97.8%, and 98.3% when the proportions requiring cardiology follow‐up were 5%, 2.5%, and 2%, respectively.
Hypothesized Effects of RHD Screening
No empirical data currently demonstrate the clinical benefits of echo screening for RHD. A randomized controlled trial is not feasible because the relatively low incidence of RHD in our target population (27.6 new cases per year) would effectively require full‐scale population screening in our region and many years of follow‐up to document outcomes such as valvular surgery. Consequently, we estimated the clinical benefits of RHD screening by using our progression model (see Sensitivity and specificity of echo) and hypothesized that screening would facilitate earlier detection and milder disease at diagnosis of RHD compared with current practice.
Earlier diagnosis of disease
Three hypothetical scenarios were modeled in which RHD was diagnosed 1, 2, and 3 years earlier for screened children (screening effectiveness scenarios 1, 2, and 3) (Table 1). Children who were not screened were assumed to present symptomatically and were diagnosed as per current practice.
Milder disease at the time of diagnosis
It is also unknown how RHD severity might be altered for children diagnosed by screening versus clinical presentation; however, it is expected that disease would be detected at an earlier (less severe) stage. In our previous analysis,9 we described the distribution of RHD severity at diagnosis, which provides an accurate picture of the severity of RHD when diagnosed according to current practice. In the group aged 5 to 15 years (n=387), we found that 59.5%, 27.1%, and 13.4% had mild, moderate, and severe RHD, respectively, at diagnosis.
In the screening context, we would expect a higher proportion of mild disease and a lower proportion of severe disease; in our own echocardiographic screening study in Australia, only 1 (5.6%) of the 18 new cases of RHD detected was classified as severe.10 Consequently, we proposed a severity distribution of 80% mild, 15% moderate, and 5% severe when RHD is diagnosed by screening. Given the uncertainty around this assumption, the severity distribution was varied in the sensitivity analysis.
Modeling Approach
Multistate model
Our multistate model for RHD progression incorporates 6 health states: inactive (past history of ARF or RHD), mild, moderate, severe without surgery, severe with surgery, and death (an absorbing state). Disease severity is defined according to the Australian RHD guidelines.15 Possible transitions between health states are illustrated in Figure 1, and examples of transition probabilities for the first year after RHD diagnosis are included.
Figure 1.
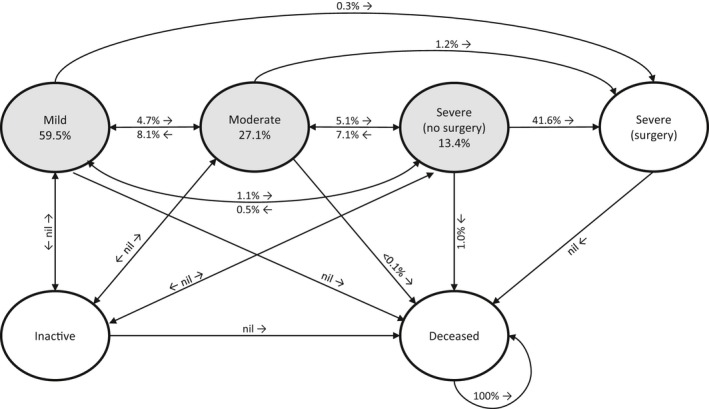
Transition probabilities between health states in the first year of rheumatic heart disease (RHD) diagnosis. The 3 shaded circles represent the proportion of children (aged 5–15 years) in each health state when they are first diagnosed with RHD according to current practice. Proportions of children who did not change health states are not shown.
Disease progression was modeled using individual patient‐level simulation. Each simulated patient was assigned an age at diagnosis between 5 and 15 years inclusive, based on the age distribution observed under current practice (Figure S1). We chose 15 years as the upper age limit so that we could compare the outcomes with those of a patient who was diagnosed up to 3 years earlier at the screening age of 12 years. For the no‐screen cohort, RHD severity at diagnosis was allocated according to current observations (Figure S1).
The same patients were simulated under each of the 2 screening strategies. In screened children, the earlier age of diagnosis was assigned according to the scenario of screening effectiveness (diagnosing RHD 1, 2, or 3 years earlier) and screening strategy (Echo A or B).
Because this study was based on hypothetical patients, human ethics approval by an institutional review committee was not required.
Time horizons
We modeled a cohort of children screened over a 5‐year period. Following diagnosis, RHD progression was modeled using monthly cycles until the minimum age of follow‐up recommended in the Australian ARF/RHD guidelines (21, 35, and 40 years for mild, moderate, and severe RHD, respectively).15 Specific monthly transition probabilities were used to simulate the rate of RHD progression during the first 10 years, as presented in our disease‐progression model.9 The average rate of progression over this 10‐year period was then used to extrapolate progression until the minimum age of follow‐up.
Software
The model was implemented in TreeAge Pro (version 15.1.0.0, 2015; TreeAge Software). The statistical software R (version 3.1.0, 2015; R Foundation for Statistical Computing) was used for plots (ggplot2, version 1.0.1, 2015) and for obtaining transition probabilities from the Aalen‐Johansen transition estimates (msSurv, version 1.1‐2, 2012).
Health Outcomes
To capture the morbidity of RHD in a composite measure, each health state was mapped to a disability weight based on the closest matching health category from the 2010 Global Burden of Disease21 (Table 2). Disability weights were aggregated on a yearly basis to calculate the patient's disability‐adjusted life‐year (DALY). The average DALY for all patients simulated under each screening strategy was subtracted from the average DALY for nonscreened patients to report the average DALYs averted by echo screening. A disability weighting of 1 was assigned in the event of death during the follow‐up period. DALYs were discounted at a rate of 5% per annum, as recommended by the Australian Pharmaceutical Benefits Advisory Council,22 and started from the age of potential diagnosis.
Table 2.
RHD Health States and Matched Disability Weights From the 2010 Global Burden of Disease21
| RHD Health State | Global Burden of Disease Category | Disability Weight (95% CI) |
|---|---|---|
| Mild | Generic uncomplicated disease: worry and daily medication | 0.031 (0.017–0.050) |
| Moderate | Heart failure: mild | 0.037 (0.021–0.058) |
| Severe, after surgery | Heart failure: moderate | 0.070 (0.044–0.102) |
| Severe, before surgery | Heart failure: severe | 0.186 (0.128–0.261) |
RHD indicates rheumatic heart disease.
Resources and Costs
Resources and costs were taken from the government health care perspective, which is useful for public policy decisions but likely underestimates the true economic value of screening because other costs to the patient, the family, and society at large are excluded. All health resources were costed in Australian dollars at 2013 price levels, and future costs were discounted at a rate of 5% per annum, as recommended in Australia.22 Costs were aggregated over the entire time horizon for each diagnosed patient. Summarized costs are presented in Table 3, and detailed breakdowns are presented in Tables S1 through S5.
All dollar amounts shown are in Australian dollars.
RHD management costs
Both inpatient and outpatient costs were incurred for each child with RHD. Hospital admission was required for initial RHD diagnosis in 90% of cases (10% were diagnosed as outpatients) and for ARF recurrence (estimated recurrence rate of 4.5% per RHD patient per year,14 regardless of RHD severity.) Inpatient costs (Table S2) were allocated according to the relevant Australian refined diagnosis‐related groups defined by the federal Australian health system.23 We used Royal Darwin Hospital admission data from 2008 to 2013 to inform the proportion of children in each diagnosis‐related group and hospital pricing data from 2012 to 2013, which calculates the average cost per diagnosis‐related group for that hospital (submitted to the National Hospital Cost Data Collection, round 17).
Additional inpatient costs were required in the event of cardiac surgery (including preoperative medical and dental management, postoperative intensive care, and interstate travel to the center that performs the surgery) (Table S3). Pricing for cardiac surgery was obtained from the referral hospital based on surgeries performed on NT indigenous children in 2014. All hospital admissions required return travel for the child plus a caregiver from their community.
Annual outpatient costs (Table S4) in the community included 4‐weekly administration of BPG by a nurse plus 1 to 4 reviews by the general practitioner. Additional consultations that could occur in or out of the community included a pediatrician, a cardiologist, an echocardiographer, and a dentist. The frequency of these consultations ranged from every 6 months to every 2 years, depending on the severity of RHD, as described by the Australian guidelines.15
We did not apply the full outpatient cost to each patient, given that disease transition probabilities were derived from a population in which adherence to secondary prophylaxis is known to be suboptimal (between 2005 and 2010, an estimated 37.8% and 24.3% patients on the NT RHD register received <50% and >80%, respectively, of the required BPG doses).24 Consequently, in our analysis, we assumed baseline adherence to follow‐up of 50% in both the screened and unscreened cohorts.
RHD screening costs
We estimated that 2 sets of screening equipment, including 2 portable echo machines (Vivid i; GE Healthcare), would be required to enable screening from the 2 major cities in the NT. Equipment costs were amortized over 5 years with the total cost calculated as the upfront costs minus a discounted resale value in the fifth year set at 10% of the purchase price. Staff costs comprised salaries for 1 full‐time‐equivalent echocardiographer, 1 full‐time‐equivalent nurse, and a cardiologist at 4 hours per week (for staff training, quality control, review of positive screens, and referral as necessary). Travel costs were for 2 staff members and included transport (commercial flights, chartered aircraft or road), accommodation, and travel allowance (Table S5).
Children requiring a face‐to‐face cardiology consultation and repeat echocardiogram would be referred to existing services (not included in the screening cardiologist's time). Costs for this follow‐up included the consultations plus travel.
Analytical Methods
Cost–utility analysis of RHD screening
We compared the outcomes of each screening strategy with current practice using a cost–utility analysis in which the incremental cost‐effectiveness ratio (ICER) was calculated as the difference in the mean aggregated cost of RHD for each strategy divided by the DALYs prevented. The ICER represents the additional cost of preventing 1 DALY, and we adopted a standard threshold value of $50 000 per DALY prevented to determine if the screening strategy was cost‐effective.25 We also looked at the effect of increasing this threshold to $70 000, which would meet the WHO definition of “very cost‐effective” (when cost per DALY averted is less than the per capita gross domestic product).
Assumptions and sensitivity analysis
For the purpose of this analysis, we made a number of assumptions, summarized below, that we subsequently evaluated in the sensitivity analysis:
The incidence of RHD is 27.6 new diagnoses per year in the target age group.
Overall, 75% of age‐eligible children are screened in each community.
Screening echocardiogram is 100% sensitive for definite RHD.
The proportion of screening echocardiograms requiring further clinical evaluation for RHD by a cardiologist was estimated to be 2.5%.
RHD severity distribution when diagnosed by screening is 80% mild, 15% moderate, and 5% severe.
Where an RHD diagnosis is made by screening, a hospital admission for diagnosis will be avoided.
Adherence to prophylaxis and clinical follow‐up was assumed to be 50% in both screened and unscreened cohorts.
Transition probabilities between RHD health states are the same in screened and unscreened children.
One‐way sensitivity analysis was performed on individual parameters listed in Table 3, and a multiway sensitivity analysis was performed on the proportions of children detected with mild, moderate, and severe RHD. Probabilistic sensitivity analysis was performed by simultaneously varying all of the model's parameter estimates. Parameters were sampled from appropriate probability distributions (Table 3) 100 times and, for each sample, 1000 individual patients were simulated for each screening year.
Cost–utility analysis of improving adherence to secondary prophylaxis
A separate analysis was done looking at the potential health effects and economic outcomes of improved adherence to secondary prophylaxis. For modeling purposes, we analyzed the potential effect of increasing adherence to 100%, which, by reducing ARF recurrences, we hypothesized may result in the following improvements in disease progression:
Mild RHD remains mild (does not progress to moderate or severe disease).
Moderate RHD: (a) A 50% reduction in progression is shown from moderate to severe disease or surgery (in our current practice model, ≈29% currently progress over 10 years since diagnosis,9 so our improved prophylaxis model allowed 15% to progress, with the other 14% remaining moderate); (b) other transitions from the moderate state remain the same.
Severe RHD showed no change to observed disease progression.
In addition to comparing outcomes of improved adherence in the screened cohort with current outcomes in the nonscreened cohort, we looked at the effect of improving prophylaxis alone, without screening. Note that for these models, we did not attempt to incorporate costs associated with improving adherence rates but calculated the potential spend that would be available to improve adherence while remaining cost‐effective.
Results
Simulated Costs and Health Burden of RHD Using Current Practice
The mean present‐day cost of RHD (any severity) for patients diagnosed in the first study year was $54 511 per case over an average of 16.7 years, whereas the total treatment cost for a cohort of 138 incident cases diagnosed over a 5‐year period was $6.9 million (Table 4). Although only 13.4% had severe RHD at diagnosis, these cases contributed to almost one‐third of the total cost. Similarly, 37.7% of children were diagnosed with or progressed to heart failure but contributed to almost three‐quarters of the total cost.
Table 4.
Economic and Health Utility Outcomes After Completion of the Minimum Recommended Duration of Secondary Prophylaxis
| Percentage of Simulated Patients in Each RHD Health State | Total Treatment Cost All Patientsa (AU$'000) | DALYs Lost Per Person | Mean Treatment Cost Per Personb (AU$'000) | Mean Duration Prophylaxis Per Person (Years) | |
|---|---|---|---|---|---|
| RHD severity at diagnosis | |||||
| Mild | 59.5% | 2695 | 0.80 | 36.0 | 14.6 |
| Moderate | 27.1% | 1961 | 1.48 | 52.4 | 18.4 |
| Severe | 13.4% | 2202 | 3.37 | 118.5 | 22.6 |
| Heart failure (any time)c | |||||
| No | 62.3% | 1816 | 0.36 | 23.2 | 12.1 |
| Yes | 37.7% | 5042 | 2.94 | 106.2 | 24.3 |
| Surgery | |||||
| No | 68.9% | 2068 | 0.51 | 23.9 | 12.6 |
| Yes | 31.1% | 4789 | 3.15 | 122.4 | 25.9 |
| Any RHD | 100% | 6858 | 1.33 | 54.5 | 16.7 |
Costs and DALYs were discounted at 5% per annum. DALY indicates disability‐adjusted life‐year; RHD, rheumatic heart disease.
Includes all new RHD diagnoses in the 5‐year study period (n=138).
Assumes diagnosis was made in the first year of the 5‐year study period (n=27.6), and assumes 50% adherence to benzathine penicillin G and outpatient management. If 100% adherence is assumed, mean treatment cost per person is $73 454.
Heart failure includes all cases diagnosed with severe RHD at some time during the follow‐up period.
The health burden in children diagnosed with RHD was an average 1.33 DALYs lost due to disease over 16.7 years. Children diagnosed with severe RHD lost 3.37 DALYs.
Cost–Utility Analysis of Echocardiographic Screening for RHD
The predicted annual cost of screening up to 2000 children under Echo A was $585 815 (range $427 184–$727 547), and the annual cost of screening up to 4000 children under Echo B was $563 944 (range $422 949–$779 100) (Table S5).
Echo B detected more RHD cases than Echo A over the first 5 years of the screening program (Table 5). Under both screening strategies, the number of RHD diagnoses increased as the hypothesized number of years of earlier diagnosis increased (scenarios 1, 2, and 3 of screening effectiveness). Earlier diagnosis resulted in reduced RHD management costs for both screening strategies compared with current practice, as well as a reduction in mean DALYs. Once screening costs were added, Echo B was cost‐effective under the assumption that RHD can be diagnosed 2 years earlier by echocardiographic screening (scenario 2), with an ICER of $47 546 per DALY averted. Echo A was not cost‐effective under any of the 3 scenarios tested (ICER greater than $50 000).
Table 5.
Clinical Outcomes and Cost–Utility Analysis of Two RHD Screening Strategies Over 5 Years, Assuming That RHD Can be Diagnosed 1, 2, or 3 Years Earlier by Screening (Scenarios 1, 2, and 3)
| Baseline | Scenario 1 | Scenario 2 | Scenario 3 | ||||
|---|---|---|---|---|---|---|---|
| No Screen | Echo A | Echo B | Echo A | Echo B | Echo A | Echo B | |
| Clinical outcomes | |||||||
| RHD severity at diagnosis | |||||||
| Mild, % | 59.5 | 61.5 | 63.9 | 62.6 | 67.2 | 64.6 | 68.2 |
| Moderate, % | 27.1 | 26.3 | 25.8 | 26.3 | 25.0 | 25.6 | 24.6 |
| Severe, % | 13.4 | 12.2 | 10.3 | 11.1 | 7.8 | 9.8 | 7.1 |
| Heart failure at any time, % | 37.7 | 36.7 | 35.2 | 35.9 | 33.5 | 34.8 | 33.2 |
| Surgery, % | 31.1 | 30.2 | 29.0 | 29.6 | 27.6 | 28.7 | 27.3 |
| Death, % | 11.3 | 10.9 | 10.4 | 10.7 | 9.9 | 10.3 | 9.8 |
| Cost–utility analysis | |||||||
| Number of diagnoses | 138 | 143 | 146 | 151 | 164 | 163 | 183 |
| Mean cost per diagnosis (AU$,000) | 49.6 | 65.4 | 61.0 | 62.5 | 55.5 | 59.3 | 53.0 |
| RHD screening cost | ··· | 18.2 | 16.5 | 17.2 | 14.8 | 15.9 | 13.2 |
| RHD management cost | 49.6 | 47.2 | 44.5 | 45.3 | 40.7 | 43.4 | 39.7 |
| Mean utility per diagnosis (DALY) | 1.33 | 1.30 | 1.25 | 1.28 | 1.21 | 1.25 | 1.20 |
| ICER (AU$/DALY saved) | ··· | 489 016 | 147 170 | 253 994 | 47 546a | 116 129 | 25 387a |
DALY indicates disability‐adjusted life‐year; ICER, incremental cost‐effectiveness ratio; RHD, rheumatic heart disease.
Cost‐effective strategy (ICER less than AU$50 000 per DALY saved).
Clinical outcomes of screened versus unscreened children are also presented in Table 5. Screening resulted in improved clinical outcomes, including fewer deaths, surgeries, and episodes of heart failure. Outcomes from screening were best using Echo B and improved further as the hypothesized number of years of earlier diagnosis increased.
The total cost of Echo B over 5 years was $2.4 million, which equates to $161 per child screened or $14 760 per case detected when our baseline assumptions are applied.
Sensitivity Analysis
Given that Echo B was the dominant screening strategy (less costly and more effective at improving clinical outcomes than Echo A), sensitivity analysis was performed only for Echo B. This was further limited to scenario 2 (RHD diagnosed 2 years earlier by screening) because scenario 3 was clearly cost‐effective (ICER $24 985) and scenario 1 was not (ICER $144 216). This is demonstrated by tornado plots of each scenario, presented in Figure S2. Only the sensitivity analysis of Echo B, scenario 2, is presented.
One‐way sensitivity analysis
One‐way sensitivity analysis was conducted on a number of parameters, as listed in Table 3. A tornado plot is presented in Figure 2 and demonstrates that the ICER of screening is sensitive to most parameters if a threshold of $50 000 per DALY averted is used. If a higher threshold is adopted ($70 000; equivalent to the Australian per capita gross domestic product26), the results are more robust, although they remain sensitive to screening attendance, RHD incidence (the underlying number of cases expected to occur in indigenous children, currently estimated at 27.6 cases per year), and the proportion of children detected with severe disease.
Multiway sensitivity analysis
Multiway sensitivity analysis was performed on the proportions of children detected with mild, moderate, and severe RHD. Screening was cost‐effective when the proportion detected with moderate RHD ranged between 5% and 25%, provided the proportion detected with severe RHD was <5%. If the proportion of children detected with severe disease increased to 10%, screening was cost‐effective only if the proportion with moderate RHD was <10%.
Probabilistic sensitivity analysis
In probabilistic sensitivity analysis using 100 random draws from the parameter distributions outlined in Table 3, screening was cost‐saving in 6% of iterations. It was cost‐effective in 41% of iterations with an ICER threshold of less than $50 000 per DALY averted and increased to 63% of the iterations with an ICER of less than $70 000 per DALY averted. More than 90% of all iterations resulted in an ICER of less than $130 000 per DALY averted. Figure 3 shows the probability that Echo B is cost‐effective for different ICER threshold values.
Figure 3.
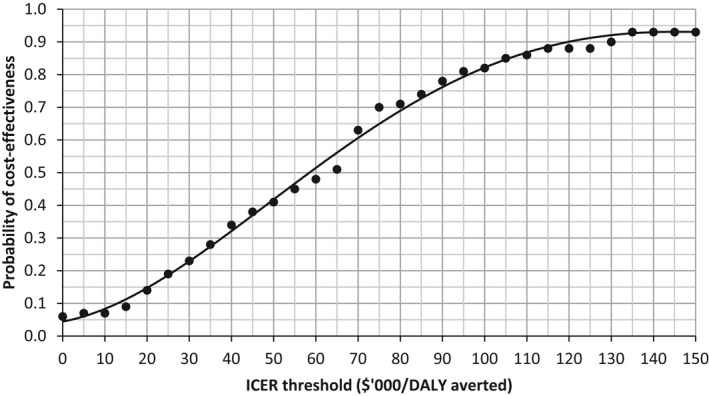
Cost‐effectiveness acceptability curve for Echo B, scenario 2. ICER indicates incremental cost‐effectiveness ratio.
Cost–Utility Analysis of Improving Adherence to Secondary Prophylaxis
Our baseline model assumed that only 50% of children in both screened and unscreened cohorts would be adherent to the recommended follow‐up (therefore, 50% of costs were applied) and that disease progression, once diagnosed, would be the same in both groups. We also modeled the possible effect of improving adherence to 100%, with or without screening. Table 6 shows that improving adherence alone could result in dramatic reductions in heart failure, surgery, and death and that predicted outcomes improved even further if improved adherence was combined with screening.
Table 6.
Clinical Outcomes and Cost–Utility Analysis of Improving BPG Adherence With and Without Screening
| No Screen | Screena | |||
|---|---|---|---|---|
| Current Progression (50% BPG) | Improved Progressionb (100% BPG) | Current Progression (50% BPG) | Improved Progressionb (100% BPG) | |
| Clinical outcomes | ||||
| RHD severity at diagnosis | ||||
| Mild, % | 59.5 | 59.5 | 67.2 | 67.2 |
| Moderate, % | 27.1 | 27.1 | 25.0 | 25.0 |
| Severe, % | 13.4 | 13.4 | 7.8 | 7.8 |
| Heart failure at any time, % | 37.7 | 18.9 | 33.5 | 12.9 |
| Surgery, % | 31.1 | 15.2 | 27.6 | 10.4 |
| Death, % | 11.3 | 6.5 | 9.9 | 4.6 |
| Cost–utility analysis | ||||
| Number of diagnoses | 138 | 138 | 164 | 164 |
| Mean cost per diagnosis (AU$,000) | 49.6 | 51.3 | 55.5 | 55.6 |
| RHD screening cost | ··· | ··· | 14.8 | 14.8 |
| RHD management cost | 49.6 | 51.3 | 40.7 | 40.8 |
| Mean utility per diagnosis (DALY) | 1.33 | 0.86 | 1.21 | 0.69 |
| ICER (AU$/DALY saved)c | ··· | 3463c | 47 546c | 9329c |
BPG indicates benzathine penicillin G; DALY, disability‐adjusted life‐year; ICER, incremental cost‐effectiveness ratio; RHD, rheumatic heart disease.
Echo B, scenario 2.
Improved progression assumes mild disease does not progress; half of the moderate disease that currently progresses to severe will not progress; all other transitions remain the same.
Compared with current progression in the no‐screen cohort.
We calculated that an additional $22 068 per diagnosis could be spent on a program to improve prophylaxis delivery over that patient's life of prophylaxis (which decreased to an average of 13.8 years with improved disease progression) and that the program would remain a cost‐effective intervention (ICER less than $50 000 per DALY averted). Based on an average of 27.6 new diagnoses per year, this equates to an additional $44 000 ($1600 per diagnosis) per year.
Discussion
This analysis of the potential cost‐effectiveness of echocardiographic screening for RHD is the most comprehensive to date. We found that echocardiographic screening alone resulted in modest improvements in clinical outcomes and that under our most plausible set of assumptions, including that RHD can be diagnosed at least 2 years earlier by screening, screening all remote indigenous children aged 5 to 12 years every second year is a potentially cost‐effective strategy for RHD detection in the NT of Australia (ICER $47 546 per DALY saved) (Table 5). If, in addition to screening, adherence to secondary prophylaxis was improved, our model predicts that clinical outcomes would be dramatically better, with corresponding improvement in the ICER. Although the costs and logistics of our proposed screening program are context‐specific, the model we present could be adapted to any other setting, provided that reasonable local data or assumptions are available for the model parameters.
A strength of our study is that we were able to use disease transition probabilities that were derived from our own contemporary population rather than relying on historical data, as previous analyses have done.7, 8 As such, we believe that the RHD transition probabilities we have used are the best available. However, our model did not estimate the risk of certain RHD complications, including stroke or endocarditis, that incur significant expense, morbidity, and mortality. Given this, the cost of undetected disease is likely to be higher than our estimate, potentially resulting in improved cost‐effectiveness of screening.
Modeling invariably requires a number of assumptions, and in our analysis we made assumptions about the disease itself as well as about parameters related to the proposed screening process. Given that there are no data to inform how much earlier RHD may be detected using echocardiographic screening, we modeled 3 scenarios of screening effectiveness and found that health and economic outcomes were best if RHD was detected 3 years earlier by screening (ICER $25 387 per DALY saved) (Table 5). Even if we assume that RHD could only be detected 2 years earlier, screening remains a cost‐effective proposition.
Another major assumption about screened cases of RHD was disease severity at diagnosis. We assumed that disease would be less severe if detected early, and we assigned screened cases according to the distribution of 80% mild, 15% moderate, and 5% severe. One‐way and multiway sensitivity analyses revealed that the ICER was not sensitive to an increase in the proportion of moderate cases up to 25%, provided the proportion of severe cases was ≤5%. We would hope that screening would reduce the proportion of children detected with severe RHD to this level but note that the ICER was sensitive to this assumption. The model was also sensitive to RHD incidence; however, it is highly unlikely that the incidence of RHD is lower than our baseline assumption (which was based on 14 years of patient data). Higher incidence is possible and would favor the cost‐effectiveness of screening.
One‐way sensitivity analysis revealed that our model was particularly sensitive to 2 parameters relating to the screening process itself: screening attendance (ie, number of children screened) and the proportion of screened children requiring cardiology follow‐up. We assumed baseline screening attendance of 75%. This is slightly higher than the average school attendance of indigenous children in the NT,18 meaning that a screening program would need to maximize efforts to recruit all school attendees and potentially use strategies to capture nonattendees. The fact that Echo B can screen twice as many children per year as Echo A is the principal reason that Echo B is the more cost‐effective option.
The sensitivity and specificity of a screening test compared with a diagnostic test are key determinants of its utility. Evaluating the performance of any proposed screening test for RHD is difficult, given that there is no gold standard diagnostic test. There is an evolving consensus, however, that the 2012 WHF criteria5 should be used for the echocardiographic diagnosis of RHD, providing a new gold standard against which a screening test can be compared.2 Our baseline assumption that only 2.5% of screened children would require face‐to‐face cardiology follow‐up is based on the premise that screening sonographers need to be well trained and familiar with the WHF criteria.
Two recent studies suggest that this is feasible. Beaton et al19 screened 4869 children in Uganda using portable echocardiography performed by an expert operator. Only 2.7% had an abnormal screening echocardiogram, and following further evaluation, nearly half of these were considered to have physiological regurgitation. In New Zealand, Cramp et al20 screened 685 children and classified 8.2% of echocardiograms as abnormal, of which 1.6% met the equivalent of the WHF criteria for definite RHD. Of note is that only 11 echocardiograms (1.6%) needed to be repeated on a hospital machine; screening image quality was sufficient to make a diagnosis for the remainder. In our model, it is anticipated that >2.5% of screens would need to be reviewed by the program cardiologist, but the considerable additional follow‐up costs would be incurred only if a consultation was required. One‐way sensitivity analysis reveals that even a small increase in the proportion of children needing review (eg, to 3%) would increase the ICER above the $50 000 threshold.
The potential expense of echocardiographic screening in resource‐poor settings has been identified as a potential barrier.27 To counter this, an emerging area of interest is screening using a hand‐held ultrasound device by local health staff with basic training, which would avoid the need for highly skilled technicians to travel to screening sites.28, 29 Although this technology certainly holds promise in some settings, it is not likely to be a cost‐saving alternative in remote Australia because the positive‐screen rate for an unskilled operator is likely to be higher, resulting in more referrals for costly cardiology review.
A major driver of cost in our model is travel, a cost that is incurred both in current practice and in a proposed screening program. The geographic context in which we set our analysis is unique and is likely to render the potential cost of screening more expensive than in some other parts of Australia and the world. Not only are the distances vast, but community populations are small, meaning that considerable resources are required to reach relatively few children. Cost‐effectiveness would be markedly improved if travel costs were reduced and/or the number of children available to be screened were greater. Screening indigenous Australian children in urban settings, for example, is likely to be highly cost‐effective because the RHD risk is still high and screening costs would be nearly halved. Screening in densely populated developing countries is also likely to be more cost‐effective than our model, provided the appropriate resources are available.
Perhaps the most important principle of screening is that treatment is available to improve outcome if disease is detected earlier. Although secondary prophylaxis with BPG is known to be effective in preventing ARF recurrences and is readily available in Australia, it is recognized that adherence rates in the indigenous population of the NT are suboptimal.24 Given that the transition probabilities for disease progression were derived from this population, in which we estimated adherence to be 50%, we modeled the hypothetical effect of improving adherence to 100%, independent of screening. Table 6 demonstrates the dramatic improvement in clinical outcomes that may result from this intervention alone: a 50% reduction in heart failure, surgery, and death over the 10 to 35 years following RHD diagnosis. Outcomes would improve even further if screening were combined with an improvement in BPG adherence. The ICERs for the 100% adherence scenarios are an underestimate, as no costs to achieve this have been included, but we calculate that $44 000 per year could be spent on improving prophylaxis delivery for new diagnoses, and it would remain a cost‐effective intervention in its own right. Improving adherence to secondary prophylaxis must remain a priority in RHD control.
Whether an ICER of $50 000 per DALY averted is appropriate for the indigenous population in Australia could be debated; this figure is a widely used but arbitrary threshold. A major Australian study looking at the cost‐effectiveness of numerous preventative strategies on health outcomes (ACE‐Prevention) discussed this and included an additional cost‐effectiveness category ($50 000–$150 000 per DALY prevented) for indigenous populations. Alternatively, the WHO defines an intervention as very cost‐effective if the cost per DALY averted is less than the gross domestic product per capita ($68 503 in Australia in 201426) and as cost‐effective if the cost per DALY averted is between 1 and 3 times per‐capita gross domestic product.30, 31 The cost‐effectiveness acceptability curve (Figure 3) shows that our screening model would have a 63% probability of being cost‐effective if the threshold were set at $70 000 compared with 41% at $50 000. Equity concerns about indigenous health in Australia may be expressed as a greater willingness to pay for the same health gain.
Economic analyses are not the only considerations in determining whether to implement a new health strategy. There may be important social and ethical reasons to tackle RHD even at great expense, given that it selectively affects the most disadvantaged and the young and has largely been eliminated from affluent populations. In contrast, there are considerations regarding the logistics required to organize mass screening programs and the opportunity costs of devoting time and dollars to this initiative at the expense of other health interventions.
Conclusions
We have demonstrated that echocardiographic screening for RHD is cost‐effective in our context if we assume that RHD can be detected ≥2 years earlier by screening. Our model is sensitive to a number of assumptions, and particular emphasis would need to be placed on screening attendance and on maximizing the specificity of the screening echocardiogram. We have also demonstrated the dramatic improvements in clinical and economic outcomes that could result if adherence to secondary prophylaxis were improved and emphasize that this remains the cornerstone of RHD control.
Sources of Funding
Cannon was supported by an Australian Postgraduate Award from the University of Western Australia. Roberts was supported by an Australian Postgraduate Award from Charles Darwin University.
Disclosures
None.
Supporting information
Table S1. Cost (AU$, 2013) of Outpatient Diagnosis of Rheumatic Heart Disease
Table S2. Cost (AU$, 2013) of Acute Rheumatic Fever and Rheumatic Heart Disease Admission to Royal Darwin Hospital
Table S3. Cost (AU$, 2013) of Cardiac Valve Surgery Including Transfer From the Royal Darwin Hospital to the Royal Children's Hospital in Melbourne, Australia, Where Surgery Is Performed
Table S4. Cost (AU$ 2013) of Outpatient Rheumatic Heart Disease (RHD) Care: Annual Cost Per Patient, Depending on RHD Severity
Table S5. Estimated Annual Cost of Portable Echocardiographic Screening for Rheumatic Heart Disease
Figure S1. Number and severity of rheumatic heart disease cases diagnosed between 1999 and 2012 in indigenous children of the Northern Territory, Australia (n=387*).
Figure S2. One‐way sensitivity analysis of Echo B, scenarios 1, 2, and 3.
(J Am Heart Assoc. 2017;6:e004515. DOI: 10.1161/JAHA.116.004515.)
References
- 1. World Health Organization . WHO Technical Report Series; 923. Rheumatic fever and rheumatic heart disease. Report of a WHO Expert Consultation, Geneva, 29 October–1 November 2001. Geneva; 2004. [PubMed] [Google Scholar]
- 2. Saxena A, Zuhlke L, Wilson N. Echocardiographic screening for rheumatic heart disease: issues for the cardiology community. Glob Heart. 2013;8:197–202. [DOI] [PubMed] [Google Scholar]
- 3. Zuhlke L, Mayosi BM. Echocardiographic screening for subclinical rheumatic heart disease remains a research tool pending studies of impact on prognosis. Curr Cardiol Rep. 2013;15:343. [DOI] [PubMed] [Google Scholar]
- 4. Roberts K, Colquhoun S, Steer A, Remenyi B, Carapetis J. Screening for rheumatic heart disease: current approaches and controversies. Nat Rev Cardiol. 2013;10:49–58. [DOI] [PubMed] [Google Scholar]
- 5. Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zuhlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease‐an evidence‐based guideline. Nat Rev Cardiol. 2012;9:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Remond M, Atkinson D, White A, Brown A, Carapetis J, Remenyi B, Roberts K, Maguire G. Are minor echocardiographic changes associated with an increased risk of acute rheumatic fever or progression to rheumatic heart disease? Int J Cardiol. 2015;198:117–122. [DOI] [PubMed] [Google Scholar]
- 7. Zachariah JP, Samnaliev M. Echo‐based screening of rheumatic heart disease in children: a cost‐effectiveness Markov model. J Med Econ. 2015;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manji RA, Witt J, Tappia PS, Jung Y, Menkis AH, Ramjiawan B. Cost‐effectiveness analysis of rheumatic heart disease prevention strategies. Expert Rev Pharmacoecon Outcomes Res. 2013;13:715–724. [DOI] [PubMed] [Google Scholar]
- 9. Cannon J, Roberts K, Milne C, Carapetis J. Rheumatic heart disease severity, progression and outcomes: a multi‐state model. J Am Heart Assoc. 2017;6:e003498 DOI: 10.1161/JAHA.116.003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts K, Maguire G, Brown A, Atkinson D, Remenyi B, Wheaton G, Kelly A, Kumar RK, Su JY, Carapetis JR. Echocardiographic screening for rheumatic heart disease in high and low risk Australian children. Circulation. 2014;129:1953–1961. [DOI] [PubMed] [Google Scholar]
- 11. Australian Bureau of Statistics . 3238.0.55.001‐ Estimates of Aboriginal and Torres Strait Islander Australians, June 2011. 2013. Available at: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3238.0.55.001Main+Features1June%202011?OpenDocument. Accessed September 9, 2015.
- 12. Department of Health and Community Services & Department of Employment Education and Training . Healthy School‐Age Kids. Darwin, Australia: Northern Territory Government; 2007. Available at: http://remotehealthatlas.nt.gov.au/hsak_manual.pdf. Accessed September 9, 2015. [Google Scholar]
- 13. Kane A, Mirabel M, Toure K, Perier MC, Fazaa S, Tafflet M, Karam N, Zourak I, Diagne D, Mbaye A, Kane M, Diack B, Jouven X, Marijon E. Echocardiographic screening for rheumatic heart disease: age matters. Int J Cardiol. 2013;168:888–891. [DOI] [PubMed] [Google Scholar]
- 14. Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. 2013;128:492–501. [DOI] [PubMed] [Google Scholar]
- 15. RHD Australia (ARF/RHD writing group) National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand . Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease. 2nd ed Darwin, Australia: Menzies School of Health Research; 2012. [Google Scholar]
- 16. Roberts KV, Brown AD, Maguire GP, Atkinson DN, Carapetis JR. Utility of auscultatory screening for detecting rheumatic heart disease in high‐risk children in Australia's Northern Territory. Med J Aust. 2013;199:196–199. [DOI] [PubMed] [Google Scholar]
- 17. Roberts K, Maguire G, Brown A, Atkinson D, Remenyi B, Wheaton G, Ilton M, Carapetis J. Rheumatic heart disease in Indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust. 2015;203:219. [DOI] [PubMed] [Google Scholar]
- 18. Purdie N, Buckley S; Australian Institute of Health and Welfare . School attendance and retention of Indigenous Australian students. Issues Paper No 1 for the Closing the gap clearinghouse 2010. Available at: http://www.aihw.gov.au/uploadedFiles/ClosingTheGap/Content/Publications/2010/ctg-ip01.pdf. Accessed September 9, 2015.
- 19. Beaton A, Okello E, Lwabi P, Mondo C, McCarter R, Sable C. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation. 2012;125:3127–3132. [DOI] [PubMed] [Google Scholar]
- 20. Cramp G, Stonehouse M, Webb R, Webb R, Chaffey‐Aupouri G, Wilson N. Undetected rheumatic heart disease revealed using portable echocardiography in a population of school students in Tairawhiti, New Zealand. N Z Med J. 2012;125:53–64. [PubMed] [Google Scholar]
- 21. Institute for Health Metrics and Evaluation (IHME) . Global Burden of Disease Study 2010 (GBD 2010) Disability Weights. Seattle: 2012. Available at: http://ghdx.healthdata.org/record/global-burden-disease-study-2010-gbd-2010-disability-weights. Accessed September 9, 2015. [Google Scholar]
- 22. Pharmaceutical Benefits Advisory Committee . Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee 2013. Version 4.4. Available at: http://www.pbac.pbs.gov.au/content/information/printable-files/pbacg-book.pdf. Accessed September 9, 2015.
- 23. Independent Hospital Pricing Authority. National Efficient Price Determination 2012‐13 . 2012. Available at: https://www.ihpa.gov.au/publications/national-efficient-price-determination-2012-2013. Accessed February 17, 2017.
- 24. Australian Institute of Health and Welfare . Rheumatic Heart Disease and Acute Rheumatic Fever in Australia: 1996–2012. Cat. no. CVD 60. Canberra: AIHW; 2013. [Google Scholar]
- 25. Vos T, Carter R, Barendregt J, Mihalopoulos C, Veerman L, Magnus A, Cobiac L, Bertram M, Wallace A. Assessing cost‐effectiveness in prevention. The University of Queensland, Brisbane, and Deakin University, Melbourne. 2010.
- 26. Organisation for Economic Co‐operation and Development . Level of GDP per capita and productivity 2014. Available at: https://stats.oecd.org/Index.aspx?DataSetCode=PDB_LV. Accessed September 9, 2015.
- 27. Carapetis JR, Zuhlke LJ. Global research priorities in rheumatic fever and rheumatic heart disease. Ann Pediatr Cardiol. 2011;4:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mirabel M, Bacquelin R, Tafflet M, Robillard C, Huon B, Corsenac P, de Fremicourt I, Narayanan K, Meunier JM, Noel B, Hagege AA, Rouchon B, Jouven X, Marijon E. Screening for rheumatic heart disease: evaluation of a focused cardiac ultrasound approach. Circ Cardiovasc Imaging. 2015;8:e00232. [DOI] [PubMed] [Google Scholar]
- 29. Lu JC, Sable C, Ensing GJ, Webb C, Scheel J, Aliku T, Lwabi P, Godown J, Beaton A. Simplified rheumatic heart disease screening criteria for handheld echocardiography. J Am Soc Echocardiogr. 2015;28:463–469. [DOI] [PubMed] [Google Scholar]
- 30. Marseille E, Bruce L, Dhruv SK, James K, Sydney R. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization . The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cost (AU$, 2013) of Outpatient Diagnosis of Rheumatic Heart Disease
Table S2. Cost (AU$, 2013) of Acute Rheumatic Fever and Rheumatic Heart Disease Admission to Royal Darwin Hospital
Table S3. Cost (AU$, 2013) of Cardiac Valve Surgery Including Transfer From the Royal Darwin Hospital to the Royal Children's Hospital in Melbourne, Australia, Where Surgery Is Performed
Table S4. Cost (AU$ 2013) of Outpatient Rheumatic Heart Disease (RHD) Care: Annual Cost Per Patient, Depending on RHD Severity
Table S5. Estimated Annual Cost of Portable Echocardiographic Screening for Rheumatic Heart Disease
Figure S1. Number and severity of rheumatic heart disease cases diagnosed between 1999 and 2012 in indigenous children of the Northern Territory, Australia (n=387*).
Figure S2. One‐way sensitivity analysis of Echo B, scenarios 1, 2, and 3.


