Abstract
Background
Children with congenital heart disease (CHD) are thought to have low levels of physical activity (PA), but few studies have used objective measures of PA in this population.
Methods and Results
We recruited patients with mild, moderate, and severe CHD and cardiac transplant recipients, aged 8 to 19 years, from pediatric cardiology clinics throughout British Columbia and Yukon, Canada. Participants were fitted with an ActiGraph accelerometer to be worn over the right hip for 7 days. Daily means were estimated for a variety of accelerometry‐derived metrics, including moderate‐to‐vigorous PA and percentage of sedentary time if they had at least 3 valid days of accelerometry data. Participants also completed a PA questionnaire. We included 90 participants (aged 13.6±2.7 years; 54% male), of which 26 had mild CHD, 26 had moderate CHD, 29 had severe CHD, and 9 were cardiac transplant recipients. Median daily moderate‐to‐vigorous PA was 43 min/day (interquartile range: 28.9–56.9 min/day), and 8% met PA guidelines of 60 minutes of moderate‐to‐vigorous PA at least 6 days a week. There were no significant differences in any accelerometry‐derived metric according to CHD severity. Boys were significantly more active and less sedentary than girls. Activity declined and sedentary behaviors increased with age in both sexes. Sports participation was common, including competitive out‐of‐school clubs (57%). PA restrictions from cardiologists were rare (15%).
Conclusions
We found normal age–sex patterns of PA in children with CHD. There were no differences in PA by CHD severity, suggesting that sociocultural factors are likely important determinants of PA in these children.
Keywords: accelerometry, congenital heart disease, pediatrics, physical activity, physical exercise
Subject Categories: Congenital Heart Disease, Pediatrics, Exercise
Introduction
Approximately 1 in 100 children are born with a congenital heart disease (CHD).1 With advances in surgical techniques and practices, survival rates and life expectancy have dramatically improved.2, 3, 4 Children with CHD, however, are at increased cardiovascular risk because traditional atherosclerotic risk factors may interact with intrinsic structural abnormalities, surgical sequelae, or disturbances in cardiac rhythm.5 The importance of physical activity (PA), which is defined as “any bodily movement produced by skeletal muscles that results in energy expenditure,”6 is increasingly recognized as a cornerstone of long‐term cardiovascular health in these patients.7, 8 It is well documented that we are confronted with a global childhood inactivity crisis,9 with North American countries fairing particularly poorly in terms of children's PA behaviors.10 Nationally representative surveys that use objective measures of PA estimate that <1 in 10 young Canadians11 and US youth12 meet PA guidelines of at least 60 minutes of moderate‐to‐vigorous PA (MVPA) daily.13, 14 Children with CHD may be even less active than their healthy peers8; however, there is conflicting evidence regarding objectively measured PA in children with CHD. Some studies reported children with severe types of CHD being significantly less active than their peers,15, 16 whereas other studies that covered a broader spectrum of CHD found activity levels that were comparable to peers.17, 18, 19 These studies have inherent limitations because they were conducted in children with specific lesions15, 16 or were small in number.17, 18, 19 In addition, very little is known about sedentary behavior in this population; sedentary behavior is increasingly recognized as a risk factor for cardiovascular disease, independent of PA.20
The aim of this analysis was (1) to describe objectively measured PA by CHD severity, (2) to describe objectively measured sedentary behavior by CHD severity, and (3) to qualitatively describe PA and sports participation and their associations with objectively measured PA.
Materials and Methods
Sample and Protocol
We included 107 children aged 8 to 19 years who had CHD or pediatric heart transplant enrolled from British Columbia Children's Hospital or traveling clinics across British Columbia and Yukon, Canada. We obtained institutional ethics approval, parental consent, and participant assent. Recruitment success was 80% overall. Data were collected during routine clinic visits between July 2014 and August 2016. We grouped cardiac diagnosis according to consensus guidelines21 as follows: (1) mild CHD (eg, atrial septal defect, mild pulmonary stenosis); (2) moderate CHD (eg, coarctation of the aorta, Tetralogy of Fallot); (3) severe CHD (eg, Fontan circulation, transposition of the great arteries); or (4) cardiac transplant recipient. Nurses measured height (0.1 cm) and weight (0.1 kg). Body mass index (BMI) was expressed as World Health Organization norms,22 and BMI was categorized according to International Obesity Task Force criteria.23 PA restrictions were obtained from the treating cardiologist.
Accelerometry
We used ActiGraph accelerometers (GT3X+, GT9X; ActiGraph LLC), which are widely used to objectively measure habitual PA in children.24 We fitted participants with the accelerometer to be worn over the right hip continuously for the next 7 days and to be removed only for water‐based activities (including showering and swimming) and during sleep. We used ActiLife v.6.13.2 (ActiGraph LLC) for accelerometer initialization (sampling set at 30 Hz) and file download, processing, and analysis. We generated 15‐second epoch .agd files from the raw .gt3x files. We identified valid accelerometry days if the device was worn for ≥600 min/day, permitting 60 minutes of ≤2 minutes of zeros to avoid discarding reasonable sedentary bouts as nonwear. Participants' overall accelerometry data were valid if they had ≥3 valid days. We compared these criteria with more conservative wear‐time criteria (≥4 valid days, composed of ≥3 weekdays and ≥1 weekend days).25 Fewer participants had valid data using this method (n=73 versus n=90), but neither sample characteristics nor group PA values differed; therefore, we opted to use the more liberal inclusion criteria (≥3 days).
For participants with valid files (ie, if they had ≥3 valid accelerometry days), we calculated summary values for each participant for a variety of PA metrics, defined as a daily mean value relative to participants' number of valid accelerometry days. Specific PA metrics included (1) daily sum of axis 1 (vertical) acceleration counts (“counts”) as a measure of total PA; (2) counts per minute (CPM) as a measure of relative PA intensity; (3) time spent in PA intensities, specifically MVPA (≥2296 CPM) and vigorous PA (≥4012 CPM).26 We estimated sedentary time as a percentage relative to valid accelerometer wear time, where percentage of sedentary time equals daily sedentary minutes (<100 CPM) divided by accelerometer wear time (min). Adherence to PA guidelines (≥60 minutes of MVPA daily13, 14) was defined as the probability of accumulating ≥60 minutes of MVPA on at least 6 of 7 days. Probability estimates are used to maximize the sample size because typically <40% of participants in accelerometry studies achieve the possible 7 days of valid accelerometer wear11, 12 (current study 44%). To calculate probabilities, a Bayesian approach was used to incorporate all participants with ≥3 valid accelerometry days; estimates are based on a β distribution, and participants' observed combination of active days (≥60 min/day MVPA) and valid wear days (≥600 min/day). This analytical approach was developed for accelerometry data collected as part of the US National Health and Nutrition Examination Survey (using a criterion of adhering to guidelines on at least 5 of 7 days),12 and a full description and specific analytical details are available elsewhere.27 To allow for comparisons with Canadian data, we applied the criteria used in the nationally representative Canadian Health Measures Survey of adhering to PA guidelines on at least 6 of 7 days.11
PA Questionnaire
A subsample completed the Physical Activity Questionnaire for Children (PAQ‐C) or Adolescents (PAQ‐A), depending on their age (≤11 versus ≥12 years, respectively). The PAQ is a self‐administered, 7‐day recall questionnaire that assesses participation in different types of activities, as well as activity during physical education.28 Each of the 8 (PAQ‐A) or 9 (PAQ‐C) questionnaire items is scored between 1 (low PA) and 5 (high PA), and a mean score of all items constitutes the overall PAQ score. We demonstrated the validity of the questionnaire against accelerometry in this population (associations between PAQ score and range of accelerometry‐derived PA metrics: ρ=0.44 to ρ=0.54, all P<0.001). We identified common sports/activity participation, defined as having participated ≥1 time during the previous 7 days. Participants also reported competitive sports participation (at school or outside of school).
Climate Records
We retrieved historical climate records for each participant during the PA measurement period (from http://climate.weather.gc.ca/index_e.html). For each participant, we identified weather stations that were geographically closest to home (except mountaintop weather stations or stations lacking weather records), and for each measurement day, we extracted relevant weather data (mean temperature [in °C], precipitation [in mm], and snow on the ground [in cm]). We calculated mean climate values for each participant based on the same days that were included in the generation of PA summary values.
Statistical Analyses
Sample descriptive statistics were calculated as frequencies (%), mean±SD, or median (interquartile range [IQR]) depending on the type of data and distribution within groups. Because of the variation in PA levels between participants, for example, medians (IQRs) were calculated for the overall sample and by sex for the accelerometry‐derived summary values. Between‐group differences were assessed by independent t tests (normal continuous data), Mann–Whitney U ranked sum or Kruskal–Wallis tests (nonnormal continuous data), the Pearson χ2 test (categorical data), or a 2‐sample test of proportion estimate (2‐tailed). Associations were assessed by Spearman rank correlation (ρ) and visual inspection of scatter plots. Multiple linear regression analyses were used to assess the association between accelerometry‐derived PA metrics—using the summary value per person across their valid measurement days as the outcome measure (eg, mean minutes of MVPA per day)—and relevant explanatory variables (sex, age, BMI z score, CHD severity, climate variables). Analyses were carried out using Stata v. 14.1 (StataCorp LLC) or SAS v. 9.1 (SAS Institute Inc; for probability estimates of adhering to PA guidelines27), and significance was set at P<0.05.
Results
Sample
We included 90 participants (aged 13.6±2.7 years, 54% male) who had sufficient accelerometry data. Excluded participants were older (aged 15.3±2.7 years) but were no different in terms of sex, CHD severity, or BMI. Cardiac diagnoses are shown in Table 1, and sample characteristics (age, sex, height, weight, BMI, disease severity, objective PA) are shown in Table 2. One quarter of children were overweight or obese, comparable to national data.11 Group median MVPA was ≈43 min/day; skewed group mean MVPA was no different from national data (≈49 versus ≈50 minutes).11 Adherence to PA guidelines, which was defined as accumulating ≥60 min/day MVPA on at least 6 of 7 days, was estimated at 8%. Adherence to guidelines overall and by sex (4% of girls, 11% of boys) was very similar to national estimates of 7% (4% of girls, 9% of boys). A detailed example of a participant's daily accelerometry data is illustrated in Figure 1.
Table 1.
Distribution and Classification of Cardiac Diagnosis in the Current Sample (n=90)
| Cardiac Diagnosis | n (%) |
|---|---|
| Mild | 26 (29) |
| Bicuspid aortic valve | 6 (7) |
| Ventricular septal defect | 5 (6) |
| Mild aortic stenosis | 4 (4) |
| Atrial septal defect | 4 (4) |
| Mixed aortic valve disease | 3 (3) |
| Mild pulmonary stenosis | 2 (2) |
| Mitral valve disease | 1 (1) |
| Small patent ductus arteriosus | 1 (1) |
| Moderate | 26 (29) |
| Tetralogy of Fallot | 11 (12) |
| Coarctation of the aorta | 7 (8) |
| Moderate/severe aortic stenosis | 2 (2) |
| Total anomalous pulmonary venous return | 1 (1) |
| Ostium primum atrial septal defect | 1 (1) |
| Sinus venosus atrial septal defect | 1 (1) |
| Ebstein's anomaly | 1 (1) |
| Shone syndrome | 1 (1) |
| Pentalogy of Cantrell | 1 (1) |
| Severe | 29 (32) |
| Fontan | 17 (19) |
| Transposition of the great arteries | 8 (9) |
| Double‐outlet ventricle | 2 (2) |
| Valved conduit | 2 (2) |
| Cardiac transplant | 9 (10) |
Congenital heart disease severity was categorized in accordance with consensus guidelines.21
Table 2.
Sample Characteristics and General Physical Activity Levels
| All (n=90) | Boys (n=49) | Girls (n=41) | P Value | |
|---|---|---|---|---|
| Participant characteristics | ||||
| Age, y | 13.6±2.7 | 13.8±2.8 | 13.5±2.7 | 0.550 |
| Height, cm | 157.0±13.2 | 158.9±14.9 | 154.8±10.5 | 0.139 |
| Weight, kg | 51.8±16.1 | 52.2±16.6 | 51.4±15.8 | 0.796 |
| BMI percentilea | 58.6±33.8 | 56.8±33.2 | 60.7±34.8 | 0.592 |
| BMI category (underweight/normal/overweight/obese)b | 12/54/19/5 | 7/32/8/2 | 5/22/11/3 | 0.539 |
| Cardiac diagnosis (mild/moderate/severe/Tx) | 26/26/29/9 | 15/12/15/7 | 11/14/14/2 | 0.408 |
| Physical activity (accelerometry‐derived)c | ||||
| Valid days (≥600 minutes wear time) | 6 (5–7) | 7 (6–7) | 6 (5–7) | 0.065 |
| Accelerometer wear time, min/day | 796 (758–846) | 802 (762–849) | 783 (754–823) | 0.215 |
| Total activity, counts×103/day | 306.0 (243.1–417.9) | 357.3 (288.0–472.4) | 256.1 (201.0–346.7) | 0.003d |
| Relative activity intensity, counts/min | 376.8 (302.6–510.2) | 433.7 (328.5–580.2) | 331.0 (252.5–432.1) | 0.005d |
| Steps, count/day | 7494 (6418–9862) | 8014 (6801–10 269) | 7194 (6087–9053) | 0.180 |
| Moderate‐to‐vigorous PA, min/daye | 42.6 (28.9–56.9) | 49.2 (33.1–67.2) | 39.2 (25.1–46.9) | 0.006d |
| Of which vigorous PA, min/day | 12.4 (6.1–20.7) | 16.8 (8.5–24.2) | 7.1 (3.3–13.3) | 0.001d |
| Sedentary time, %/day | 70.0 (61.2–75.9) | 65.2 (59.4–75.3) | 72.8 (66.5–77.8) | 0.045d |
| Adherence to PA guidelines on ≥6 day/weekf | 8% | 12% | 4% | 0.172 |
Data are mean±SD, n, or median (interquartile range). BMI indicates body mass index; PA, physical activity; Tx, cardiac transplant recipient.
BMI (kg/m2) percentiles calculated based on age–sex‐specific World Health Organization 2007 reference charts.22
International Obesity Task Force age–sex‐specific BMI weight categorization.23
ActiGraph accelerometry (GT3X+ or GT9X; 15‐second epoch), values are daily means based on ≥3 days with ≥600 minutes valid wear time.
Significantly different between sexes (P<0.05).
Based on cut points of Evenson et al (moderate‐to‐vigorous: ≥2296 counts/min; vigorous: ≥4012 counts/min).26
Figure 1.
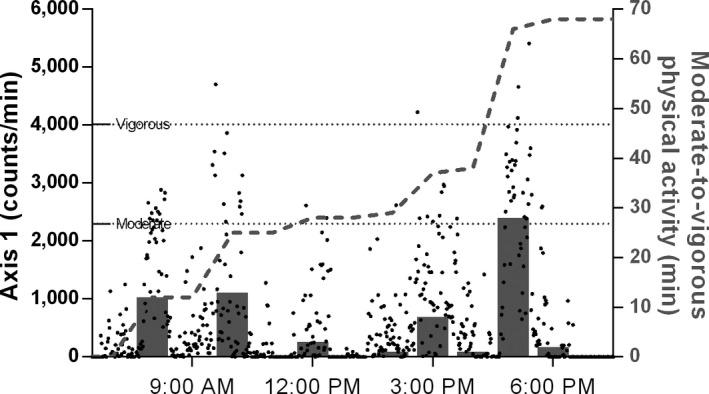
Detailed physical activity profile of a 10‐year old female Fontan patient. On this example day, the patient accrued a total of 68 minutes of moderate‐to‐vigorous physical activity (MVPA). Dots indicate detailed acceleration data, and dotted horizontal lines indicate thresholds to categorize acceleration data into moderate or vigorous intensity. Bars show MVPA per hour; the dashed line shows the cumulative MVPA for the day.
Association Between CHD Severity and Accelerometry‐Derived PA
There were no significant differences in daily MVPA by CHD severity (Figure 2). Age was inversely associated with MVPA in boys (ρ=−0.35, P=0.01) and in girls (ρ=−0.36, P=0.02) (Figure 3). Age was positively associated with sedentary time in both boys (ρ=0.55, P<0.001) and girls (ρ=0.58, P<0.001) (Figure 3). MVPA and sedentary time were closely related in both sexes (ρ=−0.74 and ρ=−0.73, respectively; all P<0.001).
Figure 2.
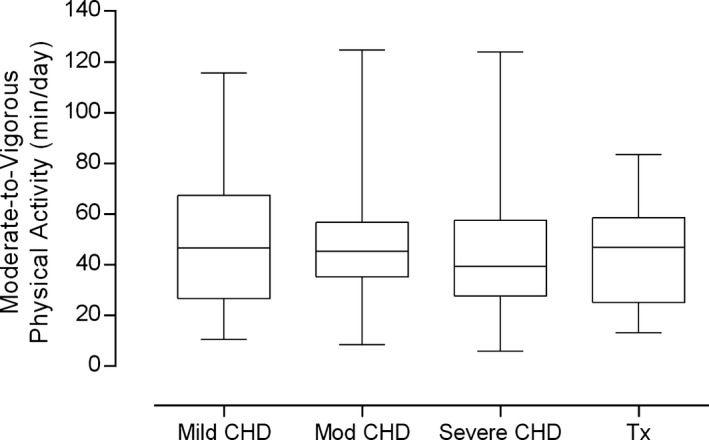
Objectively measured physical activity by CHD severity. Bars and whiskers depict medians, interquartile and absolute ranges of daily moderate‐to‐vigorous physical activity levels. There were no significant between‐group differences, with highly active participants found in all CHD groups. CHD indicates congenital heart disease; Tx, cardiac transplant recipient.
Figure 3.
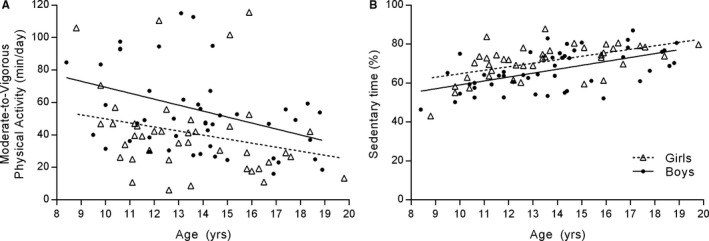
Association between age and accelerometry‐derived metrics of physical activity. A, Moderate‐to‐vigorous physical activity declined with age in boys (ρ=−0.35, P=0.01) and in girls (ρ=−0.36, P=0.02). B, Sedentary time increased in both groups (ρ=0.55–0.58, P<0.001).
In multiple linear regression analyses, age and sex were significant explanatory variables for total activity, MVPA, and sedentary behavior but not for vigorous PA in model 1 (Table 3). Age was inversely related to daily minutes of MVPA (P<0.01), and girls were significantly less active than boys (P<0.05). Model 2 also included CHD severity (reference: mild), which was not a significant explanatory variable. BMI z score was nonsignificant in all models. Overall, sex, age, BMI z score, and CHD severity explained relatively little of the variance in PA (≈15–20%) and sedentary behaviors (≈34%).
Table 3.
Multiple Regression Analyses for Accelerometrya‐Derived Physical Activity Parameters
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| β | 95% CI | β | 95% CI | |
| DV: total PA, counts×103/dayb | ||||
| Sex (ref: male) | −94.5 | (−175.7, −13.2)c | −91.6 | (−174.6, −8.7)c |
| Age, y | −24.4 | (−39.4, −9.5)d | −25.7 | (−40.9, −10.6)e |
| BMI z scoref | −12.4 | (−42.8, 18.0) | −13.9 | (−47.7, 19.9) |
| Cardiac diagnosis (ref: mild) | ||||
| Moderate | −10.6 | (−124.3, 103.1) | ||
| Severe | −69.6 | (−177.5, 38.2) | ||
| Transplant | −5.7 | (−167.2, 155.7) | ||
| r 2 | 0.154d | 0.176c | ||
| DV: MVPA, min/dayg | ||||
| Sex (ref: male) | −14.7 | (−25.9, −3.5)c | −15.4 | (−26.7, −4.1)d |
| Age, y | −3.2 | (−5.3, −1.1)d | −3.3 | (−5.4, −1.2)d |
| BMI z scoref | −2.6 | (−6.8, 1.6) | −3.8 | (−8.4, 0.8) |
| Cardiac diagnosis (ref: mild) | ||||
| Moderate | −2.3 | (−17.9, 13.2) | ||
| Severe | −11.2 | (−25.9, 3.5) | ||
| Transplant | −16.4 | (−38.5, 5.6) | ||
| r 2 | 0.165d | 0.203d | ||
| DV: vigorous PA, min/dayh | ||||
| Sex (ref: male) | −6.5 | (−13.3, 0.2) | −6.7 | (−13.6, 0.1) |
| Age, y | −0.9 | (−2.1, 0.4) | −1.0 | (−2.2, 0.3) |
| BMI z scoref | −1.8 | (−4.3, 0.8) | −2.5 | (−5.3, 0.3) |
| Cardiac diagnosis (ref: mild) | ||||
| Moderate | −2.4 | (−11.8, 7.0) | ||
| Severe | −7.4 | (−16.3, 1.5) | ||
| Transplant | −8.7 | (−22.1, 4.6) | ||
| r 2 | 0.119 | 0.119 | ||
| DV: sedentary, %/dayi | ||||
| Sex (ref: male) | 5.0 | (1.6, 8.4)d | 5.1 | (1.6, 8.6)d |
| Age, y | 1.9 | (1.3, 2.5)e | 1.9 | (1.3, 2.6)e |
| BMI z scoref | −0.3 | (−1.5, 1.1) | 0.1 | (−1.4, 1.5) |
| Cardiac diagnosis (ref: mild) | ||||
| Moderate | 0.9 | (−3.9, 5.7) | ||
| Severe | 2.6 | (−2.0, 7.1) | ||
| Transplant | 3.8 | (−3.0, 10.7) | ||
| r 2 | 0.331e | 0.347e | ||
Model 1: association between physical activity parameters and various relevant explanatory variables; Model 2: model 1 plus inclusion of cardiac diagnosis. BMI indicates body mass index; DV, dependent variable; MVPA, moderate‐to‐vigorous PA; PA, physical activity; ref, reference.
ActiGraph accelerometry (GT3X+ or GT9X; 15‐second epoch), based on ≥3 days with ≥600 minutes valid wear time.
Total PA: sum of axis 1 (or vertical axis) counts/day.
P<0.05.
P<0.01.
P<0.001.
BMI (kg/m2) z scores calculated based on age–sex‐specific World Health Organization 2007 reference charts.22
MVPA: mean daily minutes of MVPA (≥2296 counts/min).26
Vigorous PA (≥4012 counts/min).26
Sedentary time (%): mean sedentary minutes (<100 counts/min) expressed as a percentage relative to valid monitor wear time.
Association Between Climate and Accelerometry‐Derived PA
PA was measured between July 2014 and August 2016, predominantly during spring and fall months. Participants resided all across the Canadian provinces of British Columbia and the Yukon, which encompass moderate oceanic climates in the southwest to subarctic climates in the north. Detailed location‐specific climate data for individual PA measurement periods were available for all participants (proximity between home and weather station: median 6.6 km, IQR 3.9–11.5 km). Daily temperatures (9.1°C, IQR 3.5–13.6) and precipitation (1.3 mm, IQR 0.4–3.5) varied between participants according to season and location; snow cover (≥1 cm) was rare during measurement periods (n=3 participants). Few measurement days (6%) occurred on days on which temperatures fell below freezing, with PA guidelines (≥60 min/day MVPA) still met often (33% of those days). Overall, there was no significant association between MVPA and mean daily temperature (P=0.280) or mean precipitation (P=0.553). Additionally adjusting regression models (see Table 3) for mean temperature did not change the results and explained only an additional ≈1% of the variance in accelerometry‐derived PA metrics (data not shown).
Sports Participation
A subsample completed the PAQ (n=61; 49% male). The median score was 2.6 of 5 (IQR 2.1–3.1), which is somewhat lower than scores of ≈3.1 and ≈2.7 that have been reported for healthy children aged 10 and 15 years, respectively, from British Columbia.29 Commonly reported activities were running (72%), soccer (40%), swimming (34%), and dancing (31%). Soccer was more common among boys than girls (52% versus 27%), whereas the reverse was true for dance (19% versus 43%; all P<0.05). Objectively measured MVPA was higher in those who played soccer than those that did not (median 55 versus 39 min/day; P=0.01). The same was observed for swimming (median 57 versus 42 min/day; P=0.01) but not for running and dancing.
Most participants were attending school (95%). Objectively measured MVPA was significantly higher for those who participated in competitive sports outside of school (57%; 54 versus 39 min/day, P<0.01), but there was no difference for competitive school sports (30%; P>0.05).
PA Restrictions
Overall, 15% of participants received PA restrictions from their cardiologist, all of which were to avoid intense isometric contractions (ie, weightlifting). All of these participants had diagnoses that related to structural abnormalities of the left ventricular outflow tract (ie, bicuspid aortic valve) or coarctation of the aorta. None were given restrictions for aerobic exercise. There was no difference in MVPA between those with activity restrictions and those without (P>0.05).
Discussion
We objectively assessed PA in boys and girls aged 8 to 19 years across patients with a wide range of CHD disease severities and cardiac transplant recipients. Compared with national data, we found that the activity levels of our sample overall were broadly comparable to those of healthy Canadian boys and girls (≈49 versus ≈50 min/day MVPA) and not lower, as is commonly thought.8 We also found no significant differences between CHD disease severity for any accelerometry‐derived PA metric or sedentary behavior, which is increasingly being recognized as a risk factor for cardiovascular health independent of PA.20 Although it is encouraging that our findings suggest that children with CHD are no less active than their healthy peers, the results need to be viewed in the context of alarmingly low levels of PA in children generally.9, 10 In Canada, only 7% of Canadian boys and girls11 meet PA guidelines of at least 60 minutes of MVPA daily13, 14 (versus 8% in our sample and similar prevalence in US youth12)—a concerning pattern that has been observed globally and that World Health Organization global strategies and national policies have not yet been able to meaningfully address.9
Objectively Measured PA Levels in Children With CHD
Our study builds on previous studies that have assessed PA by accelerometry in children with CHD and that collectively provide a conflicting evidence base regarding the PA levels of this population. This is likely explained by variations in study setting, sample characteristics (including sample size, age range, and CHD diagnoses), and analytical approaches. Most notably, a landmark paper by McCrindle and colleagues (in Ontario, Canada) reported that the PA levels in >100 Fontan patients aged 6 to 18 years were markedly lower than those of healthy children.15 The authors used methodologies similar to ours to objectively assesses PA (ActiGraph accelerometer, but with a 1‐minute epoch and a different MVPA cut point); however, in the absence of Canadian‐normative data for objectively measured PA (published a few years later), they used data from a relatively small sample (in Massachusetts) of highly active children as their “normal” reference (of which 70% met PA guidelines of ≥60 min/day of MVPA). More recent population estimates for both Canada and the United States provide much lower normative values for MVPA,11, 12 which may have resulted in different interpretation of their findings in Fontan patients relative to healthy children.
Another small study (in Ontario, Canada) found that very young children aged 3 to 5 years with coarctation of the aorta or Tetralogy of Fallot (n=10) achieved ≈72 min/day MVPA on average, which was no different from healthy age‐matched controls.19 These high levels of activity are likely explained by a combination of the very young age range (because PA decreases with age) and the researchers' use of a much lower accelerometry sampling interval (3 seconds), to capture the sporadic high‐intensity activity typical of young children, and a lower MVPA threshold; combined, these measures will lead to higher estimates of MVPA compared with our analytical approach. A study of Fontan patients (in Ontario, Canada; n≈60) reported mean estimates of MVPA that were similar to our sample (≈50 versus 49 min/day); these data are also based on 15‐second epoch, but the investigators used a different device and MVPA threshold, and it is of note that their sample was much younger than ours (6–12 years).30, 31 Another small study (in Milwaukee, Wisconsin) assessed MVPA across a range of CHD severities and found high levels of MVPA overall (≈70 min/day),17 despite using a lower sampling interval (30‐second epoch) and higher, metabolic equivalent–based MVPA (≥4 metabolic equivalents) thresholds that would yield lower estimates of MVPA compared with our approach. Although their age range was similar to ours (6–19 years), the majority of children were much younger (mean age 10.6 versus 13.6 years), and the overall sample size was small (n=21). A study from Germany assessed MVPA in children and adults across a wide age range (8–52 years) after total cavopulmonary connection.32 They found their sample to be highly active (98 min/day), but it is of note that they used a different device, as well as a metabolic equivalent–based (≥3 metabolic equivalents) MVPA threshold. The diverse findings from these previous studies may be attributable to their evaluations of specific CHD populations and different methodologies, but it is also important to consider the importance of age, sex, and sociocultural factors in PA that have been left largely unexplored in this population.
Multifactorial Determinants of PA in Children With CHD?
We found that boys were more active and less sedentary than girls and that PA declined and sedentary behavior increased during adolescence. These age–sex patterns are as expected in healthy children11, 33, 34 and add to an emerging literature on this topic in CHD.18, 35 In healthy children, the determinants of PA are multifactorial and include factors that are intrapersonal (attitudes, motivation, self‐efficacy), sociocultural (encouragement, support, social norms), and environmental (access to opportunities and facilities).34 Several qualitative studies have identified low self‐efficacy, covert fears, discomfort, and physical fatigue as limiting factors in children with CHD.36 Some children report having been bullied by peers and perceive a lack of understanding by their teachers,37 which is important to consider for school‐based PA promotion in children with CHD. Environmental factors, such as seasonality, may affect PA of healthy children38 and this has also been observed in children with CHD,39, 40 along with findings that outdoor time is linked to more PA.39 Our results in the context of existing literature suggests that future research on PA determinants in children with CHD ought to be framed within a socioecological framework that addresses the complexity of contributing factors.
The Role of PA Restrictions
The American Heart Association recommends that children with CHD follow normal PA guidelines (≥60 min/day MVPA) because formal activity restrictions are rarely warranted in the absence of arrhythmias or significant left ventricular outflow tract pathology.8 Formal restrictions depend on individual circumstances because the evidence base for exercise restriction across the range of CHD is limited. The Association for European Paediatric Cardiology further recommends that individual PA counseling should be a priority at every clinical visit, including written recommendations for exercise (with restrictions and permissions).7 Cardiologists and parents or guardians, however, may have a tendency to restrict PA in children with CHD.36, 41 In our study, PA restrictions through the cardiologist were infrequent (≈15%) and specific to intense isometric activity, typically for patients with structural abnormalities of the left ventricular outflow tract (ie, bicuspid aortic valve) or coarctation of the aorta. We found no differences in PA between children who received restrictions compared with those who did not. Encouragement of PA by care providers may be important; one study attributed patients' high PA to the department's active encouragement of sport and PA participation.32 Structured clinical approaches to promote PA, such as exercise training or cardiac rehabilitation, are effective in children with CHD,42 but availability and/or access to such programs is limited, and effects on lifelong PA habits have not been documented. We did not assess parental restrictions or encouragement for PA or whether children restricted their own activities based on fear of adverse events.
Do Physical Limitations Affect PA Levels?
Some patients with CHD have a reduced maximal exercise capacity,7 which may result in a reduced ability to perform optimally in endurance activities. There have been reports, however, that children with severe CHD are not limited during submaximal exercise.43 This is important, given that most sports and activities of daily living rarely require a sustained maximal effort. We know of only 1 study that related objectively measured PA to ventricular dysfunction in Fontan patients, and no association was found.15 The lack of an association between CHD severity and PA in our study supports the notion that these children may not experience appreciable PA limitations as a result of their cardiac diagnosis.
Strengths and Limitations
We used objective measures to assess PA and sedentary behavior in children with a range CHD severities, and provide context for these data through supplementary use of a validated questionnaire. We obtained detailed location‐ and person‐specific climate records for the ≈500 accelerometry‐days and were able to rule out that weather had an impactful role on our overall PA results (sex and age were important explanatory factors, CHD disease severity was not). However, it is of note that our cross‐sectional study design precludes us from assessing any potential effects of seasonality within individuals. We note that the CHD classification criteria used in the present study grouped 2 participants with atrial septal defects as moderate CHD severity; however, we found no meaningful differences in any of our findings when coding these participants as having mild CHD. We did not impute missing PA data when devices where not worn (eg, during swimming), which may have slightly underestimated PA levels in some participants. We did not include physiological measures such as ventricular function or exercise capacity and cannot assess any potential effects on our findings.
Conclusion
Objectively measured PA did not differ by CHD severity but followed expected age–sex patterns, suggesting that sociocultural factors are likely important determinants of PA in these children.
Sources of Funding
We received funding from the Heart and Stroke Foundation of Canada, BC & Yukon Division (F1504, PI Harris) and the Rare Diseases Foundation (PI Harris). Funders had no involvement in the study design, data collection, analysis and interpretation of data, and the writing of this article.
Disclosures
None.
Acknowledgments
We are grateful to the children and their families for participating in this research. We thank all the nurses, administrative staff and research team at the Children's Heart Centre for their assistance.
(J Am Heart Assoc. 2017;6:e004665. DOI: 10.1161/JAHA.116.004665.)
Preliminary analyses of this work were presented as an oral presentation at the American Heart Association Scientific Sessions, November 12 to 16, 2016, in New Orleans, LA and as a poster presentation at the Canadian Cardiovascular Congress, October 22 to 25, 2016, in Montreal, Canada.
References
- 1. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos‐Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:2241–2247. [DOI] [PubMed] [Google Scholar]
- 2. Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. [DOI] [PubMed] [Google Scholar]
- 3. Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. [DOI] [PubMed] [Google Scholar]
- 4. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. [DOI] [PubMed] [Google Scholar]
- 5. Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J; American Heart Association Expert Panel on Population and Prevention Science, American Heart Association Council on Cardiovascular Disease in the Young, American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Nutrition Physical Activity and Metabolism, American Heart Association Council on High Blood Pressure Research, American Heart Association Council on Cardiovascular Nursing, American Heart Association Council on the Kidney in Heart Disease, Interdisciplinary Working Group on Quality of Care and Outcomes Research . Cardiovascular risk reduction in high‐risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. [DOI] [PubMed] [Google Scholar]
- 6. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 7. Takken T, Giardini A, Reybrouck T, Gewillig M, Hovels‐Gurich HH, Longmuir PE, McCrindle BW, Paridon SM, Hager A. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: a report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur J Prev Cardiol. 2012;19:1034–1065. [DOI] [PubMed] [Google Scholar]
- 8. Longmuir PE, Brothers JA, de Ferranti SD, Hayman LL, Van Hare GF, Matherne GP, Davis CK, Joy EA, McCrindle BW; on behalf of the American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young . Promotion of physical activity for children and adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;127:2147–2159. [DOI] [PubMed] [Google Scholar]
- 9. Sallis JF, Bull F, Guthold R, Heath GW, Inoue S, Kelly P, Oyeyemi AL, Perez LG, Richards J, Hallal PC; Lancet Physical Activity Series 2 Executive C . Progress in physical activity over the Olympic quadrennium. Lancet. 2016;388:1325–1336. [DOI] [PubMed] [Google Scholar]
- 10. Tremblay MS, Barnes JD, Gonzalez SA, Katzmarzyk PT, Onywera VO, Reilly JJ, Tomkinson GR; Global Matrix 2.0 Research T . Global Matrix 2.0: report card grades on the physical activity of children and youth comparing 38 countries. J Phys Act Health. 2016;13:S343–S366. [DOI] [PubMed] [Google Scholar]
- 11. Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian children and youth: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011;22:15–23. [PubMed] [Google Scholar]
- 12. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization . Global recommendations of physical activity for health—5‐17 years old. 2011. Available at: http://www.who.int/dietphysicalactivity/physical-activity-recommendations-5-17years.pdf?ua=1. Accessed November 23, 2016. [PubMed]
- 14. Canadian Society for Exercise Physiology . 24‐hour movement guidelines for children and youth. 2016. Available at: http://www.csep.ca/CMFiles/Guidelines/24hrGlines/Canadian24HourMovementGuidelines2016.pdf. Accessed August 9, 2016.
- 15. McCrindle BW, Williams RV, Mital S, Clark BJ, Russell JL, Klein G, Eisenmann JC. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. 2007;92:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massin MM, Hovels‐Gurich HH, Gerard P, Seghaye MC. Physical activity patterns of children after neonatal arterial switch operation. Ann Thorac Surg. 2006;81:665–670. [DOI] [PubMed] [Google Scholar]
- 17. Ewalt LA, Danduran MJ, Strath SJ, Moerchen V, Swartz AM. Objectively assessed physical activity and sedentary behaviour does not differ between children and adolescents with and without a congenital heart defect: a pilot examination. Cardiol Young. 2012;22:34–41. [DOI] [PubMed] [Google Scholar]
- 18. Kao CC, Chang PC, Chiu CW, Wu LP, Tsai JC. Physical activity levels of school‐age children with congenital heart disease in Taiwan. Appl Nurs Res. 2009;22:191–197. [DOI] [PubMed] [Google Scholar]
- 19. Stone N, Obeid J, Dillenburg R, Milenkovic J, MacDonald MJ, Timmons BW. Objectively measured physical activity levels of young children with congenital heart disease. Cardiol Young. 2015;25:520–525. [DOI] [PubMed] [Google Scholar]
- 20. Saunders TJ, Chaput JP, Tremblay MS. Sedentary behaviour as an emerging risk factor for cardiometabolic diseases in children and youth. Can J Diabetes. 2014;38:53–61. [DOI] [PubMed] [Google Scholar]
- 21. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118:e714–e833. [DOI] [PubMed] [Google Scholar]
- 22. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ. 2007;85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherar LB, Griew P, Esliger DW, Cooper AR, Ekelund U, Judge K, Riddoch C. International children's accelerometry database (ICAD): design and methods. BMC Public Health. 2011;11:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carson V, Janssen I. Volume, patterns, and types of sedentary behavior and cardio‐metabolic health in children and adolescents: a cross‐sectional study. BMC Public Health. 2011;11:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26:1557–1565. [DOI] [PubMed] [Google Scholar]
- 27. National Cancer Institute . Risk factor monitoring and methods: SAS programs for analyzing NHANES 2003‐2004 accelerometer data. Available at: http://riskfactor.cancer.gov/tools/nhanes_pam. Accessed November 23, 2016.
- 28. Kowalski KC, Crocker RE, Donen RM. The Physical Activity Questionnaire for Older Children (PAC‐C) and Adolescents (PAQ‐A) Manual. Saskatoon, Canada: University of Saskatchewan; 2004. [Google Scholar]
- 29. Voss C, Sandercock G, Wharf Higgins J, Macdonald H, Nettlefold L, Naylor PJ, McKay H. A cross‐cultural comparison of body composition, physical fitness and physical activity between regional samples of Canadian and English children and adolescents. Can J Public Health. 2014;105:e245–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Longmuir PE, Corey M, Faulkner G, Russell JL, McCrindle BW. Children after Fontan have strength and body composition similar to healthy peers and can successfully participate in daily moderate‐to‐vigorous physical activity. Pediatr Cardiol. 2015;36:759–767. [DOI] [PubMed] [Google Scholar]
- 31. Longmuir PE, Tyrrell PN, Corey M, Faulkner G, Russell JL, McCrindle BW. Home‐based rehabilitation enhances daily physical activity and motor skill in children who have undergone the Fontan procedure. Pediatr Cardiol. 2013;34:1130–1151. [DOI] [PubMed] [Google Scholar]
- 32. Muller J, Christov F, Schreiber C, Hess J, Hager A. Exercise capacity, quality of life, and daily activity in the long‐term follow‐up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J. 2009;30:2915–2920. [DOI] [PubMed] [Google Scholar]
- 33. Nader PR, Bradley RH, Houts RM, McRitchie SL, O'Brien M. Moderate‐to‐vigorous physical activity from ages 9 to 15 years. JAMA. 2008;300:295–305. [DOI] [PubMed] [Google Scholar]
- 34. Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW; Lancet Physical Activity Series Working Group . Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380:258–271. [DOI] [PubMed] [Google Scholar]
- 35. Fredriksen PM, Ingjer E, Thaulow E. Physical activity in children and adolescents with congenital heart disease. Aspects of measurements with an activity monitor. Cardiol Young. 2000;10:98–106. [DOI] [PubMed] [Google Scholar]
- 36. Moola F, Faulkner GE, Kirsh JA, Kilburn J. Physical activity and sport participation in youth with congenital heart disease: perceptions of children and parents. Adapt Phys Activ Q. 2008;25:49–70. [DOI] [PubMed] [Google Scholar]
- 37. Moola FJ, Faulkner GEJ, Kirsh JA, Schneiderman JE. Developing physical activity interventions for youth with cystic fibrosis and congenital heart disease: learning from their parents. Psychol Sport Exerc. 2011;12:599–608. [Google Scholar]
- 38. Atkin AJ, Sharp SJ, Harrison F, Brage S, Van Sluijs EM. Seasonal variation in children's physical activity and sedentary time. Med Sci Sports Exerc. 2016;48:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Longmuir PE, Russell JL, Corey M, Faulkner G, McCrindle BW. Factors associated with the physical activity level of children who have the Fontan procedure. Am Heart J. 2011;161:411–417. [DOI] [PubMed] [Google Scholar]
- 40. Chen CW, Chen YC, Gau BS, Wang JK, Hung YT, Jwo JC. Exercise behavior in adolescents with mild congenital heart disease. J Cardiovasc Nurs. 2012;27:317–324. [DOI] [PubMed] [Google Scholar]
- 41. Longmuir PE, McCrindle BW. Physical activity restrictions for children after the Fontan operation: disagreement between parent, cardiologist, and medical record reports. Am Heart J. 2009;157:853–859. [DOI] [PubMed] [Google Scholar]
- 42. Duppen N, Takken T, Hopman MTE, ten Harkel ADJ, Dulfer K, Utens EMWJ, Helbing WA. Systematic review of the effects of physical exercise training programmes in children and young adults with congenital heart disease. Int J Cardiol. 2013;168:1779. [DOI] [PubMed] [Google Scholar]
- 43. Muller J, Bohm B, Semsch S, Oberhoffer R, Hess J, Hager A. Currently, children with congenital heart disease are not limited in their submaximal exercise performance. Eur J Cardiothorac Surg. 2013;43:1096–1100. [DOI] [PubMed] [Google Scholar]


