Abstract
Background
Recent studies have demonstrated that there is a high variability of renal sympathetic nerve density distribution from proximal to distal renal artery segments. The aim of our study was to investigate the roles of renal sympathetic nerve stimulation (RSS) on atrial fibrillation and cardiac autonomic nervous activity.
Methods and Results
Twenty‐eight dogs were randomly assigned to the proximal RSS group (P‐RSS, N=7), middle RSS group (M‐RSS, N=7), distal RSS group (D‐RSS, N=7), and the control group (sham RSS, N=7). RSS was performed using electrical stimulation on the bilateral renal arteries for 3 hours. Effective refractory period and the window of vulnerability were measured at atrial and pulmonary veins sites. Superior left ganglionated plexi (SLGP) and left stellate ganglion (LSG) function and neural activity were determined. C‐fos and nerve growth factor protein expression in the SLGP and LSG were examined. Only P‐RSS (1) caused pronounced blood pressure rises, induced a significant decrease in effective refractory period, and generated a marked increase in cumulative window of vulnerability and effective refractory period dispersion; (2) increased the frequency and amplitude of the neural activity in the SLGP and LSG; (3) increased SLGP and LSG function; and (4) upregulated the level of c‐fos and nerve growth factor expression in the SLGP and LSG.
Conclusions
This study demonstrated that renal sympathetic nerve activation induced by 3 hours of P‐RSS facilitated atrial fibrillation inducibility by upregulating cardiac autonomic nervous activity, suggesting a potential autonomic cross talk between kidney and heart.
Keywords: atrial fibrillation, ganglionated plexus, left stellate ganglion, renal sympathetic nervous system
Subject Categories: Atrial Fibrillation, Autonomic Nervous System
Atrial fibrillation (AF) is a complex arrhythmia with multiple mechanisms, including electrical, structural, and neural remodeling. The importance of autonomic nerve disorder in the genesis and maintenance of AF has been well recognized.1 Recently, there has been increasing evidence showing an important association between renal sympathetic nerves (RSN) and AF.2, 3, 4 In an AF model induced by obstructive sleep apnea, Linz et al reported that RSN denervation resulted in a pronounced attenuation of atrial refractoriness shortening and spontaneous atrial arrhythmias.2 Hou et al showed that RSN denervation could reduce AF inducibility and reverse the atrial electrophysiological changes induced by sympathetic hyperactivity.3 More importantly, in clinical studies in patients with atrial fibrillation and resistant hypertension, patients who received circumferential pulmonary vein isolation combined with RSN denervation showed fewer AF recurrences than those in the pulmonary vein isolation–alone group.5, 6 These studies indicated that RSN may have a role in the generation of AF.
Previous studies from our group7, 8, 9 and from Chen et al10 have suggested that both ganglionated plexus (GP) and left stellate ganglion (LSG) play critical roles in the initiation and maintenance of AF. Recently, we showed that RSN activation by direct electrical stimulation could increase both systemic and cardiac sympathetic activity and facilitate acute ischemia‐induced ventricular arrhythmias.11 Taken together, we hypothesize that RSN activation could also increase AF inducibility by activating GP and LSG. A recent study has demonstrated that there is a high variability of renal sympathetic nerve density distribution from proximal to distal renal artery segment.12 The aim of our study was to investigate the roles of different segment renal sympathetic stimulation (RSS) on AF and cardiac autonomic nervous activity.
Methods
Animal Preparation
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Wuhan University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. Twenty‐eight male mongrel dogs (20±2 kg) with no anatomic variations in the renal arteries were supplied by the Center of Experimental Animals in the Medical College of Wuhan University. All dogs were anesthetized with Na‐pentobarbital, 40 mg/kg, and ventilated with room air by a positive‐pressure respirator (MAO01746; Harvard Apparatus, Holliston, MA). Additional maintenance doses of 2 mg/kg Na‐pentobarbital were administrated at the end of each hour during the procedure. Normal saline at 50 to 100 mL/h was infused to replace spontaneous fluid losses. Arterial blood pressure (BP) was continuously monitored from a femoral arterial sheath, and body surface electrocardiogram was recorded by using subcutaneous needle electrodes during the whole procedure. A heating pad was used to maintain the core body temperature of the dogs at 36.5±0.5°C.13, 14
The chest was entered through both left‐ and right‐sided thoracotomies at the fourth intercostal space. Eight multielectrode catheters were sutured to allow pacing and recording at the left and right superior pulmonary veins (LSPV and RSPV), left and right inferior pulmonary veins (LIPV and RIPV), left and right atrial appendage (LAA and RAA), and left and right atria (LA and RA). All tracings were amplified and digitally recorded using a computer‐based Lab System (Lead 7000; Jingjiang, Chengdu City, China), filtered at 30 to 500 Hz.
Experiment Protocol
Twenty‐eight dogs were randomly assigned to the proximal renal sympathetic stimulation (RSS) group (P‐RSS, N=7, 5‐10 mm from the renal artery ostium), middle RSS group (M‐RSS, N=7, 15‐20 mm from the renal artery ostium), distal RSS group (D‐RSS, N=7, 25‐30 mm from the renal artery ostium), and the control group (sham RSS, N=7) (Figure 1). Atrial electrophysiological function, superior left GP (SLGP), and LSG neural function and neural activity were determined at baseline and at the end of RSS. C‐fos and nerve growth factor (NGF) protein expression in the SLGP and LSG were also examined at the end of the study.
Figure 1.
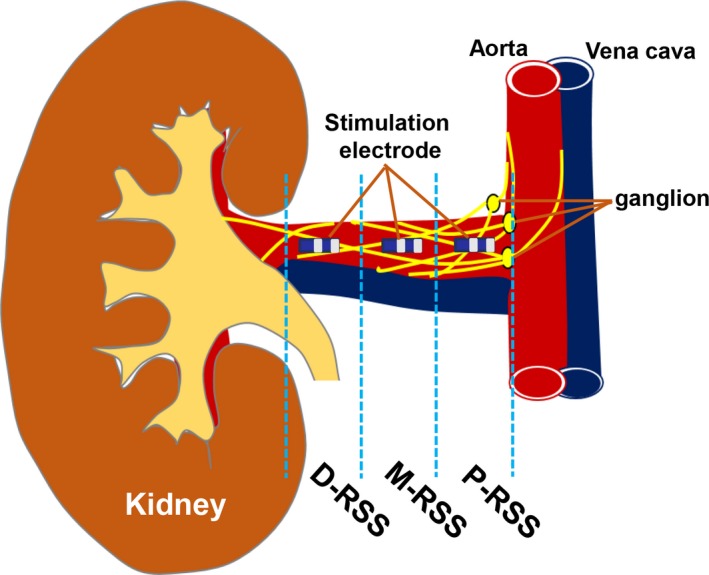
Schematic representation of location of the electrode on the adventitia of the renal artery. D‐RSS indicates distal RSS; M‐RSS, middle RSS; P‐RSS, proximal RSS; RSS, renal sympathetic stimulation.
Renal Sympathetic Stimulation
Bilateral retroperitoneal flank incisions were performed to expose the renal arteries. Two 3 Fr electrode catheters (3/2/1‐mm electrode spacing) were sutured on the adventitia of renal arteries to perform high‐frequency stimulation (frequency 20 Hz, pulse duration 2 milliseconds, intensity 10 V) with a stimulator (S88; Grass Instruments, Quincy, MA). The position of the electrode catheter sutured on the adventitia of the renal artery was 5 to 10 mm (sham group and P‐RSS group), 15 to 20 mm (M‐RSS group), or 25 to 30 mm (D‐RSS group) from the renal artery ostium (Figure 1). RSS was maintained for 3 hours.11
Atrial EP Study
As previous studies described,8, 9 programmed stimulation of atrial or pulmonary vein myocardium was performed using another stimulator (model 5328; Medtronic Inc, Minneapolis, MN). ERP was determined by programmed pacing, which consisted of 8 consecutive stimuli (S1‐S1=330 milliseconds) followed by a premature stimulus (S1‐S2), which was progressively decremented until refractoriness was achieved. Pacing was performed at 10× threshold (TH). ERP dispersion was calculated off line as the coefficient of variation (standard deviation/mean) of the ERP at all recording sites. The difference between the longest and shortest S1‐S2 interval at which AF was induced, at each bipolar pair, was defined as the window of vulnerability (WOV), which serves as a quantitative measure of AF inducibility. The cumulative WOV was counted as the sum of WOVs from all sites in each dog. AF was defined as an irregular atrial rate faster than 500 beats per minute associated with irregular atrioventricular conduction.8
Neural Activities Measurements
Direct Recording of SLGP and LSG Neural Activity
Two tungsten‐coated microelectrodes were inserted into the SLGP and LSG to contact multiple neuronal sites within the fat pad. The nerve signals were amplified with a 4‐channel AC/DC differential amplifier (DP‐304; Warner Instruments, Hamden, CT) with a high‐pass filter at 10 Hz and a low‐pass filter at 3000 Hz. The raw tracings of the neural activity were simultaneously recorded with a PowerLab data acquisition system (8/35; AD Instruments, Sydney, New South Wales, Australia). Thirty seconds of neural recording was acquired. The neural activity was characterized by its recorded amplitude and frequency. Neural activity was defined as deflections with a signal‐to‐noise ratio greater than 3:1, as previously described.8, 15, 16
Determination of SLGP and LSG Function
Based on previous studies, a bipolar plaque electrode was sutured to the epicardial surface overlying the SLGP for electrical stimulation (frequency 20 Hz; pulse duration 0.1 millisecond; intensity 10 V). Electrical stimulation was applied to the SLGP for 1 minute, and the lowest average sinus rate induced by SLGP stimulation was recorded and used as an indicator of the cholinergic influence of the SLGP.16, 17 LSG function was measured by applying electrical stimulation (frequency 20 Hz; pulse duration 0.1 millisecond, intensity 20 V). The maximal BP elevation induced by LSG stimulation was recorded as an indicator of the adrenergic influence from LSG.11
Real‐Time PCR
At the end of the experiment, SLGP and LSG were excised and washed by saline. The tissue was dissected into small portions, snap‐frozen in liquid nitrogen, and then maintained at −80°C until using. Total RNA was extracted from heart tissue with TRIzol reagent (Roche, Indianapolis, IN) as the manual instructed, and the concentration was assessed by Nanodrop 2000. A total of 2 μg RNA was used to synthesize cDNA using a Transcriptor First Stand cDNA Synthesis Kit (Roche). Quantitative real‐time PCR (qRT‐PCR) was performed with the LightCycler 480 SYBR Green I Master (Roche) and the LightCycler 480 real‐time PCR system according to the manufacturer's instructions (Roche). The primer pairs were designed online using Primer‐Blast: c‐fos forward 5′‐CAG TGC CAA CTT CAT CCC G‐3′, reverse 5′‐GCA GCC ATC TTA TTC CTT TCC‐3′; NGF forward 5′‐ACA GGA GCA AGC GGT CTT CG‐3′, reverse 5′‐TGG GTG GTG GTG CAG TAG GA‐3′. The PCR conditions used were as follows: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 10 seconds (denaturation), 60°C for 10 seconds (annealing), and 72°C for 20 seconds (extension). The relative expression levels of mRNAs were normalized to the reference gene GAPDH. All reactions were conducted in triplicate, and the data were calculated using the 2−ΔΔCT method.9
Determination of c‐fos and NGF Protein Expression in SLGP and LSG
Frozen tissues were homogenized with ice‐cold lysis buffer and prepared for total protein extraction. Protein concentration was measured by the BCA protein assay (Bio‐Rad, Hercules, CA). Denatured protein was separated by SDS‐PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked and incubated overnight at 4°C with primary antibodies against actin (Santa Cruz, Dallas, TX), NGF (Chemicon, Temecula, CA), and c‐fos (Abcam, Cambridge, UK). After 3 washes, the membranes were incubated with horseradish peroxidase‐conjugated second antibody (KPL) for 1 hour at room temperature and subsequently analyzed on an enhanced chemiluminescence detection system as previously described.11
Statistical Analysis
All continuous variables were expressed as mean±standard deviation. A paired t test was used for comparisons of continuous variables at the end of each hour versus in the baseline state. The continuous variables acquired at same time point among 4 groups were compared using 1‐way analysis of variance (ANOVA) or 2‐way repeated‐measures ANOVA with a Tukey post hoc test. SPSS 16.0 for Windows (SPSS Inc, Chicago, IL) was used for statistical analysis. Statistical significance was defined as P≤0.05.
Results
Effects of RSS on BP
The immediate BP response to RSS (20 Hz, 0.1‐millisecond pulse width, 10 V), which was determined as the highest systolic BP during 1 minute of RSS in 7 control dogs, is shown in Figure 2A. Compared with baseline (103±5 mm Hg), both P‐RSS (121±7 mm Hg) and M‐RSS (109±6 mm Hg) significantly increased the systolic BP (P=0.001 and 0.03, respectively) during 1 minute of stimulation, but D‐RSS did not affect it (Figure 2A). Similarly, the systolic BP was significantly increased in both P‐RSS (baseline 108±6 mm Hg vs 3 hours 135±9 mm Hg, P<0.001) and M‐RSS (baseline 105±5 mm Hg vs 3 hours 116±7 mm Hg, P=0.02) groups during 3 hours of stimulation when compared to baseline values (Figure 2B). However, sham RSS (control group) and D‐RSS did not change the systolic BP during 3 hours of stimulation. In addition, 3 hours of P‐RSS induced a more significant systolic BP elevation compared with M‐RSS.
Figure 2.
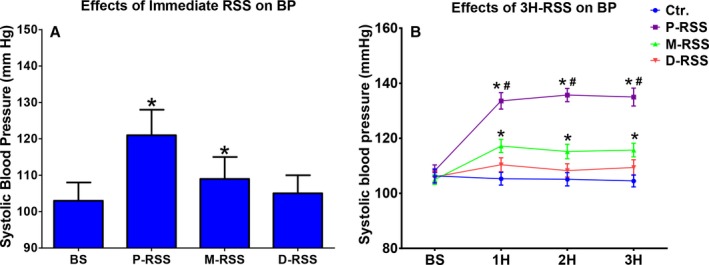
Changes in blood pressure (BP). A, Effects of immediate renal sympathetic stimulation (RSS) on BP. B, effects of 3 hours (3H) of RSS on BP. BS indicates baseline; Ctr, control; D‐RSS, distal RSS; M‐RSS, middle RSS; P‐RSS, proximal RSS. *P<0.05 vs baseline (BS); # P<0.05 vs other 3 groups at the same time point.
Effects of RSS on ERP, ERP Dispersion, and Cumulative WOV
As a result of 3 hours of P‐RSS, the ERP at all recording sites was progressively and significantly shortened (Figure 3). For example, ERP at the RSPV site was shortened from 117±9 milliseconds in the baseline state to 110±7 milliseconds at the end of the first hour, 101±9 milliseconds at the end of the second hour, and 95±10 milliseconds at the end of the third hour (P=0.03, 0.002, and <0.001, respectively). The ERP dispersion was also significantly increased by 3 hours of P‐RSS. The increase in cumulative WOV due to 3 hours of P‐RSS was significant as compared to baseline (Figure 3). However, no change in the ERP, ERP dispersion, or cumulative WOV was observed during the entire experimental period in the M‐RSS, D‐RSS, and control groups.
Figure 3.
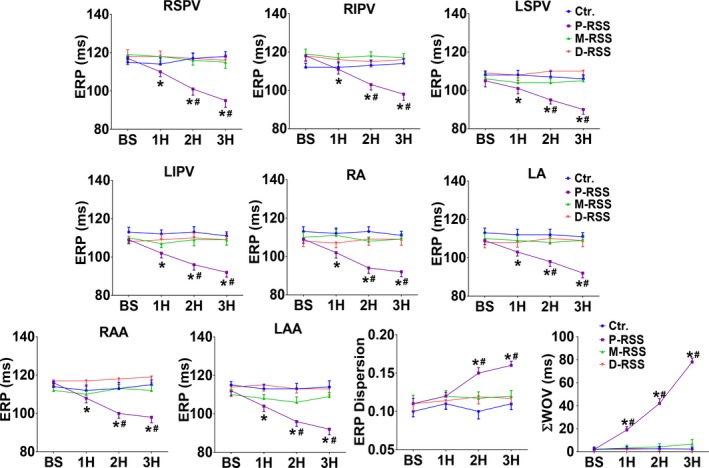
Effective refractory periods (ERP), ERP dispersion, and cumulative window of vulnerability (ΣWOV) changes in 4 groups. BS indicates baseline; Ctr, control; D‐RSS, distal RSS; M‐RSS, middle RSS; P‐RSS, proximal RSS; RA and LA, right and left atria; RAA and LAA, right and left atrial appendage; RIPV and LIPV, right and left inferior pulmonary vein; RSPV and LSPV, right and left superior pulmonary vein; RSS, renal sympathetic stimulation; 1H, 2H, 3H indicate 1, 2, and 3 hours of RSS, respectively. *P<0.05 vs BS; # P<0.05 vs other 3 groups at the same time point.
Effects of RSS on LSG and SLGP Activity
The frequency and amplitude of the neural activity directly recorded from the SLGP and LSG were markedly increased after 3 hours of P‐RSS, in contrast to the other groups (Figure 4). In the 7 dogs subjected to 3 hours of P‐RSS, there was an increase in both the frequency and the amplitude of the neural activity of SLGP (baseline 35±5 impulses/min, 0.13±0.03 mV vs 3 hours 106±7 impulses/min, 0.76±0.04 mV; P<0.001 for both when compared with the baseline) (Figure 4A and 4C). Figure 4B and 4D illustrate similar changes of both the frequency and the amplitude of the neural activity in the LSG in response to 3 hours of P‐RSS (baseline 24±5 impulses/min, 0.12±0.04 mV vs 3 hours 92±8 impulses/min, 0.45±0.05 mV; P<0.001 for both when compared with the baseline). There was no significant difference between the mean values of frequency and amplitude of the neural activity at the baseline and after 3 hours in other 3 groups.
Figure 4.
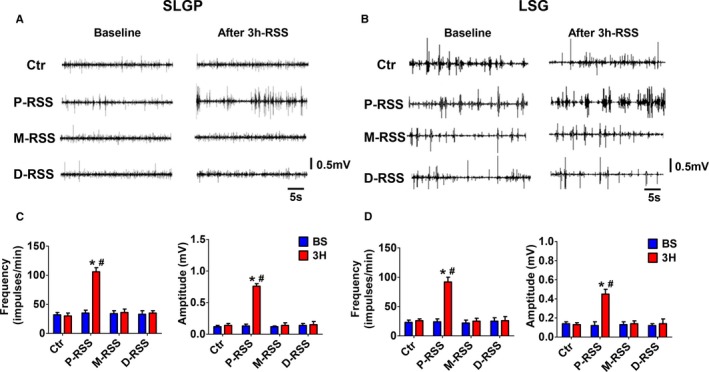
Representative examples (A and B) and quantification analysis (C and D) of autonomic neural activity in 4 groups. A and C, Neural activity in superior left ganglionated plexi (SLGP). (B and D, neural activity in left stellate ganglion (LSG). BS indicates baseline; Ctr, control; D‐RSS, distal RSS; M‐RSS, middle RSS; P‐RSS, proximal RSS; RSS, renal sympathetic stimulation. *P<0.05 vs baseline (BS); # P<0.05 vs other 3 group at the same time point.
Effects of RSS on LSG and SLGP Function
The maximal sinus rate slowing response induced by SLGP stimulation was used as a surrogate for the cholinergic influences. Figure 5A shows that the sinus rate slowing response due to SLGP stimulation was significantly increased by 3 hours of the P‐RSS (25.0±2.8% vs 37.1±5.1%, P=0.008). However, these changes were not detected in control (23.1±3.9% vs 27.3±3.2%, P=0.15), M‐RSS (25.8±4.0% vs 27.5±2.9%, P=0.42), or D‐RSS groups (24.3±3.0% vs 26.2±2.8%, P=0.29). We also found a significant activation of the ability of the LSG to increase BP during 3 hours of the P‐RSS (36.4±6.0% vs 47.5±9.6%, P=0.01) (Figure 5B). In contrast, the maximal BP elevation induced by LSG stimulation was not significantly changed in the control (33.4±7.6% vs 35.8±4.8%, P=0.65), M‐RSS (36.1±6.8% vs 37.2±4.7%, P=0.79), or D‐RSS groups (34.2±5.3% vs 35.6±5.9%, P=0.70) (Figure 5B).
Figure 5.
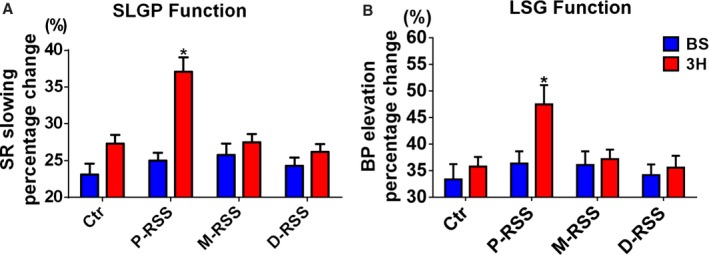
Changes of autonomic neural function by RSS in 4 groups. A, Sinus rate (SR) change induced by superior left ganglionated plexus (SLGP) stimulation. B systolic blood pressure (BP) change induced by left stellate ganglion (LSG) stimulation. BS indicates baseline; Ctr, control; D‐RSS, distal RSS; M‐RSS, middle RSS; P‐RSS, proximal RSS.; RSS, renal sympathetic stimulation. *P<0.05 vs baseline (BS).
Effects of RSS on C‐fos and NGF Expression in SLGP and LSG
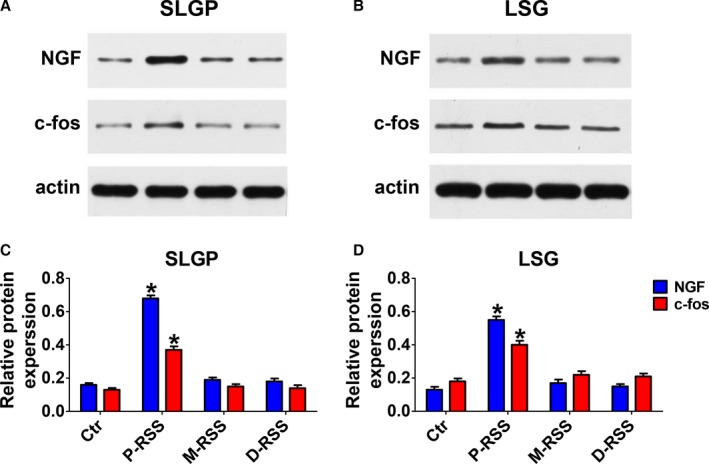
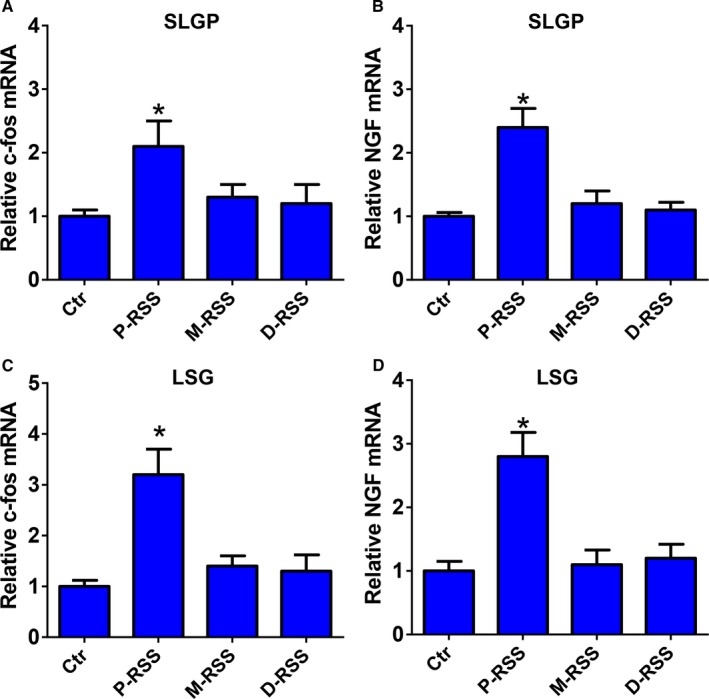
As shown in Figures 6 and 7, both the gene and protein expression of c‐fos and NGF in the SLGP and LSG were significantly increased in the P‐RSS group compared with the control group. However, no significant difference in the gene and protein expression of c‐fos and NGF in the SLGP and LSG was found in M‐RSS or D‐RSS group compared with the control group.
Figure 6.
The relative messenger RNA (mRNA) levels of c‐fos and nerve growth factor (NGF) expression in superior left ganglionated plexi (SLGP) (A and C) and left stellate ganglion (LSG) (B and D). BS indicates baseline; Ctr, control; D‐RSS, distal RSS; M‐RSS, middle RSS; P‐RSS, proximal RSS; RSS, renal sympathetic stimulation. *P<0.05 vs other 3 groups at the same time point.
Figure 7.
Representative examples (A and B) and relative protein levels (C and D) of c‐fos and NGF expression in superior left ganglionated plexi (SLGP) (A and B) and left stellate ganglion (LSG). BS indicates baseline; Ctr, control; D‐RSS, distal RSS; M‐RSS, middle RSS; P‐RSS, proximal RSS; RSS, renal sympathetic stimulation. *P<0.05 vs other 3 groups at the same time point.
Discussion
Major Findings
In this study we demonstrated that RSN activation induced by 3 hours of P‐RSS exerted profibrillatory effects on the atrium and all pulmonary veins. Specifically, RSN activation induced the shortening of ERP, significantly increased the ERP dispersion and AF inducibility, ie, WOV, at PVs and atrial sites. Several lines of evidence indicated that the profibrillatory effects of RSN activation were mediated by upregulating the neural activities in the SLGP and LSG. First, the frequency and amplitude of the neural activity directly recorded from the SLGP and LSG were markedly increased after 3 hours of P‐RSS (Figure 4). Second, the SLGP and LSG functions were increased after RSN activation (Figure 5A and 5B). Third, we found that c‐fos and NGF protein expression in the SLGP and LSG in the P‐RSS group were significantly higher (Figures 6 and 7). Therefore, we suggested that the RSN activation facilitated AF inducibility by upregulation of neural elements in the SLGP and LSG. Another major finding of this study is that only P‐RSS, but not M‐RSS or D‐RSS, increased the BP, upregulated the SLGP and LSG neural activities, and facilitated AF, indicating that proximal RSN might play a more important role in the modulation of cardiac autonomic activity and might be the optimal target for renal denervation in the management of AF.
The Relationship Between RSN and AF
It is well established that RSN is an important mediator for the central nervous system and can be a source of increased sympathetic activation,18 which plays a crucial role in the initiation and maintenance of AF.1 Clinical studies show that patients with chronic kidney disease, which is always accompanied by RSN activation, have a higher prevalence of AF, indicating that the kidney may have a role in the pathogenesis of AF.19 In recent years RSN denervation has emerged as a novel approach to decrease the sympathetic tone and as a potential therapeutic approach for AF. Both experimental and clinical studies have identified the beneficial effects of RSN denervation on AF. In a canine model of sympathetic hyperactivity induced by 3 hours of LSG stimulation plus rapid atrial pacing, Hou et al found that RSN denervation could reduce atrial fibrillation inducibility and reverse atrial electrophysiological changes.3 In a pig model of obstructive sleep apnea–induced AF, RSN denervation resulted in an even more pronounced attenuation of shortening in atrial ERP when compared to β‐blocker treatment, suggesting a superior antiarrhythmic effect of RSN denervation in this AF model.2 In a goat model of persistent AF induced by atrial burst pacing, RSN denervation reduced atrial sympathetic sprouting, structural alterations, and AF complexity independent of the changes in BP.20 Clinically, RSN denervation significantly reduced AF recurrences when combined with circumferential pulmonary vein isolation in patients with resistant hypertension.5 However, the direct influence of RSN activation on AF is still unknown. In this study we demonstrated that RSN activation could increase both SLGP and LSG neural activities and facilitate the occurrence of AF, further suggesting a causal relationship between RSN activation and AF and providing a potential mechanism underlying the anti‐AF effects of RSN denervation.
The Autonomic Cross Talk Between Kidney and Heart
Cardiac and kidney disease are common, increasingly encountered, and often coexist. The concept of cardiorenal syndrome was proposed by the Acute Dialysis Quality Initiative Wording Group in Venice, Italy, in 2008.21 The investigation on the heart‐kidney interaction is critical to understanding the cardiorenal syndrome. A prior study from our group showed that 3 hours of RSN stimulation was able to increase LSG function and facilitate the occurrence of ventricular arrhythmias during acute myocardial ischemia.11 We also found that increased ventricular arrhythmias induced by RSN stimulation were attenuated by LSG ablation.11 Based on this study, we proposed that there might be a potential link between RSN and LSG. However, the major limitation of this study is lack of direct evidence of neural activity recording. Subsequently, LSG neural activity was recorded before and after RSN denervation in ambulatory dogs, and the results showed that RSN denervation significantly decreased the 24‐hour average LSG nerve activity, which was associated with a reduction of paroxysmal atrial tachycardia episodes and duration.22 To further test the autonomic cross talk between kidney and heart, we investigated the effects of renal sympathetic stimulation on cardiac autonomic nerves, including SLGP and LSG. In this study we found that RSN activation significantly increased the neural activities and neural function in both SLGP and LSG, providing direct evidence about the autonomic cross talk between the heart and the kidney. In addition, the expressions of c‐fos and NGF protein in SLGP and LSG were significantly higher in P‐RSS group than other groups. C‐fos is a rapid indicator of extensive activation of neurons, and NGF is a biomarker of growth, survival, and differentiation of sympathetic neurons.11 The increased expression of these proteins further indicates that 3 hours of P‐RSS promotes the neuronal activation in both SLGP and LSG. Considerable evidence from experimental studies has accumulated to show that activation of GP or LSG plays a key role in the generation and maintenance of AF7, 8, 9, 10; therefore, the interaction between renal and cardiac nerves may provide a potential mechanism underlying the antiarrhythmic effects of RSN denervation.
Variation in Distribution and Density of the RSN
Recently, RSN denervation has been shown to protect against AF in both animal models and humans. However, the optimal ablation sites remain contentious. Mahfoud et al investigated the differences in renal norepinephrine and renal axon density on the basis of renal artery lesion placement.23 They found that ablation of the distal segment of the renal artery but not the proximal or middle segment of the renal artery resulted in greatest reductions of both norepinephrine and axon density. However, this study was conducted in healthy porcine models, which may be different from other animals and humans. Therefore, the optimal target for RSN denervation remains controversial. Recently, Sakakura et al12 investigated the anatomic distribution of periarterial sympathetic nerves around human renal arteries and found that the density of renal nerve fibers was higher in the proximal segment of the renal artery than in the distal segment, and both nerve and ganglion are also more abundant in the proximal segment of the renal artery. In another study, we also detected the renal nerve distribution around the canine renal artery and obtained a similar result that the renal nerve fiber density was obviously higher in the proximal segment than middle or distal segment of renal artery (the data are not shown in the present study). Scherlag et al recently reported that electrical stimulation of the aorticorenal ganglia close to the ostia of each renal artery induced more significant elevation in BP than stimulation of renal arteries.24 In the present study, we also found that only P‐RSS induced significant changes in SLGP and LSG neural activities, atrial electrophysiology, and AF inducibility, further indicating that the proximal segment of the renal artery may contribute the most to the interaction with cardiac autonomic nerves and to the development of AF. In addition, the neurons and ganglia were mainly in the proximal segment of the renal artery,25 and ablation targeted to this area may avoid RSN regeneration to the maximum extent.
Clinical Perspective
Recent clinical studies have suggested that RSN denervation is an adjunctive treatment approach for patients with AF and hypertension. In the present study we provided important information on the interaction between renal and cardiac autonomic nerves through which RSN may affect AF inducibility. It is therefore possible that RSN denervation achieves arrhythmia control through breaking the interaction between renal and cardiac autonomic nerves. In addition, we showed that only P‐RSS significantly increased the BP, upregulated the SLGP and LSG neural activities, and facilitated AF, suggesting that proximal RSN might be the optimal target for renal denervation in the management of AF.
Study Limitations
This study was conducted in young, healthy canine models. The renal arteries in young, healthy canines may be different from those in pathological conditions. We only investigated the acute (only 3 hours) effects, and long‐term effects of RSS will require future studies. The intensity of RSS used in this study is relatively high. Because different intensities of autonomic stimulation may result in different effects,26 further studies are needed to investigate the effects of low‐intensity RSS. The detailed neural analytics such as artifact removal, identification of specific neuronal subtypes and number present, and further manual validation were not performed in this study, which may to some extent limit its accuracy. In addition, we performed bilateral RSS in this study. Further studies are expected to clarify the difference between right‐side RSS and left‐side RSS.
Conclusions
In summary, we presented novel data on the cross talk between renal and cardiac autonomic nerves and its role in the development of AF (Figure 8),7, 8, 9, 10 which may provide a theoretical basis for the clinical use of RSN denervation in the management of AF.
Figure 8.
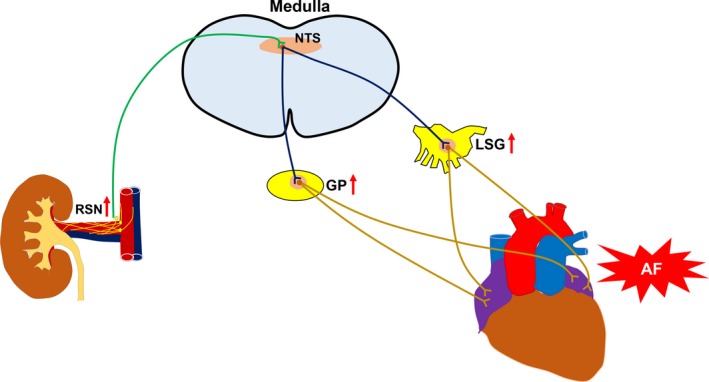
Schematic summary of potential cardiorenal neuraxial pathways through which activation of renal sympathetic nerve (RSN) could influence the atrium. Activation of RSN increases input to the nucleus of the solitary tract (NTS) in the medulla and influences the activity of NTS neurons projecting to the cardiac autonomic nervous system, thereby increasing the nerve activity of the atrial ganglionated plexi (GP) and the left stellate ganglion (LSG). Increased GP and LSG neural activities have been proved to contribute to the initiation and maintenance of atrial fibrillation (AF).7, 8, 9, 10
Sources of Funding
This work was supported by the grant from the National Key Research and Development Program of China (No. 2016YFC0104400), grants from the National Nature Science Foundation of China (Nos. 81530011, 81570463, 81600395, 81270339, and 81300182), grants from the Natural Science Foundation of Hubei Province (Nos. 2016CFA065, 2016CFA048, and 2016CFB621), grants from the Foundation of Health and Family planning Commission of Hubei Province (Nos. WJ2017C0005 and WJ2017M022) and grants from the Fundamental Research Funds for the Central Universities (Nos. 2042015kf0187 and 2042016kf0076), China, and grants from the Fundamental Research Funds of Wuhan City (No. 2016070204010134).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004716. DOI: 10.1161/JAHA.116.004716.)
References
- 1. Linz D, Ukena C, Mahfoud F, Neuberger HR, Bohm M. Atrial autonomic innervation: a target for interventional antiarrhythmic therapy? J Am Coll Cardiol. 2014;63:215–224. [DOI] [PubMed] [Google Scholar]
- 2. Linz D, Hohl M, Nickel A, Mahfoud F, Wagner M, Ewen S, Schotten U, Maack C, Wirth K, Bohm M. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767–774. [DOI] [PubMed] [Google Scholar]
- 3. Hou Y, Hu J, Po SS, Wang H, Zhang L, Zhang F, Wang K, Zhou Q. Catheter‐based renal sympathetic denervation significantly inhibits atrial fibrillation induced by electrical stimulation of the left stellate ganglion and rapid atrial pacing. PLoS One. 2013;8:e78218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low‐level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. [DOI] [PubMed] [Google Scholar]
- 6. Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Bayramova S, Losik D, Baranova V, Karaskov A, Steinberg JS. Renal denervation for improving outcomes of catheter ablation in patients with atrial fibrillation and hypertension: early experience. Heart Rhythm. 2014;11:1131–1138. [DOI] [PubMed] [Google Scholar]
- 7. Po SS, Yu L, Scherlag BJ. Cardiac autonomic nervous system: a tug of war between the big brain and little brain–friends or foes? Heart Rhythm. 2009;6:1780–1781. [DOI] [PubMed] [Google Scholar]
- 8. Yu L, Scherlag BJ, Sha Y, Li S, Sharma T, Nakagawa H, Jackman WM, Lazzara R, Jiang H, Po SS. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm. 2012;9:804–809. [DOI] [PubMed] [Google Scholar]
- 9. Wang S, Zhou X, Huang B, Wang Z, Zhou L, Chen M, Yu L, Jiang H. Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart Rhythm. 2016;13:274–281. [DOI] [PubMed] [Google Scholar]
- 10. Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang B, Yu L, Scherlag BJ, Wang S, He B, Yang K, Liao K, Lu Z, He W, Zhang L, Po SS, Jiang H. Left renal nerves stimulation facilitates ischemia‐induced ventricular arrhythmia by increasing nerve activity of left stellate ganglion. J Cardiovasc Electrophysiol. 2014;25:1249–1256. [DOI] [PubMed] [Google Scholar]
- 12. Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, Virmani R, Joner M. Anatomic assessment of sympathetic peri‐arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. [DOI] [PubMed] [Google Scholar]
- 13. Huang B, Yu L, He B, Lu Z, Wang S, He W, Yang K, Liao K, Zhang L, Jiang H. Renal sympathetic denervation modulates ventricular electrophysiology and has a protective effect on ischaemia‐induced ventricular arrhythmia. Exp Physiol. 2014;99:1467–1477. [DOI] [PubMed] [Google Scholar]
- 14. Huang B, Yu L, He B, Wang S, Lu Z, Liao K, Wang Z, Zhou X, He W, Jiang H. Sympathetic denervation of heart and kidney induces similar effects on ventricular electrophysiological properties. EuroIntervention. 2015;11:598–604. [DOI] [PubMed] [Google Scholar]
- 15. Yu L, Scherlag BJ, Li S, Fan Y, Dyer J, Male S, Varma V, Sha Y, Stavrakis S, Po SS. Low‐level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10:428–435. [DOI] [PubMed] [Google Scholar]
- 16. Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, Zhang Y, Jackman WM, Lazzara R, Jiang H, Po SS. Low‐level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2011;22:455–463. [DOI] [PubMed] [Google Scholar]
- 17. Yu L, Scherlag BJ, Dormer K, Nguyen KT, Pope C, Fung KM, Po SS. Autonomic denervation with magnetic nanoparticles. Circulation. 2010;122:2653–2659. [DOI] [PubMed] [Google Scholar]
- 18. DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41:621–624. [DOI] [PubMed] [Google Scholar]
- 19. Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, Warnock DG, Muntner P. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linz D, van Hunnik A, Hohl M, Mahfoud F, Wolf M, Neuberger HR, Casadei B, Reilly SN, Verheule S, Bohm M, Schotten U. Catheter‐based renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol. 2015;8:466–474. [DOI] [PubMed] [Google Scholar]
- 21. Cruz DN, Gheorghiade M, Palazzuoli A, Ronco C, Bagshaw SM. Epidemiology and outcome of the cardio‐renal syndrome. Heart Fail Rev. 2011;16:531–542. [DOI] [PubMed] [Google Scholar]
- 22. Huang B, Yu L, Jiang H. A potential link between left stellate ganglion and renal sympathetic nerve: an important mechanism for cardiac arrhythmias? Int J Cardiol. 2015;179:123–124. [DOI] [PubMed] [Google Scholar]
- 23. Mahfoud F, Tunev S, Ewen S, Cremers B, Ruwart J, Schulz‐Jander D, Linz D, Davies J, Kandzari DE, Whitbourn R, Bohm M, Melder RJ. Impact of lesion placement on efficacy and safety of catheter‐based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766–1775. [DOI] [PubMed] [Google Scholar]
- 24. Scherlag BJ, Sun J, He B, Po SS. Optimal sites for renal artery denervation: relation to atrial fibrillation ablation. Int J Cardiol. 2016;209:330–331. [DOI] [PubMed] [Google Scholar]
- 25. Tzafriri AR, Mahfoud F, Keating JH, Markham PM, Spognardi A, Wong G, Fuimaono K, Bohm M, Edelman ER. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linz D, Hohl M, Khoshkish S, Mahfoud F, Ukena C, Neuberger HR, Wirth K, Bohm M. Low‐level but not high‐level baroreceptor stimulation inhibits atrial fibrillation in a pig model of sleep apnea. J Cardiovasc Electrophysiol. 2016;27:1086–1092. [DOI] [PubMed] [Google Scholar]


