Abstract
Background
Plaque rupture and erosion are the 2 most common mechanisms for acute coronary syndromes. However, the outcome of these 2 distinct pathologies in patients with acute coronary syndromes has never been studied.
Methods and Results
We retrospectively studied 141 patients with acute coronary syndromes who underwent optical coherence tomography (OCT) imaging of the culprit lesion prior to stenting from the Massachusetts General Hospital OCT Registry. Management (stent versus no stent), poststent OCT findings, and outcomes were compared. Among the 141 culprit lesions, rupture was found in 79 (56%) patients and erosion in 62 (44%). Stent implantation was performed in 77 (97.5%) patients with rupture versus 49 (79.0%) in those with erosion (P<0.001). Immediately after percutaneous coronary intervention, OCT showed a higher incidence of malapposition (37.5% versus 7.3%, P<0.001), thrombus (59.4% versus 14.6%, P<0.001), and protrusion (93.8% versus 73.2%, P=0.008) in the rupture group compared with the erosion group. Plaque rupture was associated with a higher incidence of no reflow or slow flow and distal embolization. Although cardiac event rates were comparable between the two groups at the 1‐year follow‐up, none of the erosion patients who were treated conservatively without stenting had adverse cardiac events.
Conclusions
Unfavorable poststent OCT findings were more frequent in rupture patients compared with erosion patients. A subset of erosion patients who were treated conservatively without stenting remained free of adverse cardiac events for up to 1 year.
Keywords: acute coronary syndrome, optical coherence tomography, plaque erosion, plaque rupture
Subject Categories: Optical Coherence Tomography (OCT), Treatment, Thrombosis
Introduction
Acute coronary syndrome (ACS) is caused by coronary plaque rupture (PR), plaque erosion (PE), or rarely calcified nodule resulting in occlusive thrombus formation.1, 2 Ruptured plaque is characterized by a disrupted fibrous cap overlying a large necrotic core, and extensive inflammation. Eroded plaque typically shows an absence of superficial lipid, less inflammation, and less obstructive lumen. Currently, patients with ACS are uniformly treated with stenting, regardless of underlying culprit lesion pathology (PR or PE). Although an overall favorable benefit was observed in the invasive strategy group as compared with the conservative group in several randomized clinical trials, subgroup analyses suggest that this strategy does not provide an equivalent level of benefit in all patients, such as women and current smokers.3, 4, 5, 6 Limited data from the pathology and in vivo studies suggest that underlying plaque morphology other than clinical characteristics may play a critical role in determining early and long‐term outcomes in response to stent implantation.7, 8 In light of the relatively well‐preserved vascular structure and less severe luminal narrowing,1, 2, 9 it is conceivable that ACS caused by PE without significant luminal narrowing may be stabilized by effective antithrombotic treatment without stent implantation. This conservative strategy would avoid both early (vessel dissection, distal embolism, and acute stent thrombosis) and late (restenosis, neoatherosclerosis, and late and very late stent thrombosis) complications of stent implantation.10, 11, 12, 13, 14 Indeed, few reports with a small number of cases have suggested that a subset of patients with ST‐segment elevation myocardial infarction caused by PE might be treated by antithrombotic therapy with favorable outcome.2, 15, 16 However, data on the outcomes of patients with ACS caused by rupture or erosion are limited.
Optical coherence tomography (OCT) has recently been introduced as a high‐resolution intravascular imaging modality that enables in vivo diagnosis of PE as well as evaluation of vascular response to stent implantation.2, 17 In the present study, we investigated the acute stent complications (dissection, malapposition, and intrastent tissue prolapsed) and 1‐year outcomes in patients with ACS caused by PR versus erosion.
Methods
Study Populations
From August 2010 to September 2013, a total of 710 patients with ACS who underwent OCT imaging were enrolled in the Massachusetts General Hospital OCT Registry (http://www.clinicaltrials.gov: NCT01110538). A total of 421 patients were excluded for the following reasons: (1) no preprocedure OCT imaging (n=69); (2) no poststenting imaging (n=304); (3) in‐stent thrombosis or restenosis (n=33); (4) coronary artery bypass grafting (n=3); or (5) unidentifiable culprit lesions (n=12). Of 289 patients with de novo lesions, 109 patients were additionally excluded for the following reasons: (1) poor image quality (n=22); (2) covered stent (n=6); (3) predilatation before OCT imaging (n=50); (4) massive thrombus (n=6); or (5) missing clinical data (n=25). Finally, 141 patients with 141 identifiable culprit lesions were included in the analysis, including 79 patients with PR and 62 with PE (Figure 1). The culprit lesion was identified on the basis of coronary angiogram, stress test, ECG, left ventriculogram, or echocardiogram. After OCT imaging, percutaneous coronary intervention (PCI) was performed according to the local practice. The decision to perform aspiration thrombectomy, balloon angioplasty, or stent implantation was left at the operator's discretion.
Figure 1.
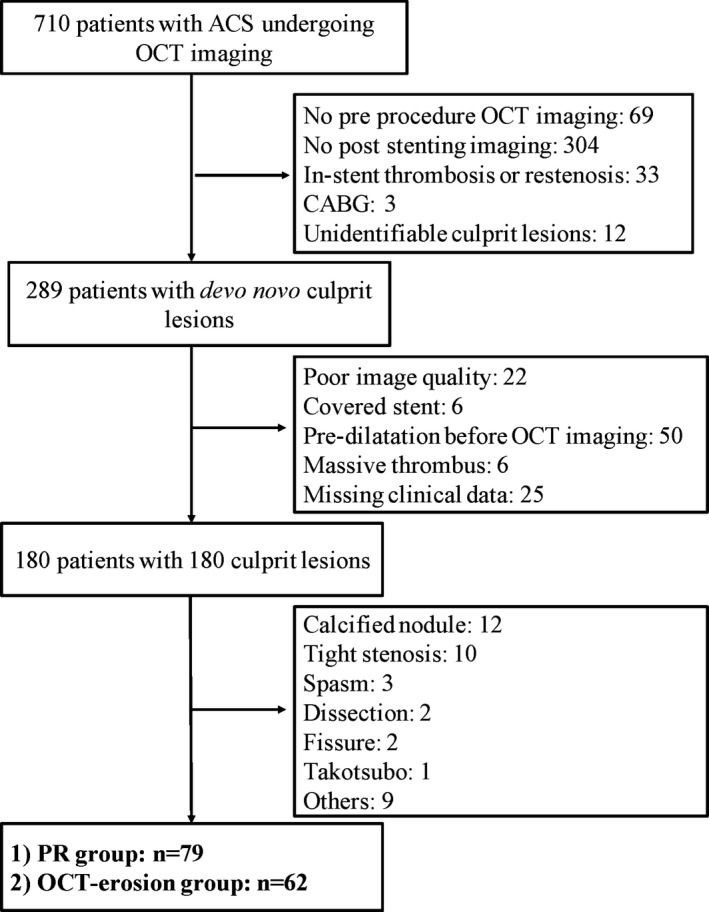
Study flow chart. ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; OCT, optical coherence tomography; PR, plaque rupture.
ST‐segment elevation myocardial infarction was defined as continuous chest pain that lasted >30 minutes, arrival at the hospital within 12 hours from the onset of symptoms, ST‐segment elevation >0.1 mV in >2 contiguous leads or new left bundle branch block on the 12‐lead ECG, and elevated cardiac markers. Non–ST‐segment elevation myocardial infarction was defined as ischemic symptoms in the absence of ST elevation on the ECG with elevated cardiac markers. Unstable angina pectoris was defined as having newly developed/accelerating chest symptoms on exertion or rest angina within 2 weeks.
Demographic and clinical data were prospectively collected. All of the images were digitally stored and submitted to the core laboratory at Massachusetts General Hospital. The protocol for the registry was approved by each site's institutional review board, and all patients provided informed consent.
PCI Procedure and OCT Image Acquisition
After intracoronary nitroglycerine administration, the OCT catheter was advanced distal to the culprit lesion. Imaging of the culprit lesion was then acquired using either the commercially available time‐domain (M2/M3 Cardiology Imaging System, Lightlab Imaging/St. Jude Medical, Westford, MA) or frequency‐domain OCT C7XR system and the Dragonfly catheter (Lightlab Imaging/St. Jude Medical, Westford, MA) as previously reported.2, 18 Aspiration thrombectomy prior to OCT imaging was allowed in patients with large occlusive thrombus or a thrombolysis in myocardial infarction (TIMI) flow grade <2. Predilatation, however, was not allowed. All treatment strategies were decided on by the operators at each institution according to local practice. At the end of the procedure, final OCT imaging was acquired in patients treated with stent implantations. The images were digitally stored and deidentified for offline analysis.
OCT Image Analysis
After the culprit lesion was identified, underlying plaque morphology was analyzed by 2 experienced reviewers using the previously reported criteria.2, 17, 18, 19 Plaques were classified as: (1) fibrous (homogeneous, high backscattering region) or (2) lipid (low‐signal region with diffuse border).17, 20 In lipid plaques, fibrous cap thickness was measured 3 times at the thinnest part, and the average value was calculated. Lipid arc was measured at 1‐mm intervals and lipid length was measured on the longitudinal view. Thin‐cap fibroatheroma was defined as a plaque with lipid content in at least 2 quadrants, with the thinnest part of the fibrous cap measuring <65 μm.21 PR was identified by the presence of fibrous cap discontinuity with a clear cavity formed inside the plaque.2, 22, 23 PE was defined as a plaque with no detectable signs of fibrous cap rupture with or without thrombus attached, including both definite and probable erosion.2, 23 Thrombus was defined as a mass floating in or protruding into the lumen with a dimension of at least 250 μm. Thrombus score was calculated as the sum of the number of quadrants with visible thrombus. The poststent OCT findings including edge dissection, in‐stent dissection, incomplete stent apposition, and tissue protrusion were assessed using the previously reported definitions.24 Tissue protrusion was defined as a bowing of the plaque into the lumen between stent struts. Edge dissection was defined as the disruption of the vessel luminal surface with a visible flap at the proximal or distal stent edge. In‐stent dissection was defined as the disruption of the vessel luminal surface with a visible flap within the stented area. Incomplete stent apposition was defined as any strut with a distance of more than the sum of strut thickness plus polymer thickness from the surface reflection of the strut to the adjacent visible surface of the vessel. Malapposition area was calculated as (lumen area−stent area) in a cross‐sectional image containing malapposed struts.
Quantitative Coronary Angiography Analysis
Angiographic images were analyzed using a quantitative coronary angiogram analysis program (CAAS 5.10.1, Pie Medical Imaging BV, Maastricht, the Netherlands). Reference diameter, minimum lumen diameter, lesion length, and percent diameter stenosis were measured.
Clinical Outcomes
Patients were followed up by telephone call or hospital visit at 6 and 12 months. The occurrence of major adverse cardiac events was recorded, which included death from any cause, nonfatal myocardial infarction, and revascularization.
Statistical Analysis
All statistical analyses were performed by an independent statistician at the core laboratory. Categorical data were compared using either chi‐square test or Fisher exact test, and are presented as counts (proportions). Continuous variables are expressed as mean±SD and compared using the independent sample t test. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc, Chicago, IL). P<0.05 was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of patients with PR and those with OCT‐erosion are summarized in Table 1. Among the 141 patients enrolled, 79 (56%) patients had PR at a culprit lesion and 62 (44%) had OCT‐erosion. The plaque characteristics of 94 patients were reported in our previous study.2 No significant difference was observed in sex, coronary risk factors, laboratory findings, and medications between the 2 groups, except younger age, higher frequency of NSTE‐ACS, and higher level of high‐density lipoprotein in the OCT‐erosion group.
Table 1.
Baseline Characteristics
| Overall (N=141) | PR (n=79) | OCT‐Erosion (n=62) | P Value | |
|---|---|---|---|---|
| Age, y | 57.9±11.5 | 60.3±10.4 | 54.8±12.1 | 0.005 |
| Male | 113 (80.1) | 65 (82.3) | 48 (77.4) | 0.473 |
| Risk factors | ||||
| Current smoker | 51 (36.2) | 26 (32.9) | 25 (40.3) | 0.363 |
| Diabetes mellitus | 33 (23.4) | 19 (24.1) | 14 (22.6) | 0.838 |
| Hyperlipidemia | 96 (68.1) | 58 (73.4) | 38 (61.3) | 0.125 |
| Hypertension | 81 (57.4) | 51 (64.6) | 30 (48.4) | 0.054 |
| Family history of CAD | 14 (9.9) | 9 (11.4) | 5 (8.1) | 0.512 |
| Obesity (BMI >25 kg/m2) | 72 (51.1) | 41 (51.9) | 31 (50) | 0.823 |
| Chronic kidney disease | 6 (4.3) | 3 (3.8) | 3 (4.8) | 1.000 |
| Previous CABG | 1 (0.7) | 1 (1.3) | 0 | 1.000 |
| Prior MI | 17 (12.1) | 11 (13.9) | 6 (9.7) | 0.442 |
| LVEF, % | 61.7±10.1 | 61.3±9.8 | 62.2±10.7 | 0.659 |
| Presentation | ||||
| STEMI | 66 (46.8) | 46 (58.2) | 20 (32.3) | 0.002 |
| NSTE‐ACS | 75 (53.2) | 33 (41.8) | 42 (67.7) | |
| Laboratory variables | ||||
| TC, mg/dL | 177.7±46.1 | 180±43.8 | 174.3±48.9 | 0.435 |
| HDL‐C, mg/dL | 46.6±20.6 | 42.5±16.7 | 51.4±23.7 | 0.013 |
| LDL‐C, mg/dL | 100.3±35.1 | 104.3±37.3 | 95.6±31.9 | 0.156 |
| TG, mg/dL | 167.5±104.4 | 182.5±119.8 | 149.4±79.5 | 0.060 |
| Creatinine, mg/dL | 0.90±0.22 | 0.93±0.21 | 0.87±0.23 | 0.100 |
| Medications at admission | ||||
| Statin | 48 (34.0) | 30 (38.0) | 18 (29.0) | 0.266 |
| β‐Blocker | 33 (23.4) | 21 (26.6) | 12 (19.4) | 0.314 |
| ARB/ACEI | 35 (24.8) | 18 (22.8) | 17 (27.4) | 0.527 |
Data are presented as mean±SD, or number (percentage). The P values were calculated using t tests for continuous variables and Fisher exact test tests for categorical variables. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome; OCT, optical coherence tomography; PR, plaque rupture; STEMI, ST‐segment elevation myocardial infarction; TC, total cholesterol; TG, triglyceride.
Angiographic Findings
The culprit lesion distribution and quantitative coronary angiogram analysis data are listed in Table 2. OCT‐erosion was most frequently detected in the left anterior descending artery. PR was equally distributed in the LAD and right coronary artery. There was no significant difference in the culprit lesion distribution between the proximal, mid, and distal segments of coronary arteries. The minimum lumen diameter was larger (P<0.001) and the diameter stenosis was less severe (P=0.001) in the OCT‐erosion group compared with the PR group. Patients with PR had a higher incidence of multivessel disease (P<0.001) and more complicated lesion type (type B2/C) (P<0.001) compared with those with OCT‐erosion.
Table 2.
Angiographic Findings
| PR (n=79) | OCT‐Erosion (n=62) | P Value | |
|---|---|---|---|
| Culprit vessel | 0.009 | ||
| LAD | 32 (40.5) | 40 (64.5) | |
| LCX | 16 (20.3) | 11 (17.7) | |
| RCA | 31 (39.2) | 11 (17.7) | |
| Lesion location | 0.082 | ||
| Proximal | 34 (43) | 20 (32.3) | |
| Mid | 16 (20.3) | 23 (37.1) | |
| Distal | 29 (36.7) | 19 (30.6) | |
| QCA data | |||
| Reference diameter, mm | 3.32±0.76 | 3.47±0.95 | 0.295 |
| Minimum lumen diameter, mm | 0.96±0.46 | 1.59±0.82 | <0.001 |
| Diameter stenosis, % | 69.22±12.3 | 55.8±17.5 | 0.001 |
| Multivessel disease | 55 (69.6) | 20 (32.3) | <0.001 |
| ACC/AHA lesion class | <0.001 | ||
| Type A/B1 | 10 (12.7) | 28 (45.2) | |
| Type B2/C | 69 (87.3) | 34 (54.8) | |
Data are presented as mean±SD or number (percentage). P values were calculated using t test for continuous variables and chi‐square test for categorical variables. ACC/AHA indicates American College of Cardiology/American Heart Association; LAD, left anterior descending coronary artery; LCX, left circumflex artery; OCT, optical coherence tomography; PR, plague rupture; QCA, quantitative coronary angiogram analysis; RCA, right coronary artery.
Procedural Characteristics
Procedural data are summarized in Table 3. Of the 79 patients with PR, 77 (97.5%) were treated with stent implantation (drug‐eluting stent=86, bare metal stent=12). Among the 62 patients with OCT‐erosion, 49 (79.0%) were treated with stent implantation (drug‐eluting stent=57, bare metal stent=4). The mean stent length and mean stent diameter were similar between the two groups. Compared with patients with PR, those with OCT‐erosion had higher baseline thrombolysis in myocardial infarction flow grade, lower incidence of thrombolysis in myocardial infarction flow grade ≤2 after stenting, and lower incidence of distal embolization.
Table 3.
Procedural Data
| PR (n=79) | OCT‐Erosion (n=62) | P Value | |
|---|---|---|---|
| PCI procedure | |||
| Stent treatment | 77 (97.5) | 49 (79.0) | <0.001 |
| No of stent/culprit lesion | 1.27±0.52 | 1.26±0.51 | 0.887 |
| Total stent length, mm | 34.2±20.7 | 30.1±18.0 | 0.192 |
| Mean stent diameter, mm | 3.2±0.43 | 3.1±0.43 | 0.275 |
| Baseline TIMI flow grade | |||
| TIMI flow grade 0 | 10 (12.7) | 2 (3.2) | 0.0013 |
| TIMI flow grade 1 | 5 (6.3) | 2 (3.2) | |
| TIMI flow grade 2 | 25 (31.6) | 8 (12.9) | |
| TIMI flow grade 3 | 39 (49.4) | 50 (80.7) | |
| Final TIMI flow grade ≤2 | 14 (17.7) | 2 (4.8) | 0.015 |
| Distal embolization | 27 (34.2) | 5 (8.1) | <0.001 |
Data are presented as number (percentage) or mean±SD. P values were calculated using t test for continuous variables and Fisher exact test for categorical variables. OCT indicates optical coherence tomography; PCI, percutaneous coronary intervention; PR, plaque rupture; TIMI, thrombolysis in myocardial infarction.
OCT Findings After PCI
Post‐PCI OCT findings in patients with PR and OCT‐erosion are listed in Table 4. Post‐PCI OCT imaging was acquired in 61 patients with PR and in 46 with OCT‐erosion. Patients with PR had a higher incidence of in‐stent dissection or thrombus, compared with those with OCT‐erosion after stent implantation. The incidence of malapposition was significantly higher and the malapposed area was significantly larger in patients with PR than in those with OCT‐erosion. The representative cases of PE and PR are shown in Figures 2 and 3.
Table 4.
Post‐PCI OCT Findings (Stent‐Based Analysis)
| PR (n=61 Stents) | OCT‐Erosion (n=46 Stents) | P Value | |
|---|---|---|---|
| Edge dissection | 9/61 (14.8) | 4/38 (10.5) | 0.764 |
| Proximal | 2/56 (3.6) | 1/38 (2.6) | 0.731 |
| Distal | 7/61 (11.5) | 3/38 (7.9) | 0.817 |
| In‐stent dissection | 35 (57.4) | 17 (37.0) | 0.036 |
| Thrombus | 35 (57.4) | 8 (17.4) | <0.001 |
| Thrombus score | 12.1±13.9 | 7.9±11.5 | 0.095 |
| Thrombus volume, mm3 | 1.23±1.86 | 0.42±0.41 | 0.027 |
| Malapposition | 27 (44.3) | 10 (21.7) | 0.015 |
| Malapposed area, mm2 | 0.83±0.39 | 0.53±0.52 | 0.045 |
| MLA, mm2 | 6.0±1.7 | 6.1±2.0 | 0.695 |
| MLD, mm | 2.6±0.4 | 2.7±0.8 | 0.688 |
| Maximum stent area, mm2 | 8.5±2.3 | 8.2±2.5 | 0.470 |
Data are presented as number (percentage) or mean±SD. P values were calculated using t test for continuous variables and Fisher exact test for categorical variables. MLA indicates minimum luminal area; MLD, minimum luminal diameter; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; PR, plaque rupture.
Figure 2.
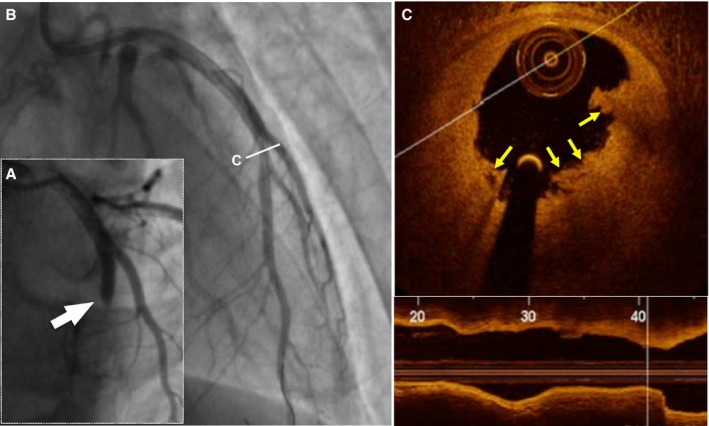
Representative optical coherence tomography (OCT) images of plaque erosion. A 28‐year‐old man with ST‐segment elevation myocardial infarction was admitted after 2 hours of chest pain. Results from index angiogram showed total occlusion in the mid–left anterior descending artery (LAD; white arrow, A). Angiogram results after thrombus aspiration showed a mild stenosis in the mid‐LAD (B). Cross‐sectional image of the culprit lesion shows residual white thrombus without evidence of ruptured fibrous cap (yellow arrows, C). The patient was treated with medical therapy without stent implantation.
Figure 3.
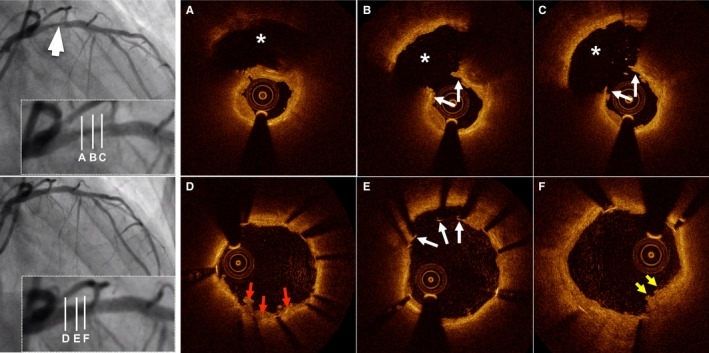
Representative optical coherence tomography (OCT) images of plaque rupture. A 60‐year‐old woman with ST‐segment elevation myocardial infarction was admitted after 11 hours of chest pain. Results from diagnostic angiogram showed total occlusion in the proximal left anterior descending artery (LAD). Angiogram results after thrombectomy demonstrates dissection lesion in the proximal LAD (white arrow, left upper panel). Cross‐sectional OCT images of the culprit lesion show disrupted fibrous cap (white arrows) and cavity (asterisk) formation (A through C). The patient was treated with a drug‐eluting stent (3.0×18 mm). Poststenting OCT revealed in‐stent thrombus (red arrows, D), malapposed struts (white arrows, E), and stent protrusion (yellow arrows, F).
Clinical Outcomes at 1‐Year Follow‐Up
Table 5 shows the clinical outcomes at the 1‐year follow up. Of the 141 enrolled patients, 134 (95.0%) were followed up to 1 year. Mean follow‐up time was 367.3±22.3 days. Statistically, no significant differences were observed between the 2 groups in rates of death, myocardial infarction, target lesion revascularization, or nontarget lesion revascularization. However, the rate of revascularization in patients with PR was numerically higher than those with OCT‐erosion (12.5% versus 6.5%, P=0.238). Two of 79 (2.5%) patients in the rupture group and 13 of 62 (21.0%) in the erosion group were treated conservatively without stent implantation. Clinical outcomes of patients with PR and OCT‐erosion stratified by treatment strategy are summarized in Table S1. At 1‐year follow‐up, 1 of 2 patients in the rupture group who was treated medically died. All 13 patients in the erosion group who were treated medically remained free of adverse events for up to 1 year.
Table 5.
Clinical Outcomes at 1 Year
| Events | Overall (N=134) | PR (n=72) | OCT‐Erosion (n=62) | P Value |
|---|---|---|---|---|
| Death | 2 (1.5) | 1 (1.4) | 1 (1.6) | 1.000 |
| Acute MI or ACS | 3 (2.2) | 1 (1.4) | 2 (3.2) | 0.596 |
| Revascularization | 13 (9.7) | 9 (12.5) | 4 (6.5) | 0.238 |
| TLR | 6 (4.6) | 5 (6.9) | 1 (1.6) | 0.216 |
| Non‐TLR | 7 (5.2) | 4 (5.6) | 3 (4.8) | 1.000 |
| Overall MACE | 17 (12.7) | 10 (13.9) | 7 (11.3) | 0.652 |
Data are presented as number (percentage). Comparisons of the events between the two groups were performed using Fisher exact test. ACS indicates acute coronary syndrome; MACE, major adverse cardiovascular events; MI, myocardial infarction; OCT, optical coherence tomography; PR, plaque rupture; TLR, target lesions revascularization.
Discussion
This study shows that, in the real world, patients with ACS caused by PE were more likely to be treated conservatively compared with those caused by PR (21.0% versus 2.5%). Among the patients who were treated with stent implantation, patients with PR had a higher incidence of malapposition, tissue protrusion, and thrombus compared with those with PE. At the 12‐month follow‐up, the rate of revascularization in patients with PR was numerically higher than those with OCT‐erosion although no statistical difference was observed. Importantly, patients with erosion who were treated conservatively were free of cardiovascular events up to the 1‐year follow‐up.
Impact of Underlying Plaque Morphology on Management of ACS
PEs are responsible for 25% to 44% of ACS cases. PE is characterized by a less occlusive plaque with relatively preserved vascular integrity.1, 2, 25, 26, 27 The autopsy study showed that ruptured plaque is often significantly obstructive and has a large plaque burden and a large necrotic core with an overlying disrupted fibrous cap, which is attenuated and inflamed. Disruption of the fibrous cap exposes a great amount of highly thrombogenic necrotic core to circulating platelets, leading to occlusive thrombus and acute ischemic events.28 In contrast, PE often has a less severe obstruction, a less superficial necrotic core, and a smaller plaque burden.26, 29 Furthermore, luminal thrombus in PE has been attributed to apoptosis or denudation of superficial endothelial cells and is rich in platelets. These distinct characteristics associated with PE provide rationale for conservative treatment with potent antithrombotic therapy without stenting. Indeed, patients with PE have a more favorable response to thrombolytic therapy compared with those with PR.23
Current treatment of ACS is routine PCI with stenting, regardless of underlying pathologies. The culprit plaque morphology is not routinely evaluated. Therefore, the impact of underlying lesion characteristics on treatment strategy selection has never been investigated in the setting of ACS. Although our investigation was a retrospective study without predefined criteria for the selection of treatment strategy and all of the patients were treated according to local practice without knowledge of underlying pathology, patients with PE were more frequently treated “conservatively” without stent implantation compared with patients with PR (21.0% versus 2.5%, P<0.001). Of the 62 patients with PE, 13 who had moderate stenosis were treated with antithrombotic therapy without stent implantation. Remarkably, those who were treated conservatively had no adverse cardiac events during the 1‐year follow‐ up. In contrast, 7 events were observed among the 49 patients who were treated with stenting. Consistent with this finding, Prati et al retrospectively investigated 31 patients with ST‐segment elevation myocardial infarction.30 After thrombus was removed by thrombectomy, OCT showed the intact fibrous cap without evidence of PR and no significant residual obstruction. Among these 31 patients, 19 were treated with stents and 12 were treated with only antithrombotic therapy without angioplasty or stenting. None of these 12 patients required an additional revascularization during the 12‐month follow‐up.30
Underlying Plaque Morphology and Clinical Outcomes After PCI
In our previous study of the mechanism responsible for ACS, lipid plaques were less frequently detected in patients with OCT‐erosion than in those with PR. When lipids were present, the overlying fibrous cap was thicker, the lipid arc was smaller, and the lipid length was shorter in patients with OCT‐erosion compared with those with PR.2 Ruptured plaque releases highly thrombogenic substrates, which may induce recurrent local thrombosis and distal embolization, perturbing the coronary microcirculation. These findings suggest that PR may be associated with worse outcomes after stenting. Higuma et al27 reported that PR was associated with worse myocardial perfusion after PCI compared with PE. In the present study, we also found that PR had a higher incidence of acute stent complications (in‐stent dissection, tissue protrusion, malapposition, and thrombus) and higher frequency of distal embolization. Recently, Niccoli et al31 demonstrated that patients with PR have a worse prognosis after 3‐year follow‐up when compared with those with PE, which is mainly driven by a higher risk of revascularization and unstable angina. In our study, we did not observe different clinical outcomes between the 2 groups, which may be attributed to the small sample size and short follow‐up period. However, the favorable clinical outcomes in patients with erosion treated conservatively highlights the new concept that in a subset of patients with ACS caused by PEs, conservative therapy without stent implantation might be feasible and safe. This concept was prospectively tested in a recent clinical trial that was performed by our group.32 If this conservative approach without a metallic stent or polymer scaffold proves to be effective and safe, it may become a new treatment paradigm for one fourth of patients with ACS. Randomized trials will be needed to reproduce this pilot data and to evaluate the long‐term outcomes of this new strategy.
Limitations
Several limitations need to be mentioned in this study. First, this is a retrospective analysis of a small number of patients. Because of the retrospective nature of the analysis, there was no systematic decision process for how to treat patients. However, although this was a retrospective study, it may provide important evidence for better individualized management of patients with ACS based on underlying lesion characteristics. In addition, the registry included only patients who underwent OCT according to the operator's discretion. Therefore, the issue of selection bias cannot be avoided. Second, the underlying plaque characteristics might be obscured by residual thrombus (specifically, red thrombus) in some cases. Although we excluded cases with massive residual thrombus after thrombus aspiration to avoid misclassification of culprit plaques, we have to acknowledge that some ruptured cases might be misdiagnosed as erosion due to invisibility of the ruptured site, which was hidden underneath the thrombus. Third, thrombus aspiration was performed to facilitate reperfusion prior to OCT imaging. Although care was taken to avoid excessive mechanical injury during thrombectomy, it is possible that this procedure may have altered the morphologic features of the underlying plaque. Therefore, we cannot exclude that in some patients, PR was iatrogenic, being caused by thrombus aspiration. Fourth, due to safety concerns, 16 (20.8%) patients in the rupture group did not undergo poststenting OCT imaging, whereas only 3 (6.1%) in the erosion group did not undergo poststenting OCT imaging (P<0.05). This difference might result in selection bias in our study. However, the main findings of the present study are unlikely changed even though poststenting OCT imaging was performed in patients with worse conditions.
Conclusions
Patients with PE are more likely to be treated with medical therapy without stent implantation compared with patients with PR. Patients with PE treated conservatively remained free of major adverse cardiac events for up to 1 year. Patients with PR had worse vascular response to stent implantation with a higher incidence of malapposition, tissue protrusion, and thrombus, compared with patients with PE.
Sources of Funding
Dr Jang's research was supported by Mr. and Mrs. Michael and Kathryn Park and by Mrs. and Mr. Gill and Allan Gray.
Disclosures
Dr Jang received an education grant and consulting fees from St. Jude Medical. Dr Yu received a grant from the National Natural Science Foundation of China (81330033) and National Science and Technology Major Projects Specialized for Prevention and Control of Major Chronic Non‐communicable Disease during the 13th Five‐Year Plan Period: Prospective Cohort Studies on Myocardial Salvage for Acute Myocardial Infarction in Whole Treating Procedures (grant 2016YFC1301100); Dr Jia received a grant from the National Natural Science Foundation of China (grant No. 81200076 and grant No. 81671763). Dr Hu received a grant from Harbin Medical university scientific research innovation fund.
Supporting information
Table S1. Clinical Outcomes of Patients With and Without Stent Implantation Stratified by Treatment Strategy
Acknowledgments
The authors thank all of the investigators and all supporting staff.
(J Am Heart Assoc. 2017;6:e004730. DOI: 10.1161/JAHA.116.004730.)
Contributor Information
Haibo Jia, Email: jhb101180@163.com.
Bo Yu, Email: yubodr@163.com.
References
- 1. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 2. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, Fox KA, Lagerqvist B, McCullough PA, Murphy SA, Spacek R, Swahn E, Wallentin L, Windhausen F, Sabatine MS. Early invasive vs conservative treatment strategies in women and men with unstable angina and non‐ST‐segment elevation myocardial infarction: a meta‐analysis. JAMA. 2008;300:71–80. [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E; TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)‐‐Thrombolysis in Myocardial Infarction 18 Investigators . Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. [DOI] [PubMed] [Google Scholar]
- 5. Invasive compared with non‐invasive treatment in unstable coronary‐artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet. 1999;354:708–715. [PubMed] [Google Scholar]
- 6. Fox KA, Poole‐Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, Wheatley DJ, Pocock SJ; Randomized Intervention Trial of unstable Angina Investigators . Interventional versus conservative treatment for patients with unstable angina or non‐ST‐elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of Unstable Angina. Lancet. 2002;360:743–751. [DOI] [PubMed] [Google Scholar]
- 7. Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug‐eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138–1145. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto M, Okamatsu K, Inami S, Takano M, Yokoyama S, Ohba T, Ibuki C, Hata N, Seino Y, Mizuno K. Relationship between neointimal coverage of sirolimus‐eluting stents and lesion characteristics: a study with serial coronary angioscopy. Am Heart J. 2009;158:99–104. [DOI] [PubMed] [Google Scholar]
- 9. Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter‐Monroe N, Kolodgie FD, van der Wal AC, Virmani R. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–132. [DOI] [PubMed] [Google Scholar]
- 10. Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Juni P. Outcomes associated with drug‐eluting and bare‐metal stents: a collaborative network meta‐analysis. Lancet. 2007;370:937–948. [DOI] [PubMed] [Google Scholar]
- 11. Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents. N Engl J Med. 2007;356:998–1008. [DOI] [PubMed] [Google Scholar]
- 12. Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug‐eluting stents. N Engl J Med. 2007;356:1020–1029. [DOI] [PubMed] [Google Scholar]
- 13. Yonetsu T, Kato K, Kim SJ, Xing L, Jia H, McNulty I, Lee H, Zhang S, Uemura S, Jang Y, Kang SJ, Park SJ, Lee S, Yu B, Kakuta T, Jang IK. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging. 2012;5:660–666. [DOI] [PubMed] [Google Scholar]
- 14. Yonetsu T, Kim JS, Kato K, Kim SJ, Xing L, Yeh RW, Sakhuja R, McNulty I, Lee H, Zhang S, Uemura S, Yu B, Kakuta T, Jang IK. Comparison of incidence and time course of neoatherosclerosis between bare metal stents and drug‐eluting stents using optical coherence tomography. Am J Cardiol. 2012;110:933–939. [DOI] [PubMed] [Google Scholar]
- 15. Prati F, Uemura S, Souteyrand G, Virmani R, Motreff P, Di Vito L, Biondi‐Zoccai G, Halperin J, Fuster V, Ozaki Y, Narula J. OCT‐based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc Imaging. 2013;6:283–287. [DOI] [PubMed] [Google Scholar]
- 16. Hu S, Jia H, Vergallo R, Abtahian F, Tian J, Soeda T, Rosenfield K, Jang IK. Plaque erosion: in vivo diagnosis and treatment guided by optical coherence tomography. JACC Cardiovasc Interv. 2014;7:e63–e64. [DOI] [PubMed] [Google Scholar]
- 17. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia‐Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G; International Working Group for Intravascular Optical Coherence T . Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. [DOI] [PubMed] [Google Scholar]
- 18. Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, Yu B, Mizuno K, Jang IK. Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3‐vessel optical coherence tomography study. JACC Cardiovasc Interv. 2012;5:1150–1158. [DOI] [PubMed] [Google Scholar]
- 19. Di Vito L, Yoon JH, Kato K, Yonetsu T, Vergallo R, Costa M, Bezerra HG, Arbustini E, Narula J, Crea F, Prati F, Jang IK; COICO group (Consortium of Investigators for Coronary OCT) . Comprehensive overview of definitions for optical coherence tomography‐based plaque and stent analyses. Coron Artery Dis. 2014;25:172–185. [DOI] [PubMed] [Google Scholar]
- 20. Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. [DOI] [PubMed] [Google Scholar]
- 21. Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. [DOI] [PubMed] [Google Scholar]
- 22. Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H, Soeda T, McNulty I, Hu S, Lee H, Yu B, Jang IK. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin‐cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63:2209–2216. [DOI] [PubMed] [Google Scholar]
- 23. Hu S, Yonetsu T, Jia H, Karanasos A, Aguirre AD, Tian J, Abtahian F, Vergallo R, Soeda T, Lee H, McNulty I, Kato K, Yu B, Mizuno K, Toutouzas K, Stefanadis C, Jang IK. Residual thrombus pattern in patients with ST‐segment elevation myocardial infarction caused by plaque erosion versus plaque rupture after successful fibrinolysis: an optical coherence tomography study. J Am Coll Cardiol. 2014;63:1336–1338. [DOI] [PubMed] [Google Scholar]
- 24. Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, Barlis P, Tearney GJ, Jang IK, Arbustini E, Bezerra HG, Ozaki Y, Bruining N, Dudek D, Radu M, Erglis A, Motreff P, Alfonso F, Toutouzas K, Gonzalo N, Tamburino C, Adriaenssens T, Pinto F, Serruys PW, Di Mario C; Expert's OCT Review Document . Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33:2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. [DOI] [PubMed] [Google Scholar]
- 26. Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. [DOI] [PubMed] [Google Scholar]
- 27. Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, Vergallo R, Minami Y, Ong DS, Lee H, Okumura K, Jang IK. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST‐segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:1166–1176. [DOI] [PubMed] [Google Scholar]
- 28. Narula J, Strauss HW. The popcorn plaques. Nat Med. 2007;13:532–534. [DOI] [PubMed] [Google Scholar]
- 29. Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, Virmani R. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prati F, Uemura S, Souteyrand G, Virmani R, Motreff P, Di Vito L, Biondi‐Zoccai G, Halperin J, Fuster V, Ozaki Y, Narula J. OCT‐based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc Imaging. 2013;6:283‐287. [DOI] [PubMed] [Google Scholar]
- 31. Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015;36:1377–1384. [DOI] [PubMed] [Google Scholar]
- 32. Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, Xu M, Yao Y, Hu S, Yamamoto E, Lee H, Zhang S, Yu B, Jang IK. Effective anti‐thrombotic therapy without stenting: intravascular optical coherence tomography‐based management in plaque erosion (the EROSION study). Eur Heart J. August 30, 2016. Available at: https://academic.oup.com/eurheartj/article-abstract/doi/10.1093/eurheartj/ehw381/2661754/Effective-anti-thrombotic-therapy-without-stenting?redirectedFrom=fulltext. Accessed February 18, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Outcomes of Patients With and Without Stent Implantation Stratified by Treatment Strategy


