Abstract
Background
Guideline recommendations on carotid endarterectomy are based predominantly on randomized, controlled trials, in which women or elderly patients are often under‐represented. This study analyzed the association of age and sex with the risk of in‐hospital stroke or death following carotid endarterectomy under routine conditions in Germany.
Methods and Results
Secondary data analysis using the Statutory German Quality Assurance Database on all carotid endarterectomy procedures (n=142 074) performed between 2009 and 2014. Primary outcome was any stroke or death until discharge; secondary outcomes were any in‐hospital stroke (alone), and death (alone). Descriptive statistics and multilevel multivariable regression analyses were applied. Patients were predominately male (68%), with mean age 71 years. Carotid stenosis was symptomatic in 40%. Primary outcome occurred in 1.8% of women and 1.9% of men. Multivariable regression analysis revealed that more‐advanced age was associated with a higher primary outcome rate (relative risk [RR] per 10‐year increase: 1.19; 95% CI, 1.14–1.24). Risk of death (alone) was associated with age (RR, 1.68; 95% CI, 1.54–1.84). Age was associated with the risk of stroke (alone; RR, 1.05; 95% CI, 1.00–1.11). Sex was not associated with primary outcome rate (1.01; 95% CI, 0.93–1.10), nor did it significantly modify the age effect.
Conclusions
This study shows that increasing age, but not sex, is associated with a higher risk of in‐hospital stroke or death following carotid endarterectomy under everyday conditions in Germany. Whereas the risk of death (alone) is significantly associated with age, the association between age and the risk of stroke (alone) can be considered of minor importance.
Keywords: aging, carotid artery, sex, stenosis, surgery
Subject Categories: Cerebrovascular Disease/Stroke, Cardiovascular Surgery, Quality and Outcomes, Aging, Women
Introduction
According to current guidelines, carotid endarterectomy (CEA) is recommended for stroke prevention in patients with a severe asymptomatic carotid stenosis (>60%,1, 2 >70%3, 4) or a moderate‐to‐severe (>50%) symptomatic carotid stenosis.1, 2, 4 Although it is suggested that patient age and sex should be taken into account in the decision‐making process,1, 2 recommendations are not yet further specified with respect to the patient's age or sex. However, a systematic review including reports on trial populations and nontrial populations, as well as symptomatic and asymptomatic patients, found that (1) women had a higher rate of perioperative stroke and death (odds ratio [OR], 1.31; 95% CI, 1.17–1.47; 25 studies, 29 345 patients) and (2) that more‐advanced age is significantly associated with an increased risk of perioperative stroke or death (“older” vs “younger”; OR, 1.17; 95% CI, 1.04–1.31; 36 studies, 55 033 patients).5 In a more‐recent systematic review, overall meta‐analysis revealed a worse outcome for female patients, but the results were considered inconsistent.6 Important shortcomings of the above‐mentioned studies are that (1) outcomes were not separately analyzed by sex and symptom status and (2) the interaction between these factors was not investigated. Additionally, the reported studies must be considered observational, because the data arise from subgroup analyses of randomized, controlled trials (RCTs) or nonexperimental studies. Although the risk of an information bias in RCTs can be considered low, strict eligibility criteria may have led to a relevant selection bias. In addition, generalizability of RCT results to everyday practice may be further limited by a referral bias5, 7, 8 and the fact that elderly persons, multimorbid patients, and women are often under‐represented in clinical trials. In Germany, all CEA procedures must be documented in a nation‐wide statutory quality assurance database. This offers the opportunity to evaluate the association of sex and age with the risk of in‐hospital stroke or death following CEA under routine conditions.
Therefore, the aim of this study was first to analyze the association between age and sex and the risk of in‐hospital stroke or death following CEA; second, to investigate whether patients' sex modifies the association between age and the outcome; and third, to describe outcome rates in subgroups (exploratory approach) based on symptomatic status and American Society of Anesthesiologists (ASA) category in a nation‐wide cohort.
Methods
The methods have already been described in detail elsewhere.9, 10 In short, this secondary data analysis is based on the German nation‐wide statutory quality assurance database, operated by the Institute for Applied Quality Improvement and Research in Health Care (AQUA Institute). Between 2009 and 2015, the AQUA Institute has been commissioned and authorized by the German Federal Joint Committee (G‐BA, legal basis §91 German Social Security Code part 520) to develop and implement external quality assurance in the German health care system pursuant to the social security code article §137a SGB V. The AQUA Institute is also mandated for data validation, data analysis, and publication of annual quality reports. In accord with the G‐BA directive concerning the measures of trans‐sectoral and inpatient quality assurance, reporting of quality assurance data is compulsory for all procedures performed in order to treat narrowed internal carotid arteries (ICAs). Therefore, because of legal obligations, the data collection covers nearly all (99.8% in 2014) CEA and carotid artery stenting procedures performed in German hospitals registered under §108 SGB V. The data were available on a procedure‐related basis only, and thus the term “case” would be more precise in specifying the unit of analysis. Nevertheless, we prefer to use the term “patient.” Because patients with recurrent stenosis were generally excluded from this study as well as the number of patients treated on both sides during the same hospital stay is low (0.19–0.62%11), we assumed that the bias attributed to intraindividual correlation of outcomes can also be considered negligible. Overall, 182 033 patients undergoing carotid revascularization between January 2009 and December 2014 were thus documented in the database.
In 2014, the authors' working group was granted access to these quality assurance data. The current study was approved by the ethics committee of the Technical University of Munich and was performed according to the Good Practice of Secondary Data Analysis (GPS12) guidelines and the recommendations for reporting observational studies conducted using routinely collected health data (STROBE and STROSA213, 14, 15). Nonanonymous patient‐level data hosted by the AQUA Institute were accessed in conformance with German data protection laws and controlled by AQUA Institute staff members (T.K., T.B.).
Patients were categorized into 5 age groups (<65, 65–69, 70–74, 75–79, and 80 years or older). Patients were classified a priori into arbitrarily selected age groups for practical reasons, in order to facilitate comparison with age groups commonly used in the literature. In general, the “<65 years” age group served as a reference.
For the purpose of this study, only CEA procedures performed electively were included, namely, patients with an asymptomatic or a symptomatic carotid stenosis who did not require emergency treatment. Symptomatic carotid stenosis was defined as having experienced neurological symptoms related to the ICA stenosis within the past 6 months. All other patients—emergency operations for crescendo transient ischemic attack (TIA) or stroke in evolution, acute ICA occlusion, recurrent stenosis, tandem stenosis, carotid aneurysms, symptomatic ICA coiling, symptomatic low‐grade (<50%) stenosis with ulcerated plaque morphology, and CEA procedures performed concurrently to cardiac or other vascular surgery—were excluded. Finally, 142 074 patients were included in this analysis (Figure 1). With respect to patients' physical status, the ASA classification system was applied.16, 17 Neurological impairments were graded according to the modified Rankin Scale (mRS).18, 19 A stroke was considered minor when graded as 0 to 2 on the mRS. A Rankin score of 3 or more was considered a major stroke.
Figure 1.
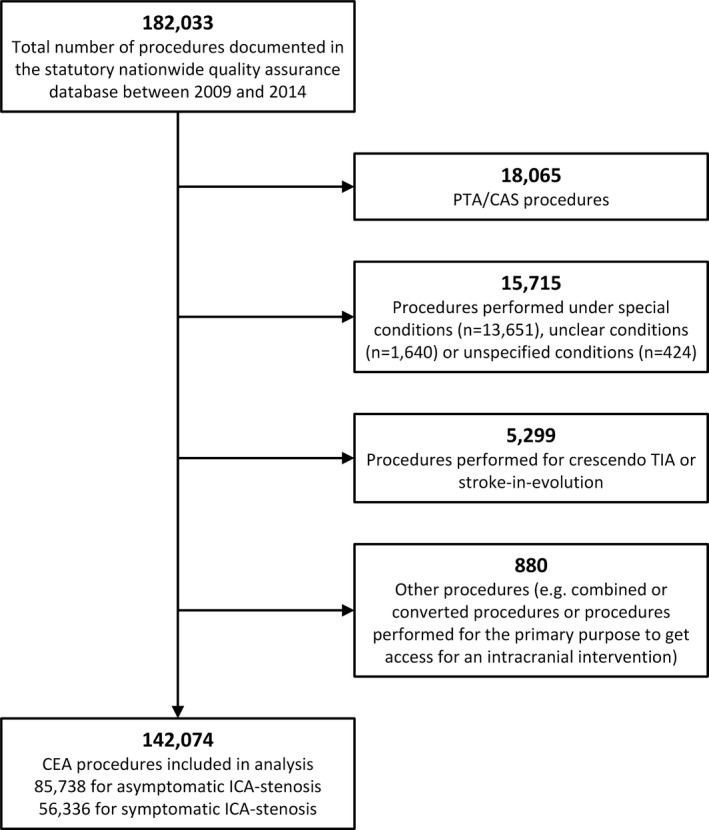
Patient selection. CAS indicates carotid artery stenting; CEA, carotid endarterectomy; ICA, internal carotid artery; PTA, percutaneous transluminal angioplasty; TIA, transient ischemic attack.
The primary outcome (dependent variable) of this study was any new stroke or death from the time of surgery until discharge from hospital. The neurological outcome was directly coded in a variable either as “none,” “TIA,” or “stroke.” Grading of neurological deficit was documented in a separate variable according to the modified Rankin scale. Diagnosis of TIA or stroke was based on clinical/neurological examination with or without further assessment by computed tomography or magnetic resonance imaging. Qualification of the physician performing the examination before and after the procedure is coded in 2 separate variables (pre‐/postprocedural neurological assessment by a specialist in neurology: yes or no, respectively). For the purpose of this study, only strokes (fixed neurological deficits occurring during or after the procedure) were considered as outcome events.
Secondary outcomes were in‐hospital occurrence of a new stroke (alone) and death (alone, in‐hospital mortality).
Statistical Analyses
Nominal and ordinal variables were analyzed using contingency tables. For normally distributed variables, the arithmetic mean and SD were calculated. For variables showing a skewed distribution, the median and the 25%/75% percentiles (Q1, Q3) were stated.
In order to calculate adjusted risk ratios (relative risk; RR) and the corresponding 95% CIs, as well as to account for confounding and clustering of patients within hospitals, a multilevel Poisson regression model was applied.20, 21, 22, 23, 24, 25 The variables, age, sex, neurological symptoms on admission, ASA category, degree of ipsi‐ and contralateral stenosis, periprocedural antiplatelet therapy, pre‐ and postprocedural assessment by a neurologist, intraprocedural neurophysiological monitoring, surgical technique, type of anesthesia, shunt use, intraoperative completion study, and clamping time, were entered into the model as fixed effects. The hospital site code specific for the year of treatment was entered as a random effect (random intercept only). The variables to be entered into the model were selected a priori according to a prespecified analysis plan that was developed with regard to the literature and theoretical considerations. The model fit was assessed by quantile–quantile plots of the random effects. Because the database can be considered complete on a national level, the calculation of CIs or P values would not actually have been necessary and the values are not directly meaningful in this context. Nevertheless, these statistical figures were calculated in order to estimate variability with regard to the future.
Because the cut‐off values for age groups were set arbitrarily, an additional analysis using age as a continuous variable was performed. On the basis of an exploratory approach, continuous age was therefore modeled adjusting for the same covariates as mentioned above. In order to assess whether sex and symptomatic status on admission modify the relationship between age and outcome, interaction terms of these variables were entered into the model.
For data processing and statistical analysis, the statistical package R was used (version 3.2.1; The R Foundation, www.r-project.org). To calculate cross‐classified tables and perform regression analyses, the R extension packages, gmodels, lme4, and gam, were used. To analyze trends for dichotomous variables, the Cochran–Armitage test was applied using SAS software (version 9.4) for Microsoft Windows (Copyright © 2015; SAS Institute Inc., Cary, NC). In general, the significance level was set to α=0.05.
Results
Baseline Characteristics and Management
In total, 142 074 individual patient data sets were available for analysis (see Figure 1 for flow chart). Two thirds of patients were male. The mean age of women and men was 72 and 70 years, respectively (Table 1). Carotid stenosis was symptomatic in 40% of both women and men. Patients were classified as ASA I/II, ASA III, and ASA IV/V in 29%, 68%, and 3%, respectively. In total, 94% of all ipsilateral stenoses were severe (70–99%; according to the method used in the North American Symptomatic Carotid Endarterectomy Trial [NASCET]) and around 18% of all patients had a severe contralateral stenosis or occlusion. See Tables 1 and 2 for further details on patient characteristics. Overall length of hospital stay was 5 days (median, Q0.25–0.75=4–6 days). By subgroups, median length of stay was 5 days in men and woman, whether symptomatic or asymptomatic (please see Figure S1 for a detailed bar chart).
Table 1.
Patient Characteristics
| Men | Women | Total (N) | |
|---|---|---|---|
| Patients (row‐%) | 96 396 (67.8%) | 45 678 (32.2%) | 142 074 |
| Age, mean (SD) | 70.4±8.9 | 71.5±9.3 | 70.7±9.0 |
| Age groups, y | |||
| <65 | 24.9% | 22.3% | 34 166 |
| 65 to 69 | 16.3% | 14.6% | 22 412 |
| 70 to 74 | 24.4% | 22.9% | 33 947 |
| 75 to 79 | 19.8% | 20.8% | 28 626 |
| ≥80 | 14.6% | 19.4% | 22 923 |
| ASA category | |||
| Category I+II | 28.4% | 31.5% | 41 751 |
| Category III | 68.8% | 66.3% | 96 638 |
| Category IV+V | 2.7% | 2.2% | 3685 |
| Symptomatic ICA stenosis | 39.4% | 40.1% | 56 336 |
| Among them AFX or TIA | 53.7%a | 54.2%a | 30 322a |
| Among them minor stroke (mRS 0–2) | 25.9%a | 24.9%a | 14 391a |
| Among them major stroke (mRS 3–5) | 15.5%a | 14.8%a | 8597a |
| Side of treatment (right side) | 50.2% | 50.4% | 71 379 |
| Severe ipsilateral stenosis (70–99%)b | 93.7% | 94.0% | 133 325 |
| Severe contralateral stenosis/occlusion (70–100%)b | 19.7% | 17.6% | 27 038 |
| Preoperative diagnostic procedures | |||
| Duplex ultrasound | 98.7% | 98.8% | 140 240 |
| Transcranial Doppler | 26.5% | 24.8% | 36 835 |
| Computed tomography angiography | 27.3% | 26.6% | 38 498 |
| Magnetic resonance angiography | 33.9% | 33.7% | 48 066 |
| Perioperative antiplatelet medication | |||
| None | 8.0% | 7.9% | 11 320 |
| Mono (acetylsalicylic acid) | 68.5% | 69.4% | 97 720 |
| Mono (others than acetylsalicylic acid) | 2.3% | 2.2% | 3236 |
| Dual | 4.3% | 3.5% | 5697 |
| Neurological assessment | |||
| Preprocedural | 69.4% | 68.5% | 98 178 |
| Postprocedural | 69.8% | 70.0% | 76 456 |
| Pre‐ and postprocedural | 54.1% | 53.2% | 70 444 |
If not otherwise stated, percentages relate to the column. AFX indicates amaurosis fugax; ASA, American Society of Anesthesiologists physical status classification system17 (ASA I=A normal healthy patient, ASA II=A patient with mild systemic disease, ASA III=A patient with severe systemic disease, ASA IV=A patient with severe systemic disease that is a constant threat to life, ASA V=A moribund patient who is not expected to survive without the operation, and ASA VI=A declared brain‐dead patient whose organs are being removed for donor purposes); ICA, internal carotid artery; TIA, transient ischemic attack.
percentages refer to the subgroup of “Symptomatic ICA stenosis”
Measured using the NASCET method19; mRS, modified Rankin Scale; (0=no symptoms, 1=no significant disability, despite symptoms; able to perform all usual duties and activities, 2=slight disability; unable to perform all previous activities but able to look after own affairs without assistance, 3=moderate disability; requires some help, but able to walk without assistance, 4=moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance, 5=severe disability; bedridden, incontinent, and requires constant nursing care and attention, 6=death).
Table 2.
Peri‐ and Intraoperative Management
| Men | Women | Total (N) | |
|---|---|---|---|
| Type of anesthesia | |||
| General anesthesia | 70.9% | 72.7% | 101 522 |
| Locoregional anesthesia | 26.9% | 25.0% | 37 355 |
| Combined or other type | 2.2% | 2.3% | 3197 |
| Intraprocedural monitoring | |||
| Any | 60.0% | 59.2.% | 85 081 |
| Electroencephalography | 9.6% | 9.8% | 8230 |
| Transcranial cerebral oximetry | 17.7% | 18.5% | 15 286 |
| Somatosensory evoked potentials | 47.9% | 49.3% | 41 144 |
| Other methods | 38.8% | 36.9% | 32 469 |
| Operation technique | |||
| CEA without patch | 1.3% | 1.1% | 1765 |
| CEA with patch | 51.5% | 48.7% | 71 920 |
| Eversion CEA | 41.3% | 44.7% | 60 297 |
| Other technique | 5.8% | 5.4% | 8092 |
| Shunt use | 42.7% | 43.6% | 61 074 |
| Clamping time, min, median (Q1–Q3) | 17 (6–28) | 15 (6–26) | n.a. |
| Intraoperative vessel patency check | 69.8% | 70.0% | 99 216 |
| Duration of operation in minutes, median (Q1–Q3) | 87 (69–108) | 83 (63–99) | n.a. |
If not otherwise stated, percentages relate to the column. CEA indicates carotid endarterectomy; Q1, first quartile; Q3, third quartile.
Crude Primary and Secondary Outcome Rates
The primary outcome occurred in 2611 of all patients (1.8%; 95% CI, 1.8–1.9%). In‐hospital stroke or death occurred in 1175 asymptomatic patients (1.4%; 95% CI, 1.3–1.5) and 1436 symptomatic patients (2.5%; 95% CI, 2.4–2.7). In patients younger than 65 years, those aged 65 to 69, 70 to 74, 75 to 79, and 80 years or older, the crude risk of any inhospital stroke or death was 1.4%, 1.7%, 1.8%, 1.9%, and 2.6%, respectively (P<0.001 for trend). With respect to the secondary outcomes, 845 deaths (0.6%; 95% CI, 0.6–0.6) and 1980 strokes (1.4%; 95% CI, 1.3–1.5) occurred in the whole cohort. The absolute risk of any in‐hospital stroke or death, any stroke (alone), and death (alone; by age, sex, and neurological status) is depicted in Figure 2. The corresponding absolute numbers are given in Table 3. With respect to ASA category, the crude primary and secondary outcome rates are stated in Figures S2 through S4.
Figure 2.
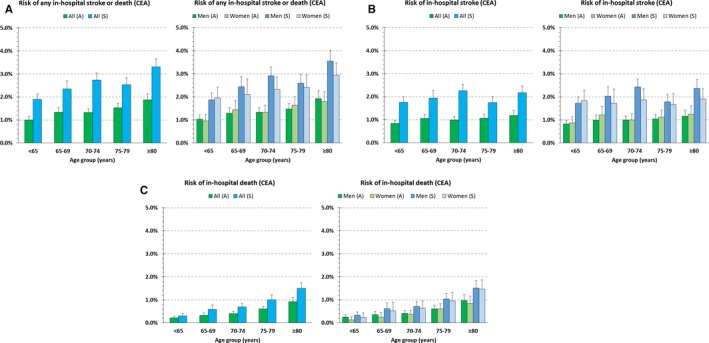
Crude risk of (A) any in‐hospital stroke or death, (B) stroke alone, and (C) death alone by age groups and neurological status on admission (left). Sex‐specific rates are depicted in the right column (see Table 3 for point estimates and 95% CIs; see Figure 3 for adjusted risk ratios and CIs). A indicates asymptomatic ICA stenosis; bars, 95% CI; CEA, carotid endarterectomy; ICA, internal carotid artery; S, symptomatic ICA stenosis (nonemergency).
Table 3.
Outcome: Crude Event Rates by Sex, Age, and Neurological Status on Admission
| n/N (%) | Asymptomatic | Symptomatic | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Any stroke or death, y | ||||
| <65 | 149/14 451 (1.0) | 57/5990 (1.0) | 178/9533 (1.9) | 82/4192 (2.0) |
| 65 to 69 | 130/10 079 (1.3) | 63/4362 (1.4) | 138/5646 (2.4) | 49/2325 (2.1) |
| 70 to 74 | 199/14 941 (1.3) | 89/6706 (1.3) | 250/8562 (2.9) | 87/3738 (2.3) |
| 75 to 79 | 171/11 577 (1.5) | 95/5798 (1.6) | 196/7553 (2.6) | 89/3698 (2.4) |
| ≥80 | 141/7326 (1.9) | 81/4508 (1.8) | 239/6728 (3.6) | 128/4361 (2.9) |
| All age groups | 790/58 374 (1.4) | 385/27 364 (1.4) | 1001/38 022 (2.6) | 435/18 314 (2.4) |
| 1175/85 738 (1.4) | 1436/56 336 (2.5) | |||
| 2611/142 074 (1.8) | ||||
| Any stroke (alone), y | ||||
| <65 | 120/14 451 (0.8) | 52/5990 (0.9) | 164/9533 (1.7) | 77/4192 (1.8) |
| 65 to 69 | 100/10 079 (1.0) | 53/4362 (1.2) | 115/5646 (2.0) | 40/2325 (1.7) |
| 70 to 74 | 149/14 941 (1.0) | 67/6706 (1.0) | 208/8562 (2.4) | 70/3738 (1.9) |
| 75 to 79 | 120/11 577 (1.0) | 65/5798 (1.1) | 135/7553 (1.8) | 62/3698 (1.7) |
| ≥80 | 85/7326 (1.2) | 56/4508 (1.2) | 159/6728 (2.4) | 83/4361 (1.9) |
| All age groups | 574/58 374 (1.0) | 293/27 364 (1.1) | 781/38 022 (2.1) | 332/18 314 (1.8) |
| 867/85 738 1.0) | 1113/56 336 (2.0) | |||
| 1980/142 074 (1.4) | ||||
| Death (alone), y | ||||
| <65 | 36/14 451 (0.2) | 8/5990 (0.1) | 32/9533 (0.3) | 10/4192 (0.2) |
| 65 to 69 | 36/10 079 (0.4) | 11/4362 (0.3) | 35/5646 (0.6) | 12/2325 (0.5) |
| 70 to 74 | 62/14 941 (0.4) | 25/6706 (0.4) | 61/8562 (0.7) | 24/3738 (0.6) |
| 75 to 79 | 70/11 577 (0.6) | 35/5798 (0.6) | 78/7553 (1.0) | 35/3698 (0.9) |
| ≥80 | 71/7326 (1.0) | 38/4508 (0.8) | 102/6728 (1.5) | 64/4361 (1.5) |
| All age groups | 275/58 374 (0.5) | 117/27 364 (0.4) | 308/38 022 (0.8) | 145/18 314 (0.8) |
| 392/85 738 (0.5) | 453/56 336 (0.8) | |||
| 845/142 074 (0.6) | ||||
Multivariable Analyses
Multivariable regression analysis of the whole cohort using age as a continuous variable revealed that age was significantly associated with a higher risk of any in‐hospital stroke or death in CEA patients (RR per 10‐year increase: 1.19; 95% CI, 1.14–1.24). With respect to the secondary outcomes, the adjusted risk of death (alone) was significantly associated with age (RR, 1.68; 95% CI, 1.54–1.84). Age was also significantly associated with the risk of stroke (alone); however, this relationship was weaker (RR, 1.05; 95% CI, 1.00–1.11). Sex was not associated with the risk of any in‐hospital stroke or death (1.01; 95% CI, 0.93–1.10), stroke (alone; 0.99; 95% CI, 0.90–1.09), or death (alone; 1.06; 95% CI, 0.91–1.23). Forest plots of multivariable regression results using age as a categorical variable are depicted in Figure 3. Adjusted RRs using age as a continuous variable are depicted in Figure 4 (left column).
Figure 3.
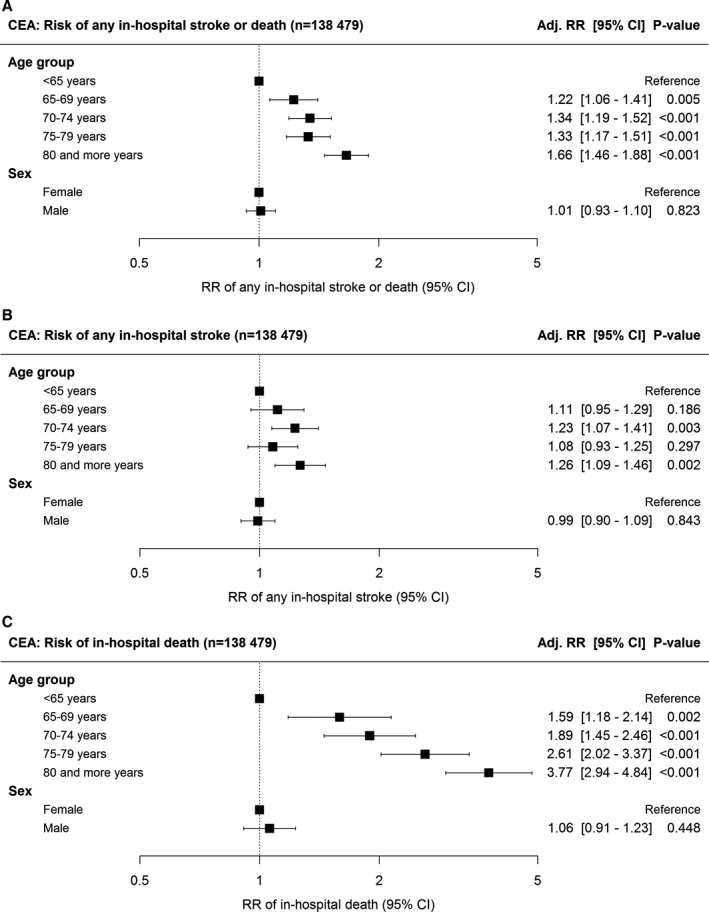
Forest plot of multilevel multivariable regression results. Association of age and sex with the in‐hospital risk of (A) any stroke or death, (B) any stroke alone, and (C) all‐cause death alone. Adj. RR=risk ratio adjusted for ASA category, neurological status on admission, ipsi‐ and contralateral degree of carotid stenosis, antiplatelet medication, pre‐ and postoperative assessment by a neurologist, intraoperative neurophysiological monitoring, technique of CEA, anesthesia, shunting, intraoperative check of technical success, clamping time, and annual hospital CEA volume (see Methods for further details on the regression model); bars indicate 95% CI. ASA indicates American Society of Anesthesiologists; CEA, carotid endarterectomy.
Figure 4.
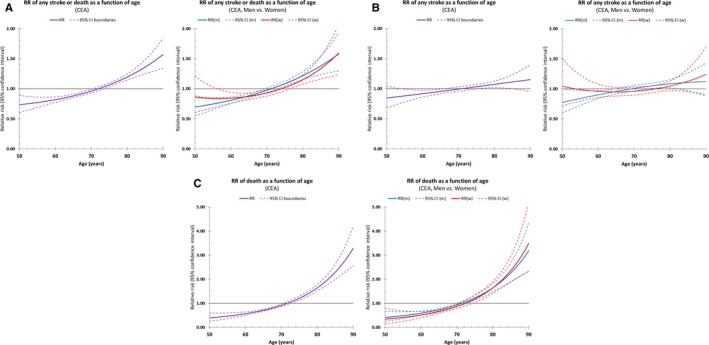
Relative risks as a function of age for (A) any in‐hospital stroke or death, (B) any stroke, and (C) all‐cause death alone (please note differing range of the y‐axis). Dotted lines indicate the 95% CI. CEA indicates carotid endarterectomy; m, men; w, women.
Sex‐Age Interaction
Linear age effects differentiated by sex and symptomatic status are given in Table 4. Sex does not significantly modify the adjusted age effects (P>0.05; Figure 4, right column). The risk of stroke or death was generally higher in symptomatic patients (see Table 3 for crude values; adjusted RR=1.61; 95% CI, 1.48–1.76), but there was no significant interaction between neurological status and the effects of age and sex (P>0.05).
Table 4.
Linear Age Effect by Sex and Neurological Status on Admission
| Linear Age Effecta Adj. RR [95% CI] | Asymptomatic | Symptomatic | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Any stroke or death | 1.21 [1.11–1.32]b | 1.21 [1.07–1.38]b | 1.23 [1.14–1.32]b | 1.09 [0.98–1.20] |
| 1.21 [1.13–1.30]b | 1.16 [1.03–1.31]b | |||
| 1.19 [1.14–1.24]b | ||||
| Any stroke (alone) | 1.06 [0.96–1.17] | 1.10 [0.95–1.27] | 1.08 [1.00–1.17] | 0.96 [0.86–1.08] |
| 1.07 [0.99–1.17] | 1.03 [0.92–1.15] | |||
| 1.05 [1.00–1.11]b | ||||
| Death (alone) | 1.65 [1.40–1.93]b | 1.75 [1.36–2.25]b | 1.69 [1.47–1.96]b | 1.68 [1.37–2.06]b |
| 1.68 [1.46–1.92]b | 1.69 [1.50–1.90]b | |||
| 1.68 [1.54–1.84]b | ||||
Adjusted relative risk of increasing age as a continuous variable on the risk of any inhospital stroke or death, any stroke, and all‐cause death.
Per 10‐year increase, adj. RR=relative risk adjusted for American Society of Anesthesiologists category, ipsi‐ and contralateral degree of carotid stenosis, antiplatelet medication, pre‐ and postoperative assessment by a specialist in neurology, intraoperative neurophysiological monitoring, technique of carotid endarterectomy (CEA), anesthesia, shunting, intraoperative check of technical success, clamping time, and annual hospital CEA volume.
P<0.05.
Discussion
The current study demonstrates that increasing age is independently associated with a higher risk of in‐hospital stroke or death following CEA under everyday conditions in Germany. This relationship is based primarily on the association between age and mortality (RR=1.68). Regarding the relationship between age and stroke (alone), both the small magnitude of association (RR=1.05) and the borderline significance indicate that age is most likely not a clinically relevant risk factor for in‐hospital stroke (alone).
Age
These findings are in accord with previously published studies.5, 26, 27 Additionally, results from the National (Nationwide) Inpatient Sample (NIS) database including more than 400 000 CEA procedures also revealed that the postoperative mortality risk, but not the postoperative risk of stroke, was higher in patients aged 70 years or older compared to younger individuals.28 Regarding the latter study, it must be mentioned that information on procedures, comorbidities, and outcomes was based on administrative records only, rather than directly documented clinical examinations.
In contrast to the current findings, in the NASCET and European Carotid Surgery Trial (ECTS) studies, it was shown that there was no significant difference in the perioperative risk of stroke or death with respect to patients' age.29 Given that patients older than 80 years had generally been excluded (NASCET30) and only 9.9% of patients were aged 75 years or older (NASCET and ECST29), the latter findings do not necessarily contradict our results. Subgroup analyses of the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) by Voeks et al also showed that there were no differences between age groups following CEA either in terms of stroke events alone or regarding the composite endpoint (stroke, death, and myocardial infarction).31 Nevertheless, the latter outcome rates referred to a 4‐year follow‐up after treatment rather than the perioperative time period. Possible reasons for the disagreement between the aforementioned reports and the current results might be that the RCTs had not been powered for the purpose of investigating age effects and the sample sizes may have thus been too small to prove an effect. In contrast to nation‐wide databases such as the NIS or the German statutory quality assurance database, strict inclusion criteria applied in RCTs may have cropped the broad age range of patients presenting with carotid stenosis under routine conditions. Finally, results from RCTs must be considered subject to referral and selection bias, as well as to performance bias.32
Sex
The present study revealed a clear difference in sex distribution (men:women=2:1). However, this finding is consistent with the general observation that carotid stenosis is more frequently found in men than in women.8, 33, 34, 35 In addition, the proportion of men observed in our cohort (67.8%) corresponds well to national‐level data from the UK (63.7–67.0%)36 and Sweden (63.8–66.6%)37 as well as to findings of multinational, randomized trials (65–71%),38 but is slightly higher than reported for the US Medicare Population (56.3–60.3%).39
One main finding of this study is that patient sex was neither associated with the risk of stroke or death (RR, 1.01; 95% CI, 0.93–1.10), nor did it influence the association between age and the primary or secondary outcomes.
In accord with our data, the largest and most recent population‐based study,40 which included more than 90 000 patients from the NIS database, also revealed that sex was not associated with the risk of stroke or death (OR, 1.01; 95% CI, 0.94–1.08; own calculation using data extracted from Luebke and Brunkwall6). In contrast, a recent systematic review and meta‐analysis based on 4 RCTs, as well as 10 population‐based database studies and 20 case series (investigating sex effect as primary aim), revealed that women had a higher risk of perioperative stroke or death (OR, 1.16; 95% CI, 1.07–1.27), which is consistent with a former meta‐analysis by Bond et al.5 Nevertheless, the latter meta‐analysis came to the conclusion that sex was not associated with the 30‐day perioperative risk of stroke and death when analyzing RCTs, database studies, and case series separately.6 However, study results were inconsistent with respect to design of the primary studies and statistical analyses (random‐effect model vs fixed‐effect model).6
With respect to our results, these differences might also be explained by the fact that the aforementioned meta‐analyses included case series and RCTs, which do not necessarily represent the real‐world situation attributed to possible selection, referral, and performance bias. Additionally, studies included in the above‐mentioned reviews traced back to the 1980s and, as has been shown by a meta‐regression,6 the risk of stroke or death has reduced over time.
Further reasons for the sex dependency of perioperative risks might be that patient‐related risk factors, such as cigarette smoking, elevated blood pressure, being overweight, hormonal disorders, pregnancy‐associated factors, and menopause, are unevenly distributed or of varying importance between men and women.41 In addition, biological factors and histomorphological plaque characteristics may also possibly account for differences in stroke or mortality risks with respect to patients' sex.42, 43, 44, 45, 46, 47 However, the current data show that sex is not significantly associated with the perioperative stroke and death risk following CEA under routine conditions in Germany.
Neurological Status and ASA Category
In the present study, symptomatic patients were more likely to suffer an outcome event than asymptomatic patients. This finding is a generally known fact and was noted in previously published RCTs as well as in observational studies and case series.2 Thus, it is not unexpected that the same risk pattern can be observed when CEA is performed under routine conditions.
With respect to ASA category, the current exploratory analysis revealed that patients classified ASA IV/V had a higher risk of stroke or death, particularly in higher age groups. This crude age effect was also noted in patients classified ASA I/II and III, but to a lesser extent. Given that there are only few patients as well as few events in the ASA IV/V category, the authors refrain from performing higher‐order interaction analyses. Nevertheless, one may cautiously build 2 hypotheses from these crude data: first, patients classified ASA I to III might be treated safely although they are old, and second, younger patients might be treated safely although they are classified ASA IV/V.
Limitations and Strengths
This study has some limitations. First, the study was retrospective and observational, given that no randomization was performed with respect to the independent variables analyzed in this study (age and sex). Thus, only associations rather than causal relationships can be inferred from the data. Additionally, a potential selection bias cannot be ruled out because only patients who received CEA were collected in the database. Regarding sex‐specific treatment selection, there are no distinct sex‐specific recommendations in the German or international guidelines. Thus, a selection bias attributed to guideline adherence issues is considered less likely. However, it is well known that benefit from CEA is lower in woman compared to men, and therefore this knowledge might have influenced medical decision making. Therefore, a potential sex‐specific selection bias could not be ruled out.
Second, because of legal reasons, follow‐up data of all patients were only collected for the in‐hospital period, and therefore the data presented in this article could not be compared to studies reporting, for example, 30‐day results. Because the majority of postoperative events occur within the first days postsurgery48 and given that the length of hospital stay is comparable with respect to age groups and sex (median, 5 days; see Figure S1), information bias is considered to be homogenous across the study groups.
Third, given that all data were self‐reported by the treating physicians or delegates of the treating departments, an information bias is possible. Misinformation may be counterbalanced by structured validity checks (performed by the so‐called Landesstellen and the AQUA Institute), as well as by hospital audits reporting results that are higher or lower than expected on a semiannual basis. Additionally, given that misclassification of outcome events (intended or not) is most likely nondifferential with respect to sex, age, and neurological symptoms on admission, the risk of bias is considered low.
Fourth, because no further data on medication or comorbidities (coronary heart disease, diabetes mellitus, hypertension, smoking habits, hyperlipidemia, statin therapy, hormone replacement therapy, etc) were documented in the database, only patients' sex, age, ASA stage, and degree of carotid stenosis were available for medical risk adjustment. Additionally, nonconsideration of unobserved confounders (eg, patency of vertebral arteries, patency of the circle of Willis, plaque morphology, contextual factors, and health‐system–specific factors) may also have influenced the indication for treatment, the selection of technique, the selective application of intra‐arterial shunting, or the decision to delegate the treatment to an experienced physician or transfer the patient to another hospital. In summary, potential bias caused by unobserved confounders cannot be ruled out, and thus generalizability of results might be limited. In the near future, it should be discussed by specialists in medical quality assurance, health services researchers, and health policy makers, which factors are needed for proper risk adjustment and control for confounding in a statutory quality assurance program in order to improve quality of vascular services in a patient‐centered and value‐based fashion.
Nevertheless, the strengths of our study are (1) patient data were collected systematically on a statutory basis with the primary aim of disease‐specific quality assurance, (2) the data were based on clinical assessment rather than administrative records, and (3) results were adjusted using multilevel multivariable regression analysis to reduce confounding as much as possible. In contrast to RCTs, data acquisition did not apply inclusion or exclusion criteria, and therefore these data reflect the provision of CEA under routine conditions.
Conclusions
This study shows that increasing age was independently associated with a higher risk of in‐hospital stroke or death following CEA under everyday conditions in Germany. This relationship is based primarily on the association between age and death (alone; RR=1.68) rather than on the weak association between age and stroke (alone; RR=1.05).
Sources of Funding
This work was funded, in part, by a research grant from the School of Medicine of the Technical University of Munich (KKF‐scholarship No. 8700000162).
Disclosures
None.
Supporting information
Figure S1. Length of postoperative hospital stay (in days). Bars indicate quartiles 1 and 3.
Figure S2. Absolute risk of any in‐hospital stroke or death in patients with ASA category I+II, III, and IV+V by age group and neurological status on admission. Bars indicate the 95% CI.
Figure S3. Absolute risk of stroke (alone) in patients with ASA category I+II, III, and IV+V by age group, sex, and neurological status on admission. Bars indicate the 95% CI.
Figure S4. Absolute risk of death (alone) in patients with ASA category I+II, III, and IV+V by age group, sex, and neurological status on admission. Bars indicate the 95% CI.
(J Am Heart Assoc. 2017;6:e004764. DOI: 10.1161/JAHA.116.004764.)
References
- 1. Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Rother J, Sievert H, van Sambeek M, Zeller T. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–2906. [DOI] [PubMed] [Google Scholar]
- 2. Eckstein HH, Kuhnl A, Dorfler A, Kopp IB, Lawall H, Ringleb PA; Multidisciplinary German‐Austrian guideline based on e, consensus . The diagnosis, treatment and follow‐up of extracranial carotid stenosis. Dtsch Arztebl Int. 2013;110:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011;42:e464–e540. [DOI] [PubMed] [Google Scholar]
- 4. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council CoC, Stroke Nursing CoCC, Council on Peripheral Vascular D . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 5. Bond R, Rerkasem K, Cuffe R, Rothwell PM. A systematic review of the associations between age and sex and the operative risks of carotid endarterectomy. Cerebrovasc Dis. 2005;20:69–77. [DOI] [PubMed] [Google Scholar]
- 6. Luebke T, Brunkwall J. Meta‐ analysis and meta‐regression analysis of the associations between sex and the operative outcomes of carotid endarterectomy. BMC Cardiovasc Disord. 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feinglass J, McDermott MM, Foroohar M, Pearce WH. Gender differences in interventional management of peripheral vascular disease: evidence from a blood flow laboratory population. Ann Vasc Surg. 1994;8:343–349. [DOI] [PubMed] [Google Scholar]
- 8. Ramani S, Byrne‐Logan S, Freund KM, Ash A, Yu W, Moskowitz MA. Gender differences in the treatment of cerebrovascular disease. J Am Geriatr Soc. 2000;48:741–745. [DOI] [PubMed] [Google Scholar]
- 9. Tsantilas P, Kuehnl A, Konig T, Breitkreuz T, Kallmayer M, Knappich C, Schmid S, Storck M, Zimmermann A, Eckstein HH. Short time interval between neurologic event and carotid surgery is not associated with an increased procedural risk. Stroke. 2016;47:2783–2790. [DOI] [PubMed] [Google Scholar]
- 10. Kuehnl A, Tsantilas P, Knappich C, Schmid S, Konig T, Breitkreuz T, Zimmermann A, Mansmann U, Eckstein HH. Significant association of annual hospital volume with the risk of inhospital stroke or death following carotid endarterectomy but likely not after carotid stenting: secondary data analysis of the statutory German carotid quality assurance database. Circ Cardiovasc Interv. 2016;9:pii: e004171. [DOI] [PubMed] [Google Scholar]
- 11. AQUA ‐ Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH . Karotis‐Revaskularisation. Bundesauswertungen 2009. ‐2014 [Carotid‐Revascularization. National annual reports 2009–2014]. Available at: https://sqg.de/front_content.php?idart=23. Accessed March 1, 2016.
- 12. Swart E, Gothe H, Geyer S, Jaunzeme J, Maier B, Grobe TG, Ihle P. Good Practice of Secondary Data Analysis (GPS): guidelines and recommendations. Gesundheitswesen. 2015;77:120–126. [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; Initiative S . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 14. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sorensen HT, von Elm E, Langan SM. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swart E, Bitzer EM, Gothe H, Harling M, Hoffmann F, Horenkamp‐Sonntag D, Maier B, March S, Petzold T, Rohrig R, Rommel A, Schink T, Wagner C, Wobbe S, Schmitt J. [A Consensus German Reporting Standard for Secondary Data Analyses, Version 2 (STROSA‐STandardisierte BerichtsROutine fur SekundardatenAnalysen)]. Gesundheitswesen. 2016;78(S 01):e145–e160. [DOI] [PubMed] [Google Scholar]
- 16. Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–243. [DOI] [PubMed] [Google Scholar]
- 17. Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. [DOI] [PubMed] [Google Scholar]
- 20. Chen W, Shi J, Qian L, Azen SP. Comparison of robustness to outliers between robust Poisson models and log‐binomial models when estimating relative risks for common binary outcomes: a simulation study. BMC Med Res Methodol. 2014;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ. 2012;184:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee J, Tan CS, Chia KS. A practical guide for multivariate analysis of dichotomous outcomes. Ann Acad Med Singapore. 2009;38:714–719. [PubMed] [Google Scholar]
- 23. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 24. Urbach DR, Austin PC. Conventional models overestimate the statistical significance of volume‐outcome associations, compared with multilevel models. J Clin Epidemiol. 2005;58:391–400. [DOI] [PubMed] [Google Scholar]
- 25. Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–670. [DOI] [PubMed] [Google Scholar]
- 26. Park H, Kwon TW, Kwon SU, Kang DW, Kim JS, Chung YS, Shin S, Han Y, Cho YP. A retrospective 10‐year, single‐institution study of carotid endarterectomy with a focus on elderly patients. J Clin Neurol. 2016;12:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller MT, Comerota AJ, Tzilinis A, Daoud Y, Hammerling J. Carotid endarterectomy in octogenarians: does increased age indicate “high risk?”. J Vasc Surg. 2005;41:231–237. [DOI] [PubMed] [Google Scholar]
- 28. Khatri R, Chaudhry SA, Vazquez G, Rodriguez GJ, Hassan AE, Suri MF, Qureshi AI. Age differential between outcomes of carotid angioplasty and stent placement and carotid endarterectomy in general practice. J Vasc Surg. 2012;55:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ; Carotid Endarterectomy Trialists C . Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. [DOI] [PubMed] [Google Scholar]
- 30. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. [DOI] [PubMed] [Google Scholar]
- 31. Voeks JH, Howard G, Roubin GS, Malas MB, Cohen DJ, Sternbergh WC III, Aronow HD, Eskandari MK, Sheffet AJ, Lal BK, Meschia JF, Brott TG. Age and outcomes after carotid stenting and endarterectomy: the carotid revascularization endarterectomy versus stenting trial. Stroke. 2011;42:3484–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delgado‐Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eckstein HH, Kühnl A, Berkefeld J, Diel R, Dörfler A, Kopp I, Langhoff R, Lawall H, Ringleb P, Sander D, Storck M. S3‐Leitlinie zur Diagnostik, Therapie und Nachsorge der extracraniellen Carotisstenose [The diagnosis, treatment and follow‐up of extracranial carotid stenosis]. AWMF Register Nr. 2012;004:028. [Google Scholar]
- 34. de Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke. 2009;40:1105–1113. [DOI] [PubMed] [Google Scholar]
- 35. de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, Rosvall M, Sitzer M, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta‐analysis. Stroke. 2010;41:1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holt PJ, Poloniecki JD, Loftus IM, Thompson MM. The relationship between hospital case volume and outcome from carotid endartectomy in England from 2000 to 2005. Eur J Vasc Endovasc Surg. 2007;34:646–654. [DOI] [PubMed] [Google Scholar]
- 37. Kragsterman B, Bjorck M, Lindback J, Bergqvist D, Parsson H; Swedish Vascular R . Long‐term survival after carotid endarterectomy for asymptomatic stenosis. Stroke. 2006;37:2886–2891. [DOI] [PubMed] [Google Scholar]
- 38. Halm EA. The good, the bad, and the about‐to‐get ugly: national trends in carotid revascularization: comment on “Geographic variation in carotid revascularization among Medicare beneficiaries, 2003–2006”. Arch Intern Med. 2010;170:1225–1227. [DOI] [PubMed] [Google Scholar]
- 39. Patel MR, Greiner MA, DiMartino LD, Schulman KA, Duncan PW, Matchar DB, Curtis LH. Geographic variation in carotid revascularization among Medicare beneficiaries, 2003–2006. Arch Intern Med. 2010;170:1218–1225. [DOI] [PubMed] [Google Scholar]
- 40. Kuy S, Dua A, Desai SS, Rossi PJ, Seabrook GR, Lewis BD, Patel B, Kuy S, Lee CJ, Subbarayan R, Brown KR. Carotid endarterectomy national trends over a decade: does sex matter? Ann Vasc Surg. 2014;28:887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241:211–218. [DOI] [PubMed] [Google Scholar]
- 42. den Hartog AG, Algra A, Moll FL, de Borst GJ. Mechanisms of gender‐related outcome differences after carotid endarterectomy. J Vasc Surg. 2010;52:1062–1071, 1071.e1‐6. [DOI] [PubMed] [Google Scholar]
- 43. Hellings WE, Pasterkamp G, Verhoeven BA, De Kleijn DP, De Vries JP, Seldenrijk KA, van den Broek T, Moll FL. Gender‐associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg. 2007;45:289–296; discussion 296‐7. [DOI] [PubMed] [Google Scholar]
- 44. Iemolo F, Martiniuk A, Steinman DA, Spence JD. Sex differences in carotid plaque and stenosis. Stroke. 2004;35:477–481. [DOI] [PubMed] [Google Scholar]
- 45. Ota H, Reeves MJ, Zhu DC, Majid A, Collar A, Yuan C, DeMarco JK. Sex differences of high‐risk carotid atherosclerotic plaque with less than 50% stenosis in asymptomatic patients: an in vivo 3T MRI study. AJNR Am J Neuroradiol. 2013;34:1049–1055, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vrijenhoek JE, Den Ruijter HM, De Borst GJ, de Kleijn DP, De Vries JP, Bots ML, Van de Weg SM, Vink A, Moll FL, Pasterkamp G. Sex is associated with the presence of atherosclerotic plaque hemorrhage and modifies the relation between plaque hemorrhage and cardiovascular outcome. Stroke. 2013;44:3318–3323. [DOI] [PubMed] [Google Scholar]
- 47. Wendorff C, Wendorff H, Pelisek J, Tsantilas P, Zimmermann A, Zernecke A, Kuehnl A, Eckstein HH. Carotid plaque morphology is significantly associated with sex, age, and history of neurological symptoms. Stroke. 2015;46:3213–3219. [DOI] [PubMed] [Google Scholar]
- 48. Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, Barnett HJ. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–1758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Length of postoperative hospital stay (in days). Bars indicate quartiles 1 and 3.
Figure S2. Absolute risk of any in‐hospital stroke or death in patients with ASA category I+II, III, and IV+V by age group and neurological status on admission. Bars indicate the 95% CI.
Figure S3. Absolute risk of stroke (alone) in patients with ASA category I+II, III, and IV+V by age group, sex, and neurological status on admission. Bars indicate the 95% CI.
Figure S4. Absolute risk of death (alone) in patients with ASA category I+II, III, and IV+V by age group, sex, and neurological status on admission. Bars indicate the 95% CI.


