Abstract
Background
The usefulness of cardiovascular disease (CVD) predictive equations in different populations is debatable. We assessed the efficacy of the Framingham‐REGICOR scale, validated for the Spanish population, to identify future CVD in participants, who were predefined as being at high‐risk in the PREvención con DIeta MEDiterránea (PREDIMED) study—a nutrition‐intervention primary prevention trial—and the impact of adherence to the Mediterranean diet on CVD across risk categories.
Methods and Results
In a post hoc analysis, we assessed the CVD predictive value of baseline estimated risk in 5966 PREDIMED participants (aged 55–74 years, 57% women; 48% with type 2 diabetes mellitus). Major CVD events, the primary PREDIMED end point, were an aggregate of myocardial infarction, stroke, and cardiovascular death. Multivariate‐adjusted Cox regression was used to calculate hazard ratios for major CVD events and effect modification from the Mediterranean diet intervention across risk strata (low, moderate, high, very high). The Framingham‐REGICOR classification of PREDIMED participants was 25.1% low risk, 44.5% moderate risk, and 30.4% high or very high risk. During 6‐year follow‐up, 188 major CVD events occurred. Hazard ratios for major CVD events increased in parallel with estimated risk (2.68, 4.24, and 6.60 for moderate, high, and very high risk), particularly in men (7.60, 13.16, and 15.85, respectively, versus 2.16, 2.28, and 3.51, respectively, in women). Yet among those with low or moderate risk, 32.2% and 74.3% of major CVD events occurred in men and women, respectively. Mediterranean diet adherence was associated with CVD risk reduction regardless of risk strata (P>0.4 for interaction).
Conclusions
Incident CVD increased in parallel with estimated risk in the PREDIMED cohort, but most events occurred in non–high‐risk categories, particularly in women. Until predictive tools are improved, promotion of the Mediterranean diet might be useful to reduce CVD independent of baseline risk.
Clinical Trial Registration
URL: http://www.Controlled-trials.com. Unique identifier: ISRCTN35739639.
Keywords: cardiovascular disease, cardiovascular risk prediction, Framingham‐REGICOR equation, Mediterranean diet, PREDIMED
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology, Lifestyle, Primary Prevention
Introduction
The current approach for cardiovascular disease (CVD) prevention uses risk estimation based on traditional cardiovascular risk factors to tailor intervention accordingly.1, 2 There are different risk assessment models depending on the geographical area and the baseline risk of the selected population.3 In European countries, the European Society of Cardiology recommends the Systematic Coronary Risk Evaluation (SCORE) strategy.4 Based on SCORE, many surveys in different European countries have been performed using either very simple5 or more complicated instruments.6, 7 In Spain, a calibrated Framingham equation (Framingham‐REGICOR [F‐R])8 shown to be effective in predicting CVD9, 10 is also commonly used.
These risk estimation systems are useful at a population level but have limitations, in particular, a strong dependence on age, short‐term (10 year) risk prediction, reliance mostly on a cross‐sectional evaluation of risk, and no consideration of nonclassical risk factors or current or past pharmacological treatment (statin, antihypertensive, and antiplatelet drugs).11, 12 Of note, although the relative risk is always higher in high‐risk persons, most CVD events (55–80%) occur in the low and moderate risk strata,10, 13 a major problem for a personalized risk reduction approach considering that usually >75% of the population is classified as non–high risk.14, 15 Consequently, the usefulness of these tools in different settings and populations and its CVD predictive ability are still a matter of debate.16
Despite the wide use of cardiovascular risk scores and the progressive improvement in risk factor control, CVD is still the leading cause of death and disability in developed countries.17, 18 Newer strategies to better identify individuals at risk and novel pharmacological and/or lifestyle treatment approaches to prevent CVD events need to be developed. The PREvención con DIeta MEDiterránea (PREDIMED) trial demonstrated that an affordable Mediterranean diet (MedDiet) supplemented with foods rich in healthy fats (extra‐virgin olive oil [EVOO] and nuts) without aiming at weight change is useful to prevent CVD in comparison with advice to follow a low‐fat diet.19 The baseline age (≥55 and ≥60 years for men and women, respectively) and the high risk (either type 2 diabetes mellitus or ≥3 major cardiovascular risk factors) of PREDIMED participants are of particular interest from an epidemiological standpoint. First, some of the limitations of predictive equations in current CVD prevention strategies11, 12 could be partly overridden. Second, the clinically relevant question of whether the MedDiet intervention tested in PREDIMED was similarly effective regardless of baseline risk could be assessed in a post hoc manner. We thus used the prespecified high‐risk PREDIMED cohort to assess baseline cardiovascular risk and its association with future CVD, to test the ability of recommended risk‐estimation strategies to identify persons who will suffer a CVD event during follow‐up, and to evaluate the effect of the intervention (MedDiet versus low‐fat diet) across the different estimated risk categories.
Material and Methods
Study Design and Participants
The PREDIMED study is a large, parallel‐group, multicenter, randomized, controlled, clinical trial designed to assess the effects of the MedDiet on the primary prevention of CVD. The design and primary results of the trial have been published in detail.19, 20 Briefly, from October 2003 to June 2009, a total of 8713 candidates were screened for eligibility at 11 recruiting centers throughout Spain, and 7447 were randomly assigned to 1 of 3 interventions: MedDiet supplemented with EVOO (MedDiet/EVOO), MedDiet supplemented with mixed nuts (MedDiet/nuts), or control diet (advice on a low‐fat diet). Participants were men aged 55 to 80 years and women aged 60 to 80 years at high cardiovascular risk but with no CVD at enrollment. Criteria for eligibility were the presence of either type 2 diabetes mellitus or at least 3 cardiovascular risk factors: current smoking, hypertension, dyslipidemia, overweight or obesity, and family history of early onset coronary heart disease. Participants were considered to have diabetes mellitus, hyperlipidemia, or hypertension if they had a previous diagnosis of these conditions and/or if they were treated with antidiabetic, cholesterol‐lowering, or antihypertensive agents, respectively. Smoking status was categorized into never, current, or past smoking, according to self‐report. Participants (n=5966, 57% women, 48% with type 2 diabetes mellitus) who were aged <74 years (the age limit of the F‐R equation) with sufficient data to estimate their cardiovascular risk were selected from the total cohort for the present analyses (Figure 1). The study was conducted in accordance with the Declaration of Helsinki. The institutional review board of all recruiting centers approved the study protocol, and all participants provided informed consent. The formal trial protocol can be found in Data S1.
Figure 1.
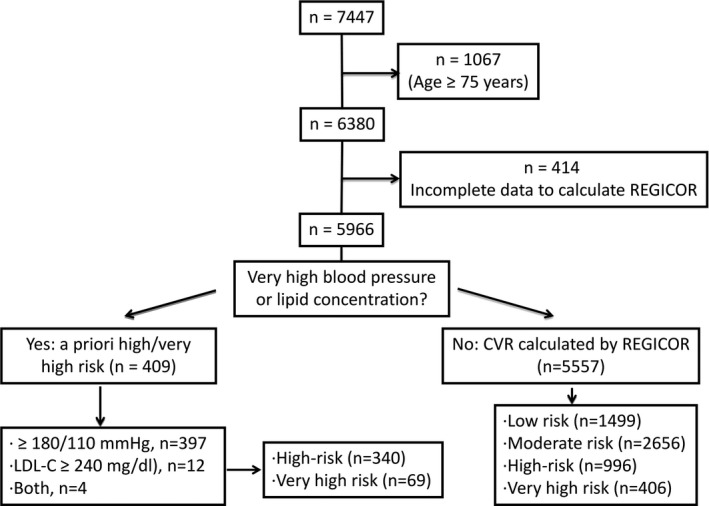
Flow‐chart of PREDIMED participants using Framingham‐REGICOR strategy. CVR indicates cardiovascular risk; LDL‐C, low‐density lipoprotein cholesterol.
Variables and Procedures
Information regarding educational level (no studies or primary, secondary or university studies) was recorded. A validated 137‐item food‐frequency questionnaire was used to estimate food, nutrients, and total energy intake.21 The validated Spanish version of the Minnesota Leisure Time Physical Activity Questionnaire was used to estimate physical activity.22 Adherence to the MedDiet was assessed with a validated 14‐item dietary screener.23 Height, weight, and waist circumference were measured with standard methods.
Fasting blood and spot urine were obtained at baseline and yearly during follow‐up. Plasma glucose, total cholesterol, high‐density lipoprotein cholesterol, and triglycerides were measured by routine laboratory tests using standardized enzymatic methods. Low‐density lipoprotein (LDL) cholesterol was calculated with the Friedewald formula. Serum creatinine was measured by enzymatic reaction using the Jaffé method, and estimated glomerular filtration rate was calculated based on creatinine using the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) equation.24 Urinary creatinine and albumin concentrations were also measured by the Jaffé method and the bromocresol green albumin method, respectively, and the urinary albumin/creatinine ratio was calculated (mg/g).
Estimation of Cardiovascular Risk
Cardiovascular risk was estimated by the F‐R strategy,8 which uses a calibrated Framingham equation validated for Spain, a low‐risk country.10 This F‐R index was calculated by using age, sex, smoking habit, presence or absence of diabetes mellitus, systolic and diastolic blood pressure, total cholesterol, and high‐density lipoprotein cholesterol.10 First, we identified participants with ≥1 markedly elevated risk factor (LDL cholesterol ≥240 mg/dL [n=12], systolic blood pressure ≥180 mm Hg and/or diastolic blood pressure ≥110 mm Hg [n=397], or both criteria [n=4]). According to the F‐R strategy, these 409 participants were classified as at least high risk; those with estimated risk ≥15%10 were classified as very high risk. We defined risk categories as very high (all participants with F‐R ≥15%), high (a priori high risk or F‐R 10–15%), moderate (a priori non–high risk and F‐R 5–10%), and low (a priori non–high risk and F‐R <5%) according to the F‐R equation. The flowchart of study participants is shown in Figure 1. Cardiovascular risk was also estimated with the stepped approach suggested in the European guidelines for persons aged <65 years, the age limit defined in this strategy.1 According to these guidelines, participants with diabetes mellitus with ≥1 risk factor, microalbuminuria, or chronic renal failure with an estimated glomerular filtration rate ≤30 mL/min were classified as very high‐risk (n=1162), and those with diabetes mellitus without other cardiovascular risk factors or microalbuminuria, an estimated glomerular filtration rate between 30 and 60 mL/min, or ≥1 markedly elevated conventional risk factor (total cholesterol >8 mmol/L [300 mg/dL], systolic blood pressure ≥180 mm Hg or diastolic blood pressure ≥110 mm Hg) were defined as high risk (n=479) (Figure S1). For the remaining participants (n=1343), cardiovascular risk was estimated with the SCORE equation for low‐risk regions (uses age, sex, smoking habit, systolic blood pressure, total cholesterol, and high‐density lipoprotein cholesterol),4 with values ≥10% representing very high risk, 5% to 10% indicating high risk, 1% to 5% showing moderate risk, and <1% noting low risk. The LDL cholesterol thresholds according to estimated risk were defined as <130 mg/dL in participants at low risk, <115 mg/dL in those at moderate risk, <100 mg/dL in those at high risk, and <70 mg/dL in persons at very high risk.1
End Point Ascertainment
The main outcome was an aggregate of major adverse cardiac events (MACE): myocardial infarction, defined by the presence of symptoms suggestive of ischemia or infarction, with either electrocardiographic evidence (new Q waves in ≥2 leads) or cardiac‐marker evidence of infarction, according to the standard American College of Cardiology definition25; stroke, defined as rapid onset of a neurological deficit lasting >24 hours, supported by imaging studies (computed tomography or magnetic resonance imaging scans); or death from cardiovascular causes. Secondary outcomes (expanded MACE) included MACE or revascularization (coronary bypass, angioplasty, or thrombolytic procedures) and newly diagnosed peripheral artery disease (defined as ankle‐brachial index of <0.9 at rest, clinical diagnosis of arterial occlusive disease based on imaging tests, or endovascular or open surgical procedure).26 Four sources of information were used to ascertain end points: repeated contacts with participants, contacts with family physicians, yearly review of medical records, and consultation of the National Death Index. All information on CVD events was evaluated by the end point adjudication committee, whose members were blind to treatment allocation. Only end points that were confirmed by the adjudication committee and that occurred between October 1, 2003, and December 1, 2010, were included in the present analyses.19
Statistical Analyses
Data are presented as mean±SD, median and interquartile range, or number (percentage), unless otherwise indicated. Normal distribution of continuous variables was assessed by the Kolmogorov–Smirnov test. The κ coefficient was used to examine the agreement between risk‐estimation strategies (F‐R and SCORE) in the classification of participants as high or very high risk versus moderate or low risk. Differences in clinical, anthropometric, sociodemographic, and laboratory variables across the different categories of estimated risk were evaluated by means of χ2 or Kruskal–Wallis tests, as appropriate. Differences in proportion of events (myocardial infarction, stroke, death from cardiovascular causes, coronary revascularization, or peripheral artery disease; merged MACE; and expanded MACE) across risk categories were also evaluated by χ2 tests.
Follow‐up time was calculated as the interval between the date of randomization and the date of incident CVD, the date of the last visit, or the last recorded clinical event of participants still alive, whichever came first. Unadjusted and adjusted Cox proportional hazard models were used to assess the association between baseline estimated risk (both F‐R and SCORE strategies) and incident CVD (MACE and expanded MACE). Adjustments were made for variables not included in the F‐R strategy: intervention group (low‐fat diet versus merged MedDiet/EVOO and MedDiet/nuts), baseline adherence to the MedDiet, physical activity, total energy intake, educational level, body mass index, baseline triglycerides, baseline diagnosis of hyperlipidemia, and treatment with antihypertensive or statin drugs. Hazard ratios (HRs) and 95% CIs were calculated, and the low‐risk stratum was used as a reference category. Finally, the effect of intervention (MedDiet as a combination of MedDiet/EVOO and MedDiet/nuts) on CVD was assessed across estimated cardiovascular risk strata (low, moderate, high, very high) in Cox proportional hazards models, both unadjusted and adjusted for the confounders mentioned previously. The Benjamini–Hochberg false discovery rate was used to adjust the main cardiovascular outcome results (HRs for MACE and expanded MACE according to F‐R risk strata and for the effect of MedDiet) for multiple comparisons.27 The 2‐sided significance level was set at P<0.05. All analyses were performed using the SPSS 20.0 statistical package (IBM Corp) and SAS software, v.9.2 (SAS Institute Inc).
Results
Estimated Baseline Cardiovascular Risk
Baseline cardiovascular risk estimated by the F‐R scale was mostly moderate (44.5%), with 30% of participants classified as high or very high risk (Figure 2). As expected, there were sex differences in risk (P<0.001). More than 80% of women were classified as being in the low/moderate risk category and <2% were very high risk, whereas close to half of men were high or very high risk and only 10% were classified as low risk. The SCORE chart (n=2982) classified only 11 men (<0.5%) as low risk, with most participants belonging to the high or very high risk category (63% of the total cohort; 53% of women and 75% of men) (Figure S2).
Figure 2.
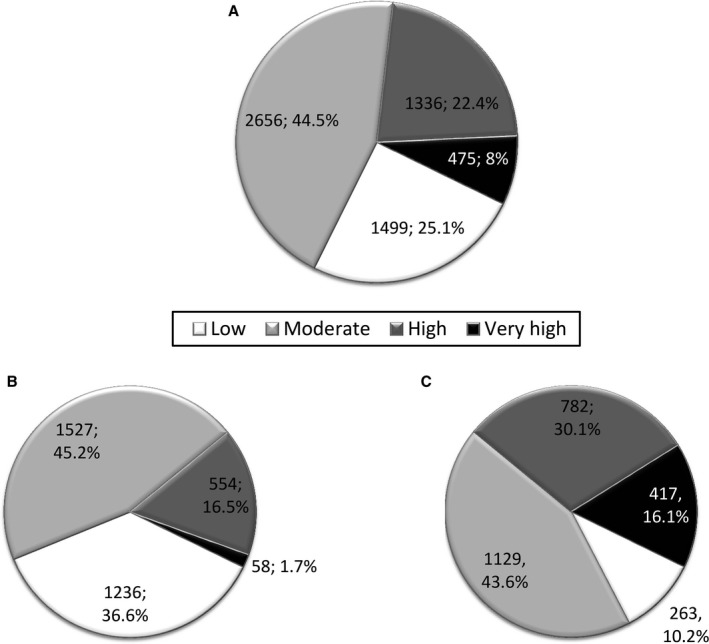
Cardiovascular risk estimated by Framingham‐REGICOR strategy in population aged 55 to 75 years in the total cohort (n=5966) (A), among women (n=3375) (B), and among men (n=2591) (C). Data are shown as number and percentage.
Concordance between the F‐R and SCORE equations in estimating cardiovascular risk was low (κ=0.321), even after excluding diabetic participants who were a priori categorized as high or very high risk only in the SCORE model (κ=0.441). The SCORE charts classified more than twice as many participants as high or very high risk compared with the F‐R model (Tables S1 and S2).
Treatment Goals and Cardiovascular Risk Factors
As expected (Table 1), LDL cholesterol, blood pressure, and the proportion of active smokers increased across cardiovascular risk categories, with no remarkable sex differences except in smoking prevalence, which was 4‐fold higher for men compared with women (31.4% versus 7.6%, P<0.001) (Table S3). Likewise, the proportion of participants with on‐goal LDL cholesterol levels (56.5%, 34.3%, 15.5%, and 1.5%) and blood pressure <140/90 mm Hg (57.8%, 31.6%, 12.1%, and 10.1%) decreased from the lowest to the highest risk categories (P<0.001, both) (Table 1). Even when considering a less strict LDL cholesterol target for the very high‐risk group (ie, <100 mg/dL), only 8.1% (10.3% of women, 7.8% of men) met this goal. Overall, fewer women at high or very high risk met LDL cholesterol or blood pressure goals or received antiplatelet treatment compared with men (Table S3).
Table 1.
Main Cardiovascular Risk Factors, Medication Use, and Treatment Goals by Framingham‐REGICOR Estimated Cardiovascular Risk Category
| Low Risk (n=1499) | Moderate Risk (n=2656) | High Risk (n=1336) | Very High Risk (n=475) | P Value | |
|---|---|---|---|---|---|
| LDL‐C | |||||
| LDL‐C, mg/dL | 126±34 | 130±33 | 134±35 | 142±34 | <0.001 |
| Statin use, n (%) | 709 (47.3) | 1117 (42.1) | 477 (35.7) | 115 (24.2) | <0.001 |
| Meeting LDL‐C goals, n (%) | 841 (56.5) | 905 (34.3) | 203 (15.5) | 7 (1.5) | <0.001 |
| BP | |||||
| SBP, mm Hg | 137.5±15.7 | 146±14.9 | 161.1±20.5 | 158.2±17.6 | <0.001 |
| DBP, mm Hg | 80.0±9.2 | 83.1±9.2 | 86.7±11.3 | 85.5±9.5 | <0.001 |
| Use of antihypertensive drugs, n (%) | 1138 (76.0) | 1978 (74.5) | 1023 (76.6) | 352 (74.1) | 0.424 |
| BP <140/90, n (%) | 867 (57.8) | 838 (31.6) | 161 (12.1) | 48 (10.1) | <0.001 |
| Smoking status | |||||
| Current smokers, n (%) | 195 (7.0) | 358 (13.5) | 326 (24.4) | 280 (59.0) | <0.001 |
| Antiplatelet drugs | |||||
| Use of antiplatelet drug, n (%) | 228 (15.2) | 504 (19.0) | 271 (20.3) | 111 (23.4) | <0.001 |
Data expressed as mean±SD or n (%). LDL‐C goals according to estimated cardiovascular risk were defined as <130 mg/dL for low risk, <115 mg/dL for moderate risk, <100 mg/dL for high risk, and <70 mg/dL for very high risk. BP indicates blood pressure; DBP, diastolic blood pressure; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure.
Table 2 shows the distribution of age, sex and selected traditional and nontraditional cardiovascular risk factors. As expected, variables considered in the F‐R strategy worsened across risk strata (P<0.001, all), with remarkably higher prevalence of diabetes mellitus in participants classified as very high risk (83% in the whole sample, 100% in women) (Table S4). Waist circumference and the prevalence of atherogenic dyslipidemia also increased from low to very high risk (P<0.001, both). As shown in Table S4, the increment in frequency of atherogenic dyslipidemia from low to very high risk was more prominent in women compared with men: 2% versus 64% and 3% versus 23%, respectively (P<0.001, both).
Table 2.
Distribution of Other Classical and Nonclassical Cardiovascular Risk Factors by Estimated Framingham‐REGICOR Risk Category
| Low Risk (n=1499) | Moderate Risk (n=2656) | High Risk (n=1336) | Very High Risk (n=475) | P Value | |
|---|---|---|---|---|---|
| Age, y | 65.8±5.2 | 64.8±4.8 | 65.9±4.7 | 67.4±4.8 | <0.001 |
| Men, n (%) | 263 (17.6) | 1129 (42.5) | 782 (58.5) | 417 (87.8) | <0.001 |
| Total cholesterol, mg/dL | 207.4±37.0 | 204.8±35.7 | 208.8±37.6 | 217.6±34.7 | <0.001 |
| HDL‐C, mg/dL | 59.3±11.8 | 49.8±9.8 | 46.9±9.1 | 42.5±8.4 | <0.001 |
| Diabetes mellitus, n (%) | 262 (17.5) | 1322 (49.8) | 888 (66.5) | 394 (83.0) | <0.001 |
| Microalbuminuria ≥30 mg/g, n (%)a | 42 (9.3) | 115 (12.6) | 116 (22.2) | 53 (27.9) | <0.001 |
| eGFR (CKD‐EPI, mL/min)b | 75.7±15.8 | 77.4±15.8 | 77.3±16.0 | 76.3±14.2 | 0.010 |
| BMI, kg/m2 | 29.6±3.8 | 30.2±3.9 | 30.3±3.8 | 30.0±4.0 | <0.001 |
| Waist, cm | 97.1±10.5 | 100.6±10.0 | 102.7±10.1 | 104.1±9.8 | <0.001 |
| Obesity, n (%) | 650 (43.4) | 1302 (49.0) | 669 (50.1) | 219 (46.1) | <0.001 |
| Central obesityc | 1053 (71.1) | 1815 (69.5) | 897 (68.4) | 279 (60.3) | <0.001 |
| Triglycerides, mg/dL | 98.3 (75.9–129.7) | 117.8 (90.5–155.9) | 129.9 (97.2–172.7) | 153.7 (111.2–216.0) | <0.001 |
| Atherogenic dyslipidemia, n (%)d | 33 (2.2) | 277 (10.5) | 201 (15.3) | 133 (28.0) | <0.001 |
| Physical activity (METS) | 210.0±213.9 | 234.5±241.0 | 261.9±277.6 | 271.8±265.4 | <0.001 |
| Baseline adherence to MedDiet (points) | 8.8±1.9 | 8.7±1.9 | 8.6±1.9 | 8.7±2.1 | 0.140 |
| Allocation to MedDiet groups, n (%) | 1009 (67.3) | 1836 (69.1) | 899 (67.3) | 318 (66.9) | 0.492 |
| Education level (%) | |||||
| No studies or primary | 1192 (79.5) | 2018 (76.0) | 998 (74.7) | 343 (72.2) | 0.020 |
| Secondary | 207 (13.8) | 433 (16.3) | 228 (17.1) | 90 (19.0) | |
| University | 100 (6.7) | 205 (7.7) | 110 (8.2) | 42 (8.8) | |
Data expressed as mean±SD, median (interquartile range), or n (%) unless indicated otherwise. In bold the variables used to estimate cardiovascular risk in Framingham‐REGICOR strategy. BMI indicates body mass index; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; MedDiet, Mediterranean diet; METS, metabolic equivalent.
Information available for 450, 912, 523, and 190 participants at low, moderate, high, and very high risk, respectively.
eGFR based on CKD‐EPI creatinine equation. Information available for 1105, 1896, 967, and 348 participants at low, moderate, high, and very high risk, respectively.
Central obesity was defined as waist >88 cm for women and >102 cm for men. Information was missing for 100 participants.
Atherogenic dyslipidemia was defined as HDL‐C <40 mg/dL and triglycerides >150 mg/dL in men and HDL‐C <45 mg/dL and triglycerides >150 mg/dL in women. Information was missing for 28 participants.
CVD Events by Estimated Cardiovascular Risk
There was low incidence of CVD events overall during the 6‐year follow‐up period in both men (n=118) and women (n=70) in this cohort of participants selected for a priori–defined high risk (Table 3), ranging from 2.36 to 17.83 events per 1000 person‐years in groups at low and very high risk, respectively (Table 4). Low‐risk women had an almost 3‐fold higher incidence of CVD events than low‐risk men (2.66 versus 0.91 events per 1000 person‐years, respectively). Men and women at very high risk had a 20‐fold (crude HR 20.22, 95% CI 2.76–148.09) and 4‐fold (HR 4.45, 95% CI 1.26–15.72) increased risk of CVD events, respectively, compared with their low‐risk counterparts. Compared with low‐risk categories, women at moderate risk (HR 2.19, 95% CI 1.19–4.05) and high risk (HR 2.51, 95% CI 1.21–5.21) had similar risk of events, whereas high‐risk men (HR 14.51, 95% CI 1.99–105.57) had almost double the risk of men at moderate risk (HR 8.43, 95% CI 1.15–61.53). Adjustment for additional risk factors not included in the F‐R strategy slightly blunted the strength of the associations (Table 4).
Table 3.
Cardiovascular Events According to Estimated CVR (Framingham‐REGICOR Strategy) by Sex
| Low Risk | Moderate Risk | High Risk | Very High Risk | Total | |
|---|---|---|---|---|---|
| Women | |||||
| Participants, n (%) | 1236 (36.6) | 1527 (45.2) | 554 (16.4) | 58 (1.7) | 3375 (100) |
| Estimated CVR | 3.2±0.8 | 6.4±1.4 | 9.8±2.7 | 16.7±2.2 | 6.0±3.1 |
| MI, n | 1 | 14 | 5 | 2 | 22 |
| Events in risk strata, % | 0.1 | 0.9 | 0.9 | 3.5a | 0.7 |
| Total events, % | 4.6 | 63.6 | 22.7 | 9.1 | 100 |
| Stroke, n | 11 | 19 | 11 | 2 | 43 |
| Events in risk strata, % | 0.9 | 1.2 | 2.0 | 3.5 | 1.3 |
| Total events, % | 25.6 | 44.2 | 25.6 | 4.7 | 100 |
| CVD death, n | 5 | 7 | 1 | 1 | 14 |
| Events in risk strata, % | 0.4 | 0.5 | 0.2 | 1.7 | 0.4 |
| Total events, % | 35.7 | 50.0 | 7.14 | 7.14 | 100 |
| MACE, n | 14 | 38 | 15 | 3 | 70 |
| Events in risk strata, % | 1.1 | 2.5 | 2.7 | 5.2b | 2.1 |
| Total events, % | 20.0 | 54.3 | 21.4 | 4.3 | 100 |
| Expanded MACE, n | 22 | 54 | 31 | 3 | 110 |
| Events in risk strata, % | 1.8 | 3.5 | 5.6 | 5.2a | 3.3 |
| Total events, % | 20.0 | 49.1 | 28.2 | 2.7 | 100 |
| Men | |||||
| Participants, n (%) | 263 (10.2) | 1129 (43.6) | 782 (30.2) | 417 (16.1) | 2591 (100) |
| Estimated CV risk | 3.7±0.7 | 7.0±1.4 | 11.2±1.8 | 18.8±3.9 | 9.8±5.0 |
| MI, n | 0 | 20 | 21 | 11 | 52 |
| Events in risk strata, % | 0 | 1.8 | 2.7 | 2.6b | 2.0 |
| Total events, % | 0 | 38.5 | 40.4 | 21.1 | 100 |
| Stroke, n | 1 | 13 | 20 | 15 | 49 |
| Events in risk strata, % | 0.4 | 1.2 | 2.6 | 3.6a | 1.9 |
| Total events, % | 2.0 | 26.5 | 40.8 | 30.6 | 100 |
| CVD death, n | 0 | 8 | 10 | 16 | 34 |
| Events in risk strata, % | 0 | 0.7 | 1.3 | 3.8a | 1.3 |
| Total events, % | 0 | 23.5 | 29.4 | 47.1 | |
| MACE, n | 1 | 37 | 45 | 35 | 118 |
| Events in risk strata, % | 0.4 | 3.3 | 5.8 | 8.4a | 4.6 |
| Total events, % | 0.9 | 31.4 | 38.1 | 29.7 | 100 |
| Expanded MACE, n | 3 | 60 | 80 | 51 | 194 |
| Events in risk strata, % | 1.1 | 5.3 | 10.2 | 12.2a | 7.5 |
| Total events, % | 1.6 | 30.9 | 41.2 | 26.3 | 100 |
Data expressed as mean±SD or n (%) unless otherwise indicated. MACE is defined as MI, stroke, or cardiovascular death. Expanded MACE is MACE plus coronary revascularization or peripheral arterial disease. CVD indicates cardiovascular disease; CVR, cardiovascular risk; MACE, major adverse cardiac event; MI, myocardial infarction.
P<0.01 between groups.
P<0.05 between groups.
Table 4.
HRs of Major Cardiovascular Events (Myocardial Infarction, Stroke, or Cardiovascular Death) According to Estimated Cardiovascular Risk (Framingham‐REGICOR Strategy) by Sex
| REGICOR | Unadjusted Incidence Rates (Per 1000 Person‐Years) | Unadjusted HR | P Value | Fully Adjusted HRa | P Value | |
|---|---|---|---|---|---|---|
| Total | Low risk | 2.36 | 1 (reference) | ··· | 1 (reference) | ··· |
| Moderate risk | 6.39 | 2.81 (1.61–4.89) | <0.001b | 2.68 (1.53–4.69) | <0.001b | |
| High risk | 10.23 | 4.58 (2.59–8.06) | <0.001b | 4.24 (2.39–7.54) | <0.001b | |
| Very high risk | 17.83 | 7.95 (4.36–14.49) | <0.001b | 6.60 (3.55–12.30) | <0.001b | |
| Women | Low risk | 2.66 | 1 (reference) | ··· | 1 (reference) | ··· |
| Moderate risk | 5.58 | 2.19 (1.19–4.05) | 0.012b | 2.16 (1.16–4.04) | 0.015b | |
| High risk | 6.14 | 2.51 (1.21–5.21) | 0.014b | 2.28 (1.07–4.89) | 0.034 | |
| Very high risk | 12.27 | 4.45 (1.26–15.72) | 0.020b | 3.51 (0.92–13.35) | 0.066 | |
| Men | Low risk | 0.91 | 1 (reference) | ··· | 1 (reference) | ··· |
| Moderate risk | 7.51 | 8.43 (1.15–61.53) | 0.036 | 7.60 (1.04–55.57) | 0.046 | |
| High risk | 13.15 | 14.51 (1.99–105.57) | 0.008b | 13.16 (1.80–95.92) | 0.011b | |
| Very high risk | 18.55 | 20.22 (2.76–148.09) | 0.003b | 15.85 (2.14–117.20) | 0.007b |
REGICOR (age, sex, smoking, total cholesterol, high‐density lipoprotein cholesterol, blood pressure, and diabetes mellitus) was additionally adjusted for group (Mediterranean diet vs low‐fat diet), baseline adherence to a Mediterranean diet, educational level, body mass index, triglycerides, treated hypertension, dyslipidemia, statin treatment, and physical activity. HR indicates hazard ratio.
P values less than or equal to a false discovery rate of q=0.024 show significant results (see Statistical Analyses).
In men, the proportion of participants with a CVD event (individually considered or merged in MACE or expanded MACE) increased gradually (0.4%, 3.3%, 5.8%, and 8.4% with low, moderate, high, and very high risk, respectively, for MACE) across estimated F‐R risk (Table 3 and Figure S3). In women, this proportion was especially high in the very high risk category, which represented only 1.7% (n=58) of female participants; the exception was expanded MACE, which was similar for high and very high risk due to peripheral artery disease event distribution (2.2% and 1.7% in women at high and very high risk, respectively). Even though the proportion of participants who had a CVD event was higher in high‐risk categories, many events actually occurred in participants at low or moderate risk, especially among women. Although in men 32.2% of MACE occurred in those not at high risk (97.4% of such MACE took place in the intermediate risk category), 74.3% of MACE occurred in non–high‐risk women. These sex differences were particularly apparent for cardiovascular death (23.5% in non–high‐risk men versus 85.7% in non–high‐risk women; P<0.001) (Table 3).
Effect of the MedDiet and Other Risk Factors on CVD Incidence
The MedDiet (MedDiet/EVOO plus MedDiet/nuts versus low‐fat diet; DietGroup variable) had a protective effect (HR 0.72, 95% CI 0.54–0.97; P=0.0329) on CVD events, as reported previously for the whole PREDIMED cohort.19 The benefits of the MedDiet were similar across all estimated risk strata, for both MACE and expanded MACE, and before and after taking into account additional risk factors not included in the F‐R strategy (P>0.4 for DietGroup×REGICOR group interaction for all comparisons) (Figure 3). In multivariate Cox proportional hazards regression analysis, we found that in addition to baseline F‐R risk and diet group (HR 0.74, 95% CI 0.55–1.00; P=0.053), baseline adherence to the MedDiet (HR 0.89, 95% CI 0.82–0.96; P=0.002), presence of dyslipidemia (HR 1.77, 95% CI 1.16–2.69; P=0.008), treatment with statins (HR 0.47, 95% CI 0.30–0.75; P=0.001), and triglyceride concentration (HR 1.002, 95% CI 1.00–1.004; P=0.069) were independently associated with MACE, with similar results by sex.
Figure 3.
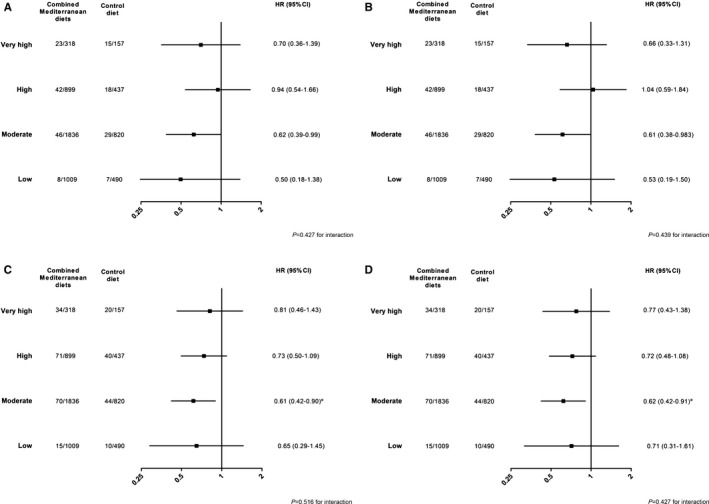
Hazards ratios of cardiovascular disease events of merged MedDiet/EVOO and MedDiet/nuts across estimated cardiovascular risk categories by the Framingham‐REGICOR strategy. Unadjusted (A) and fully adjusted (B) models for major cardiovascular events (acute myocardial infarction, stroke, and cardiovascular death). Unadjusted (C) and fully adjusted (D) models for expanded major cardiovascular events (with addition of cardiac revascularization and peripheral arterial disease). B and D, Additionally adjusted for baseline adherence to the MedDiet, educational level, body mass index, triglycerides, treated hypertension, dyslipidemia, statin treatment, and physical activity. P values less than or equal to a false discovery rate q=0.024 show significant results (see Statistical Analyses). EVOO indicates extra‐virgin olive oil; HR, hazard ratio; MedDiet, Mediterranean diet.
Discussion
In this study, the recommended strategies to evaluate cardiovascular risk1, 2 classified two‐thirds of participants from an a priori–defined high‐risk Mediterranean cohort as non–high risk. The cohort was selected based on participant age (55–74 years) and high prevalence of cardiovascular risk factors (82% hypertension, 74% dyslipidemia, and 48% with type 2 diabetes mellitus). This finding should not discourage health care professionals from using these guidelines as a first step for population‐based CVD prevention strategies. Indeed, baseline risk estimated by the F‐R strategy had good predictive power, with relative risks for major CVD events increasing 2.7‐, 4.2‐, and 6.6‐fold for moderate, high, and very high‐risk participants, respectively, compared with those at low risk. However, even though the number of CVD events increased with estimated cardiovascular risk, most events actually occurred in non–high‐risk participants, which concurs with prior reports from other populations.10, 13 This finding was particularly true in women, for which only 25% of total and 15% of fatal CVD events occurred in the groups at high or very high risk (Table 3).
In the PREDIMED cohort, cardiovascular risk estimated with the F‐R equation was mostly moderate (44.5%), with only 8% of participants identified as very high risk (Figure 2). Prevalent CVD is the main determinant of future events1, 28 and explains most of the very high‐risk category in population‐based studies.14 However, even in the setting of this primary CVD prevention trial, the high proportion of non–high‐risk persons is somewhat surprising, given the participants' characteristics, namely, 50% with diabetes mellitus and/or ≥3 cardiovascular risk factors. Indeed, the baseline cardiovascular risk distribution was similar to that observed in a nonselected nationwide population‐based sample.14 This may be due to the fact that 40% of PREDIMED participants were treated with statins and 74% received antihypertensive agents, thus blunting the potency of cholesterol concentrations and blood pressure—critical factors used to evaluate cardiovascular risk. Furthermore, cholesterol and blood pressure goals are set according to estimated risk, and partial success in achieving these goals might help explain the residual risk beyond pharmacological treatment and why most events in the PREDIMED cohort occurred in non–high‐risk participants.
Cholesterol‐lowering therapy, in particular, statins, is one of the most important strategies to reduce cardiovascular risk.29 Nevertheless, a low proportion of participants achieved LDL cholesterol goals in our cohort; for instance, only 23.6% of those at high or very high risk had LDL cholesterol <100 mg/dL. This observation concurs with findings of the European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE) IV study,30 in which <20% of coronary patients had LDL cholesterol <70 mg/dL. This underscores the fact that poor achievement of LDL cholesterol and blood pressure goals is common in cardiovascular risk prevention across different European countries.30 Statin use, which was relatively low in our cohort (40%), could partly explain the lack of LDL cholesterol goal attainment. The high proportion of participants (more than two‐thirds of the sample) (Figure 2) with low or moderate F‐R risk in our primary CVD prevention cohort31 could partly explain this observation. Yet a similar proportion of (a priori high‐risk) participants with type 2 diabetes mellitus from a large (n=28 6791) Mediterranean cohort received statin treatment (41%) despite the fact that 18% had prior CVD.32
Although male sex is a well‐established risk factor,33 the proportion of deaths from cardiovascular causes is similar among European men and women (35% and 36%, respectively) aged <75 years.18 Concurring with prior studies,14, 34 the prevalence of high or very high risk among women was half that found in men (18% versus 36%), even though women were older by study design. Consequently, the higher incidence of CVD events (MACE and expanded MACE) in men compared with women was not unexpected. Nevertheless, it is noteworthy that while 2 of 3 CVD events in men took place in those at high or very high risk, <25% events in women occurred in those at high or very high risk. Sex differences in CVD events in the low risk category were also noticeable because only 1.6% of events in men, compared with 20% of events in women, occurred in the low risk category. Compared with low‐risk strata, adjusted HRs rose in parallel with risk in men (7.60‐, 13.16‐, and 15.85‐fold for moderate, high, and very high risk, respectively) but not in women (2.16‐, 2.28‐, and 3.51‐fold for moderate, high, and very high risk, respectively) (Table 4). Clearly, our risk classification strategy performed worse in women than in men, confirming a prior observation in a population‐based study.10 These results support the proposal of the American Heart Association to address CVD‐preventive strategies specifically accounting for sex differences both in the general population35 and in persons with diabetes mellitus.36
The fact that many CVD events occur in men and women at low or moderate risk needs to be emphasized. Identification of additional risk factors and evaluation of their contribution to CVD is necessary to both to improve our prediction strategies, based mainly on traditional risk factors, and to better tailor preventive interventions at the individual and population levels.37 Results of noninvasive vascular imaging techniques have been shown to improve cardiovascular risk prediction.38 Newer evidence from large genetic studies39, 40 indicates that triglycerides, the culprit of atherogenic dyslipidemia,41 is causally related to future CVD events, and triglyceride lipoprotein content has been associated with preclinical atherosclerosis.42 Triglycerides, as well as high‐density lipoprotein cholesterol, may contribute to residual cardiovascular risk beyond LDL cholesterol levels.43, 44 No detailed lipoprotein composition studies were performed in the PREDIMED cohort, but the triglyceride levels tended to be independently associated with CVD events. More interesting, adherence to the MedDiet not only was associated with lower incidence of CVD at baseline or as a consequence of the intervention but also was beneficial regardless of the estimated cardiovascular risk. Consequently, implementation of the MedDiet appears to be a useful strategy to promote cardiovascular health at both the individual and population levels, independent of baseline risk.
The main strengths of our study are the large sample size (n=5966), the close follow‐up during the trial, and the rigorous certification of CVD events by an independent end point adjudication committee. In addition, a novel aspect of this work is the use of an a priori–defined high‐risk PREDIMED population with high prevalence of treated cardiovascular risk factors (and thus with values of blood pressure or lipid profile largely affected by cardioprotective treatment). Cohorts such as ours are usually underrepresented in studies using predictive risk equations. Our study also has limitations. Because the calculation of total risk by the F‐R strategy is in part limited (equation restriction) to persons aged 35 to 74 years, our results cannot be extended to those aged >74 years. In addition, because of the prespecified baseline high prevalence of major cardiovascular risk factors, the results are not generalizable to populations with lower baseline risk.
In conclusion, most participants in the PREDIMED cohort, prespecified as being at high cardiovascular risk, were classified as non–high risk with the F‐R scale, a population risk‐assessment scale validated in Spain.8, 10 As expected, major CVD events increased in parallel with estimated risk. However, the observation that nearly 1 of 3 events in men and 3 of 4 events in women occurred in persons classified as non–high risk points to the limitations of this population‐based strategy. CVD risk prediction would probably be improved with consideration of other risk factors not currently included in the equations and of circulating and vascular imaging biomarkers.38, 42 Pending refinement of cardiovascular risk estimation for this new personalized and predictive medicine, our finding that adherence to the MedDiet reduces the CVD burden across all risk categories adds to accumulating evidence of the benefit of this dietary pattern as a useful population‐based approach for CVD prevention.19, 45, 46
Appendix
PREDIMED Investigators
Hospital Clínic, Institut d'Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain: A. Pérez‐Heras, C. Viñas, R. Casas, L. de Santamaría, S. Romero, E. Sacanella, G. Chiva, P. Valderas, S. Arranz, J. M. Baena, M. García, M. Oller, J. Amat, I. Duaso, Y. García, C. Iglesias, C. Simón, Ll. Quinzavos, Ll. Parra, M. Liroz, J. Benavent, J. Clos, I. Pla, M. Amorós, M. T. Bonet, M. T. Martin, M. S. Sánchez, J. Altirriba, E. Manzano, A. Altés, A. Sala‐Vila, M. Cofán, C. Valls‐Pedret, T. M. Freitas‐Simoes, M. Doménech, R. Gilabert, and N. Bargalló.
University Rovira i Virgili, Reus, Spain: M. Bulló, J. Basora, R. González, A. Díaz‐López, C. Molina, G. Mena, F. Márquez, P. Martínez, N. Ibarrola, M. Sorli, J. García Roselló, F. Martín, N. Tort, A. Isach, A. Salas‐Huetos, N. Becerra‐Tomás, N. Rosique Esteban, J. J. Cabré, G. Mestres, F. Paris, M. Llaurado, R. Pedret, J. Basells, J. Vizcaino, R. Segarra, P. Hernandez‐Alonso, S. Giardina, C. Ferreira‐Pego, C. Papandreou, and L. Camacho.
University of Navarra and Osasunbidea, Servicio Navarro de Salud, Primary Care Centres, Pamplona, Spain: E. Toledo, P. Buil‐Cosiales, M. Ruiz‐Canela, J. A. Martínez, B. Sanjulian, A. Sánchez‐Tainta, J. Diez‐Espino, C. Razquin, A. Garcia‐Arellano, E. Goni, Z. Vazquez, N. Berrade, V. Extremera‐Urabayen, S. Eguaras, A. Marti, C. Arroyo‐Azpa, L. García‐Pérez, J. Villanueva Telleria, F. Cortés Ugalde, T. Sagredo Arce, M. D. García de la Noceda Montoy, M. D. Vigata López, M. T. Arceiz Campo, A. Urtasun Samper, M. V. Gueto Rubio, A. Sola, N. Goñi, MD and O. Lecea.
Institut de Recerca Hospital del Mar, Barcelona, Spain: S. Tello, J. Vila, R. de la Torre, D. Muñoz‐Aguayo, R. Elosua, J. Marrugat, H. Schröder, N. Molina, E. Maestre, O. Castañer, A. Rovira and M. Farre.
University of Valencia, Valencia, and University Jaume I, Spain: J. V. Sorli, V. Zanon‐Moreno, P. Carrasco, C. Ortega‐Azorín, E. M. Asensio, R. Osma, R. Barragán, F. Francés, M. Guillén, J. I. González, C. Saiz, O. Portolés, F. J. Giménez, O. Coltell, P. Guillem‐Saiz, L. Quiles, V. Pascual, C. Riera, M. A. Pages, D. Godoy, A. Carratala‐Calvo, M. J. Martín‐Rillo, E. Llopis‐Osorio, J. Ruiz‐Baixauli, and A. Bertolín‐Muñoz.
University Hospital of Alava, Vitoria, Spain: I. Salaverría, T. del Hierro, J. Algorta, S. Francisco, A. Alonso‐Gómez, E. Sanz, J. Rekondo, M. C. Bello, A. Loma‐Osorio.
University of Málaga, Málaga, Spain: E. Gómez‐Gracia, J. Warnberg, R. Benítez Pont, M. Bianchi Alba, R. Gómez‐Huelgas, J. Martínez‐González, V. Velasco García, J. de Diego Salas, A. Baca Osorio, J. Gil Zarzosa, J. J. Sánchez Luque and E. Vargas López.
Instituto de la Grasa, Consejo Superior de Investigaciones Científicas, Sevilla, Spain: V. Ruiz‐Gutiérrez, J. Sánchez Perona, E. Montero Romero, M. García‐García, and E. Jurado‐Ruiz.
Institute of Health Sciences IUNICS, University of Balearic Islands, and Hospital Son Espases, Palma de Mallorca, Spain: M. Fiol, M. García‐Valdueza, M. Moñino, A. Proenza, R. Prieto, G. Frontera, M. Ginard, F. Fiol, A. Jover, D. Romaguera, and J. García.
Department of Family Medicine, Distrito Sanitario Atención Primaria Sevilla, Sevilla, Spain: J. Lapetra, J. M. Santos‐Lozano, M. Ortega‐Calvo, L. Mellado, M. Leal, E. Martínez, F. José García, P. Román, P. Iglesias, Y. Corchado, L. Miró, C. Domínguez, J. M. Lozano, and E. Mayoral.
School of Pharmacy, University of Barcelona, Barcelona, Spain: R. M. Lamuela‐Raventós, M. C. López‐Sabater, A. I. Castellote‐Bargallo, A. Tresserra‐Rimbau.
University of Las Palmas de Gran Canaria, Las Palmas, Spain: J. Álvarez‐Pérez, E. M. Díaz‐Benítez, I. Bautista Castaño, A. Sánchez‐Villegas, M. J. Fernández‐Rodríguez, T. Casanas Quintana, J. Pérez‐Cabrera, M. Nissensohn, V. Díaz‐González, C. Ruano‐Rodríguez, A. P. Ortiz‐Andrelluchi, B. Macías Gutiérrez, and A. J. Santana‐Santana.
Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain: X. Pintó, E. de la Cruz, A. Galera, Y. Soler, F. Trias, I. Sarasa, E. Padres, and E. Corbella.
Primary Care Division, Catalan Institute of Health, Barcelona, Spain: C. Cabezas, E. Vinyoles, M. A. Rovira, L. García, G. Flores, J. M. Verdú, P. Baby, A. Ramos, L. Mengual, P. Roura, M. C. Yuste, A. Guarner, A. Rovira, M. I. Santamaría, M. Mata, C. de Juan, and A. Brau.
Other investigators of the PREDIMED network: J. A. Tur (University of Balearic Islands), M. P. Portillo (University of Basque Country), G. Sáez (University of Valencia). Clinical End Point Committee—M. Aldamiz, A. Alonso, J. Berjón, L. Forga, J. Gallego, A. Larrauri, MD, J. Portu, J. Timiraos, and M. Serrano‐Martínez.
Sources of Funding
This study was funded in part by Instituto de Salud Carlos III (ISCIII) (Spanish Ministry of Economy) through grants RTIC G03/140, RTIC RD 06/0045, Centro Nacional de Investigaciones Cardiovasculares CNIC 06/2007, ISCIII FIS PS09/01292, the Spanish Ministry of Science and Innovation (MICINN) AGL2010‐22319‐C03‐02 and AGL2009‐13906‐C02‐02, and an unrestricted grant from the California Walnut Commission.
Disclosures
Dr Salas‐Salvado has received research funding and is a nonpaid member of the scientific advisory committee of the International Nut Council. Dr Ros has received research funding from the California Walnut Commission (including an unrestricted educational grant) and is a nonpaid member of the Commission's Scientific Advisory Committee. Dr Estruch reports serving on the board of and receiving lecture fees from the Research Foundation on Wine and Nutrition; serving on the boards of the Beer and Health Foundation and the European Foundation for Alcohol Research; and receiving lecture fees from Fundación Dieta Mediterránea and Cerveceros de España. No other disclosures were reported.
Supporting information
Table S1. Concordance of Estimated Cardiovascular Risk Between Framingham‐REGICOR and SCORE Strategies in the Whole Population
Table S2. Concordance of Estimated Cardiovascular Risk Between Framingham‐REGICOR and SCORE Strategies in the Nondiabetic Population
Table S3. Main Cardiovascular Risk Factors, Medication Use, and Treatment Goals by Framingham‐REGICOR Estimated Cardiovascular Risk Category by Sex
Table S4. Distribution of Other Classical and Nonclassical Cardiovascular Risk Factors by Estimated Framingham‐REGICOR Risk Category by Sex
Figure S1. Flowchart of PREDIMED participants using European guidelines on cardiovascular disease prevention in clinical practice–Systematic Coronary Risk Evaluation (SCORE) strategy.
Figure S2. Cardiovascular risk estimated by the European guidelines on cardiovascular disease prevention in clinical practice–Systematic Coronary Risk Evaluation (SCORE) strategy in a population aged 55 to 65 years from the total cohort (n=2982) (A), among women (n=1569) (B), and among men (n=1415) (C). Data are shown as number and percentage.
Figure S3. Percentage of cardiovascular events across estimated cardiovascular risk by Framingham‐REGICOR strategy in the total cohort (A), among women (B), and among men (C).
Data S1. Mediterranean diet in a primary cardiovascular prevention research plan.
Acknowledgments
We thank all PREDIMED participants and personnel for their outstanding collaboration. CIBEROBN is an initiative of ISCIII, Spain. We are indebted to Emili Corbella for methodological assessment and statistical advice.
(J Am Heart Assoc. 2017;6:e004803. DOI: 10.1161/JAHA.116.004803.)
References
- 1. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M‐T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M‐L, Löllgen H, Marques‐Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;37:2315–2381.27222591 [Google Scholar]
- 2. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 3. Zhao D, Liu J, Xie W, Qi Y. Cardiovascular risk assessment: a global perspective. Nat Rev Cardiol. 2015;12:301–311. [DOI] [PubMed] [Google Scholar]
- 4. Cooney MT, Dudina A, De Bacquer D, Fitzgerald A, Conroy R, Sans S, Menotti A, De Backer G, Jousilahti P, Keil U, Thomsen T, Whincup P, Graham I. How much does HDL cholesterol add to risk estimation? A report from the SCORE Investigators. Eur J Cardiovasc Prev Rehabil. 2009;16:304–314. [DOI] [PubMed] [Google Scholar]
- 5. Zhao M, Cooney MT, Klipstein‐Grobusch K, Vaartjes I, De Bacquer D, De Sutter J, Reiner Ž, Prescott E, Faggiano P, Vanuzzo D, AlFaleh H, Menown IB, Gait D, Posogova N, Sheu WH‐H, Zhao D, Zuo H, Grobbee DE, Graham IM. Simplifying the audit of risk factor recording and control: a report from an international study in 11 countries. Eur J Prev Cardiol. 2016;23:1202–1210. [DOI] [PubMed] [Google Scholar]
- 6. Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U; EUROASPIRE Study Group . EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16:121–137. [DOI] [PubMed] [Google Scholar]
- 7. Heuschmann PU, Kircher J, Nowe T, Dittrich R, Reiner Z, Cifkova R, Malojcic B, Mayer O, Bruthans J, Wloch‐Kopec D, Prugger C, Heidrich J, Keil U. Control of main risk factors after ischaemic stroke across Europe: data from the stroke‐specific module of the EUROASPIRE III survey. Eur J Prev Cardiol. 2015;22:1354–1362. [DOI] [PubMed] [Google Scholar]
- 8. Marrugat J, Solanas P, D'Agostino R, Sullivan L, Ordovas J, Cordón F, Ramos R, Sala J, Masià R, Rohlfs I, Elosua R, Kannel WB. [Coronary risk estimation in Spain using a calibrated Framingham function]. Rev Esp Cardiol. 2003;56:253–261. [DOI] [PubMed] [Google Scholar]
- 9. Marrugat J, D'Agostino R, Sullivan L, Elosua R, Wilson P, Ordovas J, Solanas P, Cordón F, Ramos R, Sala J, Masiá R, Kannel WB. An adaptation of the Framingham coronary heart disease risk function to European Mediterranean areas. J Epidemiol Community Health. 2003;57:634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marrugat J, Vila J, Baena‐Díez JM, Grau M, Sala J, Ramos R, Subirana I, Fitó M, Elosua R. Relative validity of the 10‐year cardiovascular risk estimate in a population cohort of the REGICOR study. Rev Esp Cardiol. 2011;64:385–394. [DOI] [PubMed] [Google Scholar]
- 11. Cooney MT, Dudina AL, Graham IM. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009;54:1209–1227. [DOI] [PubMed] [Google Scholar]
- 12. Liew SM, Doust J, Glasziou P. Cardiovascular risk scores do not account for the effect of treatment: a review. Heart. 2011;97:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sposito AC, Alvarenga BF, Alexandre AS, Araújo ALR, Santos SN, Andrade JM, Ramires JAF, Quinaglia E, Silva JC, Coelho OR. Most of the patients presenting myocardial infarction would not be eligible for intensive lipid‐lowering based on clinical algorithms or plasma C‐reactive protein. Atherosclerosis. 2011;214:148–150. [DOI] [PubMed] [Google Scholar]
- 14. Amor AJ, Masana L, Soriguer F, Goday A, Calle‐Pascual A, Gaztambide S, Rojo‐Martínez G, Valdés S, Gomis R, Ortega E. Estimating cardiovascular risk in Spain by the European Guidelines on cardiovascular disease prevention in clinical practice. Rev Esp Cardiol. 2015;68:417–425. [DOI] [PubMed] [Google Scholar]
- 15. Gil‐Guillén V, Orozco‐Beltrán D, Maiques‐Galán A, Aznar‐Vicente J, Navarro J, Cea‐Calvo L, Quirce‐Andrés F, Redón J, Merino‐Sánchez J. Agreement between REGICOR and SCORE scales in identifying high cardiovascular risk in the Spanish population. Rev Esp Cardiol. 2007;60:1042–1050. [DOI] [PubMed] [Google Scholar]
- 16. Leening MJG, Berry JD, Allen NB. Lifetime perspectives on primary prevention of atherosclerotic cardiovascular disease. JAMA. 2016;315:1449–1450. [DOI] [PubMed] [Google Scholar]
- 17. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J‐P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2015;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 18. Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe—epidemiological update 2015. Eur Heart J. 2015;36:2696–2705. [DOI] [PubMed] [Google Scholar]
- 19. Estruch R, Ros E, Salas‐Salvadó J, Covas M‐I, Corella D, Arós F, Gómez‐Gracia E, Ruiz‐Gutiérrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez‐González MA. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 20. Martínez‐González MÁ, Corella D, Salas‐Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruíz‐Gutiérrez V, Lamuela‐Raventós RM, Lapetra J, Muñoz MÁ, Martínez JA, Sáez G, Serra‐Majem L, Pintó X, Mitjavila MT, Tur JA, Portillo MDP, Estruch R. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41:377–385. [DOI] [PubMed] [Google Scholar]
- 21. Fernández‐Ballart JD, Piñol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez‐Bauer M, Martínez‐González MA, Salas‐Salvadó J, Martín‐Moreno JM. Relative validity of a semi‐quantitative food‐frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. 2010;103:1808–1816. [DOI] [PubMed] [Google Scholar]
- 22. Elosua R, Marrugat J, Molina L, Pons S, Pujol E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol. 1994;139:1197–1209. [DOI] [PubMed] [Google Scholar]
- 23. Schröder H, Fitó M, Estruch R, Martínez‐González MA, Corella D, Salas‐Salvadó J, Lamuela‐Raventós R, Ros E, Salaverría I, Fiol M, Lapetra J, Vinyoles E, Gómez‐Gracia E, Lahoz C, Serra‐Majem L, Pintó X, Ruiz‐Gutierrez V, Covas M‐I. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–1145. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, Cross JT, Drozda JP, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, Gray DT, Harrington RA, Hicks KA, Hollander JE, Krumholz H, Labarthe DR, Long JB, Mascette AM, Meyer C, Peterson ED, Radford MJ, Roe MT, Richmann JB, Selker HP, Shahian DM, Shaw RE, Sprenger S, Swor R, Underberg JA, Van de Werf F, Weiner BH, Weintraub WS. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease. J Am Coll Cardiol. 2013;61:992–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruiz‐Canela M, Estruch R, Corella D, Salas‐Salvadó J, Martínez‐González MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415–417. [DOI] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 28. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 29. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kotseva K, Wood D, De Bacquer D, De Backer G, Rydén L, Jennings C, Gyberg V, Amouyel P, Bruthans J, Castro Conde A, Cífková R, Deckers JW, De Sutter J, Dilic M, Dolzhenko M, Erglis A, Fras Z, Gaita D, Gotcheva N, Goudevenos J, Heuschmann P, Laucevicius A, Lehto S, Lovic D, Miličić D, Moore D, Nicolaides E, Oganov R, Pajak A, Pogosova N, Reiner Z, Stagmo M, Störk S, Tokgözoğlu L, Vulic D. EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23:636–648. [DOI] [PubMed] [Google Scholar]
- 31. Reiner Ž, De Backer G, Fras Z, Kotseva K, Tokgözoglu L, Wood D, De Bacquer D; EUROASPIRE Investigators . Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries—findings from the EUROASPIRE IV survey. Atherosclerosis. 2016;246:243–250. [DOI] [PubMed] [Google Scholar]
- 32. Franch‐Nadal J, Mata‐Cases M, Vinagre I, Patitucci F, Hermosilla E, Casellas A, Bolivar B, Mauricio D. Differences in the cardiometabolic control in type 2 diabetes according to gender and the presence of cardiovascular disease: results from the eControl Study. Int J Endocrinol. 2014;2014:131709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mosca L, Barrett‐Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jansen‐Chaparro S, Mancera J, Cuende JI, Villalobos A, Baca AJ, Lopez‐Carmona MD, Bernal‐Lopez MR, Tinahones FJ, Gomez‐Huelgas R. Metabolic syndrome and vascular risk estimation in a Mediterranean non‐diabetic population without cardiovascular disease. Eur J Intern Med. 2012;23:558–563. [DOI] [PubMed] [Google Scholar]
- 35. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd‐Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris‐Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Sopko G, Chandra‐Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness‐based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Regensteiner JG, Golden S, Huebschmann AG, Barrett‐Connor E, Chang AY, Chyun D, Fox CS, Kim C, Mehta N, Reckelhoff JF, Reusch JEB, Rexrode KM, Sumner AE, Welty FK, Wenger NK, Anton B. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2015;132:2424–2447. [DOI] [PubMed] [Google Scholar]
- 37. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. [DOI] [PubMed] [Google Scholar]
- 38. Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima‐media thickness for cardiovascular disease prediction in the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang Z, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson J‐H, Van Zuydam N, Palmer CNA, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho‐Melander M, Ardissino D, Loos RJF, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin D‐Y, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S. Loss‐of‐function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dewey FE, Gusarova V, O'Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV, Habegger L, Buckler D, Lai K‐MV, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Amor AJ, Catalan M, Pérez A, Herreras Z, Pinyol M, Sala‐Vila A, Cofán M, Gilabert R, Ros E, Ortega E. Nuclear magnetic resonance lipoprotein abnormalities in newly‐diagnosed type 2 diabetes and their association with preclinical carotid atherosclerosis. Atherosclerosis. 2016;247:161–169. [DOI] [PubMed] [Google Scholar]
- 43. Reiner Ž. Managing the residual cardiovascular disease risk associated with HDL‐cholesterol and triglycerides in statin‐treated patients: a clinical update. Nutr Metab Cardiovasc Dis. 2013;23:799–807. [DOI] [PubMed] [Google Scholar]
- 44. Ferrari R, Catapano AL. Residual cardiovascular risk. Eur Heart J Suppl. 2016;18(suppl_C):C1. [DOI] [PubMed] [Google Scholar]
- 45. Shen J, Wilmot KA, Ghasemzadeh N, Molloy DL, Burkman G, Mekonnen G, Gongora MC, Quyyumi AA, Sperling LS. Mediterranean dietary patterns and cardiovascular health. Annu Rev Nutr. 2015;35:425–449. [DOI] [PubMed] [Google Scholar]
- 46. Martínez‐González MA, Salas‐Salvadó J, Estruch R, Corella D, Fitó M, Ros E; PREDIMED INVESTIGATORS . Benefits of the Mediterranean diet: insights from the PREDIMED Study. Prog Cardiovasc Dis. 2015;58:50–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Concordance of Estimated Cardiovascular Risk Between Framingham‐REGICOR and SCORE Strategies in the Whole Population
Table S2. Concordance of Estimated Cardiovascular Risk Between Framingham‐REGICOR and SCORE Strategies in the Nondiabetic Population
Table S3. Main Cardiovascular Risk Factors, Medication Use, and Treatment Goals by Framingham‐REGICOR Estimated Cardiovascular Risk Category by Sex
Table S4. Distribution of Other Classical and Nonclassical Cardiovascular Risk Factors by Estimated Framingham‐REGICOR Risk Category by Sex
Figure S1. Flowchart of PREDIMED participants using European guidelines on cardiovascular disease prevention in clinical practice–Systematic Coronary Risk Evaluation (SCORE) strategy.
Figure S2. Cardiovascular risk estimated by the European guidelines on cardiovascular disease prevention in clinical practice–Systematic Coronary Risk Evaluation (SCORE) strategy in a population aged 55 to 65 years from the total cohort (n=2982) (A), among women (n=1569) (B), and among men (n=1415) (C). Data are shown as number and percentage.
Figure S3. Percentage of cardiovascular events across estimated cardiovascular risk by Framingham‐REGICOR strategy in the total cohort (A), among women (B), and among men (C).
Data S1. Mediterranean diet in a primary cardiovascular prevention research plan.


