Abstract
Background
High blood pressure is thought to contribute to dementia in late life, but our understanding of the relationship between individual differences in blood pressure (BP) and cognitive functioning is incomplete. In this study, cognitive performance in nonhypertensive midlife adults was examined as a function of resting BP and regional cerebral blood flow (rCBF) responses during cognitive testing. We hypothesized that BP would be negatively related to cognitive performance and that cognitive performance would also be related to rCBF responses within areas related to BP control. We explored whether deficits related to systolic BP might be explained by rCBF responses to mental challenge.
Methods and Results
Healthy midlife participants (n=227) received neuropsychological testing and performed cognitive tasks in a magnetic resonance imaging scanner. A pseudocontinuous arterial spin labeling sequence assessed rCBF in brain areas related to BP in prior studies. Systolic BP was negatively related to 4 of 5 neuropsychological factors (standardized β>0.13): memory, working memory, executive function, and mental efficiency. The rCBF in 2 brain regions of interest was similarly related to memory, executive function, and working memory (standardized β>0.17); however, rCBF responses did not explain the relationship between resting systolic BP and cognitive performance.
Conclusions
Relationships at midlife between prehypertensive levels of systolic BP and both cognitive and brain function were modest but suggested the possible value of midlife intervention.
Keywords: cognition, magnetic resonance imaging, middle age, prehypertension, regional blood flow
Subject Categories: Autonomic Nervous System, Blood Pressure, Magnetic Resonance Imaging (MRI), Cognitive Impairment
Introduction
Age, blood pressure (BP), and metabolic factors contribute both independently and collectively to cognitive decline.1 Hypertension affects both brain structure and regional cerebral blood flow (rCBF) during cognitive performance.2 Once established, hypertension predicts stroke, vascular dementia, and indicators of brain aging.3 Evidence now suggests that BP, even in midlife, is negatively related to cognitive performance as well as to rCBF responses to physiological and cognitive challenges.3 Although relationships have been found in midlife, most evidence concurrently examining BP and cognitive and brain function is from adults aged >60 years.4, 5, 6 In this study, we asked whether cognition is associated with prehypertensive, rather than hypertensive, levels of BP in midlife and whether concurrent patterns of brain activation (assessed indirectly by rCBF) also relate to BP.
Our prior work suggested that essential hypertension might influence brain function prior to or concurrently with the progression of BP to diagnostic levels. Conceptually, chronic sympathetic nervous system activation has been associated with the onset of hypertension.7, 8 A central autonomic network9, 10 regulates sympathetic activity and shows substantial anatomical overlap with areas activated during cognitive processing. Etiological processes influencing these brain areas could jointly alter both cognitive performance and BP. During the processing of laboratory cognitive and stress challenges in normotensive participants, amygdala, insula, and cingulate areas from the central autonomic network showed regional activation correlated with concomitant increases in BP.11 Hypertensive participants responding to similar cognitive challenges also increased rCBF in amygdala and hippocampus areas related to task performance, but this was combined with decreased rCBF in parietal, thalamic, and prefrontal areas.12 This finding aligns with observations of neuropsychological deficits among midlife hypertensive patients and reduced overall CBF.6 The possibility that BP was not altering brain function but that brain function may be directly influenced by essential hypertension was raised when successful pharmacological treatment reduced BP but did not alter cognitive and brain rCBF correlates of BP observed prior to treatment.13, 14, 15 The rCBF responses during cognitive performance in the thalamus, posterior parietal, and lateral prefrontal cortex did not normalize over a 1‐year period of pharmacological treatment. Furthermore, the magnitude of BP reduction correlated positively with rCBF response at baseline, and brain indices of aging and nonspecificity of rCBF response remained constant or progressed despite successful lowering of BP.
The current work examined younger prehypertensive persons to see if BP–brain relationships were present prior to diagnostic levels of hypertension, opening a window for early intervention. Although hypertension relates negatively to neuropsychological performance at midlife,6, 16, 17 it is not known whether the rCBF response to laboratory challenge is also altered among prehypertensive persons. Accordingly, we examined cognition along with functional rCBF responses known or suggested to have a role in BP regulation. We addressed the hypothesis that among midlife adults, cognition and rCBF responses to cognitive tasks would covary with resting SBP in areas related to sympathetic function. We also explored the possibility that variation in rCBF responses with systolic BP (SBP) level would explain any negative relations between SBP and cognition.
The rCBF responses were assessed using arterial spin labeling (ASL) with magnetic resonance imaging (MRI) technology. Use of rCBF and ASL maintained continuity with our earlier work examining rCBF with positron emission tomography.12, 18 ASL has assessed total gray matter CBF at rest4 and reduced rCBF in brain regions associated with dementia in elderly hypertensive patients compared with normotensive persons.19 Although the examination of rCBF responses is rare, the feasibility of this technique is supported by studies documenting rCBF responses to breath holding and the differential rCBF response to varying cognitive tasks.20, 21
Methods
Participants
Healthy middle‐aged adults (aged 35–60 years, n=227) with resting BP <140/90 mm Hg were enrolled. None met the following exclusion criteria: (1) general medical conditions—pregnancy, ischemic coronary artery disease, cancer (treatment <12 months), chronic liver disease, chronic kidney disease (creatinine >1.2 mg/dL), or diabetes mellitus (fasting blood glucose >125 mg/dL); (2) neuropsychiatric conditions—stroke, multiple sclerosis, serious head injury, epilepsy, brain tumor and major mental illness; and (3) use of prescription medications for hypertension and psychotropic drugs. In total, 49 participants were selected as normotensive (SBP ≤120 mm Hg and diastolic BP [DBP] ≤80 mm Hg). The remaining participants were prehypertensive (SBP >120 and <140 mm Hg or DBP >80 and <90 mm Hg). Participants were recruited from the local community via newspaper advertisements, posted flyers, and databases of persons interested in research studies (eg, http://www.ctsi.pitt.edu/resrec.shtml). Eligibility was established via a telephone screen (with verbal consent), followed by BP assessment and a medical interview after written consent. All exclusions were assessed via self‐report except for the assessment of BP. Overall, 14 of the 243 consenting and eligible participants failed to participate further after the initial visit. Prehypertensive participants were followed for 2 years. This report is only on the assessments prior to follow‐up. All study procedures received local institutional review board approval.
Participants visited the lab on 3 occasions, typically within 2 weeks (see Figure 1). Visit 1 included a detailed medical history; BP measurement; height, weight, and waist measurement; 12‐hour fasting blood work; and various demographic and lifestyle questionnaires. Participants collected all urine for 16 hours following the initial visit. Concentrations were expressed relative to participants' creatinine excretions, which were also assayed. Sodium, potassium, and creatinine were separately determined using a Beckman Coulter AU680l assay.
Figure 1.
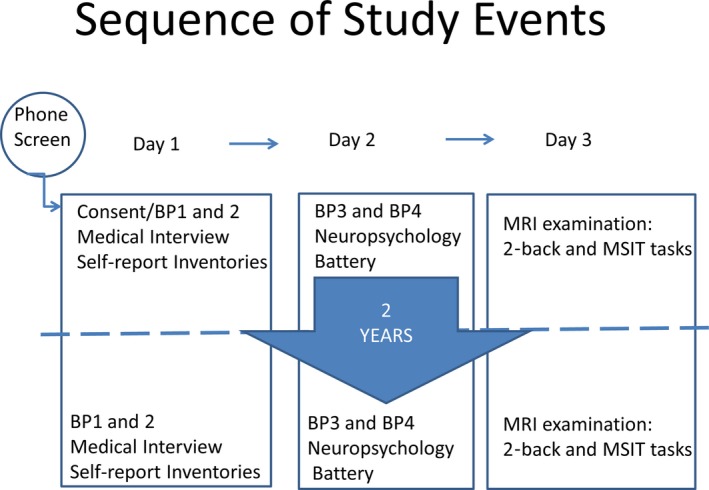
Diagram of study procedure. BP indicates blood pressure; MRI, magnetic resonance imaging; MSIT, multisource interference test.
Visit 2 consisted of BP measurement and a 2‐hour neuropsychological battery. Visit 3 was an MRI examination lasting 1 hour. After participants were seated for at least 5 minutes with back and arm supported, trained assistants recorded BP twice, separated by 60 seconds, using the ausculatory technique with cuff size based on arm circumference. Resting BP was calculated as the average of the 4 readings across 2 visits.
Neuropsychology Battery
A 2‐hour neuropsychological battery examined attention, verbal and visual memory, mental flexibility, and psychomotor ability. An estimate of intelligence was obtained from the National Adult Reading Test.22 The composition of the battery is shown in Table S1. Scores from the battery were summarized into different domains through use of principal component analysis followed by standardized varimax rotation of 5 factors. To enhance the possibility of replication, a unit‐weighted standardized combination of tests loading at >0.50 was used rather than factor scores. The derived factors assessed memory (logical and associative, short and long term), working memory, executive function, mental efficiency, and attention.
Magnetic Resonance Imaging
A 1‐hour MRI session followed training in the cognitive tasks to be performed. The neuroimaging data were acquired on a 3T Trio TIM whole‐body scanner (Siemens) equipped with a 12‐channel phased‐array head coil. The participants were positioned in a standard head coil, and a brief scout T1‐weighted image was obtained. An axial series followed, oriented to the plane connecting the anterior and posterior commissures (AC–PC line). This series included a fast spin‐echo T2‐weighted sequence (effective echo/repetition time 102/2500 ms, 1 excitation) and a FLAIR (fast fluid‐attenuated inversion recovery) sequence (effective echo/repetition time 56/9002 ms; time to inversion 2200; 1 excitation). Section thickness was 5 mm with a 1‐mm intersection gap. FLAIR images, which are highly sensitive to white matter signal abnormalities,23 were used in the assessment of white matter hyperintensity burden. A field of view of 24 cm and an image matrix of 256×192 pixels were used for all axial magnetic resonance series. T1‐weighted images were obtained using a 3‐dimensional fast‐gradient MPRAGE (magnetization prepared rapid gradient‐echo) sequence: repetition time 1540 ms, echo time 3.04 ms, 192 sagittal slices, flip angle 8°, field of view 256 mm, slice thickness 1 mm with no gap, voxel dimensions 1 mm3. The MPRAGE images were used for normalization and for morphological analyses.
The pseudocontinuous ASL (pCASL) scans interleaved perfusion images with (label) and without (control) ASL.24 These scans were obtained using gradient echo‐planar imaging (interleaved ascending, matrix 64×64, 3.75 mm in plane resolution, 6‐mm‐thick slices, 2790 Hz/pixel, echo time 18 ms, flip angle 90°, repetition time 3.6 seconds). The pCASL labeling period was 1.5 seconds with a 1.1‐second transit delay and 1‐second acquisition for 20 slices. A total of 100 pairs of label/control measurements were acquired in 12 minutes. The pCASL label was placed ≈9 cm below the center of the imaging volume. A coronal phase‐contrast magnetic resonance angiogram (single 80‐mm‐thick slice, flip angle 25°, repetition time 50 ms, matrix: 256×256) was used to ensure the pCASL plane was placed orthogonally to the internal carotids and vertebral arteries.
For preprocessing, perfusion images were realigned to the first image of the series, coregistered to each participant's MPRAGE image, spatially normalized to the International Consortium for Brain Mapping 152 template (Montreal Neurological Institute), and resliced to an isotropic voxel size of 3 mm3. Images were then smoothed with a 12‐mm full‐width at half‐maximum isotropic gaussian kernel, after which the 100 labeled and 100 unlabeled images were submitted to pairwise subtraction to obtain perfusion estimates.
Subtraction of the pCASL images yielded rCBF within gray matter (ie, voxels containing ≥80% gray matter).25 CBF maps were created from the pCASL data using the tracer kinetic model.26 Voxels with negative perfusion values and values >2.5×median were set to missing. Averaging across appropriate task segments was then done for each participant to generate separate resting and task voxelwise rCBF images and total CBF values, both in units of mL/100 g per minute.
Perfusion scanning was applied in a blocked design with 3 minutes of the control phase preceding and following 6 minutes of the active phase. The initial task was 2‐back working memory. The target requiring a button press was a letter that repeated a letter presented 2 back in the sequence. This required memory of 3 items compared with the control phase, 0‐back, in which a letter named prior to the task was the target requiring a press. The second task was a modified version of the multisource interference test (MSIT).27, 28 For the active phase, numbers 1, 2, and 3 were matched to the second, third, and fourth finger positions on a response glove. For each trial, 3 numbers were presented evenly spaced across the screen; 2 were identical (eg, 3 3 1). In the test, the finger corresponding to the differing number should execute a button press. Interference arises between the spatial correspondence of screen and fingers and the numeric mapping of fingers. The control task was the same except that the differing number always shared spatial and numeric correspondence.
Within the MRI scanner, BP was collected with a Medran Veris 8600 automated system (Bayer Health Care). BP readings were initiated every 2 minutes during a 4‐minute quiet rest period (during and following FLAIR sequence) and during the 2‐back and MSIT tasks. The task design determined that one BP would be taken during the initial control phase, 2 would be taken during the active task phase, and a final BP would be taken during the closing control phase. The obtained BP values were averaged to form a resting mean, a mean control‐phase, and a mean task‐phase BP. The difference between the task and control‐phase BPs was calculated and then averaged across the 2‐back and MSIT tasks. This “reactivity” score was used in analyses.
MRI analyses
Perfusion images were preprocessed with Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging) as well as a customized ASL toolbox.29 General linear models with a weighted least squares procedures (SPM toolbox, RobustWLS, http://www.diedrichsenlab.org/imaging/robustWLS.html) were used to assess voxelwise task effects on CBF where task conditions were modeled as boxcar functions; the CBF changes from control to task phase were evaluated from preplanned contrast analyses. We then obtained rCBF changes, namely, responses, for 16 bilateral regions of interest by extracting the first principal component of the contrast data in each of the regions across all participants. The regions of interest (ROIs) were selected based on prior literature in which BP or BP reactions to mental tasks were related to brain structure or function.13, 30, 31, 32, 33, 34, 35, 36, 37 Selected regions were the amygdala, Brodmann area 6 (BA6, prefrontal cortex), dorsolateral prefrontal cortex, midcingulate cortex, ventrolateral prefrontal cortex, ventromedial prefrontal cortex, anterior insula, cerebellum, dorsal anterior cingulate cortex, dorsal striatum, pons, posterior cingulate cortex, posterior parietal, subgenual anterior cingulate cortex, thalamus, and ventral striatum. Figure 2 shows the location of these ROIs within the brain.
Figure 2.
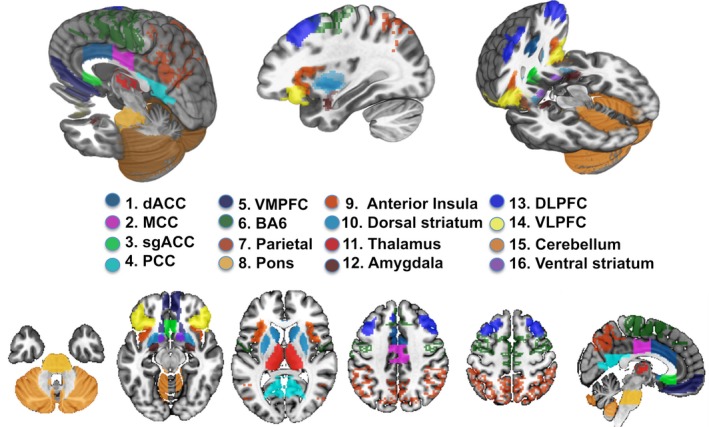
Regions of interest based on prior studies showing relationships between BP levels or BP task reactivity. BA6 indicates Brodmann area 6; BP, blood pressure; dACC; dorsal anterior cingulate cortex, DLPFC, dorsolateral prefrontal cortex; MCC, midcingulate cortex; PCC, posterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex.
Initial analyses of rCBF responses of these areas to the tasks showed substantial correlations among areas. This suggested that summary scores reflecting components of reactivity might exist and that assessment of these would substantially reduce the number of statistical comparisons used, thus acting against experimentwise (type I) error. Principal component analysis of the area intercorrelations revealed 3 components with eigenvalues >1 and consistent with a screen test applied to the eigenvalues. Identification of the components was then aided by varimax rotation. Components were highly similar between the 2 tasks performed in the scanner. Comparison of areas identified suggested areas of efferent activation.38 Areas for 2 of the components were similar to those identified in retrograde rabies virus tracing from the adrenal medulla in the Cebus monkey, that is, tracing efferent connections from the brain to a primary sympathetic efferent organ39 for a motor component and a medial prefrontal component. The motor component encompassed prefrontal motor areas and posterior parietal and midcingulate connectivity (Brodmann area 6, dorsolateral prefrontal cortex, dorsal anterior cingulate, posterior parietal) and, for our purposes, is labeled frontoparietal. The medial prefrontal component connectivity included pre‐ and subgenual cingulate areas as well as medial and ventrolateral prefrontal areas (ventrolateral prefrontal cortex, ventromedial prefrontal cortex, subgenual anterior cingulate, and ventral striatum). We termed this component frontostriatal. Our third component related to areas known to have direct and indirect connections to a primary sympathetic neural efferent area, the rostral ventrolateral medulla (amygdala, anterior insula, thalamus, pons). Reviews of neuroanatomical connectivity support connections of amygdala, insula, thalamus, and pontine nuclei.40, 41, 42 We labeled this component insular–subcortical. These labels are only descriptive summaries, as we have no direct evidence in this study of adrenal or rostral ventrolateral medullary connectivity. Component scores were generated by the combination of standardized values for the areas with unit weighting of areas loading on the component >0.48 (see Table 1).
Table 1.
Loading of Brain Areas on Factors for the 2 Tasks Administered in the Magnetic Resonance Imaging Scanner
| Component | Areas Included | Loading on Component For Interference, 2‐Back Task |
|---|---|---|
| Frontoparietal | BA6 | 0.91, 0.91 |
| DLPFC | 0.85, 0.86 | |
| dACC | 0.71, 0.69 | |
| Posterior parietal | 0.81, 0.82 | |
| Frontostriatal | VLPFC | 0.80, 0.80 |
| VMPFC | 0.86, 0.89 | |
| Ventral striatum | 0.72, 0.75 | |
| Insular–subcortical | Amygdala | 0.82, 0.78 |
| Anterior insula | 0.60, 0.49 | |
| Thalamus | 0.61, 0.60 | |
| Pons | 0.70, 0.72 |
BA6 indicates Brodmann area 6; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; VMPFC, ventromedial prefrontal cortex.
Importantly, frontoparietal and insular–subcortical areas generally increased rCBF, whereas frontostriatal areas decreased rCBF during task performance; rCBF was generally greater during the control phase relative to the active phase.
Statistical Analysis
The results were assessed with bivariate correlation and multiple regressions. Prior to analysis, the distributions of all variables were examined. Skewness of the mental efficiency and attention factors was corrected with log transformation. Extreme values were subjected to winsorizing using values of the upper and lower 1% of the distribution. Because of inability to complete the MRI session, 12 of the 227 participants did not have MRI data. These participants without MRI data were comparable in all respects to the remaining participants with the demographic characteristics shown in Table 2 except for body mass index (BMI). BMI of the those without MRI data was 32.7 compared with 28.8 for the remaining participants (P=0.02). Eight participants did not have SBP reactivity data. These participants were similar to the remaining participants except for lower educational attainment (15.3 versus 18.6 years, P<0.01). Biologically plausible covariates for the model were identified, but the large number (22 variables) required us to limit them to maintain model stability. Potential covariates were examined to determine any significant bivariate correlation with SBP, neuropsychological factor, or rCBF composite. Any variable showing a correlation was further tested in a relevant exploratory regression model to determine if any marginally significant relationship (P<0.10) was evident. Standard covariates were retained (age, sex, race, and education), and BMI and SBP reactivity to tasks were added because of their relationships. The following variables were excluded because of failure to affect primary study findings: report of medical conditions; medication use; use of nicotine; salt intake; ventricular and sulcal size; ratings of white matter hyperintensities43; perceived stress scale44; use of alcohol; activity, as estimated from the Paffenbarger scale45; and apnea, as estimated from the Berlin questionnaire.46
Table 2.
Demographics Described Separately for Participants Recruited as Normotensive or Prehypertensive
| Variable | Normotensive (n=49) | Prehypertensive (n=178) |
|---|---|---|
| Age, y | 47.1 (7.0) | 48.6 (7.0) |
| Education, years | 15.4 (3.0) | 15.3 (2.8) |
| Sex (% male) | 39 | 47 |
| Race (% white)a | 65 | 63 |
| SBP, mm Hgb | 108.2 (6.8) | 123.4 (6.8) |
| DBP, mm Hgb | 71.1 (5.2) | 81.3 (5.1) |
| Heart rate, beats/min | 69.5 (9.2) | 71.0 (9.1) |
| Mean SBP task, control, mm Hg | 2.5 (3.6) | 2.2 (4.0) |
| BMIb | 26.3 (4.9) | 29.7 (5.9) |
| Drink alcohol, % | 80 | 72 |
| Smoke, % | 20 | 27 |
| Taking prescription medications, % | 16 | 29 |
| Average number of reported medical/psychiatric conditionsb | 0.53 (0.77) | 1.12 (1.3) |
| Physical activity estimated, kcal | 1625 (1637) | 1877 (1767) |
| Apnea risk (% by Berlin questionnaire)b | 6.1 | 21.9 |
| Urinary sodium, mmol/L | 113.9 (58.9) | 106.8 (58.8) |
| Urinary potassium, mmol/L | 36.6 (21.2) | 31.8 (19.4) |
| Estimate intelligence quotient (NART) | 108.8 (10.6) | 108.6 (11.9) |
| White matter hyperintensity rating, periventricular | 1.54 (0.85) | 1.70 (0.88) |
| White matter hyperintensity rating, brainstem | 1.12 (1.13) | 1.38 (1.25) |
| APOE*E4 carrier, %b | 16 | 31 |
| Total CBF, mL/min/100 mL | 74.3 (12.9) | 71.5 (13.5) |
Data are shown as mean (SD) or percentage. Sample size varies slightly among variables because of, for example, failures in blood sampling, incomplete urine collection, and refusal to answer. Variation is between 47 and 49 for normotensive and 159 and 178 for prehypertensive participants. BMI indicates body mass index; CBF, cerebral blood flow; DBP, diastolic blood pressure; NART, National Adult Reading Test; SBP, systolic blood pressure.
Overall, 77 were black and 7 were Asian or Hispanic. Physical activity and white matter indices were transformed for purpose of analyses, but the original values are presented.
A descriptive statistical difference with a t test or χ2 test between prehypertensive and normotensive groups.
Regression models were applied to the 5 neuropsychological factors and to performance indices from the 2‐back and MSIT tasks. An accuracy index corrected for false alarms: A was used for the 2‐back task, and reaction time for correct choices was used for the MSIT task. Separate models tested whether SBP levels were related to the rCBF responses to the tasks.
Model 1 included age, race, education, sex, and SBP as predictors of neuropsychological and MRI cognitive task performance outcomes. Model 2 added BMI to test whether it altered the relationship of SBP with these outcomes. Mean SBP change (reactivity) during performance of the active task phases (2‐back and MSIT) in the scanner relative to control phases was also added in this model. If the addition of BMI or SBP change to the variables in model 1 elicited a significant reduction in the β weight for SBP, then BMI or SBP change would possibly mediate or account for SBP–cognition relationships.
Model 3 added the 3 rCBF composites as predictor variables. If SBP acted on cognitive function through any influence on these rCBF composites, then model 3 would show a significant reduction in the relationship (β weight) for SBP relative to models 1 and 2. For model 3, mean substitution was used so all participants and variables could be retained in the analysis (ie, mean values were substituted for the 12 participants without MRI data). Each component was also added singly to check any colinearity among the composites; results were essentially identical.
Models 1 and 2 were rerun with the rCBF composites as dependent measures instead of neuropsychological and MRI cognitive performance outcomes.
Results
Participants
Table 2 describes normotensive and prehypertensive participants. Normotensive and prehypertensive participants were generally comparable, although prehypertensive participants had higher BMI, risk of apnea, and prevalence of an APOE*E4 allele and were more likely to report a medical or psychiatric condition.
rCBF Response
Figure 3 shows brain images with the color code indicating the significant rCBF changes for the 2‐back and MSIT tasks compared with control based on the voxelwise analysis. The ROIs selected as relevant to BP regulation are also outlined in this image. The figure shows that pCASL yielded rCBF activation patterns similar to those in the literature.27, 47 The figure also shows activation in the ROIs selected to relate to BP; however, resting SBP and rCBF responses were unrelated (r<0.10).
Figure 3.
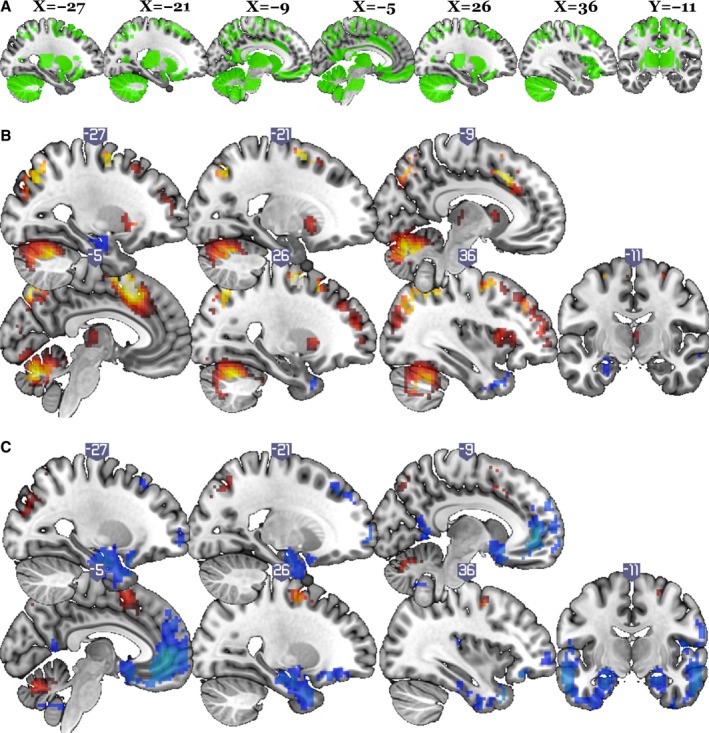
Brain activation regions for 2‐back and multisource interference test (MSIT) tasks compared with regions of interest (ROIs) in this study. X refers to the axis defining the left to right location of the sagittal sections illustrated. Y refers to the front to back location of the the verticofrontal section illustrated. A, Mask of 16 ROIs in which blood pressure or blood pressure reactions to mental tasks were related to brain structure or function based on published literature. B, Regions in which cerebral blood flow (CBF) changes in the 2‐back task were significant: random effect analysis, df=214, familywise cluster threshold with error rate of 0.05 (height threshold P<0.001, t>3.13, extent cluster size of 96). C, Regions in which CBF changes in MSIT were significant: random effect analysis, df=213, familywise cluster threshold with error rate of 0.05 (height threshold P<0.001, t>3.15, extent cluster size of 54).
Correlations
Higher resting SBP was associated with poorer performance on 4 of the 5 neuropsychological factors. Figure 4 presents scatter diagrams showing the statistically significant relationships between SBP and the factors for memory, executive function, working memory, and mental efficiency. The attention factor was unrelated to SBP (r=0.09, P value not significant).
Figure 4.
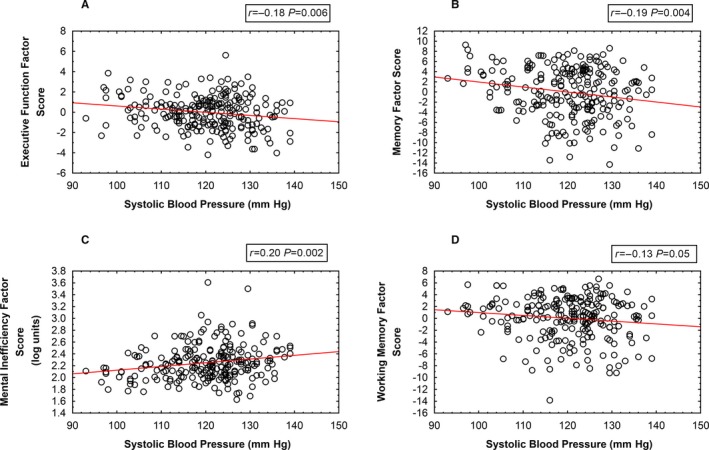
Scatter diagrams relating systolic blood pressure and the neuropsychological factors. A, Executive function. B, Memory. C, Mental inefficiency. D, Working memory.
Multiple Regression
Tables 3, 4, 5 through 6 provide multiple regression results for 3 neuropsychological factors and task performance in the scanner. SBP failed to relate to attention, so this result is not reported in the tables and subsequent models were not executed for this cognitive factor. Working memory is not reported in the tables because the marginal relationship with SBP was reduced further by model 1 covariates.
Table 3.
Multiple Regression Results for the Memory Neuropsychological Factor as a Function of SBP, BMI, and rCBF Areas
| Memory Factor | β | SE of β (95% CI) | P Value |
|---|---|---|---|
| Model 1 | |||
| SBP | −0.132 | 0.057 (−0.020 to −0.244) | 0.02 |
| Model 2 | |||
| SBP | −0.134 | 0.059 (−0.018 to −0.250) | 0.02 |
| BMI | −0.000 | 0.060 (0.118 to −0.118) | ns |
| SBP change, 2‐back | 0.042 | 0.058 (0.156 to −0.072) | ns |
| Model 3 | |||
| SBP | −0.120 | 0.058 (−0.006 to −0.234) | 0.04 |
| BMI | 0.006 | 0.059 (0.122 to −0.110) | ns |
| SBP change, 2‐back | 0.010 | 0.058 (0.124 to −0.104) | ns |
| Frontoparietal ROIs | 0.260 | 0.074 (0.405–0.115) | <0.001 |
| Frontostriatal ROIs | −0.169 | 0.079 (−0.014 to −0.324) | 0.03 |
Model 1 includes age, sex, race, and education as covariates with SBP. Model 2 adds BMI and SBP reactivity. Model 3 adds rCBF areas. Education and race were significant in all models; age was significant in model 1 only. Insular–subcortical ROIs did not contribute significantly in model 3. Model 1 R 2=0.311, model 2 R 2=0.313, model 3 R 2=0.357. BMI indicates body mass index; DBP, diastolic blood pressure; ns, not significant; rCBF, regional cerebral blood flow; ROI, region of interest; SBP, systolic blood pressure.
Table 4.
Multiple Regression Results for the Executive Function Neuropsychological Factor as a Function of SBP, BMI, and rCBF Areas
| Executive Function Factor | β | SE of β (95% CI) | P Value |
|---|---|---|---|
| Model 1 | |||
| SBP | −0.132 | 0.060 (−0.014 to −0.250) | 0.03 |
| Model 2 | |||
| SBP | −0.121 | 0.061 (−0.001 to −0.241) | <0.05 |
| BMI | −0.071 | 0.062 (0.051 to −0.193) | ns |
| SBP change, 2‐back | 0.099 | 0.061 (0.219 to −0.021) | ns |
| Model 3 | |||
| SBP | −0.118 | 0.060 (−0.000 to −0.236) | 0.05 |
| BMI | 0.098 | 0.062 (0.220 to −0.024) | ns |
| SBP change | 0.079 | 0.060 (0.200 to −0.039) | ns |
| Frontoparietal ROI's | 0.169 | 0.077 (0.320–0.018) | 0.03 |
Model 1 includes age, sex, race, and education as covariates with SBP. Model 2 adds BMI and SBP reactivity. Model 3 adds rCBF areas. Race was a significant effect in all models. Neither frontostriatal nor insular–subcortical ROIs were significantly related. Model 1 R 2=0.240, model 2 R 2=0.254, model 3 R 2=0.294. BMI indicates body mass index; DBP, diastolic blood pressure; ns, not significant; rCBF, regional cerebral blood flow; ROI, region of interest; SBP, systolic blood pressure.
Table 5.
Multiple Regression Results for the Mental Efficiency Neuropsychological Factor as a Function of SBP, BMI, and rCBF Areas
| Mental Efficiency Factor | β | SE of β (95% CI) | P Value |
|---|---|---|---|
| Model 1 | |||
| SBP | 0.134 | 0.061 (0.254–0.014) | 0.03 |
| Model 2 | |||
| SBP | 0.141 | 0.062 (0.263–0.019) | 0.02 |
| BMI | 0.001 | 0.063 (0.123 to −0.122) | ns |
| SBP reactivity | −0.152 | 0.062 (−0.030 to −0.274) | 0.01 |
| Model 3 | |||
| SBP | 0.137 | 0.062 (0.259–0.015) | 0.03 |
| BMI | −0.007 | 0.064 (0.118 to −0.132) | ns |
| SBP reactivity | −0.138 | 0.062 (−0.016 to −0.260) | 0.03 |
Model 1 includes age, sex, race, and education as covariates with SBP. Model 2 adds BMI and SBP reactivity. Model 3 adds rCBF areas. Age, race, and education contributed significantly to all models. Frontoparietal, frontostriatal, and insular–subcortical ROIs did not contribute significantly in model 3. Model 1 R 2=0.208, model 2 R 2=0.229, model 3 R 2=0.243. BMI indicates body mass index; DBP, diastolic blood pressure; ns, not significant; rCBF, regional cerebral blood flow; ROI, region of interest; SBP, systolic blood pressure.
Table 6.
Multiple Regression Results for Accuracy of Working Memory in the 2‐Back Task as a Function of SBP, BMI, and rCBF Areas
| 2‐Back Task Accuracy | β | SE of β (95% CI) | P Value |
|---|---|---|---|
| Model 1 | |||
| SBP | 0.127 | 0.060 (0.245–0.009) | 0.04 |
| Model 2 | |||
| SBP | 0.106 | 0.060 (0.224 to −0.012) | 0.08 |
| BMI | 0.033 | 0.061 (0.153 to −0.079) | ns |
| SBP reactivity | 0.247 | 0.060 (0.365–0.129) | <0.001 |
| Model 3 | |||
| SBP | 0.117 | 0.060 (0.235 to −0.001) | 0.05 |
| BMI | 0.039 | 0.060 (0.157 to −0.079) | ns |
| SBP reactivity | 0.226 | 0.060 (0.344–0.108) | <0.001 |
| Frontoparietal ROIs | 0.181 | 0.077 (0.332–0.030) | 0.02 |
Model 1 includes age, sex, race, and education as covariates with SBP. Model 2 adds BMI and SBP reactivity. Model 3 adds rCBF areas. Race and education were related significantly to 2‐back task accuracy in all models. Neither the frontostriatal nor insular–subcortical ROIs contributed significantly to model 3. Model 1 R 2=0.221, model 2 R 2=0.278, model 3 R 2=0.297. BMI indicates body mass index; DBP, diastolic blood pressure; ns, not significant; rCBF, regional cerebral blood flow; ROI, region of interest; SBP, systolic blood pressure.
Memory factor
Poorer memory was significantly related to greater SBP in all models. In model 3, both the frontoparietal and frontostriatal rCBF components were significantly related to the memory factor. Increased rCBF among the frontoparietal ROIs and decreased rCBF among the frontostriatal ROIs were related to relatively better memory performance. Addition of the rCBF areas, however, had essentially no effect on the relationship of SBP and memory.
Executive function factor
Poorer executive function was significantly related to greater SBP in model 1. This relationship was marginally reduced in model 2. In model 3, only the frontoparietal rCBF component related to executive function. Greater rCBF in the frontoparietal ROIs related to better executive function, and the relationship of SBP and executive function was retained at P=0.05.
Working memory factor
In model 1, education and race related strongly to working memory and reduced the relationship between SBP and working memory to nonsignificance (β=−0.063 [95% CI 0.166 to −0.042], SE=0.053, P value not significant). BMI did not contribute in model 2, but SBP reactivity in the 2‐back task did (β=0.171 [95% CI 0.275–0.067], SE=0.053, P=0.002). Better working memory was related to greater increase in SBP during task performance. In model 3, significant contributions to working memory were again observed for SBP reactivity (β=0.156 [95% CI 0.274–0.004], SE=0.053, P=0.004). Both the frontoparietal ROI composite (β=0.139 [95% CI 0.274–0.004], SE=0.069, P=0.04) and the frontostriatal ROI composite (β=−0.137 [95% CI 0.006 to −0.280], SE=0.073, P=0.06) showed marginal relationships to the working memory factor. Values are not shown in tables, given the absence of an SBP relationship after control of race and education (model 1 R 2=0.340, model 2 R 2=0.428, model 3 R 2=0.447).
Mental efficiency factor
Higher resting SBP remained a predictor of less mental efficiency in all 3 multivariable‐adjusted models. In model 2, less mental efficiency was related to less change in SBP during task performance. No rCBF component related significantly to mental efficiency.
MRI task performance
Better 2‐back performance was related to higher SBP level in model 1 and continued to relate, albeit at a marginal level, in all models. In model 2, BMI was not influential, but greater SBP reactivity was related to better performance. SBP and SBP‐reactivity relationships were minimally altered by the addition of brain composites in model 3. Greater frontoparietal rCBF was related to better 2‐back task accuracy in model 3. MSIT reaction times were not related to SBP or rCBF response. Greater SBP reaction to the tasks, however, related to faster MSIT performance (β=−0.140 [95% CI 0.272–0.006], SE=0.064, P=0.03).
rCBF Response and SBP
In models 1 and 2, SBP levels failed to relate significantly to rCBF in the frontoparietal, frontostriatal, or insular–subcortical area. In model 2, the insular–subcortical area replicated prior work of by showing a relationship between greater SBP reactivity to the tasks and greater rCBF (standardized β=0.139 [95% CI 0.272–0.006], SE=0.068, P=0.04; overall model R 2=0.09 with the only other significant contribution from education level).
Exploratory Analyses
All models were rerun with initial DBP rather than SBP as a predictor variable. Results completely paralleled those reported for SBP, but β weights were smaller and statistical significance was less robust. The possible separate influence of height and weight were examined as a replacement for BMI in model 2; however, comparable to the BMI results, neither height nor weight contributed significantly to the prediction of the dependent variables.
Based on a reviewer's suggestion, interactions between SBP and race and SBP and educational level were also explored. Race–SBP interactions failed to show any significant results. When added to the regression models, however, SBP–educational level interactions contributed significantly to the executive function neuropsychological factor (standardized β=0.138, SE=0.063, P=0.03, model 1 R 2=0.21), the frontoparietal rCBF composite (standardized β=0.179, SE=0.066, P=0.007, model 1 R 2=0.16), and the frontostriatal rCBF composite (standardized β=0.148, SE=0.068, P=0.03, model 1 R 2=0.10). These interactions are illustrated with arithmetic means from groups formed from combining those above and below the means for SBP and educational level. Table 7 shows these means.
Table 7.
Mean Values for Executive Function, Frontoparietal rCBF, and Frontostriatal rCBF for Groups Above and Below the Means for SBP and Educational Level
| Education <15.4 Years: SBP <120.1 mm Hg, n=43 | Education <15.4 Years: SBP >120.1 mm Hg, n=60 | Education >15.4 Years: SBP <120.1 mm Hg, n=52 | Education >15.4 Years: SBP >120.1 mm Hg, n=60 | |
|---|---|---|---|---|
| Executive function | −0.18 | −0.87 | 0.42 | 0.65 |
| Frontoparietal rCBF | 10.7 | −6.2 | 21.4 | 36.1 |
| Frontostriatal rCBF | −27.8 | −43.1 | −21.9 | 1.2 |
Comparison of means showed that the group with lower educational level and higher SBP differed from all other groups for executive function (P≤0.02) and frontoparietal rCBF (P≤0.06). The group with lower educational level and higher SBP also differed from both higher education groups for the frontostriatal rCBF (P≤0.05). rCBF indicates regional cerebral blood flow; SBP, systolic blood pressure.
Discussion
Higher BP levels spanning the normotensive to prehypertensive range during midlife were associated with decrements in memory, executive function, mental efficiency, and working memory. Brain rCBF responses to a working memory and mental interference tasks also occurred in brain areas previously related to BP or BP changes. The influence of BP on executive function and on a portion of the rCBF responses appeared to be moderated by educational level; those with less education and greater SBP showed greater decrements in executive function and less rCBF response to cognitive challenge compared with those with more education and similar SBP.
We replicated prior results relating BP to neuropsychological function in midlife5, 6, 16, 17 but did so by excluding participants with clinical hypertension (ie, SBP ≥140 mm Hg or DBP ≥90 mm Hg). Our results are cross‐sectional, but longitudinal studies have observed similar decrements with increasing BP.5, 6, 48, 49, 50 Our attention factor was unrelated to SBP. This accords with work in this area; decrements typically relate to hypertension, but different studies find decrements in different cognitive processes.5, 51, 52 Tzourio et al3 attributed such variability to age, duration and intensity of heightened BP, influence of BP‐related variables (eg, arterial stiffness), and impact on differing cerebrovascular structures. Nevertheless, we observed decrements across multiple processes, supporting the sensitivity of cognition to BP at midlife. In contrast, a number of factors typically associated with hypertension failed to relate significantly to SBP at midlife: BMI, physical activity, salt intake, sleep apnea, alcohol and tobacco use, medication use, medical conditions, and white matter hyperintensities.
The mechanisms accounting for cognitive decline with heightened BP are not completely known, and causality has not been established. Proposed mechanisms typically involve vascular impact on the brain (ie, vascular adjustments due to heightened pressure initiating brain dysfunction). Proposed vascular mechanisms include limitation of vascular reserve and vascular damage to neural structures. Novak and Hajjar53 provide a useful discussion of such vascular views. Such mechanisms are likely important with established hypertension, but our focus is on prehypertensive levels of BP. In our sample, neither total CBF nor rCBF response to task challenges among prehypertensive participants differed significantly from that among normotensive participants. This suggests that vascular reserve was not limiting cognitive processing. Similarly, controls for microvascular disease detected in our sample failed to alter the relationship between SBP and cognition. Although we did not assess all putative mechanisms (eg, disruptions of the blood–brain barrier), the hemodynamic influences of BP per se on neurovascular coupling may not underlie cognitive deficits in prehypertensive persons.
Neural dysregulation may not just accompany but actually precede the progression of BP to diagnostic levels.15, 54 In our work, this possibility was raised by continuing evidence of functional and structural indices of brain dysregulation despite successful lowering of BP in a 1‐year medication trial.14, 55 Identifying any process inducing brain dysregulation is challenging, given the complexity of essential hypertension. Hypertension continues to be recognized as a disease resulting from multiple influences, that is, a myriad of causes.56, 57 Numerous factors are implicated that either are regulated by brain function or influence brain function (eg, inflammation, reactive oxygen species, dietary intake and composition, sympathetic drive, renin angiotensin regulation, hyperuricemia).56, 58 It is unclear what initiates these influences, although psychological stress, overeating, and salt consumption have been implicated.59, 60 A combination of sympathetic nervous system drive and renal dysfunction are often viewed as the primary forces ultimately heightening BP.59, 60 Our incidental observation of a difference in the incidence of APOE*E4 polymorphism between prehypertensive and normotensive participants highlights the possible role of genetic factors. Interestingly, longitudinal observations of persons at midlife, albeit with clinical hypertension, showed a greater negative effect of word association proficiency 30 years later among those possessing APOE*E4.61 We did not see such an association in our cross‐sectional data.
Sympathetic nervous system activation has been consistently supported62 as driving the progression of BP with medullary efferent control exerted by fast‐acting amino acid neurotransmitters (GABA and glutamate) and slower and more chronic activation related to angiotension and aldosterone.63 Relatively high activation chronically and acutely in brain areas regulating sympathetic efference could relate to heightened BP and also be evident in heightened rCBF during processing, as in the current 2‐back task result. Resting SBP was negatively related to neuropsychological factors, but during the 2‐back task, heightened SBP, SBP reactivity, and frontoparietal rCBF were related to better task performance (as others have recently observed64). Heightened BP reactions to laboratory stressors predict subsequent hypertension,65, 66 and we have shown that they relate to subclinical vascular disease among those with hypertension.67 In this study, heightened BP reactivity to our cognitive tasks related to better working memory performance, executive function, and mental efficiency. Continued enhanced SBP responses to stressors might be expected to maintain performance but lead to chronically elevated BP.
Diminished rCBF in response to mental tasks12, 14 and physiological vasodilators68 have been observed previously among hypertensive compared with normotensive participants. In the present study, we selected brain areas that had previously been related in any way to BP or BP reactivity. Our hypothesis was not confirmed that rCBF responses to cognitive challenge in these areas would mediate the relative levels of cognitive function among the participants. In other words, less activation in such areas could not be shown to indicate less processing capacity and explain the neuropsychological results. The rCBF responses were significantly related to memory, working memory, and executive function, but these responses did not explain the relationships between SBP and cognition. This result raises questions about the global hypothesis that progressive levels of BP or a disease process driving these levels would exert a global influence on brain function. If this hypothesis were true, then any challenging probe of brain function would unmask a relationship between brain function and cognitive function. Our testing had methodological limits; current imaging techniques may lack spatial and temporal resolution and sensitivity necessary to detect even some widespread changes in brain function. Our technique, for example, was not adequate for the spatial resolution of important subcortical BP regulatory areas. Leaving aside these and other important concerns, namely, selection of brain areas, level of challenge, random error, the result suggests that influences are not global; specific cognitive processes and brain processes are present. Relationships may also be moderated by participant characteristics, as in our exploratory finding that rCBF responses in the frontoparietal and frontostriatal regions examined were more strongly related to SBP among these with less education. In short, the result suggests that future work must more clearly specify the persons and processes related to BP and examine these with sensitive techniques and designs such as longitudinal designs relating progression of BP to progression of relative deficit.
A speculative possibility is that chronically heightened central and peripheral sympathetic effects induce both hypertension and associated vascular changes that induce the diminished rCBF responses observed with established hypertension. With time, continued heightened cardiovascular reactivity may combine with other sympathetic nervous system–related factors (eg, obesity, apnea, genetic predisposition) to influence BP. Such progression might be expected to increase correlations with cognition and induce changes in rCBF that are detectible. This assumes that the pathogenesis of essential hypertension concurrently influences BP, brain indices, and cognition. Longitudinal observations spanning midlife are required to test this speculation. Alternatively, cognitive function may prove to be stable and independent of advancement of BP; those with relatively poor neuropsychological function may be more susceptible than others to hypertensive disease.
Further limitations should be noted. The current analysis uses cross‐sectional data and cannot determine direction of causality between SBP and cognition. In addition, our sample generalizes only to relatively healthy midlife persons without hypertension. Other measures related to BP (eg, pulse wave velocity) may relate more strongly to cognition than BP.69, 70, 71 Power analyses showed that we had a power of 0.8 with the obtained sample size to detect correlations between BP and cognitive function or rCBF of 0.19 (small effect size, 4% of variance). We were not powered to determine smaller effect sizes that might be expected in the mediation analyses further analyzing relationships of this magnitude. Technical issues and the demands of imaging led to the requirement to estimate missing data, although checks suggested minimal effects of such estimation. Ideally, we would also have verified the self‐report of study exclusions by physical examination or medical records. Ambulatory BP readings or a greater number of BP readings would also strengthen the validity of BP estimates. Strengths of the study include careful screening and detailed cognitive assessment; brain image analysis based on prior findings, limiting but not entirely controlling the risk of experimentwise error rates; and substantial measurement of factors related to hypertension.
Perspectives
These results show cognitive relationships with BP in prehypertensive persons. The emerging field of preventive neuroradiology holds promise for combating the cognitive decline associated with aging and hypertension by combining brain imaging, behavioral treatments, and neuroprotective medications.1, 72, 73, 74, 75 The current results are relevant to this effort, but demonstration of stronger and more specific relationships will be required prior to clinical applicability. The results support the value of further work relating BP, cognitive function, and the neural processes controlling cognition and BP, particularly during midlife.
Sources of Funding
This work was supported by NHLBI RO1 101959.
Disclosures
None.
Supporting information
Table S1. Neuropsychological Measures Listed Within the Derived Factor Used in Analyses
Acknowledgments
Jane Owens is thanked for her excellent management of this project. Claudiu Schirda assisted with the magnetic resonance imaging technique.
(J Am Heart Assoc. 2017;6:e004856. DOI: 10.1161/JAHA.116.004856.)
References
- 1. Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes TM, Sink KM. Hypertension and its role in cognitive function: current evidence and challenges for the future. Am J Hypertens. 2016;29:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension. 2014;63:894–903. [DOI] [PubMed] [Google Scholar]
- 4. Launer LJ, Lewis CE, Schreiner PJ, Sidney S, Battapady H, Jacobs DR, Lim KO, D'Esposito M, Zhang Q, Reis J, Davatzikos C, Bryan RN. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10:e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension. 2012;60:260–268. [DOI] [PubMed] [Google Scholar]
- 6. Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki A‐HA. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol. 2008;35:498–502. [DOI] [PubMed] [Google Scholar]
- 8. Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep. 2003;5:262–268. [DOI] [PubMed] [Google Scholar]
- 9. Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. [DOI] [PubMed] [Google Scholar]
- 10. Shoemaker JK, Goswami R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Front Physiol. 2015;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor‐evoked blood pressure reactivity: emerging evidence for a brain‐body pathway to coronary heart disease risk. Neuroimage. 2009;47:922–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton‐Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. [DOI] [PubMed] [Google Scholar]
- 13. Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, Aizenstein H. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Hum Hypertens. 2012;26:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jennings JR, Muldoon MF, Price J, Christie IC, Meltzer CC. Cerebrovascular support for cognitive processing in hypertensive patients is altered by blood pressure treatment. Hypertension. 2008;52:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. [DOI] [PubMed] [Google Scholar]
- 17. Waldstein SR, Jennings JR, Ryan CM, Muldoon MF, Shapiro AP, Pleferone JM, Fazzari TV, Manuck SB. Hypertension and neuropsychological performance in men: interactive effects of age. Health Psychol. 1996;15:102–109. [DOI] [PubMed] [Google Scholar]
- 18. Jennings JR, Muldoon MF, Ryan CM, Mintun MA, Meltzer CC, Townsend DW, Sutton‐Tyrrell K, Shapiro AP, Manuck SB. Cerebral blood flow in hypertensive patients: an initial report of reduced and compensatory blood flow responses during performance of two cognitive tasks. Hypertension. 1998;31:1216–1222. [DOI] [PubMed] [Google Scholar]
- 19. Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haight TJ, Bryan RN, Erus G, Davatzikos C, Jacobs DR, D'Esposito M, Lewis CE, Launer LJ. Vascular risk factors, cerebrovascular reactivity, and the default‐mode brain network. Neuroimage. 2015;115:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steffener J, Brickman AM, Habeck CG, Salthouse TA, Stern Y. Cerebral blood flow and gray matter volume covariance patterns of cognition in aging. Hum Brain Mapp. 2013;34:3267–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nelson HE, Willison JR, Nelson HE. National Adult Reading Test (NART) Test Manual. Windsor: NFER‐Nelson; 1991. [Google Scholar]
- 23. Bastianello S, Bozzao A, Paolillo A, Giugni E, Gasperini C, Koudriavtseva T, Millefiorini E, Horsfield MA, Colonnese C, Toni D, Fiorelli M, Pozzilli C, Bozzao L. Fast fluid‐attenuated inversion‐recovery versus conventional spin‐echo sequences for MR quantification of multiple sclerosis lesions. AJNR Am J Neuroradiol. 1997;18:699–704. [PMC free article] [PubMed] [Google Scholar]
- 24. Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49:796–802. [DOI] [PubMed] [Google Scholar]
- 26. Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–396. [DOI] [PubMed] [Google Scholar]
- 27. Bush G, Shin LM. The Multi‐Source Interference Task: an fMRI task that reliably activates the cingulo‐frontal‐parietal cognitive/attention network. Nat Protoc. 2006;1:308–313. [DOI] [PubMed] [Google Scholar]
- 28. Sheu LK, Jennings JR, Gianaros PJ. Test‐retest reliability of an fMRI paradigm for studies of cardiovascular reactivity. Psychophysiology. 2012;49:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Aguirre GK, Rao H, Wang J, Fernández‐Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beason‐Held LL, Thambisetty M, Deib G, Sojkova J, Landman BA, Zonderman AB, Ferrucci L, Kraut MA, Resnick SM. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke. 2012;43:1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta‐analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol. 1998;54:149–168. [DOI] [PubMed] [Google Scholar]
- 33. Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: consequences on short‐term information processing. Neuroimage. 2006;31:754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SW, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor‐evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gianaros PJ, Wager TD. Brain‐body pathways linking psychological stress and physical health. Curr Dir Psychol Sci. 2015;24:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baer K‐J, de la Cruz F, Schumann A, Bar KJ, Koehler S, Sauer H, Critchley H, Wagner G. Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage. 2016;134:53–63. [DOI] [PubMed] [Google Scholar]
- 39. Dum RP, Levinthal DJ, Strick PL. Motor, cognitive and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci USA. 2016;113:9922–9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Card JP, Sved AF. Central autonomic pathways In: Llewellyn‐Smith IJ, Verberne AJ, eds. Central Regulations of Autonomic Functions. New York: Oxford University Press; 2011:3–22. [Google Scholar]
- 41. Dampney RA. The hypothalamus and autonomic regulation: an overview In: Llewellyn‐Smith IJ, Verberne AJ, eds. Central Regulations of Autonomic Functions. New York: Oxford University Press; 2011:47–61. [Google Scholar]
- 42. Verberne AJM. Modulation of autonoimc function by the cerebral cortex In: Llewellyn‐Smith IJ, Verberne AJ, eds. Central Regulations of Autonomic Functions. New York: Oxford University Press; 2011:202–219. [Google Scholar]
- 43. Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright P, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 44. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 45. Winters‐Hart C, Brach SK, Trauth KA. Validity of a questionnaire to assess historical physical activity in older women. Med Sci Sports Exerc. 2004;36:2082–2087. [DOI] [PubMed] [Google Scholar]
- 46. Redline S, Strohl KP. Recognition and consequences of obstructive sleep apnea hypopnea syndrome. Clin Chest Med. 1998;19:1–19. [DOI] [PubMed] [Google Scholar]
- 47. Jennings JR, van der Veen FM, Meltzer CC. Verbal and spatial working memory in older individuals: a positron emission tomography study. Brain Res. 2006;1092:177–189. [DOI] [PubMed] [Google Scholar]
- 48. Kohler S, Baars MA, Spauwen P, Schievink S, Verhey FR, van Boxtel MJ. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension. 2014;63:245–251. [DOI] [PubMed] [Google Scholar]
- 49. Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–2340. [DOI] [PubMed] [Google Scholar]
- 50. Chen KH, Henderson VW, Stolwyk RJ, Dennerstein L, Szoeke C. Prehypertension in midlife is associated with worse cognition a decade later in middle‐aged and older women. Age Ageing. 2015;44:439–445. [DOI] [PubMed] [Google Scholar]
- 51. King H, Miller RE. Hypertension: cognitive and behavioral considerations. Neuropsychol Rev. 1990;1:31–73. [DOI] [PubMed] [Google Scholar]
- 52. Waldstein SR, Manuck SB, Ryan CM, Muldoon MF. Neuropsychological correlates of hypertension: review and methodologic considerations. Psychol Bull. 1991;110:451–468. [DOI] [PubMed] [Google Scholar]
- 53. Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Winklewski PJ, Radkowski M, Demkow U. Relevance of immune‐sympathetic nervous system interplay for the development of hypertension. Adv Exp Med Biol. 2016;884:37–43. [DOI] [PubMed] [Google Scholar]
- 55. Jennings JR, Muldoon MF, Whyte EM, Scanlon J, Price J, Meltzer CC. Brain imaging findings predict blood pressure response to pharmacological treatment. Hypertension. 2008;52:1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harrison DG. The mosaic theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J Am Soc Hypertens. 2013;7:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Page IH. The mosaic theory of arterial hypertension—its interpretation. Perspect Biol Med. 1967;10:325–333. [DOI] [PubMed] [Google Scholar]
- 58. Johnson RJ, Feig DI, Nakagawa T, Sanchez‐Lozada LG, Rodriguez‐Iturbe B. Pathogenesis of essential hypertension: historical paradigms and modern insights. J Hypertens. 2008;26:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Black HR, Elliott WJ. Hypertension: A Companion to Braunwald's Heart Disease. Philadelphia, PA: Elsevier Saunders; 2013. [Google Scholar]
- 60. Izzo JL Jr, Sica DA, Black HR. Hypertension Primer: [The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management]. 4th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 61. Nishtala A, Himali JJ, Beiser A, Murabito JM, Seshadri S, Wolf PA, Au R. Midlife hypertension risk and cognition in the non‐demented oldest old: Framingham Heart Study. J Alzheimers Dis. 2015;47:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. [DOI] [PubMed] [Google Scholar]
- 63. Gabor A, Leenen FH. Central neuromodulatory pathways regulating sympathetic activity in hypertension. J Appl Physiol. 2012;113:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yano Y, Ning H, Reis JP, Lewis CE, Launer LJ, Bryan RN, Yaffe K, Sidney S, Albanese E, Greenland P, Lloyd‐Jones D, Liu K. Blood pressure reactivity to psychological stress in young adults and cognition in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2016;5:e002718 DOI: 10.1161/JAHA.115.002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. [DOI] [PubMed] [Google Scholar]
- 66. Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. [DOI] [PubMed] [Google Scholar]
- 67. Gianaros PJ, Bleil ME, Muldoon MF, Jennings JR, Sutton‐Tyrrell K, McCaffery JM, Manuck SB. Is cardiovascular reactivity associated with atherosclerosis among hypertensives? Hypertension. 2002;40:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–H1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension‐associated cognitive decline in healthy adults. Hypertension. 2016;67:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle‐aged adults: the Framingham Third Generation Cohort Study. Hypertension. 2016;67:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta‐analysis. Neurosci Biobehav Rev. 2015;53:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Raji CA, Eyre H, Wei SH, Bredesen DE, Moylan S, Law M, Smal G, Thompson PM, Friedlander RM, Silverman DH, Baune BT, Hoang TA, Salamon N, Toga AW, Vernooij MW. Hot topics in research: preventive neuroradiology in brain aging and cognitive decline. AJNR Am J Neuroradiol. 2015;36:1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Villapol S, Saavedra JM. Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens. 2015;28:289–299. [DOI] [PubMed] [Google Scholar]
- 74. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Iadecola C. Hypertension and dementia. Hypertension. 2014;64:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Neuropsychological Measures Listed Within the Derived Factor Used in Analyses


