Abstract
Background
Few adults have ideal cardiovascular health (CVH). We studied associations of an overall CVH score with subclinical cardiovascular disease and events. We assessed whether associations varied by race/ethnicity.
Methods and Results
Among 5961 participants in the Multi‐Ethnic Study of Atherosclerosis, components of CVH were measured at baseline, 2000‐2002: systolic blood pressure, total cholesterol, fasting glucose, smoking, physical activity, diet, and body mass index. Levels were classified as ideal (2 points), intermediate (1 point), and poor (0 points) according to American Heart Association definitions. Points were summed to produce a CVH score (0‐7 low, 8‐11 moderate, 12‐14 high). Coronary artery calcium, carotid intima‐media thickness, and left ventricular mass were measured at baseline. Cardiovascular disease was defined as myocardial infarction, coronary heart disease death, resuscitated cardiac arrest, stroke, heart failure, or peripheral artery disease. Follow‐up was 10.3 years. Regression models were used to examine associations of the CVH score with subclinical disease and events, adjusting for age, sex, and education. Analyses were stratified by race/ethnicity. Adults with high or moderate CVH scores had significantly lower odds of coronary artery calcium and lower carotid intima‐media thickness and left ventricular mass than adults with low CVH scores. Adults with high or moderate CVH scores were 67% (95%CI 41% to 82%) and 37% (95%CI 22% to 49%) less likely, respectively, to experience a cardiovascular disease event than adults with low scores. There was no interaction with race/ethnicity.
Conclusions
There is a graded inverse association between CVH scores and measures of subclinical and overt cardiovascular disease that is similar across race/ethnic groups.
Keywords: cardiovascular disease prevention, disparities, subclinical atherosclerosis risk factor
Subject Categories: Epidemiology, Lifestyle, Primary Prevention, Race and Ethnicity, Vascular Disease
Introduction
Since the American Heart Association (AHA) announced its Strategic Impact Goals—“by 2020 to improve the health of all Americans by 20% while reducing deaths from cardiovascular diseases (CVDs) and stroke by 20%1”—numerous studies have confirmed that the construct of ideal cardiovascular health (CVH) has important implications for public health.2, 3, 4, 5, 6, 7, 8, 9 For example, many investigators have shown that adults with 6 to 7 ideal metrics experience a significantly lower risk of cardiovascular morbidity and mortality in the short and long term compared to adults with fewer ideal health metrics.3, 4, 8 Further, ideal CVH is also associated with lower incidence of cancer and all‐cause mortality.8, 10
Using data from the Framingham Offspring Study, Xanthakis et al showed a strong inverse association between CVH and levels of circulating biomarkers, measures of subclinical disease, and cardiovascular events (CVEs).9 The association between CVH and outcomes remained significant even after adjustment for the biomarkers and subclinical disease—intermediate markers likely to be on the causal pathway for the development of cardiovascular disease. The analyses by Xanthakis et al did not include coronary artery calcium (CAC), a potent measure of subclinical atherosclerosis. Whether the association of CVH with subsequent events is further mediated by CAC is not known. In addition, whether such findings can be replicated in a multiethnic cohort is not known.
Prior investigation by Dong et al showed a graded inverse association of the number of ideal CVH metrics and the risk of future events that was similar among white, black, and Caribbean Hispanics in the Northern Manhattan Study (NOMAS).6 However, the authors also reported that the Caribbean Hispanic participants experienced a lower risk of CVEs than whites and blacks, even after adjustment for age, sex, and the number of ideal health metrics.
Using data from the Multi‐Ethnic Study of Atherosclerosis (MESA), we examined the association of CVH with measures of subclinical disease, including CAC, and subsequent CVEs. We assessed heterogeneity of the association between CVH and outcomes by race/ethnicity and sex. We also sought to determine whether the association between CVH and outcomes can be fully explained by intermediate phenotypes or whether additional unmeasured pathways might also contribute to the risk of CVEs.
Methods
Study Participants
The study design for MESA has been published elsewhere.11 In brief, MESA is a prospective cohort study of 6814 persons aged 45 to 84 years without known CVD at baseline. Participants were recruited from July 2000 through September 2002 and identified themselves as white (38%), black (28%), Hispanic (22%), or Chinese (12%) at the time of enrollment. All imaging and risk‐factor data used for the current analyses were collected at the baseline examination. The study was approved by the institutional review boards of each site, and all participants gave written informed consent.
Measurement of Health Factors
AHA‐defined cutoffs for poor, intermediate, and ideal levels of each health factor are found in Table 1. Tobacco use was defined as current, former, or no prior use. Total cholesterol and plasma glucose were measured from blood samples obtained after a 12‐hour fast. By use of a Dinamap Pro 1000 automated oscillometric sphygmomanometer (Critikon, Tampa, FL), resting blood pressure was measured 3 times with the participant in a seated position. The mean of the last 2 measurements was used.
Table 1.
American Heart Association Definitions of Poor, Intermediate, and Ideal CVH for Each Metric
| Goal/Metric | Poor | Intermediate | Ideal |
|---|---|---|---|
| Current smoking | Yes | Former ≤12 months | Never or quit ≥12 months |
| Total cholesterol | ≥240 mg/dL | 200 to 239 mg/dL or treated to ideal levels | <200 mg/dL without medication |
| Blood pressure | SBP ≥140 or DBP ≥90 mm Hg | SBP 120 to 139 mm Hg or DBP 80 to 89 mm Hg or treated to ideal levels | <120/<80 mm Hg without medication |
| Fasting plasma glucose | ≥126 mg/dL | 100 to 125 mg/dL or treated to ideal levels | <100 mg/dL without medication |
| Body mass index | ≥30 kg/m2 | 25 to 29.9 kg/m2 | <25 kg/m2 |
| Physical activity | None | 1 to 149 min/week moderate intensity or 1 to 74 vigorous intensity | ≥150 min/week moderate intensity or ≥75 min/week vigorous intensity |
| Healthy diet scorea | 0 to 1 components | 2 to 3 components | 4 to 5 components |
CVH indicates cardiovascular health.
Fruits and vegetables ≥4.5 cups/day; fish ≥2 3.5‐oz servings/week (preferably oily fish); fiber‐rich whole grains ≥3 1‐oz‐equivalent servings/day; sodium <1500 mg/day; sugar‐sweetened beverages ≤450 kcal (36 oz)/week.
Measurement of Health Behaviors
See Table 1 for AHA‐defined cutoffs of poor, intermediate, and ideal levels of each health behavior. Weight and height were measured using standardized techniques with the participant dressed in light clothing. Body mass index was defined as the weight in kilograms divided by the square of the height in meters.
Physical activity was assessed using the Typical Week Physical Activity Survey, a self‐reporting measure that was adapted from the Cross‐Cultural Activity Participation Study.12, 13 Only intentional exercise was included in the current analysis. Activities such as low‐impact aerobics, walking, and yoga were considered moderately intense. Activities such as running, basketball, and tennis were considered vigorous.
Dietary intake over the past year was assessed using a 120‐item food frequency questionnaire.14 Validity of the questionnaire was previously reported in a sample of white, black, and Hispanic individuals, and it was subsequently amended to include dishes unique to Chinese populations.14, 15, 16 Participants recorded frequency of consumption and serving size (small, medium, and large) of specific food and beverage items. Frequency options ranged from rare/never, to 2+ times/day for foods, and 6+ times/day for beverages. We calculated servings/day for each item as the product of the reported frequency and serving size (small weighted by 0.5, medium by 1.0, and large by 1.5).
The structure of the food frequency questionnaire did not permit calculation of sodium content or fruit/vegetable consumption beyond 2 servings/day. As a result, we could not calculate a diet score as defined in the Strategic Impact Goals. Instead, dietary patterns were derived using a principal‐components analysis. The Whole Grains and Fruits pattern was shown to have a strong inverse association with CVD, and so it was used for the current analysis.17 High scores for the Whole Grains and Fruits pattern reflect high consumption of fruit, whole grains, green leafy vegetables, nuts, and low‐fat dairy and low consumption of red meat, refined grains, and sugar‐sweetened beverages, which resembles the ideal diet recommended in the Strategic Impact Goals. Dietary scores were adjusted for caloric intake. For these analyses, the distribution of potential scores was divided into quartiles. The top quartile was considered ideal, the middle quartiles intermediate, and the bottom quartile poor.
Measurement of Subclinical Disease
Carr et al have reported the details of the MESA computed tomographic scanning and interpretation methods for measurement of CAC.18 The protocol for measuring carotid intima‐media thickness (CIMT) was previously validated in the Cardiovascular Health Study.19 The protocols for the magnetic resonance imaging acquisition and analysis to calculate left ventricular (LV) mass were described previously.20, 21
Ascertainment of Events
A detailed description of the follow‐up methods is available at www.mesa-nhlbi.org. Briefly, at intervals of 9 to 12 months, interviewers telephoned participants or family members to inquire about interim hospital admissions, outpatient diagnoses of CVD, and deaths. Follow‐up for this analysis extended through December 2013. To verify self‐reported diagnoses, trained personnel abstracted data from hospital records for an estimated 96% of hospitalized CVEs; records were available for 95% of outpatient diagnostic encounters. Next of kin and physicians were interviewed for participants who experienced out‐of‐hospital cardiovascular deaths. Two physician members of the MESA morbidity and mortality review committee independently classified events. If they disagreed, then the full committee made the final classification.
We classified coronary heart disease (CHD) events as definite or probable myocardial infarction, death due to CHD, resuscitated cardiac arrest, and coronary revascularization. We classified CVD events as CHD plus fatal or nonfatal stroke, definite or probable heart failure (HF), peripheral arterial disease, and CVD death. Stroke events included ischemic and hemorrhagic subtypes.
HF events included adults with both preserved and reduced ejection fraction. Definite and probable HF required clinical symptoms, such as shortness of breath, or signs, such as edema. Probable HF further required a physician diagnosis of HF and medical treatment for HF. Definite HF also required pulmonary edema/congestion by chest radiograph and/or a dilated ventricle or poor LV function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction.
Incident peripheral arterial disease was defined as lower extremity revascularization or the development of an ankle‐brachial index ≤0.90, among participants whose baseline ankle‐brachial index was >0.90 but <1.4. The ankle‐brachial index was measured at exams 3 and 5. Details of ankle‐brachial index measurement have been reported previously.22
Statistical Analysis
We defined ideal, intermediate, and poor levels of each health factor and behavior according to the definitions outlined in the 2020 Impact Goals,1 with adjustment of the classification of diet as described above. In our preliminary analyses we found that <1% of MESA participants met all 7 criteria for ideal CVH. To examine the spectrum of CVH we calculated an overall CVH score based on the health level of each factor and behavior. Ideal levels were given 2 points, intermediate 1 point, and poor 0 points. Each participant was then given a cumulative score by adding points for each health factor and behavior. The lowest possible score was zero (poor levels of all criteria), and the highest score was 14 (ideal levels of all 7 criteria). As with prior analyses, we stratified participants into 3 mutually exclusive categories of overall CVH: high (12‐14 points), moderate (8‐11 points), and low (0‐7 points).23, 24 The CVH score was intended to provide a comprehensive assessment of CVH but was not intended as a tool for risk prediction.
Baseline characteristics were compared according to the overall CVH score using general linear models for continuous variables and cross‐tabulations for categorical variables. The dependent variables for measures of subclinical disease were baseline prevalence of CAC >0, mean CIMT, and mean LV mass. Logistic regression was used to compute odds ratios for CAC >0 at baseline. General linear models were used to examine the association of the CVH score with CIMT and LV mass. The low CVH group served as the reference. All analyses were performed for the entire cohort and stratified by race/ethnic group.
We examined the association of the CVH score with CHD and CVD events using Cox proportional hazards models. We verified that the proportional hazards assumption was appropriate. All of the models were fitted unadjusted or adjusted for age, sex, race/ethnicity, and education. Additionally, to assess the extent to which the associations were explained by potential intermediate phenotypes of subclinical disease, we then adjusted each model for CAC, CIMT, and LV mass. CAC scores were expressed as ln(CAC+1). We tested interactions between the CVH score and sex and race/ethnicity in multivariate models of CVD and CHD events. Effect modification among race/ethnicity, sex, and CVH was tested using a 2‐way interaction. We included the product of the CVH×race/ethnicity and CVH×sex terms in the model, and no significant interaction (P for interaction <0.05) was present. To examine further the dose‐response relationship between continuous CVH scores and the risk of events, we added penalized cubic splines to our Cox proportional hazards models. Penalized cubic splines provide nonparametric estimates for the hazard ratio associated with each cardiovascular score. All analyses were performed for the entire cohort and stratified by race/ethnic group.
All analyses were performed using SAS version 9.2 (SAS institute, Cary, NC). A P<0.05 was considered statistically significant. The penalized cubic spline model was implemented using coxph and pspline functions in R version 2.14.1.
Results
After excluding participants with missing data on CVH metrics, the final study sample for analyses related to the CVH score and CVD events included 5961 men and women. The sample sizes for analyses that included measures of subclinical disease were 5956 (CAC), 5889 (CIMT), 4384 (LV mass), and 4343 (CAC, CIMT, and LV mass). Baseline characteristics according to overall CVH score are shown in Table 2. The majority of participants had moderate CVH scores (52%), followed by low (41.2%) and high (6.8%) CVH scores. Compared to those with low health scores, adults with high CVH scores were younger and more likely to be women, whites, and Chinese. Higher median income and years of education were also associated with higher CVH.
Table 2.
Baseline Characteristics According to CVH Score—Multi‐Ethnic Study of Atherosclerosis, 2000‐2002a
| Low CVH (0‐7 Points) N=2456 | Moderate CVH (8‐11 Points) N=3097 | High CVH (12‐14 Points) N=408 | |
|---|---|---|---|
| Mean age, y | 64.1 | 62.1 | 59.5 |
| Male, % | 48.5 | 46.3 | 39.2 |
| Race/ethnicity, % | |||
| White | 34.6 | 43.4 | 49.5 |
| Black | 34.8 | 22.0 | 11.0 |
| Hispanic | 26.0 | 20.8 | 10.5 |
| Chinese | 4.6 | 13.8 | 28.9 |
| Bachelor's degree or higher, % | 26.1 | 40.9 | 59.6 |
| Annual income >$50 000, % | 36.2 | 45.9 | 55.2 |
| Systolic blood pressure, mm Hg (SD) | 134 (21) | 123 (20) | 108 (12) |
| Use of antihypertensive medication, % | 52.12 | 21.6 | 2.9 |
| Total cholesterol, mg/dL (SD) | 198 (39.5) | 193 (32.8) | 179 (24.1) |
| Use of lipid‐lowering medication, % | 32.6 | 9.3 | 1.5 |
| High‐density lipoprotein cholesterol, mg/dL (SD) | 48.4 (13.4) | 52.5 (15.5) | 56.2 (16.7) |
| Fasting glucose, mg/dL (SD) | 108.3 (39.8) | 89.9 (18.0) | 84.4 (8.7) |
| Use of glucose‐lowering medication, % | 25.2 | 3.7 | 0.3 |
| Current smoker, % | 19.8 | 8.7 | 0.5 |
| Body mass index, kg/m2 (SD) | 31.1 (5.4) | 26.7 (4.5) | 23.2 (2.7) |
| Minutes of moderate or vigorous physical activity/week | 272 | 472 | 614 |
| Ideal diet, n (%)b | 36 (1.5) | 169 (5.5) | 69 (16.9) |
Whole grains and fruits pattern was used for this analysis, reflecting high consumption of fruit, whole grains, green leafy vegetables, nuts, and low‐fat dairy and low consumption of red meat, refined grains, and sugar‐sweetened beverages. The top quartile of the possible diet scores was considered ideal. CVH indicates cardiovascular health.
There is a statistically significant difference in all baseline characteristics between categories, P<0.001.
Diet scores were calculated using a principal‐components analysis of the food frequency questionnaire.
Adults with high CVH had a significantly lower burden of subclinical CVD for all measures studied, as shown in Table 3. Those with high and moderate CVH scores had 71% and 43% lower odds, respectively, of having CAC >0 compared to adults with low CVH. Adults with high CVH scores also had a 15% lower mean CIMT and a 9% lower mean LV mass than adults with low CVH scores. The associations of CVH with subclinical disease were similar for all races/ethnicities and both men and women. Formal testing showed no interaction with sex or race/ethnicity.
Table 3.
Measures of Subclinical CVD According to CVH Score—Multi‐Ethnic Study of Atherosclerosis, 2000‐2002a
| Low CVH (0‐7 Points) | Moderate CVH (8‐11 Points) | High CVH (12‐14 Points) | P Valueb | |
|---|---|---|---|---|
| Overall prevalence of CAC >0, n (%) | 1442/2456 (58.7) | 1403/3097 (45.3) | 120/408 (29.4) | <0.001 |
| White | 586/850 (68.9) | 699/1343 (52.0) | 69/202 (34.2) | <0.001 |
| Black | 422/854 (49.4) | 258/681 (37.9) | 6/45 (13.3) | <0.001 |
| Hispanic | 360/639 (56.3) | 231/646 (35.8) | 11/43 (25.6) | <0.001 |
| Chinese | 74/113 (65.5) | 215/427 (50.3) | 34/118 (28.8) | <0.001 |
| Overall multivariable‐adjusted odds ratio of CAC >0 (95%CI) | 1.0 | 0.57 (0.50‐0.65) | 0.29 (0.22‐0.38) | <0.001 |
| White | 1.0 | 0.56 (0.45‐0.69) | 0.33 (0.23‐0.49) | <0.001 |
| Black | 1.0 | 0.66 (0.52‐0.82) | 0.20 (0.08‐0.52) | <0.001 |
| Hispanic | 1.0 | 0.49 (0.38‐0.64) | 0.28 (0.12‐0.63) | <0.001 |
| Chinese | 1.0 | 0.70 (0.44‐1.12) | 0.30 (0.16‐0.54) | <0.001 |
| Overall median CAC score among adults with CAC >0, (25th, 75th percentiles) | 105 (27, 368) | 75 (19, 267) | 49 (11, 147) | <0.001 |
| White | 150 (39, 456) | 107 (20, 346) | 34 (9, 156) | <0.001 |
| Black | 90 (23, 301) | 59 (17, 215) | 15 (4, 50) | <0.001 |
| Hispanic | 77 (19, 291) | 61 (18, 204) | 28 (13, 151) | <0.001 |
| Chinese | 65 (27, 256) | 59 (17, 186) | 89 (40, 155) | <0.001 |
| Overall mean common CIMT, mm (SD)c | 0.91 (0.20) | 0.85 (0.18) | 0.77 (0.14) | <0.001 |
| White | 0.91 (0.20) | 0.85 (0.19) | 0.76 (0.14) | <0.001 |
| Black | 0.92 (0.19) | 0.88 (0.18) | 0.81 (0.17) | <0.001 |
| Hispanic | 0.90 (0.20) | 0.82 (0.18) | 0.77 (0.15) | <0.001 |
| Chinese | 0.89 (0.18) | 0.83 (0.17) | 0.76 (0.14) | <0.001 |
| Overall mean LV mass g/m2, indexed for BSAd | 80.2 | 77.0 | 73.3 | <0.001 |
| White | 78.2 (15.4) | 74.7 (14.5) | 72.2 (12.4) | <0.001 |
| Black | 82.4 (19.2) | 80.4 (16.9) | 75.6 (17.1) | <0.001 |
| Hispanic | 83.4 (17.8) | 78.9 (15.3) | 76.6 (11.9) | <0.001 |
| Chinese | 77.7 (12.2) | 74.6 (12.9) | 67.3 (11.2) | <0.001 |
BSA indicates body surface area; CAC, coronary artery calcium; CIMT, carotid intima‐media thickness; CVD, cardiovascular disease; CVH, cardiovascular health; LV, left ventricular.
All models were adjusted for age, sex, race/ethnicity, and education.
P values are for linear trends.
5889 participants had complete data for CIMT.
4384 participants had complete data for LV mass.
Over a mean follow‐up of 10.3 years there were 837 CVD events, of which 210 were strokes and 462 were coronary events: 220 myocardial infarctions, 101 CHD deaths, 306 revascularizations, and 26 resuscitated cardiac arrests. Peripheral arterial disease developed in 83 participants, and 238 participants developed heart failure. A disproportionate number of events occurred among participants with low CVH scores; they comprised 41.2% of the MESA cohort but experienced more than half of the cardiovascular (57.2%) and coronary (58.4%) events (Tables 4 and 5). Adults with moderate CVH scores experienced 40.6% of the CVD events and 38.7% of the CHD events. CVD events were rare in those with high CVH scores. Compared to adults with a low CVH score, those with a high score were 67% less likely to experience a CVD event and 66% less likely to experience a CHD event.
Table 4.
Risk of CVD Events According to the CVH Score—Multi‐Ethnic Study of Atherosclerosis, 2000‐2013a
| Low CVH (0‐7 Points) N=2456 | Moderate CVH (8‐11 Points) N=3097 | High CVH (12‐14 Points) N=408 | |
|---|---|---|---|
| CVEs, n (%) | 479 (19.5) | 340 (11.0) | 18 (4.4) |
| White | 185 (21.8) | 175 (13.0) | 12 (5.9) |
| Black | 162 (19.0) | 63 (9.3) | 0 (0) |
| Hispanic | 118 (18.5) | 65 (10.1) | 1 (0.6) |
| Chinese | 14 (12.4) | 37 (8.7) | 5 |
| Overall HR (95%CI) | 1.0 | 0.63 (0.51‐0.78) | 0.33 (0.18‐0.59) |
| White | 1.0 | 0.69 (0.55‐0.87) | 0.36 (0.19‐0.68) |
| Black | 1.0 | 0.46 (0.35‐0.62) | ···b |
| Hispanic | 1.0 | 0.59 (0.44‐0.80) | 0.14 (0.02‐0.97) |
| Chinese | 1.0 | 0.90 (0.48‐1.67) | 0.50 (0.18‐1.42) |
| Overall CAC‐adjusted HR (95%CI) | 1.0 | 0.72 (0.59‐0.88) | 0.28 (0.14‐0.57) |
| White | 1.0 | 0.70 (0.57‐0.87) | 0.40 (0.22‐0.72) |
| Black | 1.0 | 0.50 (0.37‐0.67) | ···b |
| Hispanic | 1.0 | 0.68 (0.50‐0.93) | 0.16 (0.02‐1.15) |
| Chinese | 1.0 | 1.0 (0.54‐1.86) | 0.71 (0.25‐2.0) |
| Overall CIMT‐adjusted HR (95%CI)c | 1.0 | 0.63 (0.52‐0.77) | 0.21 (0.10‐0.43) |
| White | 1.0 | 0.65 (0.53‐0.81) | 0.35 (0.19‐0.63) |
| Black | 1.0 | 0.45 (0.34‐0.7) | ···b |
| Hispanic | 1.0 | 0.62 (0.45‐0.84) | 0.15 (0.02‐1.05) |
| Chinese | 1.0 | 0.92 (0.49‐1.71) | 0.54 (0.19‐1.55) |
| Overall LV mass‐adjusted HR (95%CI)d | 1.0 | 0.60 (0.47‐0.76) | 0.23 (0.11‐0.50) |
| White | 1.0 | 0.67 (0.52‐0.86) | 0.41 (0.22‐0.77) |
| Black | 1.0 | 0.46 (0.32‐0.66) | ···b |
| Hispanic | 1.0 | 0.71 (0.49‐1.03) | 0.22 (0.03‐1.59) |
| Chinese | 1.0 | 0.84 (0.42‐1.70) | 0.76 (0.25‐2.28) |
| Overall CAC‐CIMT‐LV mass–adjusted HR (95%CI)e | 1.0 | 0.73 (0.59‐0.89) | 0.49 (0.28‐0.88) |
| White | 1.0 | 0.74 (0.57‐0.95) | 0.51 (0.27‐0.96) |
| Black | 1.0 | 0.46 (0.32‐0.67) | ···b |
| Hispanic | 1.0 | 0.82 (0.56‐1.20) | 0.25 (0.04‐1.83) |
| Chinese | 1.0 | 0.96 (0.47‐1.95) | 1.01 (0.33‐3.08) |
Cardiovascular disease was defined as definite or probable myocardial infarction, death due to coronary heart disease, resuscitated cardiac arrest, coronary revascularization, fatal or nonfatal stroke, definite or probable heart failure, peripheral arterial disease, and cardiovascular disease death. CAC indicates coronary artery calcium; CIMT, carotid intima‐media thickness; CVD, cardiovascular disease; CVEs, cardiovascular events; CVH, cardiovascular health; HR, hazard ratio; LV, left ventricular.
All models were adjusted for age, sex, race/ethnicity, and education.
Unable to calculate HR because there were no events in this category.
5889 participants had complete data for CIMT.
4384 participants had complete data for LV mass.
4343 participants had complete data for CAC, CIMT, and LV mass.
Table 5.
Risk of Coronary Heart Disease Events According to the CVH Score—Multi‐Ethnic Study of Atherosclerosis, 2000‐2013a
| Low CVH (0‐7 Points) N=2456 | Moderate CVH (8‐11 Points) N=3097 | High CVH (12‐14 Points) N=408 | |
|---|---|---|---|
| Overall coronary events, n (%) | 249 (10.1) | 165 (5.3) | 12 (2.9) |
| White | 114 (13.4) | 96 (7.2) | 9 (4.5) |
| Black | 78 (9.1) | 31 (4.6) | ···b |
| Hispanic | 63 (9.9) | 34 (5.3) | 1 (2.3) |
| Chinese | 10 (8.9) | 23 (5.4) | 3 (2.5) |
| Overall HR (95%CI) | 1.0 | 0.54 (0.44‐0.66) | 0.34 (0.18‐0.61) |
| White | 1.0 | 0.56 (0.43‐0.74) | 0.39 (0.20‐0.78) |
| Black | 1.0 | 0.50 (0.33‐0.76) | ···b |
| Hispanic | 1.0 | 0.59 (0.39‐0.90) | 0.28 (0.04‐2.05) |
| Chinese | 1.0 | 0.78 (0.37‐1.63) | 0.41 (0.11‐1.51) |
| Overall CAC‐adjusted HR (95%CI) | 1.0 | 0.64 (0.52‐0.78) | 0.48 (0.27‐0.87) |
| White | 1.0 | 0.67 (0.51‐0.89) | 0.56 (0.28‐1.11) |
| Black | 1.0 | 0.56 (0.37‐0.86) | ···b |
| Hispanic | 1.0 | 0.74 (0.48‐1.13) | 0.37 (0.05‐2.7) |
| Chinese | 1.0 | 0.92 (0.44‐1.94) | 0.59 (0.16‐2.19) |
| Overall CIMT‐adjusted HR (95%CI)c | 1.0 | 0.55 (0.45‐0.68) | 0.38 (0.21‐0.68) |
| White | 1.0 | 0.58 (0.44‐0.77) | 0.43 (0.21‐0.85) |
| Black | 1.0 | 0.50 (0.32‐0.77) | ···b |
| Hispanic | 1.0 | 0.60 (0.39‐0.92) | 0.30 (0.04‐2.17) |
| Chinese | 1.0 | 0.81 (0.38‐1.71) | 0.46 (0.12‐1.72) |
| Overall LV mass‐adjusted HR (95%CI)d | 1.0 | 0.55 (0.43‐0.70) | 0.40 (0.21‐0.75) |
| White | 1.0 | 0.53 (0.38‐0.74) | 0.42 (0.20‐0.89) |
| Black | 1.0 | 0.52 (0.30‐0.87) | ···b |
| Hispanic | 1.0 | 0.77 (0.47‐1.27) | 0.46 (0.06‐3.37) |
| Chinese | 1.0 | 0.91 (0.38‐2.15) | 0.69 (0.17‐2.76) |
| Overall CAC‐CIMT‐LV mass–adjusted HR (95% CI)e | 1.0 | 0.64 (0.50‐0.82) | 0.57 (0.30‐1.07) |
| White | 1.0 | 0.62 (0.44‐0.87) | 0.58 (0.27‐1.24) |
| Black | 1.0 | 0.56 (0.32‐0.97) | ···b |
| Hispanic | 1.0 | 0.91 (0.54‐1.52) | 0.55 (0.07‐4.06) |
| Chinese | 1.0 | 1.11 (0.46‐2.67) | 0.95 (0.23‐3.90) |
Coronary heart disease was defined as definite or probable myocardial infarction, death due to coronary heart disease, resuscitated cardiac arrest, and coronary revascularization. CAC indicates coronary artery calcium; CIMT, carotid intima‐media thickness; CVH, cardiovascular health; HR, hazard ratio; LV, left ventricular.
All models were adjusted for age, sex, race/ethnicity, and education.
We are unable to calculate HR because there were no events in this category.
5889 participants had complete data for CIMT.
4384 participants had complete data for LV mass.
4343 participants had complete data for CAC, CIMT, and LV mass.
Adjusting individually for CAC, CIMT, and LV mass—potential mediators of the association between CVH and CVD events—only slightly attenuated the significant graded association between the CVH score and the risk of a CVD event (see Table 4). Adjustment for all 3 measures of subclinical disease in the same model (Table 4) attenuated the association, but it nonetheless remained significant for CVD. For CHD the association remained significant with adjustment for the individual measures of subclinical disease, but remained significant for moderate scores only with adjustment for CAC, CIMT, and LV mass together (Table 5). However, the number of CHD events available for analysis decreased by 34% because only adults with all 3 measures were included. We did not find significant interactions between CVH scores and race/ethnicity or sex (data not shown). When the analyses were stratified by race/ethnicity, the results were limited by small sample size—CVD events occurred among only 1 Hispanic, 5 Chinese, and 0 black participants with high CVH scores. The associations between CVH scores and CVD and CHD events remained significant among white participants with moderate and high CVH scores and among black participants with moderate CVH scores.
When the CVH score was examined as a continuous measure, we observed an approximately linear association with CVD and CHD risks for every racial/ethnic group (see Figures 1 and 2). The risk of an event was progressively lower with higher CV health scores without evidence of a plateau, although the confidence intervals widened with higher scores.
Figure 1.
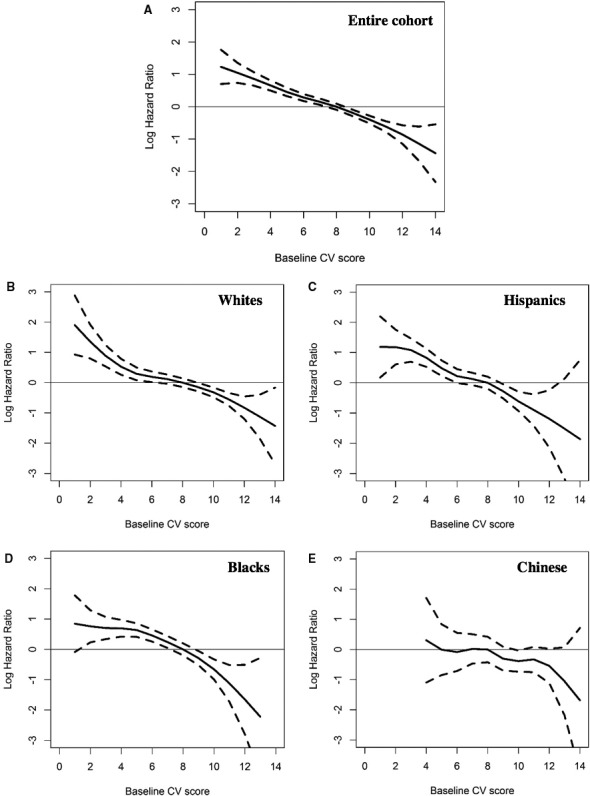
Association of the cardiovascular health score with the log hazard ratio of a cardiovascular disease event, Multi‐Ethnic Study of Atherosclerosis, 2000‐2013. A, The association for the entire cohort; subsequent panels are stratified by race/ethnicity. B, White participants. C, Hispanic participants. D, Black participants. E, Chinese participants. CV indicates cardiovascular.
Figure 2.
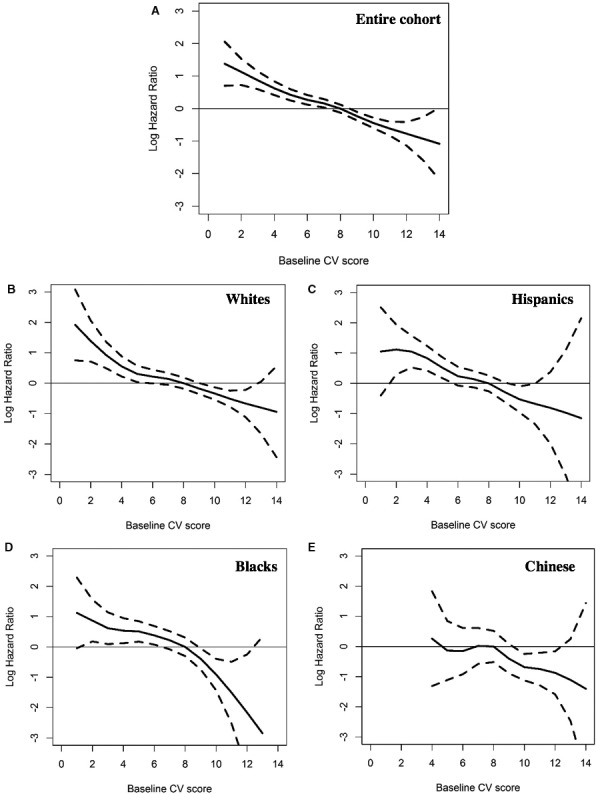
Association of the cardiovascular health score with the log hazard ratio of a coronary heart disease event, Multi‐Ethnic Study of Atherosclerosis, 2000‐2013. Data are presented for the entire cohort and stratified by race/ethnicity. A, The association for the entire cohort; subsequent panels are stratified by race/ethnicity. B, White participants. C, Hispanic participants. D, Black participants. E, Chinese participants. CV indicates cardiovascular.
Discussion
In the large community‐based MESA cohort we found a strong inverse association between overall CVH, as defined by the AHA's 7 metrics, and subclinical CVD and subsequent CVEs. Compared with individuals who had low CVH scores, those with high and moderate levels of CVH had a significantly lower burden of subclinical CVD and markedly lower risks for CVD and CHD events over 10 years of follow‐up. Importantly, we found that the association of CVH with future events and subclinical disease was similar across racial and ethnic groups and between men and women.
Of note, the significant and substantial associations of high and moderate levels of CVH with lower risk for CVD were maintained after adjustment for CAC, CIMT, and LV mass—measures of subclinical CVD that would be expected to link CVH status with subsequent risk. It is possible that CAC, CIMT, and LV mass do not fully encompass subclinical disease, and so we have not adjusted for it completely. Alternatively, our results may suggest that the protection afforded by greater CVH involves pathways other than those related to atherosclerosis and cardiac stress. Thus, the additional benefits of high CVH may reflect aspects of a healthy lifestyle or physical fitness that are not measured in the CVH score and warrant further study.25
Also of note is that more than 25% of adults with high CVH had CAC >0. One potential explanation is that the CVH score may have a stronger association with factors such as plaque morphology than with the presence or absence of atherosclerosis. For example, autopsy and imaging studies demonstrate that the pattern and degree of calcification vary widely based on plaque morphology and suggest that calcification alone is not an adequate marker of plaque vulnerability.26 Another consideration is that we classified adults with nonideal levels for 1 or 2 metrics as high CVH because so few MESA participants met all 7 ideal health criteria. As a result, we may have underestimated the strength of association between ideal CVH and a lower burden of subclinical CVD and events.
Our findings complement those of Xanthakis et al, who examined the association of CVH with clinical events and multiple serum and imaging biomarkers of neurohormonal activation, cardiac stress, and atherosclerosis in the Framingham Offspring study.9 The Framingham investigators similarly found an inverse linear association between higher CVH scores and lower risk of future events after adjusting for the biomarkers that could mediate the association between CVH and clinical events. Our study adds to the literature by demonstrating that the association of CVH and CVD remains significant in a multiethnic cohort after adjustment for CAC.
Our results highlight significant socioeconomic and racial/ethnic disparities within the spectrum of CVH, as have other studies.3, 5, 6 In MESA, adults with high CVH scores had a higher income and education compared to those with moderate and low scores. Blacks and Hispanics were substantially more likely to have low and moderate scores compared to high scores. Addressing such disparities will be an important component of successful interventions, such as improving access to healthy food and places to exercise in all communities. Importantly, we found that the association of the CVH score and CVEs was similar across race/ethnic groups, as did investigators from NOMAS.6 Thus, improving CVH is likely to be a means for reducing disparities in CVD outcomes. Our results are also consistent with those of the INTERHEART study, which showed that modifiable risk factors similarly accounted for >90% of the risk of myocardial infarction across 52 countries, regardless of age or sex.27
Our results differ slightly from those of NOMAS in relation to the risk of events among Hispanic participants. In NOMAS, Hispanic participants experienced a slightly lower risk of events than white or black participants despite having less favorable control of their cardiovascular risk factors, which has been termed the Hispanic paradox.6, 28 The NOMAS investigators suggested that factors beyond the 7 metrics of CVH, such as genetic and sociocultural factors, may contribute to the risk of CVD events.6 In MESA, the event rates among Hispanic participants were similar to those of other racial/ethnic groups after adjustment for CVH scores. One potential explanation is that in NOMAS the Hispanic participants represented a more homogeneous population than in MESA. NOMAS participants were primarily foreign‐born from countries in the Caribbean (62% from the Dominican Republic). Of the Hispanic participants in MESA, 68.5% were born outside of the United States; many were from Mexico and South America.29
Similar to other population‐based studies the prevalence of ideal levels of all 7 metrics was <1% among MESA participants.3, 4, 5, 6 In addition, about 40% of MESA participants had poor levels of multiple health factors and behaviors. Importantly, we found that adults who had primarily intermediate levels of health factors and behaviors—meaning they had prehypertension, impaired glucose tolerance, borderline hypercholesterolemia, or medically controlled levels of these risk factors—experienced a lower burden of subclinical disease and fewer CVEs than those with primarily poor levels. Although there have been encouraging population‐level trends in the control of certain health factors, such as decreases in smoking, blood pressure, and cholesterol levels, these improvements have been offset by a dramatic increase in obesity and diabetes.4 It is possible that the rise in prevalence of obesity and diabetes at least partially explains why men and women less than age 55 have experienced a significantly smaller decline in CHD mortality since the year 2000, compared to adults above age 65.30 If current trends continue, population‐level CVH in the United States is projected to improve by only 6% between 2006 and 2020, far short of the target set in the AHA's Strategic Impact Goal. Our results suggest that public health efforts should not focus solely on achieving ideal levels of CVH metrics. Even shifting individuals from poor to intermediate health could translate into a reduction in cardiovascular morbidity and mortality over time.
Improvements in CVH would also have downstream effects on far more than just cardiovascular morbidity and mortality. Higher levels of CVH are associated with a broad range of health benefits, including fewer symptoms of depression,31 improved cognition,32 better quality of life,33, 34 lower incidence of cancer,10 lower health care expenditures,35 and lower all‐cause mortality.7
Our analysis has limitations that should be acknowledged. Physical activity and diet were assessed by self‐report and so are susceptible to recall and social desirability bias.13 We were not able to calculate the diet score as described in the Strategic Impact Goals because of the way the food frequency questionnaire was structured. We reported a prevalence of an ideal diet of 4.6%, which is higher than that in other cohort studies.3, 6 It is therefore possible that some participants were misclassified as having an ideal diet, which would have biased our results to the null. As recommended by the AHA, we weighted all health factors and behaviors equally when calculating the CVH scores. However, we do not feel this diminishes the score's value, given that it is meant as a tool for monitoring the population's CVH and not for risk prediction. Prior studies have demonstrated a stepwise reduction in risk for each additional metric at ideal levels without differentiating between the different metrics, suggesting that our approach was valid.3 This was additionally confirmed in our spline analysis.
Conclusions
Using a CVH score derived from the AHA's construct of CVH, we found a strong, inverse graded association between CVH scores and both subclinical disease and CVD and CHD events in MESA. We found that the association of CVH scores with subclinical and clinical disease was similar across racial/ethnic groups and between men and women. Adults with higher CVH scores had lower CAC prevalence, CIMT, and LV mass. Adjusting for CAC, CIMT, and LV mass only partially attenuated the strong associations between higher CVH scores and a lower incidence of CVD. Our results underscore the need to adopt interventions that address all levels of CVH across men and women and the diverse subgroups of our population.
Sources of Funding
This research was supported by contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from the National Center for Research Resources.
Disclosures
None.
Acknowledgments
The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions.
(J Am Heart Assoc. 2017;6:e004894. DOI: 10.1161/JAHA.116.004894.)
References
- 1. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2. Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015;132:1979–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community‐based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed HM, Blaha MJ, Nasir K, Jones SR, Rivera JJ, Agatston A, Blankstein R, Wong ND, Lakoski S, Budoff MJ, Burke GL, Sibley CT, Ouyang P, Blumenthal RS. Low‐risk lifestyle, coronary calcium, cardiovascular events, and mortality: results from MESA. Am J Epidemiol. 2013;178:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. [DOI] [PubMed] [Google Scholar]
- 10. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 12. Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross‐Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8:805–813. [DOI] [PubMed] [Google Scholar]
- 13. Bertoni AG, Whitt‐Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nettleton JA, Steffen LM, Mayer‐Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR Jr. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Block G, Woods M, Potosky A, Clifford C. Validation of a self‐administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 16. Mayer‐Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi‐Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–324. [DOI] [PubMed] [Google Scholar]
- 17. Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR Jr. Dietary patterns and incident cardiovascular disease in the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 19. O'Leary DH, Polak JF, Wolfson SK Jr, Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. [DOI] [PubMed] [Google Scholar]
- 20. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi‐ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–S365. [DOI] [PubMed] [Google Scholar]
- 22. Wassel CL, Berardi C, Pankow JS, Larson NB, Decker PA, Hanson NQ, Tsai MY, Criqui MH, Allison MA, Bielinski SJ. Soluble P‐selectin predicts lower extremity peripheral artery disease incidence and change in the ankle brachial index: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd‐Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005‐2010. Circulation. 2013;127:1369–1376. [DOI] [PubMed] [Google Scholar]
- 24. Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd‐Jones DM. Cardiovascular health behavior and health factor changes (1988‐2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lloyd‐Jones DM. Cardiovascular health and protection against CVD: more than the sum of the parts? Circulation. 2014;130:1671–1673. [DOI] [PubMed] [Google Scholar]
- 26. Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol. 2014;34:724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez CJ. Disparities in ideal cardiovascular health: a challenge or an opportunity? Circulation. 2012;125:2963–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diez Roux AV, Detrano R, Jackson S, Jacobs DR Jr, Schreiner PJ, Shea S, Szklo M. Acculturation and socioeconomic position as predictors of coronary calcification in a multiethnic sample. Circulation. 2005;112:1557–1565. [DOI] [PubMed] [Google Scholar]
- 30. Wilmot KA, O'Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association's definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS One. 2012;7:e52771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association life's simple 7 and incident cognitive impairment: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635 DOI: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allen NB, Badon S, Greenlund KJ, Huffman M, Hong Y, Lloyd‐Jones DM. The association between cardiovascular health and health‐related quality of life and health status measures among U.S. adults: a cross‐sectional study of the National Health and Nutrition Examination Surveys, 2001‐2010. Health Qual Life Outcomes. 2015;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Feinglass J, Guralnik JM, Greenland P, Stamler J. Favorable cardiovascular risk profile in middle age and health‐related quality of life in older age. Arch Intern Med. 2003;163:2460–2468. [DOI] [PubMed] [Google Scholar]
- 35. Daviglus ML, Liu K, Greenland P, Dyer AR, Garside DB, Manheim L, Lowe LP, Rodin M, Lubitz J, Stamler J. Benefit of a favorable cardiovascular risk‐factor profile in middle age with respect to Medicare costs. N Engl J Med. 1998;339:1122–1129. [DOI] [PubMed] [Google Scholar]


