Abstract
Background
It is unknown whether renal dysfunction conveys poor anticoagulation control in warfarin‐treated patients with atrial fibrillation and whether poor anticoagulation control associates with the risk of adverse outcomes in these patients.
Methods and Results
This was an observational study from the Stockholm CREatinine Measurements (SCREAM) cohort including all newly diagnosed atrial fibrillation patients initiating treatment with warfarin (n=7738) in Stockholm, Sweden, between 2006 and 2011. Estimated glomerular filtration rate (eGFR; mL/min per 1.73 m2) was calculated from serum creatinine. Time‐in‐therapeutic range (TTR) was assessed from international normalized ratio (INR) measurements up to warfarin cessation, adverse event, or end of follow‐up (2 years). Adverse events considered a composite of intracranial hemorrhage, ischemic stroke, myocardial infarction, or death. During median 254 days, TTR was 83%, based on median 21 INR measurements per patient. TTR was 70% among patients with eGFR <30, around 10% lower than in those with normal renal function. During observation, adverse events occurred in 4.0% of patients, and those with TTR ≤75% were at higher adverse event risk. This was independent of patient characteristics, comorbidities, number of INR tests, days exposed to warfarin, and, notably, independent of eGFR: adjusted odds ratio (OR) 1.84 (95% CI, 1.41–2.40) for TTR 75% to 60% and adjusted OR 2.09 (1.59–2.74) for TTR <60%. No interaction was observed between eGFR and TTR in association to adverse events (P=0.2).
Conclusion
Severe chronic kidney disease (eGFR <30) patients with atrial fibrillation have worse INR control while on warfarin. An optimal TTR (>75%) is associated with lower risk of adverse events, independently of underlying renal function.
Keywords: all‐cause death, anticoagulant, atrial fibrillation, bleeding, ischemic stroke, renal function
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Cardiovascular Disease, Intracranial Hemorrhage, Ischemic Stroke, Anticoagulants
Introduction
Atrial fibrillation (AF) is a common cardiovascular complication associated to poor outcomes, including an increased risk of stroke. Anticoagulant therapy with warfarin can effectively reduce stroke risk by 60% at the cost, however, of an increased risk of intracranial hemorrhage (ICH) and bleeding.1 The success of preventing adverse events (both ischemic and bleeding events), with warfarin is dependent on maintaining an optimal anticoagulation management, namely, achieving international normalized ratio (INR) between 2.0 and 3.0. The time in therapeutic range (TTR) quantifies the percentage of time within this range, and optimal TTR has been associated with better outcomes.2 TTR is typically affected by patient‐related factors (including comorbidities and genetic predisposition), warfarin dose, drugs known to interact with warfarin, as well as center‐ and country/health care–related factors.3, 4
Patients with chronic kidney disease (CKD) often develop AF. At the same time, CKD confers increased risk of ischemic stroke and bleeding.5, 6 Anticoagulation management in these patients is challenging, and some observational studies have raised concerns regarding the safety and effectiveness of warfarin in AF patients with CKD, particularly those with end‐stage renal disease and undergoing dialysis.7, 8 A limitation of those studies is, however, the lack of information on the patient's INR control, which could explain the observed increased risk of adverse outcomes in warfarin‐treated patients with CKD.
In this study, we hypothesized that patients with CKD have worse anticoagulant control (poor TTR), and that it is a worse TTR that associates with poor outcomes. We tested this hypothesis in a real‐world setting of newly diagnosed AF patients initiating warfarin therapy.
Methods
Study Population and Exposure
Patients were selected from the Stockholm CREatinine Measurements (SCREAM) project,9 a health care utilization cohort for the region of Stockholm, Sweden. SCREAM collected laboratory tests and health care use data from all individuals ≥18 years who had serum creatinine measured at least once between 2006 and 2011. SCREAM covers 98% of all cardiovascular disease cases registered in the region.9
Eligible patients for this study were newly diagnosed AF patients initiating warfarin treatment (see Figure 1, flow chart). Diagnosis of AF and other comorbidities was obtained from International Classification of Diseases, Tenth Revision (ICD‐10) codes (see Tables S1 through S4 for definitions). AF has been shown to have a high diagnostic validity, with 95% having AF on electrocardiogram when based on ICD codes.10 Information on pharmacy‐dispensed medications was obtained from the Swedish Dispensed Drug registry, which records all dispensations from any Swedish pharmacy (Table S2).
Figure 1.
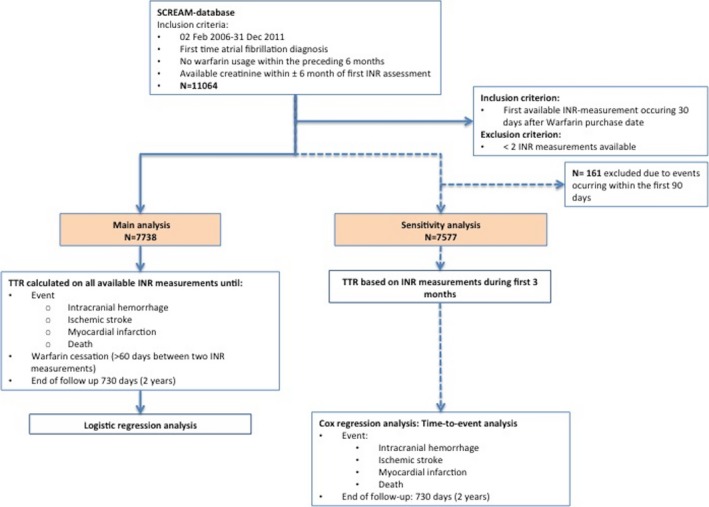
Flow chart. INR, international normalized ratio.
The index date was the day of the first warfarin dispensation after a new AF diagnosis. Demographics, comorbid history, and ongoing/recent medication (dispensations during the preceding 6 months) were calculated at that point. All available INR measurements from day 30 and up to 730 days (2 years) from the first warfarin purchase were used to estimate TTR. TTR was calculated as the percentage of time that INR was therapeutic (an INR between 2 and 3), assuming a linear association between 2 measurements.11 Patients were followed until INR measurements stopped (defined as lack of INR measurements within 60 days), occurrence of an adverse event (ICH/ischemic stroke/myocardial infarction [MI]/death), or 2 years from warfarin initiation.
The serum creatinine measured closest (within ±6 months) to index date was used to calculate eGFR by the Chronic Kidney Disease Epidemiology Collaboration formula,12 which is based on creatinine, age, sex, and race. All creatinines were isotope dilution mass spectrometry standardized, and renal function was categorized according to Kidney Disease: Improving Global Outcomes staging13 as follows: estimated glomerular filtration rate (eGFR) ≥60 mL/min per 1.73 m2, 45 to 59, 30 to 44, and <30 or treated with dialysis. Patients undergoing dialysis were ascertained by linkage with the Swedish Renal Register. Given that albuminuria is less routinely measured in health care, differentiation of early CKD stages was not possible.
The requirement for informed consent was waived in this study. The study was approved by the local ethics committee in Stockholm, Sweden.
Outcome
The study outcome considered a composite of ICH, ischemic stroke, MI, or death. Events were ascertained through ICD‐10 codes (Table S4) in connection with a health care consultation and by linkage with the Swedish Population registry, which records deaths and ICD‐10 causes of death for all Swedish citizens with no loss to follow‐up. The validity of ICH in the Swedish registry is very high at 99.4%.14 The validity of other ICD diagnoses derived from the patient register is between 85% and 95%.15 Events were included if occurring within 30 days from the last INR measurement (30‐day lag phase).
Statistical Analysis
Continuous data are presented as mean (±SD) or median (interquartile interval; IQR). Categorical data are presented as number and percentage. Fractional regression analysis was used to assess whether eGFR and CKD stages associated with TTR. Analyses were adjusted for clinically relevant factors and factors reported to be associated with TTR in previous studies.3, 16 These were: age (in categories: <65, 65–74, 75–85, and ≥85 years), sex, diabetes mellitus, liver disease, hypertension, vascular disease, heart failure, valvular disease, amiodarone use, aspirin use, cancer, and renal function (as 4 eGFR categories eGFR ≥60, 45–59, 30–44, and <30/dialysis).
The association between TTR, renal function, and adverse outcomes was assessed in a logistic regression model. Covariates included renal function categories (same as above), TTR (categories >75, 60–75, and <60%), age (<65, 65–74, 75–84, and ≥85 years), sex, diabetes mellitus, hypertension, vascular disease, heart failure, valvular disease, cancer, known coagulation/platelet defect, anemia, past ischemic stroke, past venous thromboembolism, past intracranial bleeding, past gastrointestinal bleeding, antiplatelet use, number of INR measurements, and number of days on warfarin. Interactions were tested between renal function and TTR, renal function and age, and age and TTR.
As a sensitivity analysis, we recomputed TTR using only INR measurements during the first 180 days (3 months) of therapy (Figure 1) and then estimated time‐to‐event from day 180 onward. In this setting, we followed patients for up to 2 years regardless of whether warfarin was discontinued. A Kaplan–Meier curve was used to graphically display the unadjusted association between an adverse outcome and TTR. A multivariable Cox regression analysis assessed the association between renal function, TTR, and the composite outcome. Covariates included the same as mentioned above. The proportional hazards assumption for the Cox model was tested with the Schoenfeld residuals, and overall fit of the Cox model was evaluated by plotting the Cox‐Snell residuals. All analyses were performed using STATA software (version 14.1; StataCorp LP, College Station, TX).
Results
Study Population
Between 2006 and 2011, 11 064 new AF cases were registered in the region of Stockholm. Of those, 7738 patients initiated warfarin treatment and had a recent creatinine measured to estimate their eGFR (Figure 1, Table 1). The median TTR (IQR) was 83% (71–92). The median eGFR was 73 (59–86) mL/min per 1.73 m2. There were 11 patients treated with dialysis.
Table 1.
Baseline Characteristics of Study Participants
| All | eGFR ≥60 | eGFR 45 to 59 | eGFR 30 to 44 | eGFR <30 or Dialysis | P Value | |
|---|---|---|---|---|---|---|
| N | 7738 | 5692 | 1353 | 512 | 181 | |
| eGFR, median (IQR) | 73 (59–86) | 80 (71–89) | 54 (50–57) | 39 (36–42) | 23 (15–27) | |
| Age, median (IQR), y | 73 (65–80) | 70 (63–78) | 78 (72–83) | 81 (76–85) | 80 (71–85) | <0.001 |
| <65 | 2006 (25.9%) | 1846 (32.4%) | 108 (8.0%) | 30 (5.9%) | 22 (12.2%) | <0.001 |
| 65 to 74 | 2461 (31.8%) | 1953 (34.3%) | 382 (28.2%) | 88 (17.2%) | 38 (21.0%) | |
| 75 to 84 | 2565 (33.1%) | 1603 (28.2%) | 631 (46.6%) | 253 (49.4%) | 78 (43.1%) | |
| ≥85 | 706 (9.1%) | 290 (5.1%) | 232 (17.1%) | 141 (27.5%) | 43 (23.8%) | |
| Female | 3153 (40.7%) | 2112 (37.1%) | 676 (50.0%) | 273 (53.3%) | 92 (50.8%) | <0.001 |
| CHA2DS2‐VASc, mean (SD) | 2.9 (1.8) | 2.6 (1.8) | 3.7 (1.6) | 4.4 (1.6) | 4.2 (1.7) | <0.001 |
| HAS‐BLED, mean (SD) | 2.1 (1.1) | 1.9 (1.1) | 2.4 (1.0) | 2.6 (0.9) | 3.1 (1.0) | <0.001 |
| Comorbid history | ||||||
| MI | 690 (8.9%) | 402 (7.1%) | 146 (10.8%) | 105 (20.5%) | 37 (20.4%) | <0.001 |
| Ischemic heart disease | 1423 (18.4%) | 892 (15.7%) | 309 (22.8%) | 168 (32.8%) | 54 (29.8%) | <0.001 |
| Peripheral arterial disease | 382 (4.9%) | 227 (4.0%) | 83 (6.1%) | 49 (9.6%) | 23 (12.7%) | <0.001 |
| PCI | 216 (2.8%) | 134 (2.4%) | 48 (3.5%) | 31 (6.1%) | 3 (1.7%) | <0.001 |
| CABG | 112 (1.4%) | 73 (1.3%) | 26 (1.9%) | 12 (2.3%) | 1 (0.6%) | 0.068 |
| Hypertension | 4014 (51.9%) | 2693 (47.3%) | 820 (60.6%) | 364 (71.1%) | 137 (75.7%) | <0.001 |
| Diabetes mellitus | 1153 (14.9%) | 741 (13.0%) | 241 (17.8%) | 122 (23.8%) | 49 (27.1%) | <0.001 |
| Heart failure | 716 (9.3%) | 315 (5.5%) | 203 (15.0%) | 137 (26.8%) | 61 (33.7%) | <0.001 |
| Valvular disease | 94 (1.2%) | 60 (1.1%) | 18 (1.3%) | 11 (2.1%) | 5 (2.8%) | 0.033 |
| Biological valve prosthesis | 43 (0.6%) | 25 (0.4%) | 9 (0.7%) | 6 (1.2%) | 3 (1.7%) | 0.027 |
| Mechanical valve prosthesis | 65 (0.8%) | 38 (0.7%) | 17 (1.3%) | 7 (1.4%) | 3 (1.7%) | 0.046 |
| Pacemaker/ICD | 367 (4.7%) | 226 (4.0%) | 80 (5.9%) | 47 (9.2%) | 14 (7.7%) | <0.001 |
| Known liver disease | 31 (0.4%) | 23 (0.4%) | 2 (0.1%) | 3 (0.6%) | 3 (1.7%) | 0.021 |
| Chronic obstructive pulmonary disease | 489 (6.3%) | 332 (5.8%) | 95 (7.0%) | 46 (9.0%) | 16 (8.8%) | 0.009 |
| Cancer (within last 3 years) | 971 (12.5%) | 631 (11.1%) | 211 (15.6%) | 94 (18.4%) | 35 (19.3%) | <0.001 |
| Alcohol abuse | 151 (2.0%) | 123 (2.2%) | 16 (1.2%) | 7 (1.4%) | 5 (2.8%) | 0.071 |
| Dementia | 33 (0.4%) | 17 (0.3%) | 10 (0.7%) | 6 (1.2%) | 0 (0.0%) | 0.005 |
| Gastrointestinal bleeding | 124 (1.6%) | 85 (1.5%) | 23 (1.7%) | 9 (1.8%) | 7 (3.9%) | 0.091 |
| Known coagulation/platelet defect | 58 (0.7%) | 37 (0.7%) | 13 (1.0%) | 6 (1.2%) | 2 (1.1%) | <0.001 |
| Known anemia | 311 (4.0%) | 178 (3.1%) | 65 (4.8%) | 41 (8.0%) | 27 (14.9%) | <0.001 |
| Ischemic stroke | 616 (8.0%) | 421 (7.4%) | 122 (9.0%) | 59 (11.5%) | 14 (7.7%) | 0.004 |
| Transient ischemic attack | 289 (3.7%) | 201 (3.5%) | 55 (4.1%) | 27 (5.3%) | 6 (3.3%) | 0.21 |
| Peripheral systemic embolism | 66 (0.9%) | 31 (0.5%) | 15 (1.1%) | 15 (2.9%) | 5 (2.8%) | <0.001 |
| Pulmonary embolism | 308 (4.0%) | 191 (3.4%) | 64 (4.7%) | 38 (7.4%) | 15 (8.3%) | <0.001 |
| Deep venous thrombosis | 191 (2.5%) | 138 (2.4%) | 30 (2.2%) | 19 (3.7%) | 4 (2.2%) | 0.29 |
| Medication history (last 6 months) | ||||||
| Aspirin | 2782 (36.0%) | 1870 (32.9%) | 598 (44.2%) | 241 (47.1%) | 73 (40.3%) | <0.001 |
| Clopidogrel | 158 (2.0%) | 94 (1.7%) | 34 (2.5%) | 24 (4.7%) | 6 (3.3%) | <0.001 |
| NSAID | 1250 (16.2%) | 908 (16.0%) | 217 (16.0%) | 94 (18.4%) | 31 (17.1%) | 0.54 |
| Acetaminophen | 971 (12.5%) | 642 (11.3%) | 185 (13.7%) | 99 (19.3%) | 45 (24.9%) | <0.001 |
| Statins | 1927 (24.9%) | 1299 (22.8%) | 395 (29.2%) | 176 (34.4%) | 57 (31.5%) | <0.001 |
| SSRI | 337 (4.4%) | 233 (4.1%) | 71 (5.2%) | 25 (4.9%) | 8 (4.4%) | 0.28 |
| Proton pump inhibitor | 1023 (13.2%) | 666 (11.7%) | 212 (15.7%) | 104 (20.3%) | 41 (22.7%) | <0.001 |
| Amiodarone | 12 (0.2%) | 8 (0.1%) | 2 (0.1%) | 1 (0.2%) | 1 (0.6%) | 0.58 |
| Macrolides | 52 (0.7%) | 33 (0.6%) | 12 (0.9%) | 2 (0.4%) | 5 (2.8%) | 0.003 |
| Quinolones | 260 (3.4%) | 171 (3.0%) | 54 (4.0%) | 22 (4.3%) | 13 (7.2%) | 0.004 |
| Cotrimoxazole | 24 (0.3%) | 17 (0.3%) | 4 (0.3%) | 0 (0.0%) | 3 (1.7%) | 0.007 |
Data are presented as n (%), median (IQR) or mean (SD). CABG indicates coronary artery bypass graft; ICD, intracardiac defibrillator; IQR, interquartile range; MI, myocardial infarction; NSAID, nonsteroidal anti‐inflammatory drugs; PCI, percutaneous coronary intervention; SSRI, selective serotonin reuptake inhibitors.
As compared to patients with normal renal function (eGFR ≥60 mL/min per 1.73 m2), those within CKD were older. Across lower eGFR strata, there was a higher proportion of women and a more‐frequent history of hypertension and MI. Both the CHA2DS2‐VASC and HAS‐BLED scores were higher. Patients with lower eGFR categories more often used medications that could increase the risk of bleeding (eg, aspirin or a combined antiplatelet therapy; Table 1) or drugs known to interact with warfarin (ie, antibiotics).
eGFR Strata and TTR
TTR was poorer across lower eGFR strata (Figure 2, Table 2). This association remained after multivariable adjustment (Figure 3, Table 3). As shown in Table 3, patients with eGFR of 45 to 59 had mean predicted TTR of 79%, which, albeit significantly (P<0.05) lower than the reference category (eGFR ≥60), was only 1% higher (95% CI, 0–25). Patients with eGFR of 30 to 44 had mean predicted TTR of 77%, which was 1% lower (95% CI, −3 to −10; P=0.3) than the reference category. On the other hand, patients with an eGFR <30/dialysis had a mean predicted TTR of 68% (95% CI, 65–72), which was 10% lower than the reference category. The fully adjusted multivariable model is shown in Table 4. Other covariates independently associated with worse TTR were, besides eGFR strata, female sex (weak association), higher age (weak association), presence of diabetes mellitus, vascular disease, or heart failure, and concomitant use of aspirin (Table 4).
Figure 2.
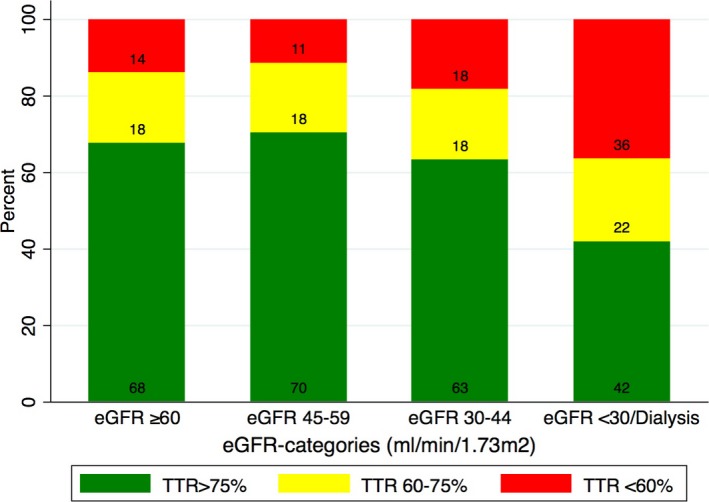
Proportion of patients in different time‐in‐therapeutic ranges (TTR) across worsening eGFR strata. eGFR indicates estimated glomerular filtration rate.
Table 2.
TTR Across eGFR Strata
| All | eGFR ≥60 | eGFR 45 to 59 | eGFR 30 to 44 | eGFR <30 or Dialysis | P Value | |
|---|---|---|---|---|---|---|
| N | 7738 | 5692 | 1353 | 512 | 181 | |
| TTR %, median (IQR) | 83 (71–92) | 83 (71–92) | 83 (72–91) | 82 (68–90) | 70 (50–82) | <0.001 |
| TTR %, mean (SD) | 78 (20) | 78 (20) | 79 (19) | 76 (21) | 66 (23) | <0.001 |
| TTR in categories | ||||||
| TTR >75%, n (%) | 5204 (67.3) | 3853 (67.7) | 951 (70.3) | 324 (63.3) | 76 (42.0) | <0.001 |
| TTR 60% to 75%, n (%) | 1423 (18.4) | 1042 (18.3) | 248 (18.3) | 94 (18.4) | 39 (21.5) | |
| TTR <60%, n (%) | 1111 (14.4) | 797 (14.0) | 154 (11.4) | 94 (18.4) | 66 (36.5) | |
| Number of INR measurements, median (IQR) | 21 (9–39) | 20 (9–38) | 25 (10–41) | 24 (11–42) | 21 (9–43) | <0.001 |
| Median (IQR) days on warfarin | 254 (91–691) | 244 (91–671) | 329 (99–708) | 287 (96–704) | 175 (57–589) | <0.001 |
| Median (IQR) days between INRs | 12 (8–17) | 12 (8–17) | 13 (8–17) | 12 (8–16) | 9 (5–14) | <0.001 |
| Percent (IQR) of INRs >3.0 | 11% (0–19) | 11 (0–19) | 12 (3.7–20) | 13 (5.8–21) | 14 (6.5–23) | <0.001 |
| Percent (IQR) of INRs <2.0 | 19% (9–31) | 18 (9–30) | 19 (10–30) | 20 (10–31) | 29 (18–43) | <0.001 |
IQR indicates interquartile range; INRs, international normalized ratios; TTR, time‐in‐therapeutic range.
Figure 3.
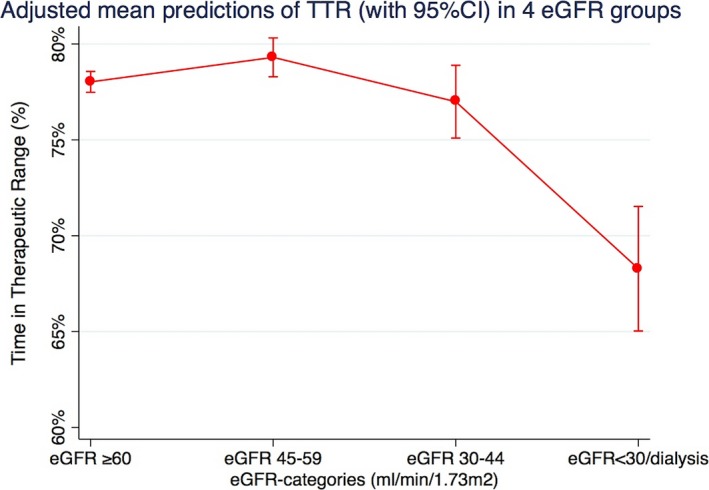
Adjusted mean predictions of time‐in‐therapeutic range (TTR) with 95% confidence intervals in 4 eGFR strata. Output from a multivariable fractional regression analysis including eGFR strata, age (in categories: <65, 65–74, 75–85, and ≥85 years), sex, diabetes mellitus, liver disease, hypertension, vascular disease, heart failure, valvular disease, amiodarone use, aspirin use, and cancer. eGFR indicates estimated glomerular filtration rate.
Table 3.
Predictors of TTR
| CKD Stagea (mL/min per 1.73 m2) | TTR (95% CI) | P Value | Change in TTR (95% CI) | P Value |
|---|---|---|---|---|
| ≥60 | 78% (77–79) | <0.001 | (Ref) | ··· |
| 45 to 59 | 79% (78–80) | <0.001 | 1% (0–25) | 0.033 |
| 30 to 44 | 77% (75–79) | <0.001 | −1% (−3 to 10) | 0.313 |
| <30 or dialysis | 68% (65–72) | <0.001 | −10% (−13 to −60) | <0.001 |
Simplified fractional regression analysis showing the mean predicted TTR across renal function categories and the relative change (in proportion) from the reference category.
Fractional regression analysis adjusted for: age (in categories: <65, 65–74, 75–85, ≥85 years), sex, diabetes mellitus, liver disease, hypertension, vascular disease (myocardial infarction, ischemic heart disease, peripheral arterial disease), heart failure, valvular disease, amiodarone use, aspirin use, and cancer. CKD indicates chronic kidney disease; TTR, time‐in‐therapeutic range.
Table 4.
Full Fractional Regression Analysis Showing the Coefficients (and 95% Confidence Intervals) of All Available Covariates Considered to Influence TTR
| Predictors of TTR | Coefficient (95% CI) | P Value |
|---|---|---|
| Renal function categories | ||
| eGFR ≥60 | Ref | |
| eGFR 45 to 59 | 7.6% (0.5–14.7) | 0.035 |
| eGFR 30 to 44 | −5.9% (−17.3 to 5.4) | 0.307 |
| eGFR <30 or dialysis | −50.1% (−65.5 to −34.6) | <0.001 |
| Age, y | ||
| Age <65 | Ref | |
| Age 65 to 74 | 7.7% (0.6–14.7) | 0.034 |
| Age 75 to 84 | 3.1% (−4.2 to 10.5) | 0.404 |
| Age ≥85 | −2.4% (−13.3 to 8.6) | 0.671 |
| Women | −5.3% (−10.8 to 1.8) | 0.058 |
| Diabetes mellitus | −13.2% (−20.6 to − 5.8) | <0.001 |
| Liver disease | −21.9% (−66.0 to 22.2) | 0.330 |
| Hypertension | 1.5% (−4.0 to 7.0) | 0.596 |
| Vascular disease (past MI, ischemic heart disease, peripheral arterial disease) | −10.5 (−18.5 to −2.5) | 0.010 |
| Heart failure | −17.1% (−26.4 to −7.7) | <0.001 |
| Valvular disease | 4.4% (−19.3 to 28.0) | 0.717 |
| Amiodarone | −35.9% (−111 to 39.6) | 0.351 |
| Aspirin | 6.3% (5.4–11.9) | 0.032 |
| Cancer within last 3 years | −4.9% (−12.8 to 3.0) | 0.226 |
eGFR indicates estimated glomerular filtration rate; MI, myocardial infarction; TTR, time‐in‐therapeutic range.
TTR, eGFR Strata, and Risk of Adverse Outcomes
A total of 402 (5.1%) adverse events occurred during 254 days (IQR, 91–691; Table 5). The most common adverse event was death (2.6%), followed by ischemic stroke (1.7%), ICH (0.5%), and MI (0.4%). In adjusted logistic regression analyses, both renal function and TTR were independently associated with the odds of adverse events (Table 6). The association between TTR and adverse events was not modified by differing eGFR (P for interaction, 0.169). Patients with TTR 60% to 75% (odds ratio [OR], 1.84; 95% CI, 1.41–2.40) and TTR <60% (OR, 2.09; CI, 1.59–2.74) had higher odds of adverse events than patients with TTR >75%.
Table 5.
Proportion of Survivors as Well as Single and Composite Study Outcomes Across eGFR Strata
| eGFR ≥60 | eGFR 45 to 59 | eGFR 30 to 44 | eGFR <30 or Dialysis | P Value | |
|---|---|---|---|---|---|
| N | 5692 | 1353 | 512 | 181 | |
| Single endpoints | |||||
| ICH | 26 (0.5%) | 9 (0.7%) | 2 (0.4%) | 2 (1.1%) | <0.001 |
| Ischemic stroke | 86 (1.5%) | 31 (2.3%) | 11 (2.2%) | 5 (2.8%) | |
| MI | 12 (0.2%) | 10 (0.7%) | 9 (1.8%) | 1 (0.6%) | |
| Death | 102 (1.8%) | 42 (3.1%) | 33 (6.5%) | 21 (11.6%) | |
| Combined endpoint | |||||
| ICH/ischemic stroke/MI/death | 226 (4.0%) | 92 (6.8%) | 55 (10.7%) | 29 (16.0%) | <0.001 |
Data presented as n (%). eGFR indicates estimated glomerular filtration rate; ICH, intractranial hemorrhage; MI, myocardial infarction.
Table 6.
Multivariable Logistic Regression of Factors Associated With the Composite Endpoint of ICH, Ischemic Stroke, MI, and Death (n=7738) for All Included Patients
| OR (95% CI) | P Value | |
|---|---|---|
| Renal function, mL/min per 1.73 m2 | ||
| eGFR ≥60 | 1.0 (ref) | |
| eGFR 45 to 59 | 1.32 (1.01–1.72) | 0.045 |
| eGFR 30 to 44 | 1.54 (1.09–2.18) | 0.025 |
| eGFR <30, or dialysis | 1.70 (1.06–2.72) | 0.27 |
| TTR | ||
| TTR >75% | 1.0 (ref) | |
| TTR 60% to 75% | 1.84 (1.41–2.40) | <0.001 |
| TTR <60% | 2.09 (1.59–2.74) | <0.001 |
| Age, y | ||
| <65 | 1.0 (ref) | |
| 65 to 74 | 2.03 (1.41–2.92) | <0.001 |
| 75 to 84 | 3.40 (2.38–4.87) | <0.001 |
| ≥85 | 3.14 (2.02–4.89) | <0.001 |
| Female | 0.92 (0.74–1.14) | 0.427 |
| Diabetes mellitus | 1.08 (0.82–1.43) | 0.574 |
| Hypertension | 0.96 (0.77–1.21) | 0.740 |
| Vascular disease (past MI, ischemic heart disease, or peripheral arterial disease) | 1.40 (1.06–1.83) | 0.017 |
| Heart failure | 1.78 (1.33–2.36) | <0.001 |
| Valvular disease | 1.27 (0.59–2.75) | 0.542 |
| Cancer within last 3 years | 0.85 (0.62–1.15) | 0.292 |
| Coagulation/platelet defect | 3.06 (1.33–7.07) | 0.009 |
| Anemia | 1.10 (0.71–1.69) | 0.681 |
| Ischemic stroke | 0.71 (0.43–1.20) | 0.200 |
| Past systemic emboli | 1.70 (1.13–2.54) | 0.010 |
| Deep vein thrombosis/pulmonary embolism | 1.51 (1.05–2.16) | 0.025 |
| Past ICH | 1.12 (0.29–4.35) | 0.865 |
| Past gastrointestinal bleeding | 1.37 (0.70–2.68) | 0.356 |
| Antiplatelet therapy | 1.07 (0.85–1.35) | 0.543 |
| No. of INR measurements | 1.03 (1.02–1.04) | <0.001 |
| No. of days on warfarin | 1.00 (0.99–1.00) | <0.001 |
P values for the interaction terms for the outcome is presented below the table. Interaction terms tested: age and eGFR: P=0.398; age and TTR, P=0.721; eGFR and TTR, P=0.169. eGFR indicates estimated glomerular filtration rate; ICH, intractranial hemorrhage; INR, international normalized ratio; MI, myocardial infarction; TTR, time‐in‐therapeutic range.
Sensitivity Analyses
There were 7577 (98%) event‐free patients during the first 3 months of warfarin therapy (Figure 1). Survival is graphically displayed after the first 3 months according to TTR strata (Figure S1) and in relation to renal function (Figure S2). We estimated TTR from the first 3 months of INR measurement (Table S5) and modeled time to event from month 3 onward by Cox proportional models without censoring at warfarin cessation. During follow‐up, 683 patients (9.0%) had an event (Table S6). In adjusted Cox regression analysis (Table S7), both a lower TTR and a lower renal function predicted adverse outcomes, with no interaction terms (P for interaction=0.8). Patients with TTR 60% to 75% (hazard ratio [HR], 1.52; CI, 1.25–1.83) and with TTR <60% (HR, 1.89; CI, 1.58–2.25) had a 52% and 89% higher risk of adverse events, respectively, as compared with a TTR >75%.
Discussion
This study shows a clinically relevant association between renal dysfunction and poor TTR among new AF patients on warfarin. An adequate TTR was less frequently achieved in CKD patients, especially among those with severe CKD. This study also shows that fewer adverse events are observed in patients with adequate TTR, irrespective of underlying renal function.
TTR is a measure of long‐term INR control, which is frequently used in clinical trials and recommended by current National Institute for Health and Care Excellence guidelines.17 However, we acknowledge that it is probably still rarely used in clinical practice. TTR gives a percentage of time of the treatment period that the INR was therapeutic, but it does not tell whether values were sub‐ or supratherapeutic. Adverse events are closely related to achieved TTR, with an optimal threshold of TTR somewhere above 58% to 65%.2, 17, 18, 19, 20 In our study, the observed TTR was exceptionally high, in accord with Sweden's renowned good INR control (with a mean over 75% in several randomized, controlled, clinical trials18, 19). Yet, our study did observe that despite extensive adjustment for confounders, those with eGFR <30/dialysis had a clinically worse TTR. The reasons behind the worse TTR in CKD patients cannot be inferred from our observational design, but may be attributed to renal function per se, as well as factors/conditions associated with CKD. It is notable that patients with severe CKD had more‐frequent INR measurements, possibly attributed to difficulties in achieving optimal INR, more‐frequent therapy discontinuations attributed to procedures/intervention, or by the more‐frequent use of drugs known to interact with warfarin. Our study expands to a real‐life North European setting the series of studies from Limdi et al, showing, in the US Warfarin Pharmacogenetics Cohort, that patients with CKD not requiring dialysis require lower warfarin doses, more often had supratherapeutic INRs (INR ≥4), and have a higher risk of hemorrhage, as compared to patients with normal kidney function.7, 21, 22, 23 The difficulty of CKD patients in keeping optimal INR was also reported by Quinn et al24 in 46 US dialysis patients with weekly INR measurements and an achieved mean TTR of 49.2%.
There is strong evidence that the risk of ischemic stroke caused by AF can be substantially reduced with adequate warfarin therapy. Subtherapeutic INR (below 2.0) increases the risk of ischemic stroke, and supratherapeutic INR (above 3.0 and particularly above 4.0) sharply increases the risk of intracranial bleeding.25 A recent study indicated that ICH risk associated with INR ≥4.0 increased by several fold in individuals with advanced CKD.7 In most reports, as well as in our study, subtherapeutic INRs (19% of measurements) were more common than supratherapeutic ones (11%). We speculated that poor TTR may, in part, explain the worse outcome and higher bleeding rate described in observational studies of CKD patients on warfarin, particularly among those undergoing dialysis.26, 27 We observed no interaction between TTR and eGFR and outcome in our study, suggesting that both factors affect outcome independently of each other, and that adequate TTR reduces the adverse event risk also in patients with advanced CKD/dialysis. Despite being the largest study of its kind, we could only identify 11 patients on dialysis satisfying inclusion criteria, and we are therefore underpowered to report TTR‐associated outcomes in this particular population. However, our findings accord with an earlier small, retrospective study indicating that no dialysis patient with adequate INR control had a stroke or a fatal bleeding event.28 Further, Kooiman et al29 observed that both less time spent within therapeutic range and high INR variability were factors associated with increased risk of stroke and bleeding in warfarin‐treated CKD patients. Within our study design, we were concerned that patients who were critically ill/moribund would be taken off warfarin and died shortly after warfarin discontinuation. For that reason, our sensitivity analysis estimated TTR on the first 3 months and analyzed outcome risk emulating an “intention to treat” design. The fact that results were comparable to our main analysis provides robustness to our conclusions.
Strengths of this study are the large real‐life cohort with information on INR control and eGFR. In addition, the inclusion of newly diagnosed AF patients with complete information on warfarin therapy and outcomes provides more‐unbiased associations. However, this study also has limitations: Our analysis is based on repeated warfarin dispensations, but we lack information on short‐term therapy discontinuations or indications for it. This probably would have prompted the physician to order more INR measurements during that period of time, but likely in the long term would have less effect on TTR. Finally, we only accounted for comorbidities and drugs interacting with warfarin at index date, but not during follow‐up. In the multivariable fractional regression analysis, we have included all available variables. However, there could still be residual confounding, given that unmeasured factors associated with a worse TTR are not accounted for. However, it is unknown whether renal function is associated with additional harmful factors.
Conclusion
In real‐life newly diagnosed AF patients on warfarin, those with eGFR <30/dialysis have a significantly worse INR control. An optimal TTR (>75%) is associated with lower risk of adverse events, independently of underlying renal function. Identifying the reasons behind, and applying more‐stringent efforts to improve, the TTR of these patients is necessary to ensure warfarin's net clinical benefit.
Sources of Funding
This study was funded by The Swedish Society of Medicine (Svenska Läkaresällskapet), the Swedish Heart and Lung Foundation, the Stockholm County Council (ALF project), and the Martin Rind and Westman Foundations. Furthermore, the Stockholm County Council funded the clinical postdoctoral positions of Szummer and Evans.
Disclosures
Szummer has received lecture honoraria from AstraZeneca, Aspen, and St Jude Medical. Friberg has received lecture honoraria/research funding/aced as a consultant for Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Pfizer, Sanofi, and St Jude. The remaining authors have no disclosures to report.
Supporting information
Table S1. Definition of Comorbidities
Table S2. Medication Collected From the Pharmacy Obtained From the Swedish Drug Prescription Registry
Table S3. Definition of CHA2DS2‐VASc and HAS‐BLED Score
Table S4. Definition of Events During Follow‐up
Table S5. Sensitivity Analysis (TTR Based on First 90 Days): Time‐in‐Therapeutic Range (TTR) in the Different Renal Function Categories
Table S6. Sensitivity Analysis (TTR Based on First 90 Days): Proportion of Single and Composite Study Outcomes Across eGFR Strata
Table S7. Sensitivity Analysis (TTR Based on First 90 Days): Multivariable Cox Regression Analysis of Factors Associated With the Composite Endpoint of Intracranial Hemorrhage, Ischemic Stroke, Myocardial Infarction, and Death (n=7577)
Figure S1. Sensitivity analysis: association of TTR and renal function to outcome. Kaplan–Meier curve: time‐in‐therapeutic range and association to outcome. Outcome is a composite of intracranial hemorrhage/ischemic stroke/myocardial infarction/death.
Figure S2. Kaplan–Meier curve for combined eGFR and TTR groups.
(J Am Heart Assoc. 2017;6:e004925. DOI: 10.1161/JAHA.116.004925.)
References
- 1. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; Guidelines ESCCfP . 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S; Investigators AW . Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–2037. [DOI] [PubMed] [Google Scholar]
- 3. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe‐TT(2)R(2) score. Chest. 2013;144:1555–1563. [DOI] [PubMed] [Google Scholar]
- 4. Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE. Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA. 1998;279:657–662. [DOI] [PubMed] [Google Scholar]
- 5. Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, Lindhardsen J, Gislason GH, Torp‐Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–635. [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L, Singer DE; Investigators AS . Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Limdi NA, Nolin TD, Booth SL, Centi A, Marques MB, Crowley MR, Allon M, Beasley TM. Influence of kidney function on risk of supratherapeutic international normalized ratio‐related hemorrhage in warfarin users: a prospective cohort study. Am J Kidney Dis. 2015;65:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen JI, Montez‐Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Runesson B, Gasparini A, Qureshi AR, Norin O, Evans M, Barany P, Wettermark B, Elinder CG, Carrero JJ. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J. 2016;9:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. [DOI] [PubMed] [Google Scholar]
- 11. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 12. Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS. Comparative performance of the CKD Epidemiology Collaboration (CKD‐EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 14. Friberg L, Skeppholm M. Usefulness of Health Registers for detection of bleeding events in outcome studies. Thromb Haemost. 2016;116:1131–1139. [DOI] [PubMed] [Google Scholar]
- 15. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335:540–546. [DOI] [PubMed] [Google Scholar]
- 17. Guidelines N . NICE guidelines 2014: management. 2014.
- 18. Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, Pogue J, Reilly PA, Yang S, Connolly SJ; Investigators R‐L Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983. [DOI] [PubMed] [Google Scholar]
- 19. Wallentin L, Lopes RD, Hanna M, Thomas L, Hellkamp A, Nepal S, Hylek EM, Al‐Khatib SM, Alexander JH, Alings M, Amerena J, Ansell J, Aylward P, Bartunek J, Commerford P, De Caterina R, Erol C, Harjola VP, Held C, Horowitz JD, Huber K, Husted S, Keltai M, Lanas F, Lisheng L, McMurray JJ, Oh BH, Rosenqvist M, Ruzyllo W, Steg PG, Vinereanu D, Xavier D, Granger CB; Apixaban for Reduction in S and Other Thromboembolic Events in Atrial Fibrillation I . Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127:2166–2176. [DOI] [PubMed] [Google Scholar]
- 20. Piccini JP, Hellkamp AS, Lokhnygina Y, Patel MR, Harrell FE, Singer DE, Becker RC, Breithardt G, Halperin JL, Hankey GJ, Berkowitz SD, Nessel CC, Mahaffey KW, Fox KA, Califf RM; Investigators RA . Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trial. J Am Heart Assoc. 2014;3:e000521 DOI: 10.1161/JAHA.113.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Limdi MA, Crowley MR, Beasley TM, Limdi NA, Allon M. Influence of kidney function on risk of hemorrhage among patients taking warfarin: a cohort study. Am J Kidney Dis. 2013;61:354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Limdi NA, Beasley TM, Baird MF, Goldstein JA, McGwin G, Arnett DK, Acton RT, Allon M. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol. 2009;20:912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Limdi NA, Limdi MA, Cavallari L, Anderson AM, Crowley MR, Baird MF, Allon M, Beasley TM. Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis. 2010;56:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quinn LM, Richardson R, Cameron KJ, Battistella M. Evaluating time in therapeutic range for hemodialysis patients taking warfarin. Clin Nephrol. 2015;83:80–85. [DOI] [PubMed] [Google Scholar]
- 25. Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. [DOI] [PubMed] [Google Scholar]
- 26. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knoll F, Sturm G, Lamina C, Zitt E, Lins F, Freistatter O, Kronenberg F, Lhotta K, Neyer U. Coumarins and survival in incident dialysis patients. Nephrol Dial Transplant. 2012;27:332–337. [DOI] [PubMed] [Google Scholar]
- 29. Kooiman J, van Rein N, Spaans B, van Beers KA, Bank JR, van de Peppel WR, del Sol AI, Cannegieter SC, Rabelink TJ, Lip GY, Klok FA, Huisman MV. Efficacy and safety of vitamin K‐antagonists (VKA) for atrial fibrillation in non‐dialysis dependent chronic kidney disease. PLoS One. 2014;9:e94420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of Comorbidities
Table S2. Medication Collected From the Pharmacy Obtained From the Swedish Drug Prescription Registry
Table S3. Definition of CHA2DS2‐VASc and HAS‐BLED Score
Table S4. Definition of Events During Follow‐up
Table S5. Sensitivity Analysis (TTR Based on First 90 Days): Time‐in‐Therapeutic Range (TTR) in the Different Renal Function Categories
Table S6. Sensitivity Analysis (TTR Based on First 90 Days): Proportion of Single and Composite Study Outcomes Across eGFR Strata
Table S7. Sensitivity Analysis (TTR Based on First 90 Days): Multivariable Cox Regression Analysis of Factors Associated With the Composite Endpoint of Intracranial Hemorrhage, Ischemic Stroke, Myocardial Infarction, and Death (n=7577)
Figure S1. Sensitivity analysis: association of TTR and renal function to outcome. Kaplan–Meier curve: time‐in‐therapeutic range and association to outcome. Outcome is a composite of intracranial hemorrhage/ischemic stroke/myocardial infarction/death.
Figure S2. Kaplan–Meier curve for combined eGFR and TTR groups.


