Abstract
Background
Treatment with renin‐angiotensin system (RAS) inhibitors might restrain the structural/electrical remodeling associated with atrial fibrillation (AF). Limited evidence exists regarding the potential benefits of RAS inhibition post‐acute myocardial infarction (AMI) in patients with AF. This study sought to assess the association between RAS inhibition and all‐cause mortality and new‐onset AF in patients with/without congestive heart failure (CHF) post‐AMI.
Methods and Results
Patients hospitalized for AMI between 2006 and 2012 were identified in Swedish registries. Patients were stratified in 4 subgroups; patients with CHF and AF (n=11 489); patients with CHF without AF (n=31 676); patients with AF without CHF (n=10 066); and patients without both CHF and AF (n=59 417). Patients exposed to RAS inhibition were compared to nontreated. Three‐year risk of all‐cause mortality and new‐onset AF was assessed using adjusted Cox regression analyses. At discharge, 83 291 (73.9%) patients received RAS inhibition. RAS inhibition was associated with lower 3‐year risk of all‐cause mortality in CHF patients with AF, adjusted hazard ratio (HR) with 95% CI 0.75 (0.70–0.81), CHF patients without AF, HR 0.65 (0.60–0.69), AF patients without CHF, HR 0.82 (0.75–0.90), and in patients without CHF and AF, HR 0.76 (0.72–0.81), respectively. RAS inhibition was not associated with lower 3‐year risk of new‐onset AF in patients without AF but with/without CHF; HR 0.96 (0.84–1.10) and 1.12 (1.02–1.22), respectively.
Conclusions
RAS inhibition post‐AMI was associated with lower risk of all‐cause mortality. In patients with/without CHF, RAS inhibition was not associated with lower incidence of new‐onset AF.
Keywords: atrial fibrillation, myocardial infarction
Subject Categories: Acute Coronary Syndromes, Arrhythmias, ACE/Angiotension Receptors/Renin Angiotensin System
Introduction
Inhibition of the renin‐angiotensin system (RAS) with angiotensin‐converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) have been shown to reduce the risk of death, reinfarction, and hospitalization for congestive heart failure (CHF) post‐acute myocardial infarction (AMI) in patients with CHF and/or left ventricular dysfunction.1, 2, 3, 4, 5 Also, ACEIs and ARBs have, in previous studies, shown to have antiarrhythmic properties by restraining the structural and electrical remodeling associated with atrial fibrillation (AF).6, 7, 8, 9, 10
Current non‐ST‐segment elevation myocardial infarction (NSTEMI) guidelines from the United States11 and Europe12 recommend the use of RAS inhibitors in patients with CHF, left ventricular dysfunction, and in those with hypertension or diabetes mellitus. Also, the American guidelines suggest that such therapy might be reasonable for all other patients with cardiac or vascular disease. ARBs are stated as a noninferior alternative in ACEI‐intolerant patients. Similarly, the ST‐segment elevation myocardial infarction (STEMI) guidelines from the United States13 and Europe14 state that ACEIs or ARBs are recommended for all patients with CHF and left ventricular dysfunction, and that it is reasonable for all other patients without contraindications. AF guidelines from the United States15 and Europe16 recommend RAS inhibition as a reasonable upstream therapy that might be considered in patients with CHF and left ventricular dysfunction to prevent new‐onset AF. At the same time, these guidelines stress that RAS inhibition is not beneficial for primary prevention of new‐onset AF in patients without cardiovascular disease.
It is still unclear whether or not ACEIs and ARBs are beneficial for patients with AF post‐AMI. Moreover, it is uncertain whether ACEIs and ARBs are effective upstream therapies for prevention of new‐onset AF post‐AMI. With this study we sought to answer the following questions: (1) Is RAS inhibition associated with lower risk of all‐cause mortality, cardiovascular mortality, recurrent AMI, and stroke in patients with and without CHF and/or AF post‐AMI? (2) In patients with no history or in‐hospital diagnosis of AF post‐AMI, is RAS inhibition associated with reduced risk of new‐onset AF?
Methods
Study Population
This is a retrospective, observational cohort study including all patients admitted to all coronary care units in Sweden (n=72). Data in this study were obtained from the Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry.17 SWEDEHEART is nationwide and includes all consecutive patients admitted to coronary care units because of symptoms indicative of acute coronary syndrome. Patients included in the registry do not provide written consent, but are informed about their inclusion in the registry and have the possibility to opt out. In the present study, patients aged above 18 years discharged alive after their first AMI between 2006 and 2012 were included. Patients with no history of CHF and with missing data regarding left ventricular ejection fraction (LVEF) were excluded (see Figure 1). The last follow‐up date for included patients was on December 31, 2013.
Figure 1.
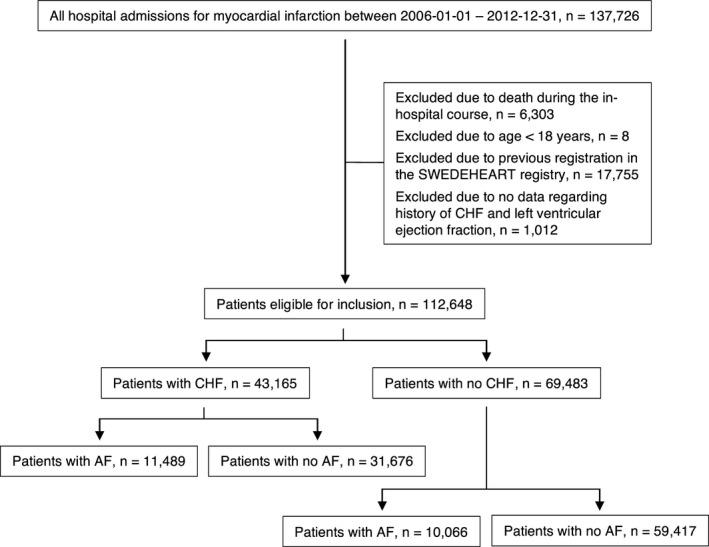
Selection of study population. AF indicates atrial fibrillation; CHF, congestive heart failure; SWEDEHEART, Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies.
Data Collection and Data Linkage With Other Registries
Information about comorbidities at arrival, patient presentation at admission, hospital course, and medication at arrival and discharge was obtained from SWEDEHEART and was enriched with data from the National Patient Registry and the National Dispensed Drug Registry. The National Patient Registry, which has previously been shown to have high validity, is a mandatory registry for all hospitals in Sweden and includes diagnoses for all patients seeking hospital care since 1987.18 The National Dispensed Drug Registry includes data about all prescribed drug purchases in Sweden, including anatomical therapeutic chemical classification codes, dates, dosages, and quantities for every prescription since July 2005.19 The data linkage was approved and performed by the National Board of Health and Welfare in Sweden using the unique 10‐digit civic registration number. The ethics committee at Karolinska Institute, Stockholm, approved the study protocol. See Table S1 for more details regarding data collection, data linkage, and variable definition.
Definition of Exposure
The main exposure was treatment with ACEI and/or ARB, either at hospital discharge as registered in the SWEDEHEART registry, or if an ACEI or ARB was dispensed within 7 days from discharge according to the National Dispensed Drug Registry. For ACEI and ARB, the duration of treatment after discharge was estimated to last 3 months. Thereafter, each drug purchase from the pharmacy within 6 months, as captured in the National Dispensed Drug Registry, resulted in the patient being considered exposed to the medication for an additional 6 months. An interruption gap of 6 months or above resulted in an assumption of drug discontinuation and a censoring from the on‐treatment analysis. In accord with this, a drug dispense of ACEI or ARB in a previously unexposed patient resulted in censoring from the on‐treatment analysis. Ongoing treatment with ACEI or ARB before index AMI was based on data entered in SWEDEHEART at hospital arrival, or if an ACEI or ARB was dispensed within 6 months before admission.
Definition of Outcomes
The efficacy outcome in this study was all‐cause mortality. Additional outcomes included cardiovascular mortality, recurrent AMI, and stroke (ischemic or hemorrhagic) within 3 years from discharge. Also, the risk of new‐onset AF was estimated in a subgroup of patients with no history of AF before discharge. Data on outcome were based on observations in the National Patient Registry and in the National Cause of Death Registry (see Table S2). When analyzing outcome, patients were censored after 3 years from discharge or at the end of follow‐up. For all‐cause mortality, no other censoring scheme was applied. For cardiovascular mortality, recurrent AMI, stroke, and new‐onset AF, patients were also censored for all‐cause mortality.
Statistical Analyses
Statistical analyses were performed on all patients meeting the inclusion criteria. Results were described according to treatment with ACEI and/or ARB versus no such treatment, with follow‐up started at the time of discharge. Patients were stratified and analyzed in 4 different subgroups; patients with CHF and AF; patients with CHF without AF; patients without CHF and with AF; and patients without both CHF and AF. Demographic and baseline characteristics were reported using percentages for categorical variables and with median and interquartile range (IQR) for continuous variables. Categorical variables were compared using Pearson's χ2 test, and continuous variables were compared using the Wilcoxon signed‐rank test. The association between drug treatment and outcome was studied in an on‐treatment analysis. Kaplan–Meier survival plots were used to illustrate outcome in relation to drug treatment. Unadjusted and adjusted Cox proportional hazards models were reported using hazard ratios (HR) with 95% confidence interval (CI). In the adjusted model, potential clinical cofounders were accounted for and included: age (3‐knot restricted cubic spline); sex; diabetes mellitus; hypertension; peripheral vascular disease; previous stroke; type of infarction (STEMI or NSTEMI); Killip class on admission; new‐onset AF during hospital course; LVEF; creatinine level (3‐knot restricted cubic spline); discharge hospital (as a Γ distributed random frailty effect); percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) during the in‐hospital course; and concomitant medication at discharge (oral anticoagulants, aspirin, P2Y12‐inhibitors, β‐blockers, diuretics, digoxin, and statins). Tests for interactions were performed to determine whether the association of ACEI and/or ARB treatment with outcome was similar in patients with AF and those without AF and were calculated using z‐tests based on the results from the two separate cohorts in the Cox proportional hazards models. For missing data, multiple imputation with chained equations was performed, generating 25 imputed data sets using the above adjustment variables, including outcome, as predictors.20
Four sensitivity analyses were conducted. In the first sensitivity analysis, an intention‐to‐treat analysis was performed in which drug treatment with ACEI and/or ARB at discharge was accounted for, censoring only at the end of follow‐up or for mortality. Second, a complete case analysis was performed excluding patients with any missing data (n=75 547). Third, a propensity‐score–matched analysis was conducted in which propensity scores were estimated using random‐effects logistic regression models with ACEI and/or ARB at discharge as outcome and all other variables above as explanatory variables. In the fourth sensitivity analysis, ACEI and ARB were analyzed separately. Finally, we also assessed for differences in the following subgroups; age ≤75 years versus age >75 years; NSTEMI versus STEMI; and hypertension versus no hypertension.
All analyses were performed using R statistics software (version 3.1.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population and Baseline Characteristics
In this study, all consecutive patients admitted because of a first AMI between 2006 and 2012 in all coronary care units in Sweden were included (n=137 726). A proportion of patients were excluded from the final study population because of in‐hospital mortality (n=6303), age under 18 years (n=8), previous registration in SWEDEHEART (n=17 755), and because of missing data regarding both CHF and LVEF (n=1012). After all exclusions, a total of 112 648 patients remained (see Figure 1).
The study population had a median age of 72 years, and 35.5% were women. At discharge, a total of 83 291 (73.9%) were treated with ACEI and/or ARB. Table 1 shows the characteristics of the patients included stratified by use of ACEI and/or ARB. Compared to patients not discharged on treatment with RAS inhibition, those receiving such treatment were more often male and more likely to have a history of diabetes mellitus, hypertension, AMI, previous PCI, and CABG. Patients discharged on RAS inhibition were more likely to have an STEMI, left ventricular dysfunction, or a discharge diagnosis of CHF. Also, patients on ACEI and/or ARB more often underwent PCI during the index hospitalization. At discharge, patients on RAS inhibition were more often prescribed aspirin, P2Y12 inhibitors, oral anticoagulants, β‐blockers, calcium‐channel blockers, diuretics, and statins.
Table 1.
Baseline Table
| All Patients (n=112 648) | No ACEI/ARB (n=29 357) | ACEI/ARB (n=83 291) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR), y | 72 (62–81) | 74 (62–83) | 71 (62–79) | <0.001 |
| Sex, women | 39 987 (35.5) | 11 729 (40.0) | 28 258 (33.9) | <0.001 |
| Smoking, n=106 886 | 25 714 (24.1) | 6451 (23.8) | 19 263 (24.1) | <0.001 |
| Body mass index, median (IQR), n=97 204 | 26 (24–29) | 25 (23–28) | 27 (24–29) | <0.001 |
| Admission year | <0.001 | |||
| 2006 | 16 949 (15.0) | 5783 (19.7) | 11 166 (13.4) | |
| 2007 | 17 264 (15.3) | 5177 (17.6) | 12 087 (14.5) | |
| 2008 | 16 311 (14.5) | 4453 (15.2) | 11 858 (14.2) | |
| 2009 | 15 387 (13.7) | 3877 (13.2) | 11 510 (13.8) | |
| 2010 | 15 466 (13.7) | 3469 (11.8) | 11 997 (14.4) | |
| 2011 | 15 616 (13.9) | 3287 (11.2) | 12 329 (14.8) | |
| 2012 | 15 655 (13.9) | 3311 (11.3) | 12 344 (14.8) | |
| Comorbidities and presentation at admission | ||||
| Diabetes mellitus | 27 149 (24.1) | 4959 (16.9) | 22 190 (26.6) | <0.001 |
| Hypertension | 67 436 (59.9) | 13 571 (46.2) | 53 865 (64.7) | <0.001 |
| MI | 29 330 (26.0) | 7087 (24.1) | 22 243 (26.7) | <0.001 |
| CHF | 12 039 (10.7) | 2903 (9.9) | 9136 (11.0) | <0.001 |
| Peripheral vascular disease | 6143 (5.5) | 1618 (5.5) | 4525 (5.4) | 0.61 |
| Stroke | 13 038 (11.6) | 3618 (12.3) | 9420 (11.3) | <0.001 |
| Any bleeding | 7390 (6.6) | 2174 (7.4) | 5216 (6.3) | <0.001 |
| Chronic obstructive pulmonary disease | 7855 (7.0) | 2262 (7.7) | 5593 (6.7) | <0.001 |
| Dementia | 616 (0.5) | 282 (1.0) | 334 (0.4) | <0.001 |
| Cancer diagnosis within 3 years | 3160 (2.8) | 1033 (3.5) | 2127 (2.6) | <0.001 |
| Previous PCI | 12 727 (11.3) | 2667 (9.1) | 10 060 (12.1) | <0.001 |
| Previous CABG | 9461 (8.4) | 2100 (7.2) | 7361 (8.8) | <0.001 |
| Hospital course | ||||
| STEMI | 36 472 (32.4) | 6949 (23.7) | 29 523 (35.4) | <0.001 |
| PCI | 63 821 (56.7) | 12 621 (43.0) | 51 200 (61.5) | <0.001 |
| CABG | 3935 (3.5) | 1181 (4.0) | 2754 (3.3) | <0.001 |
| Creatinine, median (IQR), μmol/L, n=110 143 | 83 (70–102) | 84 (70–106) | 83 (70–100) | <0.001 |
| Decompensated heart failure (Killip ≥II), n=108 078 | 15 524 (14.4) | 3886 (13.8) | 11 638 (14.6) | 0.003 |
| LVEF <50%, n=82 026 | 35 679 (43.5) | 4924 (27.1) | 30 755 (48.2) | <0.001 |
| CHF (including history of) | 43 165 (38.3) | 7136 (24.3) | 36 029 (43.3) | <0.001 |
| New‐onset AF | 9853 (8.9) | 2635 (9.2) | 7218 (8.9) | 0.08 |
| AF (including history of) | 21 555 (19.1) | 5936 (20.2) | 15 619 (18.8) | <0.001 |
| Discharge medication | ||||
| Aspirin | 104 283 (92.8) | 26 459 (90.5) | 77 824 (93.6) | <0.001 |
| P2Y12 inhibitors | 86 889 (77.2) | 19 770 (67.6) | 67 119 (80.6) | <0.001 |
| Oral anticoagulants | 7921 (7.0) | 1655 (5.7) | 6266 (7.5) | <0.001 |
| β‐blockers | 100 285 (89.1) | 24 537 (83.8) | 75 748 (91.0) | <0.001 |
| Calcium‐channel blockers | 17 711 (15.7) | 4327 (14.8) | 13 384 (16.1) | <0.001 |
| Digoxin | 3613 (3.2) | 1016 (3.5) | 2597 (3.1) | 0.003 |
| Diuretics | 37 220 (33.1) | 8834 (30.2) | 28 386 (34.1) | <0.001 |
| Nitrates | 19 301 (17.2) | 5874 (20.1) | 13 427 (16.2) | <0.001 |
| Statins | 94 871 (84.3) | 21 382 (73.1) | 73 489 (88.3) | <0.001 |
Patient characteristics, admission year, and clinical and in‐hospital characteristics of acute myocardial infarction patients in relation to treatment with ACEI and/or ARB at discharge. Demographic and baseline characteristics were reported using percentages for categorical variables or with median and IQR for continuous variables (as noted). ACEI indicates angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; CABG, coronary artery bypass grafting; CHF, congestive heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
All‐Cause Mortality, Cardiovascular Mortality, Recurrent AMI, and Stroke
A total of 17 121 patients died within 3 years from discharge. Figure 2 illustrates unadjusted Kaplan–Meier curves for all‐cause mortality within 3 years postdischarge. For all‐cause mortality, the cumulative incidence rate per 100 person years for patients treated with ACEI/ARB versus nontreated was 18.8 and 9.0, respectively. In patients with CHF at baseline, treatment with RAS inhibition was associated with a lower 3‐year risk of all‐cause mortality, both in patients with a diagnosis of AF and in those with no diagnosis of AF, adjusted HR with 95% CI 0.75 (0.70–0.81) and 0.65 (0.60–0.69), respectively. Similar associations were observed in patients with no diagnosis of CHF, with or without AF at baseline, adjusted HR 0.82 (0.75–0.90) and 0.76 (0.72–0.81), respectively.
Figure 2.
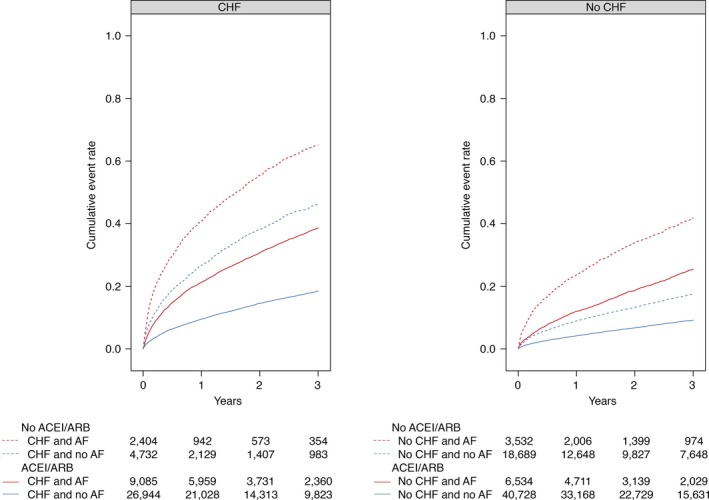
Kaplan–Meier plots depicting outcome for patients with and without CHF and AF. Kaplan–Meier plots depicting the cumulative incidence curve for all‐cause mortality stratified by use of ACEI and/or ARB. ACEI indicates angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; CHF, congestive heart failure.
The unadjusted event rate of 3‐year cardiovascular mortality is presented in Table 2. After adjustment for confounding factors, treatment with ACEI/ARB was associated with a lower risk of cardiovascular mortality, HR 0.81 (0.78–0.85). Similar findings were observed in patients with CHF at baseline. For patients with no diagnosis of CHF and with AF at baseline, there was a borderline trend toward lower cardiovascular mortality after adjustments, HR 0.91 (0.82–1.02). For patients without both CHF and AF, there was a significant association with reduced cardiovascular mortality, adjusted HR 0.91 (0.84–0.99).
Table 2.
Outcome in Relation to Treatment
| Outcomes, No. of Events/Person Time in units of 100 years (Event Rate) | All Patients (n=112 648) | Stratification | |||||
|---|---|---|---|---|---|---|---|
| CHF and AF (n=11 489) | CHF and No AF (n=31 676) | P Value for Interaction | No CHF and AF (n=10 066) | No CHF and No AF (n=59 417) | P Value for Interaction | ||
| All‐cause mortality | 17 121/2121.4 (8.1) | 3964/176.1 (22.5) | 5474/590.7 (9.3) | ··· | 2383/173.5 (13.7) | 5300/1181.1 (4.5) | ··· |
| No ACEI/ARB | 6115/477.9 (12.8) | 1134/25.3 (44.9) | 1450/57.1 (25.4) | ··· | 1119/53.2 (21.0) | 2412/342.3 (7.0) | ··· |
| ACEI/ARB | 11 006/1643.5 (6.7) | 2830/150.8 (18.8) | 4024/533.6 (7.5) | ··· | 1264/120.3 (10.5) | 2888/838.8 (3.4) | ··· |
| Unadjusted HR | 0.53 (0.52–0.55) | 0.45 (0.42–0.49) | 0.32 (0.30–0.34) | <0.001 | 0.51 (0.47–0.55) | 0.49 (0.46–0.52) | 0.42 |
| Adjusted HR | 0.73 (0.71–0.76) | 0.75 (0.70–0.81) | 0.65 (0.60–0.69) | 0.003 | 0.82 (0.75–0.90) | 0.76 (0.72–0.81) | 0.18 |
| Cardiovascular mortality | 11 015/2121.4 (5.2) | 2854/176.1 (16.2) | 3660/590.7 (6.2) | ··· | 1546/173.5 (8.9) | 2955/1181.1 (2.5) | ··· |
| No ACEI/ARB | 3732/477.9 (7.8) | 809/25.3 (32.0) | 970/57.1 (17.0) | ··· | 696/53.2 (13.1) | 1257/342.3 (3.7) | ··· |
| ACEI/ARB | 7283/1643.5 (4.4) | 2045/150.8 (13.6) | 2690/533.6 (5.0) | ··· | 850/120.3 (7.1) | 1698/838.8 (2.0) | ··· |
| Unadjusted HR | 0.58 (0.56–0.60) | 0.47 (0.43–0.50) | 0.33 (0.31–0.35) | <0.001 | 0.56 (0.50–0.61) | 0.56 (0.52–0.60) | 0.98 |
| Adjusted HR | 0.81 (0.78–0.85) | 0.78 (0.71–0.86) | 0.67 (0.62–0.73) | 0.02 | 0.91 (0.82–1.02) | 0.91 (0.84–0.99) | 0.94 |
| MI | 20 802/1889.7 (11.0) | 3092/150.2 (20.6) | 6551/518.8 (12.6) | ··· | 2427/149.8 (16.2) | 8732/1070.9 (8.2) | ··· |
| No ACEI/ARB | 5609/432.4 (13.0) | 713/21.1 (33.7) | 1179/50.1 (23.5) | ··· | 898/46.6 (19.3) | 2819/314.6 (9.0) | ··· |
| ACEI/ARB | 15 193/1457.2 (10.4) | 2379/129.0 (18.4) | 5372/468.7 (11.5) | ··· | 1529/103.3 (14.8) | 5913/756.2 (7.8) | ··· |
| Unadjusted HR | 0.85 (0.82–0.87) | 0.68 (0.62–0.73) | 0.61 (0.57–0.65) | 0.05 | 0.81 (0.75–0.88) | 0.89 (0.85–0.93) | 0.06 |
| Adjusted HR | 0.95 (0.92–0.98) | 0.86 (0.78–0.94) | 0.84 (0.79–0.90) | 0.73 | 0.97 (0.88–1.06) | 1.02 (0.97–1.07) | 0.35 |
| Stroke | 4620/2080.6 (2.2) | 910/169.1 (5.4) | 1244/579.3 (2.1) | ··· | 848/166.7 (5.1) | 1618/1165.4 (1.4) | ··· |
| No ACEI/ARB | 1198/468.9 (2.6) | 170/24.2 (7.0) | 208/55.7 (3.7) | ··· | 297/51.1 (5.8) | 523/337.9 (1.5) | ··· |
| ACEI/ARB | 3422/1611.7 (2.1) | 740/145.0 (5.1) | 1036/523.7 (2.0) | ··· | 551/115.6 (4.8) | 1095/827.5 (1.3) | ··· |
| Unadjusted HR | 0.84 (0.79–0.90) | 0.79 (0.67–0.94) | 0.57 (0.49–0.67) | 0.01 | 0.84 (0.73–0.97) | 0.85 (0.77–0.95) | 0.83 |
| Adjusted HR | 0.96 (0.89–1.03) | 1.02 (0.85–1.22) | 0.80 (0.68–0.95) | 0.06 | 1.03 (0.88–1.20) | 0.98 (0.87–1.10) | 0.58 |
| New‐onset AF | 4928/1713.3 (2.9) | 2105/566.3 (3.7) | 2823/1147.0 (2.5) | ||||
| No ACEI/ARB | 1110/388.7 (2.9) | 303/54.8 (5.5) | 807/333.9 (2.4) | ||||
| ACEI/ARB | 3818/1324.6 (2.9) | 1802/511.5 (3.5) | 2016/813.1 (2.5) | ||||
| Unadjusted HR | 1.03 (0.96–1.10) | 0.71 (0.63–0.80) | 1.03 (0.95–1.12) | ||||
| Adjusted HR | 1.07 (1.00–1.15) | 0.96 (0.84–1.10) | 1.12 (1.02–1.22) | ||||
Number and incidence rate of events and crude and adjusted hazard ratios for outcomes stratified by ACEI and/or ARB treatment in patients with and without congestive heart failure and atrial fibrillation. Crude event rates were calculated according to the number of events per 100 person‐years. Unadjusted and adjusted HR is given with a 95% confidence interval. ACEI indicates angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; CHF, congestive heart failure; HR, hazard ratio; MI, myocardial infarction.
The 3‐year event rate for AMI is presented in Table 2. After adjustments, treatment with ACEI/ARB was significantly associated with a lower rate of AMI, HR 0.95 (0.92–0.98). A similar association was noted among a subgroup of patients with CHF at baseline, irrespectively if they had AF or no AF. For patients without CHF at baseline, no beneficial association was observed with ACEI/ARB in regard to recurrent AMI.
The unadjusted cumulative incidence rate per 100 person‐years of 3‐year stroke was 2.1 in patients exposed to ACEI/ARB versus 2.6 in nontreated patients. After adjustments, the association of ACEI/ARB with risk of 3‐year stroke was similar to nontreated patients, adjusted HR 0.96 (0.89–1.03). In a subgroup of patients with CHF and without AF at baseline, ACEI/ARB was associated with a lower risk of stroke, adjusted HR 0.80 (0.68–0.95). However, this association was not observed among the other subgroup of patients.
New‐Onset AF
In patients with no history of AF or in‐hospital diagnosis of AF, the cumulative incidence rate per 100 person‐years of 3‐year new‐onset atrial fibrillation was 2.9 in patients treated ACEI/ARB versus 2.9 in nontreated patients (see Figure 3; Table 2). After adjustment for patient, hospital, and treatment characteristics, ACEI/ARB was not associated with a lower risk of new‐onset AF, adjusted HR 1.07 (1.00–1.15). A similar lack of association was observed among patients with or without CHF.
Figure 3.
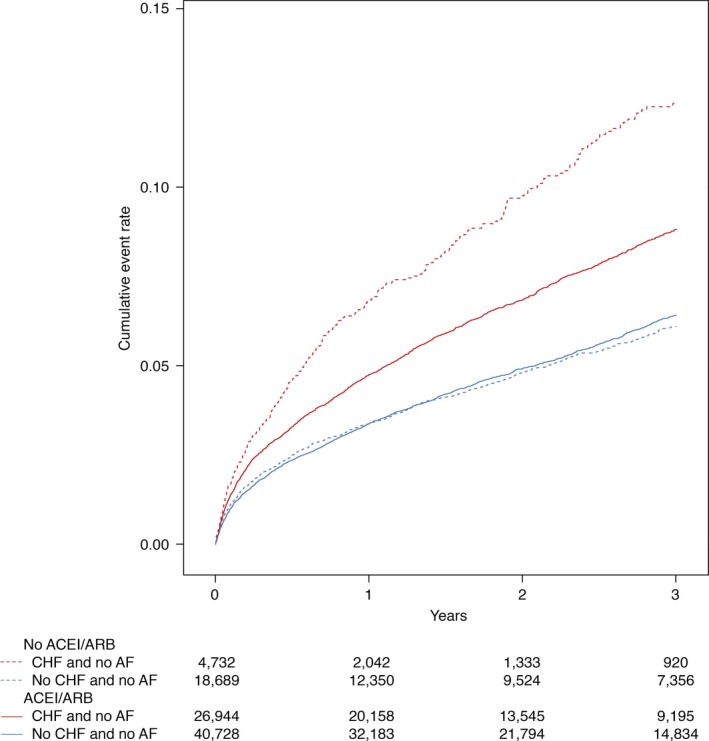
Kaplan–Meier plot depicting new‐onset AF for patients with and without CHF. Kaplan–Meier plot depicting the cumulative incidence curve for new‐onset AF stratified by use of ACEI and/or ARB. ACEI indicates angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; CHF, congestive heart failure.
Sensitivity Analyses
Similar results to the main analyses were observed in an intention‐to‐treat analysis (Table S3), a complete case analysis (Table S4), a propensity score analysis (Table S4), and when ACEI and ARB were analyzed separately (Table S5).
Subgroup Analysis
All‐cause mortality did not show significant heterogeneity in subgroup analysis, with 2 exceptions. The association between lower risk of all‐cause mortality and treatment with ACEI/ARB appeared to be diminished in 2 subgroup of patients without CHF and with AF at baseline, in patients with STEMI and in those with no hypertension (see Figure 4).
Figure 4.
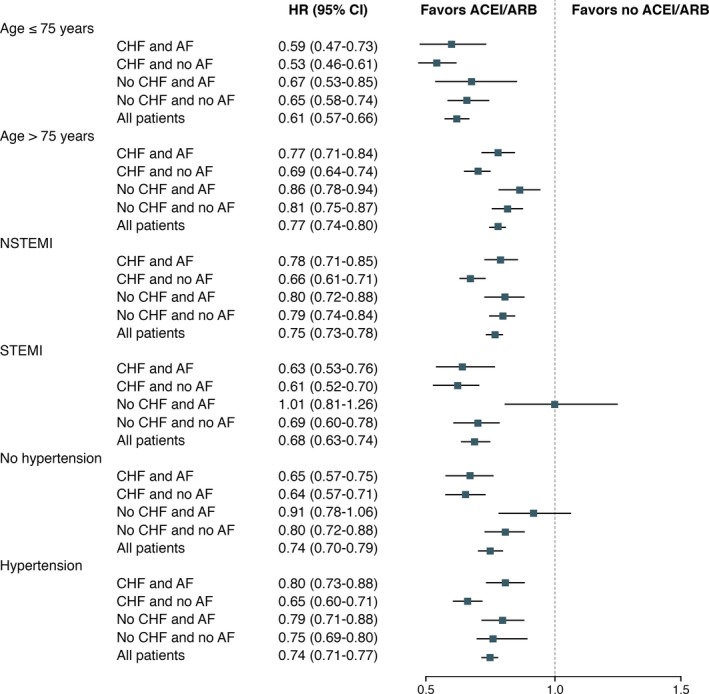
Risk of all‐cause mortality in prespecified subgroups. Risk of all‐cause mortality in subgroup of patients stratified by use of ACEI and/or ARB. ACEI indicates angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; CHF, congestive heart failure; HR, hazard ratio; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
Discussion
This study shows that RAS inhibition in patients with AMI was associated with a lower risk of all‐cause mortality. This association was consistent among patients with or without CHF and with or without AF at baseline. A similar beneficial association was observed for cardiovascular mortality in all patients, and for recurrent AMI in patients with CHF, irrespective of AF. No favorable association was observed with ACEI and/or ARB in regard to new‐onset AF.
Several trials, such as the Survival and Ventricular Enlargement (SAVE) trial,1 the Acute Infarction Ramipril Efficacy (AIRE) trial,2 and the Trandolapril Cardiac Evaluation (TRACE) trial,3, 4 have shown that ACEI lower rates of mortality and cardiovascular mortality in patients with CHF post‐AMI. This observation has also been supported by a meta‐analysis based on the 3 aforementioned trials.21 Similar beneficial results have been shown with ARB in the Valsartan in Acute Myocardial Infarction (VALIENT) trial.5 Moreover, there are data supporting that RAS inhibition might be beneficial for all post‐AMI patients, not only those suffering from CHF.22, 23 The lower risk of mortality observed in our study, and in previous trials, might be a result of reduction in myocardial infarct expansion, ventricular remodeling, and ventricular dilatation observed with RAS inhibtion.24, 25
The association between RAS inhibition and recurrent AMI is still controversial. The SAVE trial showed a lower risk,1 and the TRACE trial showed no association between RAS inhibition and recurrent AMI.3 In our study, the observed risk of recurrent AMI was lower among patients with CHF treated with ACEI and/or ARB. Studies have suggested that ACEIs do not appear to be associated with a reduced process of atherosclerosis.26 However, the reduced risk of AMI might be attributed to other possible mechanisms, such as improved endothelial function, a reduced risk of left ventricular hypertrophy, and renal protection.27, 28
In this study, we found no association between ACEI/ARB and risk of stroke among patients without CHF and in those with both CHF and AF. This is similar to, and in contrast to, other trials on patients with coronary artery disease.29, 30, 31 In a meta‐analysis32 based on the 3 cited trials on patients with coronary artery disease, a significant reduction in stroke rate was observed, although this was mainly driven by one of the trials, the Heart Outcomes Prevention Evaluation (HOPE) trial.29 Moreover, the divergence between our results and the meta‐analysis could be because of different inclusions criteria and because of differences between patient characteristics in trials and in routine care.
In this study, RAS inhibition in patients with no history of AF, or in‐hospital finding/diagnosis of AF, was not associated with a lower risk of new‐onset AF during a 3‐year follow‐up. A similar finding was observed in patients with and without CHF at baseline. In comparison, post‐hoc analyzes of randomized, controlled trials in patients with CHF but without known AF have suggested that RAS inhibition might reduce the incidence of new‐onset AF.33, 34, 35 In contrast, 2 prospective, randomized trials on patients with AF did not show beneficial effects of RAS inhibition with regard to future AF burden similar to our findings.36, 37 Explanatory mechanisms for the possible antiarrhythmic property of RAS inhibition, such as restrained electrical and structural remodeling, have been proposed in preclinical studies.7, 8, 9 To our knowledge, 2 trials have studied RAS inhibition and risk of AF post‐AMI. In the TRACE trial,38 a lower risk of new‐onset AF was observed among patients discharged with trandolapril. In contrast, the Gruppo Italiano per lo Studio Della Sopravvivenza Nell'Infarto Miocardico‐3 (GISSI‐3) trial39 reported no significant reduction in the risk of new‐onset AF with RAS inhibition. In a meta‐analysis including these 2 trials, no association was observed between RAS inhibition and risk of new‐onset AF in a subgroup of post‐AMI patients.40 Moreover, a similar lack of association between ACE inhibition and new‐onset AF was noted in the HOPE trial.41
Current practice guidelines on NSTEMI,11, 12 STEMI,13, 14 and AF15, 16 recommend ACEI or ARB to patients with CHF and left ventricular dysfunction. Also, the guidelines suggest that RAS inhibition might be useful for other patients with cardiac disease but without signs or symptoms of CHF. However, treatment with RAS inhibition is not stated as beneficial for the primary prevention of AF.11, 12, 13, 14, 15, 16 Our study confirms that ACEI and ARBs are beneficial post‐AMI and associated with a lower risk of all‐cause mortality, cardiovascular mortality, and, in patients with CHF, lower risk of recurrent AMI. Moreover, our study suggests that RAS inhibition is not associated with a lower risk of new‐onset AF post‐AMI.
The present study has limitations that have to be considered when interpreting the results. First, this is a retrospective, observational study and patients were not randomized to RAS inhibition. It is inevitable that residual confounding exists. However, we have a large sample size and access to detailed clinical information and were able to include many important confounders in our statistical analyses. Also, our results were confirmed in several sensitivity analyses, including complete case analysis and propensity‐score–matching analysis. Second, although we were able to follow drug dispense postdischarge and could in an on‐treatment analysis censor for drug initiation or discontinuation, we had no information on reasons for drug initiation or withdrawal after discharge. Also, we lack information on frequency of adverse effects, such as hyperkalemia. Finally, it is plausible that 3 years of follow‐up might be too short to capture long‐term effects of RAS inhibition in preventing new‐onset AF.
Conclusions
Treatment with ACEI and/or ARB was associated with a lower risk of all‐cause mortality and cardiovascular mortality in all patients post‐AMI, and a lower risk of recurrent AMI was observed in patients with CHF. In patients with no AF at baseline, RAS inhibition was not associated with a lower risk of new‐onset AF.
Sources of Funding
This work was supported by a grant from the Swedish Foundation for Strategic Research, Stockholm, Sweden (Grant No. KF10‐0024 to Tailoring of treatment in all comers with AMI).
Disclosures
Andell reports speaker fees from AstraZeneca. Spaak reports speaker honoraria from Abbott/AbbVie, MSD, AstraZeneca, and Bristol‐Myers Squibb. Oldgren reports consultant and lecture fees from Bayer, Boehringer Ingelheim, and Bristol‐Myers Squibb/Pfizer. The remaining authors have no disclosures to report.
Supporting information
Table S1. Definition of Baseline Characteristics, In‐Hospital Course, Procedures and Discharge Medication—History of Comorbidities was Collected From the Protocol‐Standardized SWEDEHEART Questionnaires and was Enriched With Data From the National Patient Registry Using the International Classification of Diseases, 9th and 10th Revision. Information About Angiotensin‐Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers at Discharge was Derived From the SWEDEHEART Questionnaire and the National Dispensed Drug Registry Using Anatomical Therapeutic Chemical Classification Codes
Table S2. Definition of Outcomes—The National Patient Registry, The Swedish Cause of Death Registry and the International Classification of Diseases, 10th Revision, was Used to Define the Outcomes
Table S3. Intention‐to‐Treat Analysis—Number and Incidence Rate of Events and Crude and Adjusted Hazard Ratios for Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors and/or Angiotensin II Receptor Blockers Treatment in Patients With and Without Congestive Heart Failure and Atrial Fibrillation in an Intention‐to‐Treat Analysis
Table S4. Complete Case and Propensity Score Analyses—Complete Case and Propensity Score Analyses With Hazard Ratios for Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors and/or Angiotensin II Receptor Blockers Treatment in Patients With and Without Congestive Heart Failure and Atrial Fibrillation
Table S5. Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers Treatment—Number and Incidence Rate of Events and Crude and Adjusted Hazard Ratios for Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors or Angiotensin II Receptor Blockers Treatment in Patients With and Without Congestive Heart Failure and Atrial Fibrillation
Acknowledgments
We thank all the staff in all coronary care units in Sweden for their help and cooperation in contributing data to the SWEDEHEART register. All participating centers, medical doctors, nurses, and the details of the SWEDEHEART register are presented on the registry's website (www.ucr.uu.se/swedeheart).
(J Am Heart Assoc. 2017;6:e005165. DOI: 10.1161/JAHA.116.005165.)
An abstract based on the preliminary results of this work was presented as a poster at the European Society of Cardiology Congress, August 27–31, 2016, in Rome, Italy.
References
- 1. Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau JLJ, Rouleau JLJ, Rutherford J, Wertheimer JH, Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1992;327:669–677. [DOI] [PubMed] [Google Scholar]
- 2. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators . Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet. 1993;342:821–828. [PubMed] [Google Scholar]
- 3. Køber L, Torp‐Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbæk J, Cole DS, Auclert L, Pauly NC, Aliot E, Persson S, Camm AJ. A clinical trial of the angiotensin‐converting‐enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1995;333:1670–1676. [DOI] [PubMed] [Google Scholar]
- 4. Torp‐Pedersen C, Køber L. Effect of ACE inhibitor trandolapril on life expectancy of patients with reduced left‐ventricular function after acute myocardial infarction. Lancet. 1999;354:9–12. [DOI] [PubMed] [Google Scholar]
- 5. Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau J‐L, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 6. Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–2345. [DOI] [PubMed] [Google Scholar]
- 7. Solti F, Vecsey T, Kékesi V, Juhasz‐Nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc Res. 1989;23:882–886. [DOI] [PubMed] [Google Scholar]
- 8. Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff‐perfused rabbit heart. Circulation. 1997;96:1686–1695. [DOI] [PubMed] [Google Scholar]
- 9. Sapna S, Ranjith SK, Shivakumar K. Cardiac fibrogenesis in magnesium deficiency: a role for circulating angiotensin II and aldosterone. Am J Physiol Heart Circ Physiol. 2006;291:H436–H440. [DOI] [PubMed] [Google Scholar]
- 10. Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin–angiotensin‐aldosterone system (RAAS) for primary prevention of non‐valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 11. Amsterdam EA, Wenger NK, Brindis RG, Casey Donald EJ, Ganiats TG, Holmes David RJ, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 12. Roffi M, Patrono C, Collet J‐P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GYH, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 13. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 14. Steg PG, James SK, Atar D, Badano LP, Blömstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van‘t Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 15. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland Joseph CJ, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary—a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 16. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; Available at: http://eurheartj.oxfordjournals.org/content/early/2016/08/26/eurheartj.ehw210. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J‐L, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wettermark B, Zoëga H, Furu K, Korhonen M, Hallas J, Nørgaard M, Almarsdottir AB, Andersen M, Andersson Sundell K, Bergman U, Helin‐Salmivaara A, Hoffmann M, Kieler H, Martikainen JE, Mortensen M, Petzold M, Wallach‐Kildemoes H, Wallin C, Sørensen HT. The Nordic prescription databases as a resource for pharmacoepidemiological research—a literature review. Pharmacoepidemiol Drug Saf. 2013;22:691–699. [DOI] [PubMed] [Google Scholar]
- 20. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 21. Flather MD, Yusuf S, Køber L, Pfeffer M, Hall A, Murray G, Torp‐Pedersen C, Ball S, Pogue J, Moyé L, Braunwald E. Long‐term ACE‐inhibitor therapy in patients with heart failure or left‐ventricular dysfunction: a systematic overview of data from individual patients. Lancet. 2016;355:1575–1581. [DOI] [PubMed] [Google Scholar]
- 22. ISIS‐4 (Fourth International Study of Infarct Survival) Collaborative Group . ISIS‐4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarction. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 23. Gruppo Italiano per lo Studio della Soprawivenza nell'Infarto Miocardico . GISSI‐3: effects of lisiriopril and transdermal glyceryl trinitrate singly and together on 6‐week mortality and ventricular function after acute myocardial infarction. Lancet. 1994;343:1115–1122. [PubMed] [Google Scholar]
- 24. Pfeffer MA, Greaves SC, Arnold JMO, Glynn RJ, LaMotte FS, Lee RT, Menapace FJ, Rapaport E, Ridker PM, Rouleau J‐L, Solomon SD, Hennekens CH. Early versus delayed angiotensin‐converting enzyme inhibition therapy in acute myocardial infarction. Circulation. 1997;95:2643–2651. [DOI] [PubMed] [Google Scholar]
- 25. Hokimoto S, Yasue H, Fujimoto K, Yamamoto H, Nakao K, Kaikita K, Sakata R, Miyamoto E. Expression of angiotensin‐converting enzyme in remaining viable myocytes of human ventricles after myocardial infarction. Circulation. 1996;94:1513–1518. [DOI] [PubMed] [Google Scholar]
- 26. MacMahon S, Sharpe N, Gamble G, Clague A, Ni Mhurchu C, Clark T, Hart H, Scott J, White H. Randomized, placebo‐controlled trial of the angiotensin‐converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. J Am Coll Cardiol. 2000;36:438–443. [DOI] [PubMed] [Google Scholar]
- 27. O'Keefe JH, Wetzel M, Moe RR, Brosnahan K, Lavie CJ. Should an angiotensin‐converting enzyme inhibitor be standard therapy for patients with atherosclerotic disease? J Am Coll Cardiol. 2001;37:1–8. [DOI] [PubMed] [Google Scholar]
- 28. Evans M, Carrero J‐J, Szummer K, Åkerblom A, Edfors R, Spaak J, Jacobson SH, Andell P, Lindhagen L, Jernberg T. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in myocardial infarction patients with renal dysfunction. J Am Coll Cardiol. 2016;67:1687–1697. [DOI] [PubMed] [Google Scholar]
- 29. Effects of an angiotensin‐converting–enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 30. The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators . Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double‐blind, placebo‐controlled, multicentre trial (the EUROPA study). Lancet. 2003;362:782–788. [DOI] [PubMed] [Google Scholar]
- 31. The PEACE Trial Investigators . Angiotensin‐converting‐enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin‐converting‐enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–588. [DOI] [PubMed] [Google Scholar]
- 33. Vermes E, Tardif J‐C, Bourassa MG, Racine N, Levesque S, White M, Guerra PG, Ducharme A. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the studies of left ventricular dysfunction (SOLVD) trials. Circulation. 2003;107:2926–2931. [DOI] [PubMed] [Google Scholar]
- 34. Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, Masson S, Cerè E, Tognoni G, Cohn JN. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT). Am Heart J. 2005;149:548–557. [DOI] [PubMed] [Google Scholar]
- 35. Ducharme A, Swedberg K, Pfeffer MA, Cohen‐Solal A, Granger CB, Maggioni AP, Michelson EL, McMurray JJV, Olsson L, Rouleau JL, Young JB, Olofsson B, Puu M, Yusuf S. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. 2006;152:86–92. [PubMed] [Google Scholar]
- 36. Goette A, Schön N, Kirchhof P, Breithardt G, Fetsch T, Häusler KG, Klein HU, Steinbeck G, Wegscheider K, Meinertz T. Angiotensin II‐antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol. 2012;5:43–51. [DOI] [PubMed] [Google Scholar]
- 37. The ACTIVE I Investigators . Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011;364:928–938. [DOI] [PubMed] [Google Scholar]
- 38. Pedersen OD, Bagger H, Køber L, Torp‐Pedersen C; Group on behalf of the TS . Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999;100:376–380. [DOI] [PubMed] [Google Scholar]
- 39. Pizzetti F, Turazza FM, Franzosi MG, Barlera S, Ledda A, Maggioni AP, Santoro L, Tognoni G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI‐3 data. Heart. 2001;86:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin‐angiotensin system inhibition: a meta‐analysis. J Am Coll Cardiol. 2010;55:2299–2307. [DOI] [PubMed] [Google Scholar]
- 41. Salehian O, Healey J, Stambler B, Alnemer K, Almerri K, Grover J, Bata I, Mann J, Matthew J, Pogue J, Yusuf S, Dagenais G, Lonn E. Impact of ramipril on the incidence of atrial fibrillation: results of the Heart Outcomes Prevention Evaluation study. Am Heart J. 2007;154:448–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of Baseline Characteristics, In‐Hospital Course, Procedures and Discharge Medication—History of Comorbidities was Collected From the Protocol‐Standardized SWEDEHEART Questionnaires and was Enriched With Data From the National Patient Registry Using the International Classification of Diseases, 9th and 10th Revision. Information About Angiotensin‐Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers at Discharge was Derived From the SWEDEHEART Questionnaire and the National Dispensed Drug Registry Using Anatomical Therapeutic Chemical Classification Codes
Table S2. Definition of Outcomes—The National Patient Registry, The Swedish Cause of Death Registry and the International Classification of Diseases, 10th Revision, was Used to Define the Outcomes
Table S3. Intention‐to‐Treat Analysis—Number and Incidence Rate of Events and Crude and Adjusted Hazard Ratios for Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors and/or Angiotensin II Receptor Blockers Treatment in Patients With and Without Congestive Heart Failure and Atrial Fibrillation in an Intention‐to‐Treat Analysis
Table S4. Complete Case and Propensity Score Analyses—Complete Case and Propensity Score Analyses With Hazard Ratios for Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors and/or Angiotensin II Receptor Blockers Treatment in Patients With and Without Congestive Heart Failure and Atrial Fibrillation
Table S5. Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers Treatment—Number and Incidence Rate of Events and Crude and Adjusted Hazard Ratios for Outcomes Stratified by Angiotensin‐Converting Enzyme Inhibitors or Angiotensin II Receptor Blockers Treatment in Patients With and Without Congestive Heart Failure and Atrial Fibrillation


