Abstract
Background
We aimed to test the impact of achieved blood pressure (BP) on first stroke among patients with grade 1 hypertension and without cardiovascular diseases in the China Stroke Primary Prevention Trial.
Methods and Results
A total of 3187 patients with uncomplicated grade 1 hypertension were included. The risk of outcomes was assessed according to: (1) the proportion of visits in which BP was reduced to <140/90 mm Hg, and (2) the time‐averaged systolic BP (SBP) or diastolic BP levels during the study treatment period. The median antihypertensive treatment duration was 4.6 years. Only 1.5% of the participants discontinued the treatments because of adverse reaction. Overall, the risk of stroke decreased with the increase of the proportion of study visits with BP <140/90 mm Hg (for per 5% increase; hazard ratio, 0.92 [95% CI, 0.87–0.98]). Consistently, compared with patients with time‐averaged SBP ≥140 or diastolic BP ≥90 mm Hg, the risk of stroke was lower in patients with time‐averaged SBP of 120 to <140 mm Hg (1.1% versus 2.9%; hazard ratio, 0.39 [95% CI, 0.22–0.69]) or diastolic BP <90 mm Hg (1.5% versus 2.7%; hazard ratio, 0.41 [95% CI, 0.17–0.98]). The beneficial results were consistent across age (<60 versus ≥60 years), sex, baseline SBP (<150 versus 150 to <160 mm Hg), study treatment groups (enalapril or enalapril‐folic acid), and hypertension subtypes (isolated systolic hypertension or systolic‐diastolic hypertension). However, a time‐averaged SBP <120 mm Hg (versus 120–140 mm Hg) was associated with an increased risk for stroke. Similar results were observed for composite cardiovascular events or all‐cause death.
Conclusions
Achieved BP <140/90 mm Hg was significantly associated with a decreased risk of stroke or all‐cause death in patients with uncomplicated grade 1 hypertension.
Keywords: achieved blood pressure, all‐cause death, cardiovascular disease prevention, grade 1 hypertension, high blood pressure, hypertension, primary prevention, stroke
Subject Categories: High Blood Pressure, Cerebrovascular Disease/Stroke, Primary Prevention
Introduction
Hypertension has become a highly important public health challenge, affecting more than 1 billion adults worldwide.1 More important, most hypertensive patients have grade 1 hypertension (untreated systolic blood pressure [SBP] of 140–159 mm Hg and/or diastolic blood pressure [DBP] of 90–99 mm Hg) and no previous cardiovascular diseases.2 The decision to treat this population has important clinical (eg, adverse drug effects, lifetime of drug therapy) and public health (eg, high cost of drugs, medical services) implications.3 However, whether these patients should be treated remains controversial.
Antihypertensive drugs are recommended for patients with grade 1 hypertension at low to moderate risk after months of unsuccessful lifestyle measures according to the 2013 European Society of Hypertension/European Society of Cardiology guidelines (class IIa, level B),4 the 2014 American Society of Hypertension/International Society of Hypertension guidelines,5 and the 2014 Eighth Joint National Committee report (expert opinion, grade E).6 However, the 2011 National Institute for Health and Care Excellence7 guideline recommended antihypertensive treatment only for patients with grade 1 hypertension at high total cardiovascular risk. The 2016 Hypertension Canada's Canadian Hypertension Education Program guidelines8 also recommended that antihypertensive therapy should be considered for patients with grade 1 hypertension only in those with macrovascular target organ damage or other independent cardiovascular risk factors. The inconsistency in the recommendations among these major guidelines shows that the evidence concerning drug treatment in uncomplicated grade 1 hypertension is scanty and controversial.4, 9
The China Stroke Primary Prevention Trial (CSPPT) found that the combined use of enalapril and folic acid, compared with enalapril alone, significantly reduced the risk of first stroke by 21% (hazard ratio [HR], 0.79; 95% CI, 0.68–0.93) in Chinese hypertensive patients without a history of cardiovascular diseases.10 The CSPPT included about half of hypertensive patients without previously antihypertensive treatment and with different on‐treatment blood pressure (BP) levels, which offers us an exceptional opportunity to investigate the relationship between achieved BP and cardiovascular outcomes or all‐cause death in patients with grade 1 hypertension. Therefore, the current study, a post hoc analysis of the CSPPT, aimed to test the impact of achieved BP on first stroke among patients with grade 1 hypertension and without cardiovascular diseases.
Methods
Study Design
The rationale and study design for the CSPPT have previously been reported in detail.10 Briefly, the CSPPT was a multicommunity, randomized, double‐blind, controlled trial conducted from May 19, 2008, to August 24, 2013, in 32 communities in Jiangsu (20 communities) and Anhui (12 communities) provinces. The CSPPT was approved by the ethics committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number: FWA00001263) and registered with ClinicalTrials.gov (NCT00794885). All participants gave written informed consent. The study enrolled a total of 20 702 hypertensive adults without a history of cardiovascular disease.
Study Population
This study enrolled patients with grade 1 hypertension among the previously untreated hypertensive adults in the CSPPT. Untreated hypertension in the CSPPT was defined as not receiving antihypertensive medication within the past 2 weeks.
Detailed inclusion and exclusion criteria for the CSPPT are described elsewhere. Eligible participants were men and women aged 45 to 75 years with hypertension, defined as seated resting SBP ≥140 mm Hg or DBP ≥90 mm Hg at both the screening and recruitment visits, or patients who were taking antihypertensive medication. The major exclusion criteria included history of physician‐diagnosed stroke, myocardial infarction, heart failure, post‐coronary revascularization, and/or congenital heart disease.
Intervention and Follow‐Up
Eligible participants were randomly assigned in a 1:1 ratio to 1 of 2 treatment groups: a daily oral dose of 1 tablet containing 10 mg enalapril and 0.8 mg folic acid (single‐tablet combination, the enalapril‐folic acid group); or a daily oral dose of 1 tablet containing 10 mg enalapril only (the enalapril only group). Other classes of antihypertensive medications, mostly dihydropyridine calcium channel blockers and hydrochlorothiazide, could be prescribed concomitantly if necessary. BP control within a normal range (SBP <140 mm Hg and DBP <90 mm Hg) was not mandatory.
Participants were followed up every 3 months. At each visit, BP and pulse rates of the participants were measured, the number of pills taken between visits were counted, and concomitant medications and adverse events were recorded.
At each visit, seated BP measurements were obtained by trained research staff using a mercury manometer after the patients had been seated for 10 minutes. The standard method and appropriately sized cuffs were used. Triplicate measurements on the same arm were taken, with at least 2 minutes between readings. The mean SBP and DBP values of the 3 independent measures were used in the analysis.
Outcomes
The primary outcome was a first nonfatal or fatal stroke (ischemic or hemorrhagic), excluding subarachnoid hemorrhage and silent stroke. Stroke was defined as rapidly developed clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 hours or longer (unless interrupted by surgery or death) or a demonstrable lesion on computed tomography or magnetic resonance imaging scan that was consistent with acute stroke, with no apparent causes other than of vascular origin. Computed tomography or magnetic resonance imaging were not essential for diagnosis of stroke, but were necessary for differentiating the subtypes of stroke (ischemic or hemorrhagic). Without imaging data, stroke was still diagnosed in the presence of specific signs and symptoms of focal disturbance of cerebral function and the subtype defined as “uncertain” (see the protocol the CSPPT1).
Secondary outcomes included a composite of cardiovascular events (cardiovascular death, myocardial infarction, and stroke) and all‐cause death.
All of the study outcomes were reviewed and adjudicated according to standard criteria by an independent end point adjudication committee.
Statistical Analysis
The current study included a total of 3187 patients with grade 1 hypertension from the CSPPT (Figure 1). There were missing values on serum total cholesterol (n=56), body mass index (n=1), smoking status (n=3), drinking status (n=4), serum folate (n=24), serum homocysteine (n=43), serum vitamin B12 (n=24), serum fasting glucose (n=57), and estimated glomerular filtration rate levels (n=58) at baseline. Multiple imputations, with a number of 5 imputed data sets and an imputation method of predictive mean matching, were used to deal with missing values in the outcome analyses by fitting a model to each of the imputed data sets and then pooling the results together in a “mice” package of R (R software, http://www.R-project.org/).
Figure 1.
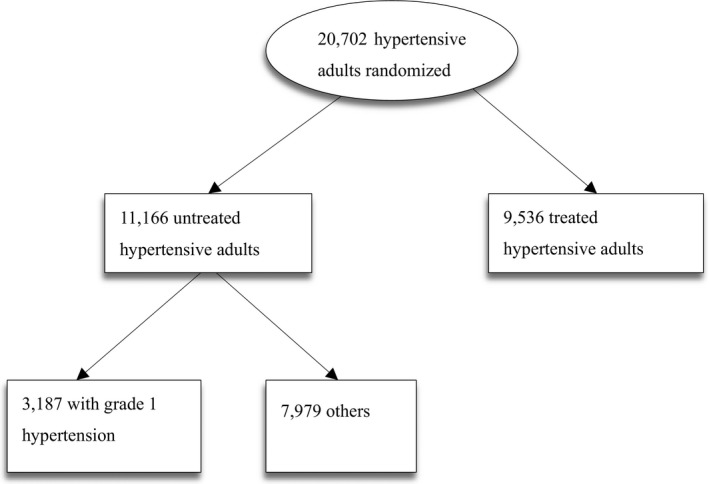
Flow chart of the participants.
Time‐averaged BP levels during the treatment period were calculated for each participant using all postbaseline results up to the last visit before the date of an event or the end of follow‐up in those without an event (times of BP measurement during the treatment period: median, 16; interquartile range, 12–18 times). Diabetes mellitus was defined as having a history of diabetes mellitus or a fasting glucose ≥7 mmol/L at baseline or under glucose‐lowering therapy. Hypertension subtypes were defined as follows: systolic‐diastolic hypertension (SBP ≥140 and DBP ≥90 mm Hg), isolated systolic hypertension (SBP ≥140 and DBP <90 mm Hg), or isolated diastolic hypertension (SBP <140 and DBP ≥90 mm Hg). Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Participants were divided into 4 groups according to the proportion of study visits (<25 [reference], 25–<50, 50–<75, and ≥75%) in which BP was reduced to <140/90 mm Hg up to the occurrence of an event or, in those without an event, up to study end. No minimum number of visits was required for patients to be included in the analysis. Furthermore, the participants were divided into groups according to the time‐averaged SBP (<120, 120–<140 [reference], and ≥140 mm Hg) or DBP (<75, 75–<80, 80–<90, and ≥90 [reference] mm Hg) levels during the treatment period.
Cox proportional hazards models were used to estimate the HRs and 95% CIs for the risk of study outcomes associated with the proportion (continuous and categorical) of study visits in which BP was <140/90 mm Hg and time‐averaged BP levels, without and with adjustment for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking status, SBP, DBP, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
A 2‐tailed P<0.05 was considered statistically significant in all analyses. R software version 3.3.1 was used for all statistical analyses.
Results
Cohort in Analysis and Baseline Characteristics
A total of 3187 participants with grade 1 hypertension at baseline were included in the analyses. The flow of the participants is presented in Figure 1.
Baseline characteristics of the 4 groups of participants according to the proportion of study visits in which BP was reduced to <140/90 mm Hg are summarized in Table 1. From the lowest to the highest proportion of study visits with BP <140/90 mm Hg, participants were less likely to be men and tended to have lower percentages of cigarette smoking and alcohol drinking and lower levels of creatinine and homocysteine. Similar trends were observed when participants were grouped by the time‐averaged SBP or DBP levels during the treatment period (Tables 2 and 3).
Table 1.
Characteristics of the Study Participants According to the Proportion of Visits in Which BP was Reduced to <140/90 mm Hga
| Total (N=3187) | Proportions | P Value | ||||
|---|---|---|---|---|---|---|
| <25% (n=295) | 25% to <50% (n=722) | 50% to <75% (n=1257) | ≥75% (n=913) | |||
| Age, y | 59.4 (7.4) | 58.6 (8.3) | 59.5 (7.5) | 59.7 (7.2) | 59.3 (7.1) | 0.129 |
| Male, No. (%) | 1316 (41.3) | 136 (46.1) | 305 (42.2) | 553 (44.0) | 322 (35.3) | <0.001 |
| Body mass index, kg/m2 | 24.0 (3.6) | 24.2 (3.9) | 24.2 (3.6) | 24.0 (3.5) | 24.0 (3.5) | 0.555 |
| Current smoking, No. (%) | 816 (25.6) | 95 (32.2) | 181 (25.1) | 354 (28.2) | 186 (20.4) | <0.001 |
| Current alcohol drinking, No. (%) | 869 (27.3) | 99 (33.4) | 207 (28.7) | 358 (28.5) | 205 (22.5) | 0.005 |
| MTHFR C677T polymorphisms, No. (%) | ||||||
| CC | 941 (29.5) | 89 (30.2) | 213 (29.5) | 357 (28.4) | 282 (30.9) | 0.648 |
| CT | 1553 (48.7) | 144 (48.8) | 338 (46.8) | 634 (50.4) | 437 (47.9) | |
| TT | 693 (21.8) | 62 (21.0) | 171 (23.7) | 266 (21.2) | 194 (21.2) | |
| Treatment group, No. (%) | ||||||
| Enalapril | 1579 (49.6) | 144 (48.8) | 357 (49.5) | 620 (49.3) | 458 (50.2) | 0.973 |
| Enalapril‐folic acid | 1608 (50.4) | 151 (51.2) | 365 (50.5) | 637 (50.7) | 455 (49.8) | |
| Baseline BP, mm Hg | ||||||
| Systolic | 150.9 (6.4) | 150.8 (6.9) | 151.5 (6.4) | 151.0 (6.3) | 150.1 (6.3) | <0.001 |
| Diastolic | 87.6 (7.9) | 89.2 (8.0) | 88.4 (7.9) | 87.4 (8.1) | 86.8 (7.5) | <0.001 |
| Time‐averaged BP during the treatment period, mm Hg | ||||||
| Systolic | 135.4 (9.6) | 151.6 (8.4) | 142.0 (6.0) | 134.4 (5.0) | 126.2 (5.2) | <0.001 |
| Diastolic | 81.0 (6.6) | 88.5 (7.5) | 84.0 (6.1) | 80.5 (5.5) | 76.9 (4.6) | <0.001 |
| Self‐reported hyperlipidemia | 59 (1.9) | 2 (0.7) | 15 (2.1) | 26 (2.1) | 16 (1.8) | 0.421 |
| Self‐reported diabetes mellitus | 67 (2.1) | 6 (2.0) | 12 (1.7) | 28 (2.2) | 21 (2.3) | 0.812 |
| Laboratory results | ||||||
| Fasting glucose, mmol/L | 5.6 (1.6) | 5.7 (1.8) | 5.6 (1.6) | 5.6 (1.6) | 5.5 (1.4) | 0.342 |
| Total cholesterol, mmol/L | 5.4 (1.2) | 5.2 (1.1) | 5.3 (1.1) | 5.4 (1.2) | 5.5 (1.1) | <0.001 |
| Creatinine, μmol/L | 64.4 (15.5) | 66.7 (20.4) | 64.6 (14.4) | 64.4 (14.3) | 63.6 (16.2) | 0.029 |
| Homocysteine, μmol/L | 13.8 (7.2) | 14.4 (9.1) | 14.2 (8.6) | 13.7 (6.7) | 13.2 (6.0) | 0.027 |
| Folate, ng/mL | 9.3 (4.4) | 9.2 (4.2) | 9.3 (4.2) | 9.4 (4.9) | 9.2 (4.0) | 0.856 |
| Medication use, No. (%) | ||||||
| Lipid‐lowering drugs | 7 (0.2) | 1 (0.3) | 1 (0.1) | 4 (0.3) | 1 (0.1) | 0.689 |
| Glucose‐lowering drugs | 32 (1.0) | 2 (0.7) | 7 (1.0) | 15 (1.2) | 8 (0.9) | 0.819 |
| Antiplatelet drugs | 10 (0.3) | 0 (0) | 1 (0.3) | 6 (0.5) | 3 (0.3) | 0.437 |
BP indicates blood pressure; MTHFR, methylenetetrahydrofolate reductase.
Values are presented as mean (SD) for continuous variables.
Table 2.
Characteristics of the Study Participants by Time‐Averaged SBP Levelsa During the Treatment Period
| Time‐Averaged SBP, mm Hg | P Value | |||
|---|---|---|---|---|
| ≥140 (n=870) | 120 to <140 (n=2215) | <120 (n=102) | ||
| Age, y | 60.2 (7.6) | 59.2 (7.2) | 57.8 (7.3) | <0.001 |
| Male, No. (%) | 374 (43.0) | 916 (41.4) | 26 (25.5) | 0.003 |
| Body mass index, kg/m2 | 23.8 (3.6) | 24.1 (3.5) | 24.0 (3.5) | 0.052 |
| Current smoking, No. (%) | 243 (28.0) | 559 (25.3) | 14 (13.7) | 0.008 |
| Current alcohol drinking, No. (%) | 259 (29.8) | 597 (27.0) | 13 (12.8) | 0.001 |
| MTHFR C677T polymorphisms, No. (%) | ||||
| CC | 261 (30.0) | 656 (29.6) | 24 (23.5) | 0.745 |
| CT | 423 (48.6) | 1077 (48.6) | 53 (52.0) | |
| TT | 186 (21.4) | 482 (21.8) | 25 (24.5) | |
| Treatment group, No. (%) | ||||
| Enalapril | 424 (48.7) | 1109 (50.1) | 46 (45.1) | 0.528 |
| Enalapril‐folic acid | 446 (51.3) | 1106 (49.9) | 56 (54.9) | |
| Baseline BP, mm Hg | ||||
| Systolic | 152.0 (5.9) | 150.6 (6.5) | 148.0 (7.6) | <0.001 |
| Diastolic | 87.4 (8.2) | 87.7 (7.8) | 88.4 (7.2) | 0.384 |
| Time‐averaged BP during the treatment period, mm Hg | ||||
| Systolic | 147.3 (6.6) | 131.5 (5.2) | 116.7 (3.0) | <0.001 |
| Diastolic | 84.6 (7.1) | 79.8 (5.8) | 75.2 (5.2) | <0.001 |
| Self‐reported hyperlipidemia | 15 (1.7) | 41 (1.9) | 3 (2.9) | 0.689 |
| Self‐reported diabetes mellitus | 21 (2.4) | 43 (1.9) | 3 (2.9) | 0.595 |
| Laboratory results | ||||
| Fasting glucose, mmol/L | 5.7 (1.9) | 5.6 (1.4) | 5.5 (1.6) | 0.114 |
| Total cholesterol, mmol/L | 5.3 (1.1) | 5.4 (1.2) | 5.4 (1.3) | 0.007 |
| Creatinine, μmol/L | 64.8 (16.2) | 64.3 (15.4) | 62.7 (12.6) | 0.416 |
| Homocysteine, μmol/L | 14.4 (8.3) | 13.6 (6.8) | 12.9 (5.6) | 0.011 |
| Folate, ng/mL | 9.4 (4.7) | 9.3 (4.3) | 9.4 (4.0) | 0.591 |
| Medication use, No. (%) | ||||
| Lipid‐lowering drugs | 1 (0.1) | 6 (0.3) | 0 (0) | 0.630 |
| Glucose‐lowering drugs | 10 (1.2) | 21 (1.0) | 1 (1.0) | 0.880 |
| Antiplatelet drugs | 0 (0) | 9 (0.4) | 1 (1.0) | 0.091 |
BP indicates blood pressure; MTHFR, methylenetetrahydrofolate reductase; SBP, systolic blood pressure.
Values are presented as mean (SD) for continuous variables.
Table 3.
Characteristics of the Study Participants by Time‐Averaged DBP Levelsa During the Treatment Period
| Time‐Averaged DBP, mm Hg | P Value | ||||
|---|---|---|---|---|---|
| ≥90 (n=262) | 80 to <90 (n=1503) | 75 to <80 (n=869) | <75 (n=553) | ||
| Age, y | 54.4 (6.6) | 57.8 (7.1) | 60.9 (6.8) | 63.9 (6.4) | <0.001 |
| Male, No. (%) | 134 (51.2) | 633 (42.1) | 321 (36.9) | 228 (41.2) | <0.001 |
| Body mass index, kg/m2 | 25.1 (3.8) | 24.6 (3.5) | 23.7 (3.5) | 22.7 (3.1) | <0.001 |
| Current smoking, No. (%) | 80 (30.5) | 373 (24.9) | 213 (24.5) | 150 (27.1) | 0.027 |
| Current alcohol drinking, No. (%) | 93 (35.5) | 428 (28.5) | 201 (23.2) | 147 (26.6) | 0.005 |
| MTHFR C677T polymorphisms, No. (%) | |||||
| CC | 79 (30.2) | 414 (27.6) | 268 (30.8) | 180 (32.6) | 0.098 |
| CT | 119 (45.4) | 740 (49.2) | 423 (48.7) | 271 (49.0) | |
| TT | 64 (24.4) | 349 (23.2) | 178 (20.5) | 102 (18.4) | |
| Treatment group, No. (%) | |||||
| Enalapril | 132 (50.4) | 726 (48.3) | 439 (50.5) | 282 (51.0) | 0.616 |
| Enalapril‐folic acid | 130 (49.6) | 777 (51.7) | 430 (49.5) | 271 (49.0) | |
| Baseline BP, mm Hg | |||||
| Systolic | 148.4 (8.2) | 150.6 (6.6) | 151.4 (5.7) | 151.8 (5.4) | <0.001 |
| Diastolic | 94.1 (4.6) | 90.3 (6.3) | 86.0 (7.1) | 79.8 (7.7) | <0.001 |
| Time‐averaged BP during the treatment period, mm Hg | |||||
| Systolic | 145.7 (10.0) | 136.8 (8.7) | 132.8 (8.5) | 130.6 (8.5) | <0.001 |
| Diastolic | 93.9 (3.7) | 84.2 (2.7) | 77.7 (1.4) | 71.5 (3.0) | <0.001 |
| Self‐reported hyperlipidemia | 2 (0.8) | 36 (2.4) | 15 (1.7) | 6 (1.1) | 0.111 |
| Self‐reported diabetes mellitus | 2 (0.8) | 29 (1.9) | 22 (2.5) | 14 (2.5) | 0.287 |
| Laboratory results | |||||
| Fasting glucose, mmol/L | 5.5 (1.4) | 5.6 (1.4) | 5.7 (1.7) | 5.6 (1.9) | 0.240 |
| Total cholesterol, mmol/L | 5.3 (1.1) | 5.5 (1.2) | 5.4 (1.1) | 5.4 (1.1) | <0.001 |
| Creatinine, μmol/L | 65.6 (14.7) | 64.8 (15.0) | 63.9 (16.2) | 63.9 (16.2) | 0.112 |
| Homocysteine, μmol/L | 14.1 (10.4) | 13.9 (7.7) | 13.6 (6.1) | 13.5 (5.5) | 0.516 |
| Folate, ng/mL | 9.2 (4.0) | 9.0 (4.4) | 9.6 (4.4) | 9.8 (4.5) | <0.001 |
| Medication use, No. (%) | |||||
| Lipid‐lowering drugs | 0 (0) | 5 (0.3) | 2 (0.2) | 0 (0) | 0.445 |
| Glucose‐lowering drugs | 1 (0.4) | 15 (1.0) | 9 (1.0) | 7 (1.3) | 0.703 |
| Antiplatelet drugs | 0 (0) | 5 (0.3) | 3 (0.4) | 2 (0.4) | 0.823 |
BP indicates blood pressure; DBP, diastolic blood pressure; MTHFR, methylenetetrahydrofolate reductase.
Values are presented as mean (SD) for continuous variables.
Primary and Secondary Outcomes According to the Proportion of Visits With BP <140/90 mm Hg
During the study treatment period, a total of 49 participants (1.5%) discontinued the treatments because of adverse reactions. For the primary outcome, the median length of follow‐up was 4.6 years (interquartile range, 4.2–4.9). First stroke occurred in 52 participants. Stroke cases could be classified into ischemic (n=36) or hemorrhagic stroke (n=16) based on computed tomographic or magnetic resonance imaging findings. Furthermore, a total of 3 cases were fatal stroke.
A median of 16 (interquartile range, 12–18) BP measurements were taken during the treatment period. The association between the proportion of study visits in which BP was <140/90 mm Hg and risk of first stroke are presented in Figure 2. Overall, the risk of stroke decreased with the increase of the proportion of visits with BP <140/90 mm Hg (for per 5% increase; HR,0.92; 95% CI, 0.87–0.98) (Table 4).
Figure 2.
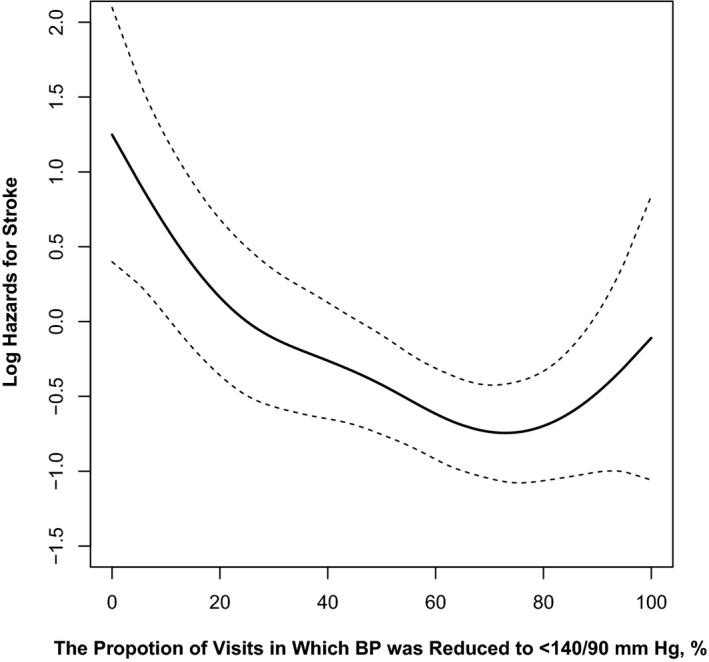
The association between the proportion of study visits in which blood pressure (BP) was <140/90 mm Hg and the risk of first stroke. Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, systolic and diastolic blood pressure, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
Table 4.
Primary and Secondary Outcomes According to the Proportion of Visits in Which BP was Reduced to <140/90 mm Hg
| Proportion of Visits With BP <140/90 mm Hg | Outcome, No. (%) | HR (95% CI) | Adjusteda HR (95% CI) |
|---|---|---|---|
| Primary outcome | |||
| First stroke | |||
| Continuous: per 5% increase | 52 (1.6) | 0.92 (0.87–0.97) | 0.92 (0.87–0.98) |
| Categorical | |||
| <25% | 11 (3.7) | Ref | Ref |
| 25% to <50% | 13 (1.8) | 0.46 (0.20–1.02) | 0.47 (0.21–1.05) |
| 50% to <75% | 16 (1.3) | 0.32 (0.15–0.69) | 0.33 (0.15–0.72) |
| ≥75% | 12 (1.3) | 0.33 (0.15–0.76) | 0.35 (0.15–0.80) |
| P for trend | 0.011 | 0.015 | |
| Secondary outcomes | |||
| Composite of stroke, myocardial infarction, or death from cardiovascular causes | |||
| Continuous: per 5% increase | 61 (1.9) | 0.92 (0.88–0.97) | 0.92 (0.88–0.97) |
| Categorical | |||
| <25% | 13 (4.4) | Ref | Ref |
| 25% to <50% | 15 (2.1) | 0.45 (0.21–0.94) | 0.45 (0.21–0.95) |
| 50% to <75% | 19 (1.5) | 0.32 (0.16–0.65) | 0.32 (0.16–0.65) |
| ≥75% | 14 (1.5) | 0.33 (0.15–0.70) | 0.33 (0.15–0.71) |
| P for trend | 0.006 | 0.007 | |
| All‐cause death | |||
| Continuous: per 5% increase | 87 (2.7) | 0.94 (0.90–0.98) | 0.95 (0.90–0.99) |
| Categorical | |||
| <25% | 15 (5.2) | Ref | Ref |
| 25% to <50% | 21 (2.9) | 0.54 (0.28–1.05) | 0.55 (0.28–1.08) |
| 50% to <75% | 31 (2.5) | 0.46 (0.25–0.84) | 0.46 (0.24–0.86) |
| ≥75% | 20 (2.2) | 0.41 (0.21–0.80) | 0.46 (0.23–0.91) |
| P for trend | 0.016 | 0.040 | |
BP indicates blood pressure; HR, hazard ratio.
Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, systolic and diastolic blood pressure, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
The time‐averaged SBPs were 151.6, 142.0, 134.4, and 126.1 mm Hg, respectively, in patients with <25%, 25% to 50%, 50% to 75%, and ≥75% of the study visits in which BP was <140/90 mm Hg (Table 1). Compared with participants with <25% of the visits in which BP was <140/90 mm Hg (stroke incidence: 3.7%), the incidence of stroke decreased significantly in those with 25% to 50% (1.8%; HR, 0.47 [95% CI, 0.21–1.05]), 50% to 75% (1.3%; HR, 0.33 [95% CI, 0.15–0.72]), and ≥75% (1.3%; HR, 0.35 [95% CI, 0.15–0.80]) of the visits with BP <140/90 mm Hg (P for trend=0.015). Excluding participants with diabetes mellitus (n=321) or estimated glomerular filtration rate <60 mL/min per 1.73 m2 (n=27) at baseline did not substantially change the results (<25%, 3.8% [reference]; 25–<50%, 1.7%; HR, 0.45 [95% CI, 0.19–1.07]; 50–<75%, 1.3%; HR, 0.34 [95% CI, 0.15–0.78]; ≥75%, 1.2%; HR, 0.35 [95% CI, 0.14–0.87]; P for trend=0.030). Similar results were observed for the composite cardiovascular events or all‐cause death (Table 4).
Primary and Secondary Outcomes According to Time‐Averaged BP During the Treatment Period
The association between time‐averaged SBP during the treatment period and risk of first stroke followed a U shape, with an increased risk above or below the reference range of 120 to <140 mm Hg (Figure 3). Compared with patients with time‐averaged SBP of 120 to <140 mm Hg (mean SBP: 131.5±5.2 mm Hg), the risk of first stroke was significantly higher in participants with time‐averaged SBP ≥140 mm Hg (mean SBP: 147.3±6.6 mm Hg; HR, 2.58 [95% CI, 1.46–4.56]) or <120 mm Hg (mean SBP: 116.7±3.0 mm Hg; HR, 3.43 [95% CI, 1.01–11.63]) (Table 5).
Figure 3.
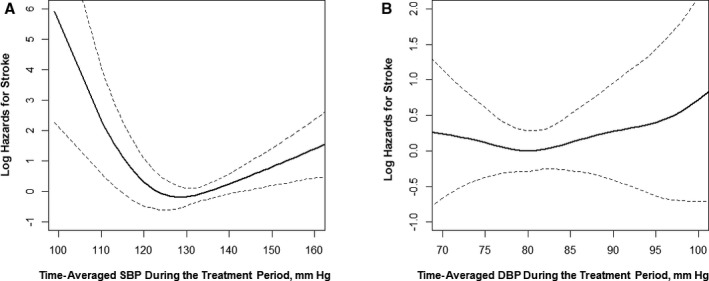
The association between time‐averaged systolic blood pressure (SBP) (A) or diastolic blood pressure (DBP) (B) during the treatment period and risk of first stroke. Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, systolic and diastolic blood pressure, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
Table 5.
Primary and Secondary Outcomes by Time‐Averaged SBP Levels During the Treatment Period
| Time‐Averaged SBP Category, mm Hg | Outcome, No. (%) | HR (95% CI) | Adjusteda HR (95% CI) |
|---|---|---|---|
| Primary outcome | |||
| First stroke | |||
| <120 | 3 (2.9) | 2.84 (0.85–9.43) | 3.43 (1.01–11.63) |
| 120 to <140b | 24 (1.1) | Ref | Ref |
| ≥140 | 25 (2.9) | 2.73 (1.56–4.77) | 2.58 (1.46–4.56) |
| Secondary outcomes | |||
| Composite of stroke, myocardial infarction, or death from cardiovascular causes | |||
| <120 | 4 (3.9) | 3.12 (1.10–8.87) | 3.80 (1.31–11.01) |
| 120 to <140 | 29 (1.3) | Ref | Ref |
| ≥140 | 28 (3.2) | 2.52 (1.50–4.24) | 2.37 (1.40–4.02) |
| All‐cause death | |||
| <120 | 5 (5.0) | 2.46 (0.98–6.18) | 3.41 (1.34–8.67) |
| 120 to <140 | 47 (2.1) | Ref | Ref |
| ≥140 | 35 (4.1) | 1.94 (1.25–3.01) | 1.63 (1.05–2.55) |
SBP indicates systolic blood pressure.
Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, systolic and diastolic blood pressure, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
Versus ≥140 mm Hg; adjusted hazard ratio (HR), 0.39; 95% CI, 0.22–0.69.
Furthermore, compared with patients with time‐averaged DBP ≥90 mm Hg (mean DBP: 93.9±3.7 mm Hg), the risk of first stroke was lower in participants with time‐averaged DBP <90 mm Hg (mean DBP: 79.8±5.5 mm Hg; HR, 0.41 [95% CI, 0.17–0.98]) (Table 6).
Table 6.
Primary and Secondary Outcomes by Time‐Averaged DBP During the Treatment Period
| Time‐Averaged DBP Category, mm Hg | Outcome, No. (%) | HR (95% CI) | Adjusteda HR (95% CI) |
|---|---|---|---|
| Primary outcome | |||
| First stroke | |||
| ≥90 | 7 (2.7) | Ref | Ref |
| <90 | 45 (1.5) | 0.56 (0.25–1.24) | 0.41 (0.17–0.98) |
| 80 to <90 | 23 (1.5) | 0.56 (0.24–1.30) | 0.45 (0.18–1.09) |
| 75 to <80 | 10 (1.2) | 0.41 (0.16–1.09) | 0.29 (0.10–0.83) |
| <75 | 12 (2.2) | 0.78 (0.31–1.99) | 0.46 (0.14–1.50) |
| Secondary outcomes | |||
| Composite of stroke, myocardial infarction, or death from cardiovascular causes | |||
| ≥90 | 8 (3.0) | Ref | Ref |
| <90 | 53 (1.8) | 0.57 (0.27–1.20) | 0.37 (0.16–0.84) |
| 80 to <90 | 26 (1.7) | 0.55 (0.25–1.21) | 0.41 (0.18–0.94) |
| 75 to <80 | 11 (1.3) | 0.40 (0.16–0.99) | 0.25 (0.09–0.69) |
| <75 | 16 (2.9) | 0.90 (0.39–2.11) | 0.49 (0.17–1.41) |
| All‐cause death | |||
| ≥90 | 7 (2.7) | Ref | Ref |
| <90 | 80 (2.7) | 0.98 (0.45–2.13) | 0.66 (0.29–1.50) |
| 80 to <90 | 35 (2.3) | 0.85 (0.38–1.91) | 0.69 (0.30–1.59) |
| 75 to <80 | 24 (2.7) | 0.98 (0.42–2.28) | 0.60 (0.24–1.49) |
| <75 | 21 (3.8) | 1.34 (0.57–3.17) | 0.60 (0.22–1.61) |
DBP indicates diastolic blood pressure; HR, hazard ratio.
Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, systolic and diastolic blood pressure, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
Similar results were observed for the composite cardiovascular events or all‐cause death (Figure 4, Tables 5 and 6).
Figure 4.
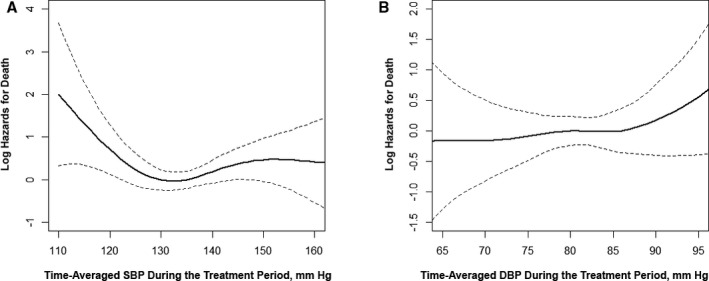
The association between time‐averaged systolic blood pressure (SBP) (A) or diastolic blood pressure (DBP) (B) during the treatment period and risk of all‐cause death. Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, SBP and DBP, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
Stratified Analyses for the Primary Outcome
Stratified analyses were performed by sex, age (<60 versus ≥60 years), baseline SBP levels (<150 [mean baseline SBP: 144.9±4.1 mm Hg] versus ≥150 mm Hg [mean: 155.1±3.1 mm Hg]), hypertension subtypes (isolated systolic hypertension or systolic‐diastolic hypertension), and study treatment groups (enalapril or enalapril‐folic acid group). The lower risk of first stroke was observed in participants with ≥25% of visits in which BP was <140/90 mm Hg (versus <25%) or time‐averaged SBP during the treatment period of 120 to <140 mm Hg (versus ≥140 mm Hg) across all subgroups (Figure 5).
Figure 5.
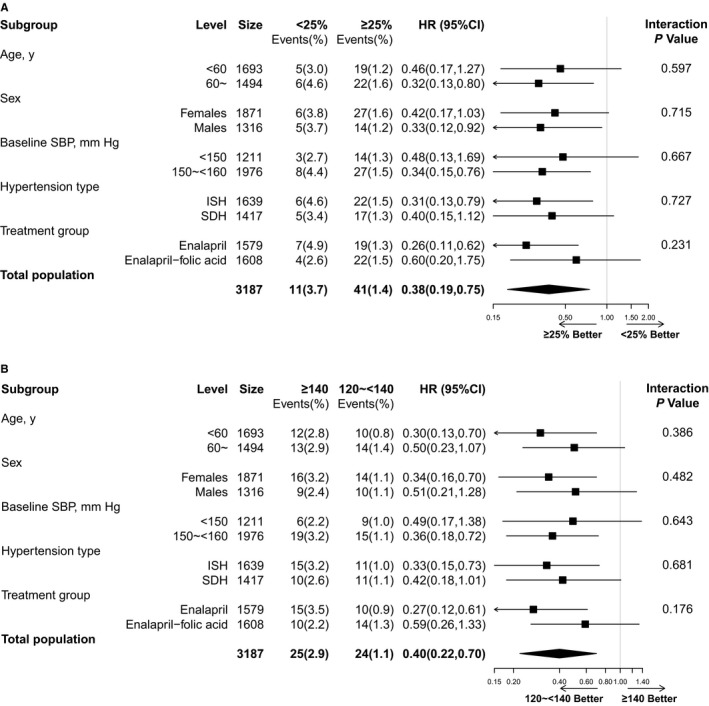
Primary outcome according to the proportion of visits in which blood pressure was <140/90 mm Hg (≥25% vs <25%) (A) or time‐averaged systolic blood pressure (SBP) during the treatment period (120 to <140 mm Hg vs ≥140 mm Hg) (B) in various subgroups. Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, SBP and DBP, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline. HR indicates hazard ratio; ISH, isolated systolic hypertension; SDH, systolic‐diastolic hypertension.
Regular concomitant medication was defined as ≥180 cumulative days of taking the drug of interest. The most common concomitant other antihypertensive drugs during the treatment period were dihydropyridine and hydrochlorothiazide, which were used in about 60% and 29% of the participants, respectively. Further adjustment of the concomitant other antihypertensive drugs during treatment period did not substantially change the results (Table 7). Moreover, although multiple imputations were used to deal with missing values in the current study, because of the relatively small number of missing data, the results did not vary meaningfully when missing data were treated as missing without the imputation (Table 8).
Table 7.
Impact of Achieved BP on First Stroke in Multivariate‐Adjusted Models Including Concomitant Other Antihypertensive Drugs During the Treatment Period
| Variables | Outcome, No. (%) | Adjusteda HR (95% CI) |
|---|---|---|
| Proportion of visits with BP <140/90 mm Hg | ||
| <25% | 11 (3.7) | Ref |
| 25% to <50% | 13 (1.8) | 0.46 (0.21–1.04) |
| 50% to <75% | 16 (1.3) | 0.32 (0.15–0.70) |
| ≥75% | 12 (1.3) | 0.34 (0.14–0.80) |
| P for trend | 0.015 | |
| Time‐averaged SBP, mm Hg | ||
| <120 | 3 (2.9) | 3.48 (1.00–12.06) |
| 120 to <140 | 24 (1.1) | Ref |
| ≥140 | 25 (2.9) | 2.63 (1.47–4.73) |
| Time‐averaged DBP, mm Hg | ||
| ≥90 | 7 (2.7) | Ref |
| <90 | 45 (1.5) | 0.40 (0.17–0.98) |
| 80 to <90 | 23 (1.5) | 0.46 (0.14–1.50) |
| 75 to <80 | 10 (1.2) | 0.28 (0.09–0.83) |
| <75 | 12 (2.2) | 0.44 (0.18–1.09) |
BP indicates blood pressure; DBP, diastolic blood pressure; HR, hazard ratio; SBP, systolic blood pressure.
Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, systolic and diastolic blood pressure, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline, as well as concomitant use of calcium channel blockers and diuretics during the treatment period.
Table 8.
Impact of Achieved BP on First Stroke Without Missing Data Imputation
| Variables | Outcome, No. (%) | Adjusteda HR (95% CI) |
|---|---|---|
| Proportion of visits with BP<140/90 mm Hg | ||
| <25% | 11 (3.8) | Ref |
| 25% to <50% | 13 (1.8) | 0.48 (0.21–1.07) |
| 50% to <75% | 16 (1.3) | 0.33 (0.15–0.73) |
| ≥75% | 12 (1.4) | 0.36 (0.16–0.83) |
| P for trend | 0.018 | |
| Time‐averaged SBP, mm Hg | ||
| <120 | 3 (3.1) | 3.44 (1.02–11.64) |
| 120 to <140 | 24 (1.1) | Ref |
| ≥140 | 25 (2.9) | 2.56 (1.45–4.52) |
| Time‐averaged DBP, mm Hg | ||
| ≥90 | 7 (2.7) | Ref |
| <90 | 45 (1.6) | 0.41 (0.17–1.00) |
| 80 to <90 | 23 (1.6) | 0.48 (0.15–1.54) |
| 75 to <80 | 10 (1.2) | 0.29 (0.10–0.86) |
| <75 | 12 (2.2) | 0.46 (0.19–1.12) |
BP indicates blood pressure; DBP, diastolic blood pressure; HR, hazard ratio; SBP, systolic blood pressure.
Adjusted for age, sex, study centers, methylenetetrahydrofolate reductase C677T polymorphism, study treatment groups, body mass index, smoking, alcohol drinking, systolic and diastolic blood pressure, estimated glomerular filtration rate, fasting glucose, total cholesterol, folate, vitamin B12, and homocysteine levels at baseline.
Discussion
Two types of data analysis11 were employed in the current study. First, the incidence and risk of outcomes were assessed according to the proportion of study visits in which BP was reduced to <140/90 mm Hg, to reflect the way physicians determine BP control and modify treatment strategy in real clinical practice. Second, outcomes were calculated according to the time‐averaged BP levels during the treatment period. The results of all of these analyses suggested that achieved BP <140/90 mm Hg was significantly associated with decreased risks of stroke and composite of cardiovascular events or all‐cause death in patients with grade 1 hypertension and without a history of major cardiovascular diseases. The absolute rate of stroke was relatively small (about 0.35% per year) in our current study. However, compared with participants with <25% of the visits in which BP was <140/90 mm Hg (stroke incidence: 3.7%), the incidence of stroke decreased significantly in those with ≥25% (absolute stroke risk reduction: 2.3%; HR, 0.38 [95% CI, 0.19–0.75]) of the visits with BP<140/90 mm Hg (Figure 5). Both the absolute and the relative risk reductions were considerable. More important, the beneficial results were consistent across age, sex, baseline SBP, hypertension subtypes, and treatment groups. In addition, only 1.5% of the participants discontinued the treatments because of adverse reaction. Since a large proportion of hypertensive patients are individuals with grade 1 hypertension and without cardiovascular disease, our findings have important implications.
A previous meta‐analysis3 of 4 randomized trials, including 8912 participants, found that antihypertensive treatment for adults with grade 1 hypertension did not reduce mortality or cardiovascular disease risk. However, a recent meta‐analysis12 including 6361 additional participants from the Blood Pressure Lowering Treatment Trialists’ Collaboration (BPLTTC) data sets concluded that BP‐lowering therapy was likely to prevent stroke and death in patients with uncomplicated grade 1 hypertension. Nevertheless, most of the patients in BPLTTC trials had diabetes mellitus and were under antihypertensive treatment at baseline. These results indicate that many of the high‐risk patients (with diabetes mellitus) in BPLTTC trials—if untreated—may have been above the range defining grade 1 hypertension. The recent meta‐analysis conducted by Thomopoulos et al13 included trials or trial subgroups with mean baseline SBP/DBP levels in grade 1 hypertension's definition range and a low to moderate risk (<5% cardiovascular deaths in 10 years in controls): Felodipine Event Reduction (FEVER) stratum with baseline SBP below the median (mean baseline SBP/DBP: 144/89 mm Hg and with baseline antihypertensive treatments)14; Hypertension Detection and Follow‐up Program [HDFP] stratum with baseline DBP 90 to 94 mm Hg15; OSLO16; Treatment of Mild Hypertension Study (TOMHS); mean baseline SBP/DBP: 140/91 mm Hg)17; and US Public Health Service (USPHS).18 This meta‐analysis suggested that BP‐lowering treatment significantly decreased the risk of stroke, coronary events, and all‐cause death. These results provided stronger support to the recommendation to initiate drug treatment in patients with grade 1 low to moderate risk hypertension. However, stratification of trials in grades according to the mean baseline SBP/DBP values was just an approximation.19 The trials included in the meta‐analyses, especially the Oslo (mean baseline SBP/DBP: 157/97 mm Hg)16 and USPHS (mean baseline SBP/DBP: 147/99 mm Hg)18 studies may include a number of patients with baseline BP higher than the current definition for grade 1 hypertension (untreated SBP of 140–159 mm Hg and/or DBP of 90–99 mm Hg). Therefore, extrapolation of the findings from these meta‐analyses to uncomplicated grade 1 hypertension patients remains to be determined.
The time‐averaged SBPs during the treatment period were 151.6, 142.0, 134.4, and 126.1 mm Hg in patients with <25%, 25% to 50%, 50% to 75%, and ≥75%, respectively, of the study visits in which BP was reduced to <140/90 mm Hg (Table 1). The greatest reduction in risk (primary or secondary outcomes, about 2% of the absolute risk reduction) always occurred when the proportion of visits with BP <140/90 mm Hg progressed from <25% to 25–<50%, with a further modest reduction when the proportion increased from 25–<50% to 50–<75% (about 0.5% of the absolute risk reduction), and with no further obvious reduction or a slight increase when the proportion increased to from 50–<75% to ≥75%. These results suggested that in patients with grade 1 hypertension and without cardiovascular diseases, more aggressive SBP reduction may not offer substantial advantages. Accordingly, our study also suggested that the risk of first stoke, composite of cardiovascular events, or all‐cause death were all significantly increased in participants with time‐averaged SBPs <120 mm Hg, compared with those with time‐averaged SBPs of 120 to <140 mm Hg. Consistent with our results, some of the previous studies found that an SBP <120 mm Hg was associated with an increased risk of stroke (versus 130–<140 mm Hg; Prevention Regimen For Effectively Avoiding Second Strokes [PROFESS] trial20) or all‐cause death (versus 120–130 mm Hg; the International Verapamil SR‐Trandolapril Study [INVEST]21). However, targeting an SBP of <120 mm Hg (intensive group), compared with <140 mm Hg (standard group), has been reported to result in lower rates of stroke in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial22 and lower rates of composite cardiovascular events in Systolic Blood Pressure Intervention Trial (SPRINT).23 The possible explanations for these inconsistent results may be that, first, the average SBP in the intensive treatment group were 119.3 and 121.4 mm Hg in the ACCORD and SPRINT trials, respectively, which indicated that many of the participants in the intensive group did not reach the goal of an SBP <120 mm Hg, and instead may have had an SBP of 120 to <130 mm Hg. Second, it has been argued that, if measured by the usual office technique, the SPRINT BP values would likely be higher than those reported.19, 24 Third, common sense indicates that a J curve must exist for BP. However, the “nadir” SBP values may possibly vary in hypertensive patients with different ethnic backgrounds or concomitant baseline diseases. We agree that the controversy of “the lower the better” versus the J curve can only be solved by a series of well‐designed randomized trials, such as the ongoing Stroke in Hypertension Optimal Treatment (SHOT) study.25 Nevertheless, our current findings emphasize that patients with grade 1 hypertension should possibly avoid excessive SBP reduction during treatment.
Furthermore, it has been recognized that most of the previous trials showing the benefits of antihypertensive treatment in the elderly or in patients with isolated systolic hypertension have enrolled participants with baseline SBP ≥160 mm Hg (grade 2 or 3 hypertension).4 In our current analysis, the lower risk of first stroke was found in participants with ≥25% of the visits in which BP was <140/90 mm Hg (versus <25%) or time‐averaged SBP during the treatment period at 120 to <140 mm Hg (versus ≥140 mm Hg) in subgroups with different ages (<60 versus ≥60 years), different baseline SBP levels (<150 versus ≥150 mm Hg), and different hypertension subtypes (isolated systolic hypertension or systolic‐diastolic hypertension). However, these results still warrant further confirmation.
Study Limitations
This study has several limitations. First, this was a post hoc analysis of the CSPPT. Despite extensive adjustments for known factors and the benefits being consistent across different outcomes, we could not exclude the possibility that unrecorded risk factors may explain some of our findings. Second, only a small number of patients had a time‐averaged SBP <120 mm Hg or a time‐averaged DBP ≥90 mm Hg, which means that comparisons of the risk of study outcomes involved groups of markedly different sizes, some of which were small. Third, because of the small numbers of events, we could not determine the optimal BP levels below 140/90 mm Hg in grade 1 hypertension. Furthermore, the time‐averaged BP reflects the effect of long‐term control of BP. The major problem with time‐averaged BP is the difficulty in implementing it shortly after the start of treatment. It will take some time to have a good idea of a patient's time‐averaged BP in a prospective context. Overall, the CSPPT was not specifically designed to determine the BP goal for preventing cardiovascular diseases. Our current results indicate the possible beneficial or detrimental effect when the long‐term averaging BP was reduced to a certain level. Therefore, confirmation of our findings in a large‐scale clinical trial of randomized participants with uncomplicated grade 1 hypertension to different BP targets is essential.
Conclusions
Among patients with grade 1 hypertension and without cardiovascular diseases, achieved BP <140/90 mm Hg was significantly associated with a decreased risk of stroke or all‐cause death both when data were calculated as a proportion of visits with BP <140/90 mm Hg or as on‐treatment time‐averaged BP.
Sources of Funding
The study was supported by funding from the following: the Projects of National Natural Science Foundation of China (81473052, 81402735); National Key Research and Development Program (2016YFC0903100); National Key Technologies R&D Program (2016 YFC0904900); NSFC Innovative Group grant (81521003); the National Clinical Research Center for Kidney Disease, Nanfang Hospital, Nanfang Medical University, Guangzhou, China; the State Key Laboratory for Organ Failure Research, Nanfang Hospital, Nanfang Medical University, Guangzhou, China; the Department of Development and Reform, Shenzhen Municipal Government (grant SFG 20201744); the Special Project on the Integration of Industry, Education and Research of Guangdong Province (2011A091000031); and the Science and Technology Planning Project of Guangdong Province, China (2014B090904040, 201604020003). The funders had no role in the design and/or conduct of the study (data collection, management, analysis, and interpretation) or in the preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
Disclosures
Dr Qin reports grants from the National Science Foundation and consulting fees from AUSA Research Institute, Shenzhen AUSA. Dr Sun reports grants from the Ministry of Science and Technology of the People's Republic of China, and Major State Basic Research Development Program of China. Dr JiGuang Wang reports receiving lecture and consultation fees from Daiichi‐Sankyo, MSD, Novartis, Pfizer, Sanofi, and Servier. Dr Binyan Wang reports consulting fees from AUSA Research Institute, Shenzhen AUSA. Dr Huo reports grants from the National Major Scientific and Technological Special Project and nonfinancial support from Shenzhen AUSA. Dr Xin Xu reports grants from the Major Scientific and Technological Planning Project of Guangzhou; the Science, Technology and Innovation Committee of Shenzhen; and personal fees from AUSA Research Institute, Shenzhen AUSA. Professor Xiping Xu reports grants from National Science and Technology Major Projects of China and grants from the Natural Science Foundation of China. Dr Hou reports grants from the Major State Basic Research Development Program of China; Ministry of Science and Technology of the People's Republic of China; the National Clinical Research Center for Kidney Disease; and the State Key Laboratory for Organ Failure Research, Guangzhou, China. No other disclosures were reported.
(J Am Heart Assoc. 2017;6:e005247. DOI: 10.1161/JAHA.116.005247.)
Contributor Information
Xiping Xu, Email: xipingxu126@126.com.
Fan Fan Hou, Email: ffhouguangzhou@163.com.
References
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Franklin SS, Jacobs MJ, Wong ND, L'Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle‐aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. [DOI] [PubMed] [Google Scholar]
- 3. Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev. 2012;8:CD006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members . 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 5. Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet JC, Jean‐Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. [DOI] [PubMed] [Google Scholar]
- 6. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 7. National Institute for Health and Clinical Excellence . Hypertension (CG127): clinical management of primary hypertension in adults. Available at: http://www.nice.org.uk/guidance/cg127. Accessed August, 2011.
- 8. Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, Cloutier L, Gelfer M, Lamarre‐Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tobe SW, Ruzicka M, Burns KD, Vallée M, Prasad GV, Lebel M, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E, Herman RJ, Bacon SL, Rabkin SW, Gilbert RE, Campbell TS, Grover S, Honos G, Lindsay P, Hill MD, Coutts SB, Gubitz G, Campbell NR, Moe GW, Howlett JG, Boulanger JM, Prebtani A, Larochelle P, Leiter LA, Jones C, Ogilvie RI, Woo V, Kaczorowski J, Trudeau L, Petrella RJ, Hiremath S, Drouin D, Lavoie KL, Hamet P, Fodor G, Grégoire JC, Lewanczuk R, Dresser GK, Sharma M, Reid D, Lear SA, Moullec G, Gupta M, Magee LA, Logan AG, Harris KC, Dionne J, Fournier A, Benoit G, Feber J, Poirier L, Padwal RS, Rabi DM; CHEP Guidelines Task Force . Hypertension Canada's 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569–588. [DOI] [PubMed] [Google Scholar]
- 9. Zanchetti A. Do we over treat mild hypertension? Expert Opin Pharmacother. 2015;16:1121–1126. [DOI] [PubMed] [Google Scholar]
- 10. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, Yang T, Xiao C, Zhao G, Dong Q, Zhu D, Wang X, Ge J, Zhao L, Hu D, Liu L, Hou FF; CSPPT Investigators . Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Kjeldsen SE, Zappe DH, Holzhauer B, Hua TA, Zanchetti A, Julius S, Weber MA. Cardiovascular outcomes at different on‐treatment blood pressures in the hypertensive patients of the VALUE trial. Eur Heart J. 2016;37:955–964. [DOI] [PubMed] [Google Scholar]
- 12. Sundström J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, Woodward M, Neal B; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of blood pressure reduction in mild hypertension: a systematic review and meta‐analysis. Ann Intern Med. 2015;162:184–191. [DOI] [PubMed] [Google Scholar]
- 13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 2. Effects at different baseline and achieved blood pressure levels—overview and meta‐analyses of randomized trials. J Hypertens. 2014;32:2296–2304. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Zhang X, Liu L, Zanchetti A. Is a systolic blood pressure target <140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J. 2011;32:1500–1508. [DOI] [PubMed] [Google Scholar]
- 15. Davis BR, Ford CE. The hypertension detection follow‐up program In: Black HR, ed. Clinical Trials in Hypertension. New York, NY: Marcel Dekker, Inc.; 2001:27–60. [Google Scholar]
- 16. Helgeland A. Treatment of mild hypertension: a five year controlled drug trial. The Oslo study. Am J Med. 1980;69:725–732. [DOI] [PubMed] [Google Scholar]
- 17. Neaton JD, Grimm RH Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, Cutler JA, Flack JM, Schoenberger JA, McDonald R. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270:713–724. [PubMed] [Google Scholar]
- 18. Smith WM. Treatment of mild hypertension: results of a ten‐year intervention trial. Circ Res. 1977;40:I98–I105. [PubMed] [Google Scholar]
- 19. López‐Jaramillo P, Coca A, Sánchez R, Zanchetti A; Latin American Society of Hypertension . Hypertension guidelines: is it time to reappraise blood pressure thresholds and targets? Position statement of the Latin American Society of Hypertension. Hypertension. 2016;68:257–262. [DOI] [PubMed] [Google Scholar]
- 20. Ovbiagele B, Diener HC, Yusuf S, Martin RH, Cotton D, Vinisko R, Donnan GA, Bath PM; PROFESS Investigators . Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA. 2011;306:2137–2144. [DOI] [PubMed] [Google Scholar]
- 21. Cooper‐DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ACCORD Study Group , Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail‐Beigi F. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. SPRINT Research Group , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kjeldsen SE, Lund‐Johansen P, Nilsson PM, Mancia G. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension. 2016;67:808–812. [DOI] [PubMed] [Google Scholar]
- 25. Zanchetti A, Liu L, Mancia G, Parati G, Grassi G, Stramba‐Badiale M, Silani V, Bilo G, Corrao G, Zambon A, Scotti L, Zhang X, Wang H, Zhang Y, Zhang X, Guan TR, Berge E, Redon J, Narkiewicz K, Dominiczak A, Nilsson P, Viigimaa M, Laurent S, Agabiti‐Rosei E, Wu Z, Zhu D, Rodicio JL, Ruilope LM, Martell‐Claros N, Pinto F, Schmieder RE, Burnier M, Banach M, Cifkova R, Farsang C, Konradi A, Lazareva I, Sirenko Y, Dorobantu M, Postadzhiyan A, Accetto R, Jelakovic B, Lovic D, Manolis AJ, Stylianou P, Erdine S, Dicker D, Wei G, Xu C, Xie H, Coca A, O'Brien J, Ford G. Blood pressure and LDL‐cholesterol targets for prevention of recurrent strokes and cognitive decline in the hypertensive patient: design of the European Society of Hypertension‐Chinese Hypertension League Stroke in Hypertension Optimal Treatment randomized trial. J Hypertens. 2014;32:1888–1897. [DOI] [PubMed] [Google Scholar]


