Abstract
Background
The incidence, predictors, and impact of atrial arrhythmias along with left atrial structural changes in patients with left ventricular assist devices (LVADs) remain undetermined.
Methods and Results
All patients who underwent LVAD implantation from 2008 to 2015 at the University of Chicago Medical Center were included. Electronic medical records, electrocardiograms, echocardiograms, and cardiac electrical device interrogations were reviewed. The association of arrhythmias and clinical covariates with survival was evaluated by Kaplan–Meier and Cox proportional hazards analyses. A total of 331 patients were followed for a median of 330 days (range 0–2306 days). Mean age was 57.8±12.8 years, 256 participants (77.3%) were male, mean left ventricular ejection fraction was 20±6.6%, and 124 (37.5%) had ischemic cardiomyopathy. Atrial arrhythmias (53.8%) were highly prevalent and frequently coexisted before LVAD implantation: atrial fibrillation (AF) in 45.9%, atrial flutter in 13.9%, atrial tachycardia in 6.9%, and atrioventricular nodal reentrant tachycardia in 1.2%. New‐onset AF was documented in 14 patients (7.8% of patients without prior AF) after the first 30 days with an LVAD. Increasing age, renal insufficiency, and lung disease were predictors of new‐onset AF after LVAD implantation. Of patients with paroxysmal AF, 43% had no further AF after LVAD. Left atrial size and volume index improved with LVAD (P<0.005). History of persistent AF, atrial tachycardia, ventricular arrhythmia, coronary artery bypass, and low albumin were associated with decreased survival.
Conclusions
Atrial arrhythmias are significantly prevalent in patients who require LVAD and are associated with increased mortality; however, LVADs induce favorable atrial structural and electrical remodeling.
Keywords: atrial tachyarrhythmia, atrium, left ventricular assist device, remodeling
Subject Categories: Heart Failure, Catheter Ablation and Implantable Cardioverter-Defibrillator, Remodeling
Introduction
Atrial arrhythmias (AAs), particularly atrial fibrillation (AF), are common in patients with heart failure.1, 2 Recently, the Framingham Heart Study demonstrated that AF occurs in more than half of persons with heart failure with reduced ejection fraction.3 Although the exact mechanism of AA in heart failure is not known, it is recognized that AAs can be initiated or maintained by the complex electroanatomical remodeling in atria that is triggered by stretch, neurohormonal activation, and oxidative stress.4, 5, 6, 7, 8, 9
Left ventricular assist devices (LVADs) have become the mainstay of therapy for patients with advanced heart failure, either as a bridge to transplantation or as a destination therapy.10 Currently, a majority of the LVADs in the United States are implanted as destination therapy. Although mechanical decompression of the left ventricle via LVAD is known to induce significant ventricular remodeling, the effect of LVADs on AAs and the atrial structural and electrical substrate is not well described.11, 12
In the current study, we hypothesized that LVAD implantation may have a significant impact on AAs with associated atrial remodeling through alteration in atrial filling pressures and perfusion. Conversely, AAs may affect overall clinical outcome including long‐term survival in patients with heart failure. To test these hypotheses, the incidence and prevalence of AAs, left atrial (LA) sizes, and atrial conduction intervals were evaluated before and after LVAD implantation. The long‐term survival in patients with and without AA was estimated, and predictors of the development of AAs and mortality were determined.
Methods
All consecutive patients (n=331) who underwent LVAD implantation at the University of Chicago Medical Center between January 2008 and April 2015 were included in this retrospective analysis. The study was approved by the hospital institutional review committee, and patients provided informed consent.
Data Collection
Data were abstracted from a centralized electronic medical record at the University of Chicago Medical Center. Medical records, ECGs, cardiac implantable electrical device interrogations, and echocardiograms were reviewed to determine arrhythmia incidence, atrial conduction intervals, atrial structure, and clinical outcome. Surface ECGs (12‐lead) were reviewed for underlying rhythm and conduction intervals. P‐wave duration and PR interval were measured in leads II and V1. A PR interval >200 ms was considered prolonged. Echocardiograms in the year prior to LVAD implantation and at latest follow‐up were reviewed for LA size and volume. LA sizes were graded from 0 to 3 based on 2‐dimensional LA volume (0=normal, 1=mildly enlarged, 2=moderately enlarged, and 3=severely enlarged).13 Incident AA during follow‐up was adjudicated by review of all ECGs, review of cardiac implantable device interrogations by the patient's treating electrophysiologist, and review of inpatient telemetry by the patient's inpatient treating cardiologist. Baseline history of arrhythmia was abstracted from medical history obtained by the patient's treating cardiologist. When available, this information was confirmed with prior ECGs and cardiac implantable device interrogations. Using the standard definition, AF was categorized as paroxysmal AF (PAF) and nonparoxysmal AF including persistent, long‐standing persistent, and permanent AF (PeAF).2 AF was considered postoperative if it occurred within 30 days of implantation in patients with no prior history of AF. Recurrence of PAF in patients with PAF was defined as AF occurring >30 days after LVAD implantation.
Follow‐up
Patients were followed from the time of LVAD implantation until death, transplantation, or the end of the review period in December 2015. A minority of the patients (<5%) had abbreviated follow‐up after initial monitoring due to transfer to another center.
Statistical Analysis
Baseline categorical variables were investigated using χ2 testing, and continuous variables were analyzed with ANOVA. Survival analysis after LVAD implantation was conducted by Kaplan–Meier time‐to‐death curves, which were stratified by pre‐ and postoperative history of arrhythmias. Right‐sided censoring was used to evaluate patients who were transplanted or explanted. The association of arrhythmias and other clinical covariates with survival was further evaluated using Cox proportional hazards regression. Observed survival in the main cohort and subgroups was compared by means of the 1‐sample log‐rank test. Univariate and multivariate associations between baseline variables and survival were assessed by means of the log‐rank test and a Cox regression model. The following variables were considered potential predictors of development of AF: age, sex, ischemic heart disease, valvular heart disease, enlarged LA, conduction intervals, left ventricle dysfunction, coronary artery bypass grafting, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, thyroid disease, creatinine, albumin, and obstructive sleep apnea. All significant univariate predictors were included as potential predictors in multivariate models. Multivariate models are presented in the form of point estimates of the hazard ratios, with 95% CIs.
Results
Patient Characteristics
A total of 331 consecutive patients (75 women and 256 men) underwent LVAD implantation during the study period. A majority of the patients (57.8%) received LVAD as destination therapy. The median follow‐up period was 330 days (range 0–2306 days). At baseline, patients with AF were older and had a higher incidence of ischemic cardiomyopathy. Less than 2% of patients had pulsatile LVAD. Detailed baseline characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Study Cohort n=331 | No AF n=179 (54%) | PAF n=129 (39%) | PeAF n=23 (6.9%) |
|---|---|---|---|---|
| Female, n (%) | 75 (22.7) | 43 (24) | 30 (23) | 2 (8.6) |
| Age, mean yearsa | 57.8±12.8 | 54.3±13.6 | 61.3±10.5 | 66.0±9.2 |
| Left ventricular ejection fraction, %a | 20.0±6.6 | 19.2±7.0 | 20.6±5.9 | 22.9±6.2 |
| LA volume >40 mL/m2, n (%) | 253 (76.4) | 128 (72.0) | 105 (81.4) | 20 (87.0) |
| PR interval >200 ms, %a | 11 | 8.7 | 14.8 | 30.4 |
| Ischemic cardiomyopathy, n (%)a | 124 (37.5) | 56 (31.2) | 54 (41.9) | 14 (60.9) |
| Valvular heart disease, n (%) | 225 (68.0) | 117 (65.3) | 91 (70.5) | 17 (73.9) |
| Hypertension, n (%) | 250 (75.5) | 135 (75.4) | 99 (76.7) | 16 (69.6) |
| Diabetes mellitus, n (%) | 163 (49.2) | 82 (45.8) | 69 (53.4) | 12 (52.1) |
| Thyroid disease, n (%) | 83 (24.8) | 36 (20.6) | 38 (29.5) | 8 (34.8) |
| Obstructive sleep apnea, n (%) | 99 (30.0) | 53 (30.0) | 38 (29.5) | 8 (34.8) |
| COPD, n (%) | 90 (27) | 44 (24.6) | 38 (29.5) | 8 (34.8) |
| CABG, n (%) | 111 (33.5) | 52 (29.1) | 48 (37.2) | 11 (47.8) |
| Baseline creatinine, mg/dL | 1.6±0.9 | 1.6±1.0 | 1.7±0.9 | 1.6±0.6 |
| Baseline albumin, g/dL | 3.5±0.5 | 3.5±0.6 | 3.6±0.5 | 3.6±0.5 |
AF indicates atrial fibrillation; CABG, coronary arterial bypass grafting; COPD, chronic obstructive pulmonary disease; LA, left atria; PAF, paroxysmal atrial fibrillation; PeAF, persistent or permanent atrial fibrillation.
P<0.05 for χ2 or ANOVA comparison between groups.
Cardiac implantable electrical device interrogations were available for a majority of patients with cardiac resynchronization therapy, with defibrillator in 35.6%, dual chamber implantable cardioverter‐defibrillator in 14.2%, single‐chamber defibrillator in 13.9%, subcutaneous defibrillator in 0.3%, and pacemaker‐only devices in 1.2% of patients. In the remaining patients, cardiac device interrogation did not occur during follow‐up, the patient did not have a cardiac implantable electrical device, or the device was removed at the time of LVAD implantation.
Prevalence of AAs Before LVAD
AAs were highly prevalent prior to LVAD implantation and were present in 178 patients (53.8%) in this cohort (Figure 1). The most common AA was AF, seen in 152 (45.9%) patients. AF was predominantly paroxysmal in these patients (84.9%). The second most common AA was atrial flutter (AFL), experienced by 46 patients (13.9%). Atrial tachycardia (AT) was documented in 23 patients (6.9%). Atrioventricular nodal reentrant tachycardia was the least common AA, found in 4 patients (1.2%). There was no documented atrioventricular reentrant tachycardia. AAs demonstrated significant coexistence in this cohort before LVAD implantation (Table 2).
Figure 1.
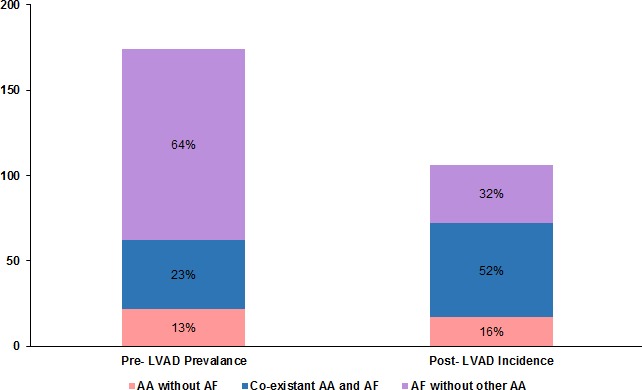
Prevalence of AAs before LVAD implantation and incidence of AAs after LVAD. AAs including AF, atrial flutter, and atrial tachycardia were highly prevalent and coexistent prior to LVAD implantation. The incidence of new‐onset AAs and AF after LVAD was relatively decreased but significant. AAs indicates atrial arrhythmias; AF, atrial fibrillation; LVAD, left ventricular assist device.
Table 2.
Predictors of Recurrence and New‐Onset AF
| Factor | Univariate Analysis | Multivariate Analyses | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Predictors of new‐onset AF | ||||||
| Increasing age | 1.04 | 1.01–1.07 | 0.007 | 1.04 | 1.01–1.07 | 0.01 |
| COPD | 2.20 | 1.05–4.57 | 0.03 | 2.28 | 1.05–4.89 | 0.03 |
| High creatinine | 1.40 | 1.00–2.03 | 0.049 | 1.50 | 1.07–2.22 | 0.03 |
| Predictors of AF recurrence | ||||||
| Increasing age | 1.06 | 1.03–1.08 | <0.00001 | 1.04 | 1.02–1.07 | 0.001 |
| PR >200 ms | 2.35 | 1.12–4.99 | 0.02 | 2.46 | 1.11–5.48 | 0.03 |
| Ischemic cardiomyopathy | 2.25 | 1.43–3.57 | 0.0005 | … | … | … |
| CABG | 1.60 | 1.01–2.54 | 0.046 | … | … | … |
| COPD | 1.97 | 1.21–3.23 | 0.007 | … | … | … |
| Thyroid disease | 1.70 | 1.03–2.83 | 0.04 | … | … | … |
AF indicates atrial fibrillation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Incidence of AAs Following LVAD
New‐onset AAs were documented in 104 patients (31%) following LVAD implantation, including postoperative AAs in first 30 days after surgery (Figure 1). A total of 26 patients with new‐onset arrhythmias had coexistence of ≥2 AAs. New‐onset AF was documented in 14 patients after the first 30 days of LVAD. Postoperative AF occurred in 37 patients with no previous history of AF. AT was the second most common new‐onset AA after LVAD and was detected in 41 patients. New‐onset AFL was documented in 31 patients, and atrioventricular nodal reentrant tachycardia was diagnosed in 4 patients after LVAD. In patients with PAF, either de novo or recurrent, 8.5% progressed to PeAF during the study period.
Notably, in 43% of patients with pre‐LVAD history of AF, no further evidence of AF was demonstrated during post‐LVAD follow‐up. This was also the case in the subgroup of patients with atrial leads (n=152) in which only 24% of patients with history of AF before LVAD had episodes of AF in follow‐up device interrogation. The incidence and proportion of new‐onset AAs in this subset were also similar to the entire cohort with the exception of new atrioventricular nodal reentrant tachycardia, which was detected entirely within the subgroup of patients with atrial leads. The subgroup of patients with atrial leads did not differ from those without, other than with respect to age (mean age 60 versus 56 years, P=0.005), AFL history (18% versus 10%, P=0.04), history of ventricular tachycardia or fibrillation (34% versus 23%, P=0.04), and history of chronic obstructive pulmonary disease (22 versus 34, P=0.02).
Predictors of New‐Onset and Recurrent AF
All baseline characteristics at the time of LVAD implantation were assessed as potential predictors of the development or recurrence of AF. Among those coexisting conditions, in univariate and multivariate analyses, predictors of the development of new‐onset AF were increasing age, increasing baseline creatinine, and history of chronic obstructive pulmonary disease (Table 2). Univariate analysis also demonstrated that increasing age, ischemic cardiomyopathy, history of coronary artery bypass grafting, chronic obstructive pulmonary disease, thyroid disease, and PR interval >200 ms were predictors of recurrent AF episodes after LVAD implant in patients with prior AF history. In multivariate analyses, increasing age at implant and PR interval >200 ms remained predictors of recurrent AF (Table 2).
Atrial Remodeling
Transthoracic echocardiograms showed considerably enlarged left atria in a majority of patients before LVAD implantation, with a mean baseline LA volume index of 56.8 mL/m2 (Figure 2). LVAD caused significant changes in atrial structure by reducing LA size and volume index, as shown in Figure 2. Follow‐up echocardiogram at a median of 290 days after LVAD implantation showed that mean LA volume index was decreased to 46.8 mL/m2 (P=0.0005). Mean baseline LA descriptions decreased from 2.6±0.04 (2=moderately dilated, 3=severely dilated) to 2.0±0.1 (P=0.005).
Figure 2.
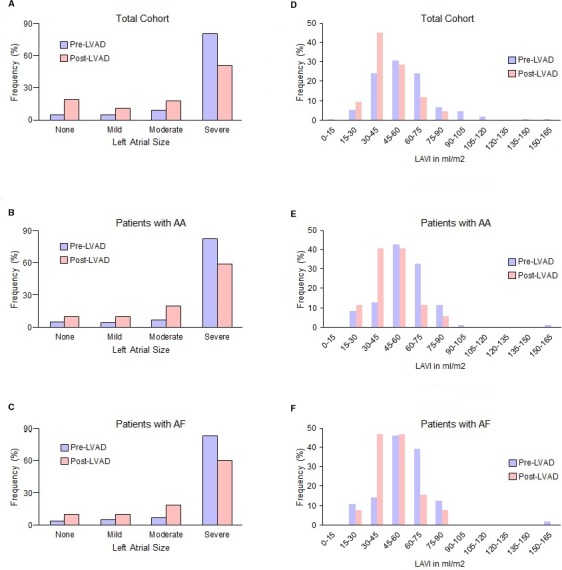
Impact of LVAD on LA structure. LA size and LAVI were evaluated by transthoracic echocardiogram before and after LVAD implantation. LA sizes were graded as 0 (normal), 1 (mild), 2 (moderate), and 3 (severe). The patients with end‐stage heart failure had significant LA enlargement before LVAD implantation; however, LA size (A through C) and LAVI (D through F) significantly decreased with LVAD in the overall cohort and in subgroups of patients with atrial fibrillation (AF) or any AAs. AAs indicates atrial arrhythmias; LA, left atrial; LVAD, left ventricular assist device; LAVI, left atrial volume index.
Although follow‐up echocardiogram occurred in most patients, LA volumes were not directly evaluated in all cases because of technical reasons. LA descriptions were available for 314 patients (98%) prior to LVAD and 170 (51.4%) after LVAD. LA volume index was available for 149 patients (45%) prior to LVAD and 42 patients (12.7%) after LVAD. Patients with LA echocardiogram follow‐up were more often female (32% versus 18%, P=0.03) and had less ischemic cardiomyopathy (31% versus 44%, P=0.02) than those without but were otherwise similar in baseline characteristics.
LA descriptions and LA volume index data were consistent among patients with both measurements in >95% of cases. LA volume index and LA size descriptions from patients followed for <1 year were not significantly different from those of patients followed for >1 year. This change in LA geometry was also consistent when only patients with echocardiogram data before and after LVAD were considered. Atrial conduction intervals including P‐wave duration and PR interval were also measured by using surface ECG. P‐wave duration (114±25 ms) was comparable in study patients with and without AF. A total of 11% of the overall cohort demonstrated PR prolongation before LVAD (Table 1). The PR interval was significantly prolonged in patients with AF, progressing as the AF burden increased, with more PR prolongation in PAF (14.8%) and PeAF (30.4%) than in patients with no AF (8.7%; P<0.05).
Coexistence of AAs
In this study cohort, AAs commonly coexisted before and after LVAD implantation, with AF being the most common (Table 3). AFL was the second most common AA and was 3 times more common in patients with AF compared with those without history of AF (P<0.05). AFL co‐occurred with AF in 34 patients (10.5%), whereas AFL without AF was present in 12 patients (3.6%). AT and atrioventricular nodal reentrant tachycardia had a similar but nonsignificant association with AF. Prior to LVAD, AT was documented in 23 patients (6.9%) and was coexistent with AF in 11 patients (Table 3).
Table 3.
Prevalence and Coexistence of Other Atrial Arrhythmias With AF Before and After LVAD Implantation
| No AF | PAF | PeAF | |
|---|---|---|---|
| Pre‐LVAD, n (%) | 179 (54.1) | 129 (39) | 23 (6.9) |
| AFL, n (%)* | 12 (6.7) | 31 (24) | 3 (13) |
| AT, n (%) | 11 (6.1) | 11 (8.5) | 1 (4.3) |
| AVNRT, n (%) | 3 (1.7) | 1 (0.8) | 0 (0) |
| Post‐LVAD, n (%) | 132 (39.9) | 165 (49.8) | 34 (10.3) |
| AFL, n (%)* | 15 (11.4) | 56 (33.9) | 6 (17.6) |
| AT, n (%) | 21 (15.9) | 38 (23.0) | 5 (14.7) |
| AVNRT, n (%) | 5 (3.8) | 3 (1.8) | 0 (0.0) |
AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; AVNRT, atrioventricular nodal reentrant tachycardia; LVAD, left ventricular assist device; PAF, paroxysmal atrial fibrillation; PeAF, persistent/permanent atrial fibrillation.
*P<0.05.
Impact of AAs on Survival
In the overall cohort, the median duration of survival was 660 days of mechanical support. A history of any AA prior to LVAD implantation was not associated with decreased survival in Kaplan–Meier analysis; however, when subdivided into PAF and PeAF, increasing arrhythmia burden demonstrated a nonsignificant trend toward reduced survival (Figure 3A, P=0.057). Furthermore, when compared with all other patients, patients with PeAF prior to LVAD had reduced survival (P=0.02, 1‐year survival 63% versus 43%) (Figure 3A). Similarly, patients with a history of AT prior to LVAD had reduced survival (P=0.004, 1‐year survival 63% versus 41%) (Figure 3B). A history of AFL did not demonstrate an impact on survival. After LVAD implantation, the development of AF in patients with no prior history of AF was associated worsened survival in a multivariate Cox regression that treated AF status as a time‐dependent variable. This finding remained significant when postoperative AF was not included.
Figure 3.
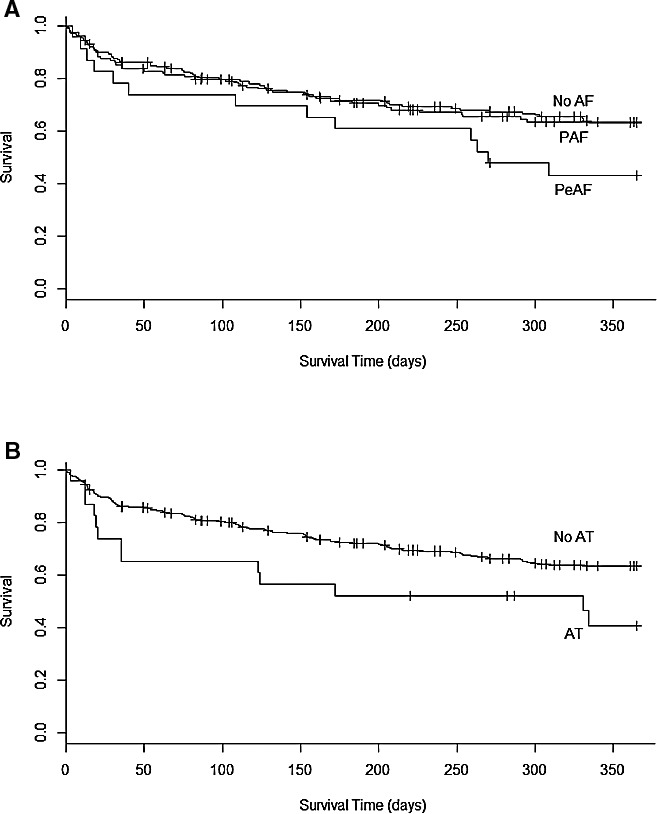
Long‐term survival in patients with LVAD. Kaplan–Meier analysis shows the impact of atrial arrhythmias on long‐term survival of patients with LVAD. As shown, survival of patients who had PAF and PeAF at baseline was significantly worse than patients without AF (A). Patients with AT showed significantly worse survival than patients with no AT (B). AF indicates atrial fibrillation; AT, atrial tachycardia; LVAD, left ventricular assist device; PAF, paroxysmal atrial fibrillation; PeAF, persistent/permanent atrial fibrillation.
In Cox proportional hazards multivariable analysis adjusting for all baseline characteristics, history of PeAF or AT remained an independent predictor of mortality (P=0.03 and P=0.002, respectively). Other independent predictors of mortality were a history of ventricular arrhythmia, decreasing baseline albumin and history of coronary artery bypass grafting. The hazard ratios for independent predictors of mortality are given in Table 4. Increasing AF burden at baseline from no AF to PAF to PeAF demonstrated a trend toward increased mortality in a similar multivariate model (P=0.07; hazard ratio 1.2, 95% CI 0.98–1.65).
Table 4.
Predictors of Mortality in Patients With a Left Ventricular Assist Device
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Baseline albumin | 0.6 (0.5–0.8) | 0.0002 |
| History of AT | 2.3 (1.4–4.0) | 0.002 |
| History of PeAF | 1.8 (1.0–3.0) | 0.03 |
| History of CABG | 1.6 (1.0–2.5) | 0.02 |
| History of ventricular arrhythmia | 1.4 (1.1–2.1) | 0.01 |
AT indicates atrial tachycardia; CABG, coronary artery bypass grafting; PeAF, persistent or permanent atrial fibrillation.
Discussion
This study evaluated the incidence, predictors, and clinical impact of AAs along with LA structural changes in a large cohort of patients who underwent LVAD implantation. We found high prevalence and incidence of AAs, particularly AF, before and after LVAD. However, LA size and volume index were significantly improved after LVAD implantation, and a significant proportion of patients with PAF had no further documented AF. In addition, we found that a history of PeAF or AT prior to LVAD and the development of new AF after LVAD were associated with increased mortality. Together, these findings suggest that LVADs induce reverse electroanatomical remodeling of the atria, but AAs may still significantly affect clinical outcome of patients with LVADs.
There is limited literature regarding AAs before and after LVAD implantation; however, the prevalence of AF in our study was comparable to prior investigations.14, 15, 16 We further documented a considerable prevalence of AFL, AT, and atrioventricular nodal reentrant tachycardia in the study cohort. These AAs frequently coexisted, particularly with AF. The presence of cardiac implantable electrical devices in a large proportion of patients provided an accurate data source to assess the incidence of AAs.
Despite the high burden of AAs, a significant percentage of patients with PAF in our cohort had no further evidence of AF after LVAD implantation. A large percentage of new‐onset AF occurred in the postoperative period. In addition, LA size and volume indices improved significantly at follow‐up. These observations suggest that LVADs induce reverse remodeling of the atria. It is known that LVADs cause significant ventricular remodeling both through direct unloading of the left ventricle and through partial normalization of the neurohormonal response to the lowered cardiac output of a failing heart.11, 12 Our findings suggest that reduced atrial pressures and an improved neurohormonal status may have the same impact on the atria. This is consistent with findings in which neurohormonal blockade decreases the incidence and recurrence of AF in patients with heart failure, and response to cardiac resynchronization therapy is associated with structural reverse remodeling of the LA and decreased AF incidence.17, 18, 19, 20, 21 Consequently, in both patients with LVAD and those with noninvasively treated heart failure, management of heart failure is likely an important component of a rhythm control strategy.
Given the interaction of AF, heart failure, and other comorbidities, extensive baseline characteristics were evaluated as potential predictors of new‐onset AF after LVAD implant. Of these parameters, increasing age, history of chronic obstructive pulmonary disease, and increasing baseline creatinine were significant predictors. Increasing age and reduced lung function are well known to increase the risk of AAs.3, 8, 22 In recent studies examining the risk of new‐onset AF in heart failure patients without LVAD, increasing age, decreased left ventricular diastolic elastance, diabetes mellitus, hypertension, increased diuretic use, and renal impairment were found to be predictors of AF.23, 24 Therefore, the development of new AF in patients with LVADs may similarly be related to the impact of comorbid conditions and impaired management of volume status related to renal dysfunction.
Conversely, AAs were shown to affect survival in this cohort. A history of PeAF or AT prior to LVAD was associated with increased mortality. Although this was not the case in patients with AFL, many patients with this history had likely undergone curative therapy with cavotricuspid isthmus ablation. An increasing burden of AF demonstrated a nonsignificant trend toward mortality. These findings are consistent with existing literature in which preoperative AF alone did not affect mortality,14 but persistent AF was associated with an increased risk of death or heart failure hospitalization.15 Because AT is typically paroxysmal, it is notable that its association with mortality is more pronounced than PAF. This difference may be due to a more sustained high ventricular rate with AT or an undetermined confounding comorbidity. Finally, development of AF episodes after LVAD implantation was also independently associated with increased mortality.
The mechanisms by which AF and other AAs affect survival in patients with LVAD are not well understood. Although not directly evaluated by this study, AF may be a reflection of inadequate mechanical support. As shown in a recent study, only 42.9% of patients were found to have normal central venous or pulmonary capillary wedge pressure at baseline speed settings.25 AA may have an effect on right ventricular function as well. Consistent with this hypothesis, a small case series demonstrated improvement of right ventricular failure after ablation of AFL in patients with LVADs.26 Consequently, AAs and LA volume may be useful noninvasive parameters for the chronic optimization of LVADs. Finally, although AAs may be a marker of overall disease burden, this appears less likely given the independence from other comorbidities in predicting decreased survival.15, 16, 27, 28
The present study is limited by its retrospective nature and is a single‐center cohort. The incidence of AAs may be underreported because of a lack of systematic surveillance in this population; however, we found similar incidence of AAs within the subgroup of patients with atrial leads. With regard to echocardiographic parameters, follow‐up LA dimension was not available for all patients; however, patients with follow‐up were similar to those without follow‐up in most baseline characteristics. Although the impact of AAs on mortality was evaluated, we did not evaluate the quality‐of‐life measures, which are known to affect AA in other populations.29 Our results may not be applicable to the broader population of patients with end‐stage heart failure who received different circulatory assist devices or with fewer comorbidities; the prevalence of comorbidities was higher in our study cohort as a result of referral bias to a tertiary center. This bias is manifest in the higher mortality rate of our cohort compared with historical INTERMACS registries.
In conclusion, patients who require LVAD have increased incidence and prevalence of AAs, particularly AF. In this population, a history of PeAF and AT or the development of AAs after LVAD implantation are associated with reduced long‐term survival. However, LVAD implantation suppresses AAs in a subset of the patients with reverse remodeling of atrial electroanatomical substrate. AAs may be an important marker of inadequate mechanical unloading of the ventricle. In addition, atrial geometry may be a useful noninvasive parameter for the chronic optimization of LVADs. Maintenance of sinus rhythm may provide better clinical outcomes in patients with advanced heart failure and LVADs.
Sources of Funding
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute K Award (Grant number 1K08HL117082‐01A1), awarded to Ozcan.
Disclosures
Uriel receives consulting fees and research grant support from St. Jude Medical and Medtronic. Jeevanandam receives consulting fees from St. Jude, Medtronic, and Reliant Heart.
(J Am Heart Assoc. 2017;6:e005340. DOI: 10.1161/JAHA.116.005340.)
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 3. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved vs. reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozcan C, Battaglia E, Young R, Suzuki G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J Am Heart Assoc. 2015;4:e001733 DOI: 10.1161/JAHA.114.001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 6. Segerson NM, Sharma N, Smith ML, Wasmund SL, Kowal RC, Abedin M, Macgregor JF, Pai RK, Freedman RA, Klein RC, Wall TS, Stoddard G, Hamdan MH. The effects of rate and irregularity on sympathetic nerve activity in human subjects. Heart Rhythm. 2007;4:20–26. [DOI] [PubMed] [Google Scholar]
- 7. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J‐P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela B, Lichtman J, Lisabeth LD, Liu S, Mackey R, Magid D, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani S, Woo D, Yeh RW, Turner MB. Executive summary: heart disease and stroke statistics—2015 update. Circulation. 2015;131:434–441. [DOI] [PubMed] [Google Scholar]
- 8. Estes NAM, Sacco RL, Al‐Khatib SM, Ellinor PT, Bezanson J, Alonso A, Antzelevitch C, Brockman RG, Chen P‐S, Chugh SS, Curtis AB, DiMarco JP, Ellenbogen KA, Epstein AE, Ezekowitz MD, Fayad P, Gage BF, Go AS, Hlatky MA, Hylek EM, Jerosch‐Herold M, Konstam MA, Lee R, Packer DL, Po SS, Prystowsky EN, Redline S, Rosenberg Y, Van Wagoner DR, Wood KA, Yue L, Benjamin EJ. American Heart Association atrial fibrillation research summit: a conference report from the American Heart Association. Circulation. 2011;124:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weil BR, Ozcan C. Cardiomyocyte remodeling in atrial fibrillation and hibernating myocardium: shared pathophysiologic traits identify novel treatment strategies? Biomed Res Int. doi: 10.1155/2015/587361. Available at: https://www.hindawi.com/journals/bmri/2015/587361/. Accessed February 28, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. [DOI] [PubMed] [Google Scholar]
- 11. Burkhoff D, Klotz S, Mancini DM. LVAD‐induced reverse remodeling: basic and clinical implications for myocardial recovery. J Cardiac Fail. 2006;12:227–239. [DOI] [PubMed] [Google Scholar]
- 12. McCarthy PM, Nakatani S, Vargo R, Kottke‐Marchant K, Harasaki H, James KB, Savage RM, Thomas JD. Structural and left ventricular histologic changes after implantable LVAD insertion. Ann Thorac Surg. 1995;59:609–613. [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer K, Tsang W, Voigt J. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 14. Stulak JM, Deo S, Schirger J, Aaronson KD, Park SJ, Joyce LD, Daly RC, Pagani FD. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg. 2013;96:2161–2167. [DOI] [PubMed] [Google Scholar]
- 15. Enriquez AD, Calenda B, Gandhi PU, Nair AP, Anyanwu AC, Pinney SP. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J Am Coll Cardiol. 2014;64:1883–1890. [DOI] [PubMed] [Google Scholar]
- 16. Yoruk A, Sherazi S, Massey HT, Kutyifa V, McNitt S, Hallinan W, Huang DT, Chen L, Aktas MK. Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm. 2016;13:1052–1056. [DOI] [PubMed] [Google Scholar]
- 17. Murray KT, Rottman JN, Arbogast PG, Shemanski L, Primm RK, Campbell WB, Solomon AJ, Olgin JE, Wilson MJ, DiMarco JP, Beckman KJ, Dennish G, Naccarelli GV, Ray WA. Inhibition of angiotensin II signaling and recurrence of atrial fibrillation in AFFIRM. Heart Rhythm. 2004;1:669–675. [DOI] [PubMed] [Google Scholar]
- 18. Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, Masson S, Cerè E, Tognoni G, Cohn JN. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val‐HeFT). Am Heart J. 2005;149:548–557. [DOI] [PubMed] [Google Scholar]
- 19. Donal E, Tan K, Leclercq C, Ollivier R, Derumeaux G, Bernard M, de Place C, Mabo P, Daubert J‐C. Left atrial reverse remodeling and cardiac resynchronization therapy for chronic heart failure patients in sinus rhythm. J Am Soc Echocardiogr. 2009;22:1152–1158. [DOI] [PubMed] [Google Scholar]
- 20. Yu C‐M, Fang F, Zhang Q, Yip GWK, Li CM, Chan JY‐S, Wu L, Fung JW‐H. Improvement of atrial function and atrial reverse remodeling after cardiac resynchronization therapy for heart failure. J Am Coll Cardiol. 2007;50:778–785. [DOI] [PubMed] [Google Scholar]
- 21. Fung JWH, Yip GWK, Zhang Q, Fang F, Chan JYS, Li CM, Wu LW, Chan GCP, Chan HCK, Yu C‐M. Improvement of left atrial function is associated with lower incidence of atrial fibrillation and mortality after cardiac resynchronization therapy. Heart Rhythm. 2008;5:780–786. [DOI] [PubMed] [Google Scholar]
- 22. Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012–1016. [DOI] [PubMed] [Google Scholar]
- 23. Campbell NG, Cantor EJ, Sawhney V, Duncan ER, DeMartini C, Baker V, Diab IG, Dhinoja M, Earley MJ, Sporton S, Davies LC, Schilling RJ. Predictors of new onset atrial fibrillation in patients with heart failure. Int J Cardiol. 2014;175:328–332. [DOI] [PubMed] [Google Scholar]
- 24. Yoon JH, Kim M‐H, Chung H, Choi E‐Y, Min P‐K, Yoon YW, Lee BK, Hong B‐K, Rim S‐J, Kwon HM, Kim J‐Y. Echo‐Doppler‐derived indexes of ventricular stiffness and ventriculo‐arterial interaction as predictors of new‐onset atrial fibrillation in patients with heart failure. Cardiovasc Ultrasound. 2016;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, Jorde UP, Juricek C, Ota T, Jeevanandam V, Burkhoff D, Lang RM. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016;4:208–217. [DOI] [PubMed] [Google Scholar]
- 26. Hottigoudar RU, Deam AG, Birks EJ, McCants KC, Slaughter MS, Gopinathannair R. Catheter ablation of atrial flutter in patients with left ventricular assist device improves symptoms of right heart failure. Congest Heart Fail. 2013;19:165–171. [DOI] [PubMed] [Google Scholar]
- 27. Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–321. [DOI] [PubMed] [Google Scholar]
- 28. Brenyo A, Rao M, Koneru S, Hallinan W, Shah S, Massey HT, Chen L, Polonsky B, McNitt S, Huang DT, Goldenberg I, Aktas M. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J Cardiovasc Electrophysiol. 2012;23:515–520. [DOI] [PubMed] [Google Scholar]
- 29. Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NAM, Field ME, Goldberger ZD, Hammill SC, Indik JH, Lindsay BD, Olshansky B, Russo AM, Shen W‐K, Tracy CM, Al‐Khatib SM. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia. J Am Coll Cardiol. 2016;67:e27–e115. [DOI] [PubMed] [Google Scholar]


