Abstract
Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder that predominantly affects women and is associated with prevalent hypertension, renal injury, and cardiovascular disease. Immune system dysfunction is recognized as an important factor in the pathogenesis of hypertension. We recently showed that preventing autoimmunity prevents the development of hypertension in an experimental model of SLE (female NZBWF1 mice). The present study tests the hypothesis that mycophenolate mofetil (MMF), an immunosuppressive therapy used clinically to treat SLE by depleting proliferating B and T lymphocytes, can improve blood pressure control.
Methods and Results
Female SLE and control (NZW/LacJ) mice were treated daily for 8 weeks with 60 mg/kg MMF. Circulating CD45R+ B cells were lower in MMF‐treated SLE mice after 4 weeks of treatment, but neither CD4+ nor CD8+ T cells were reduced by MMF. Plasma anti–double‐stranded DNA IgG autoantibodies, a marker of SLE disease activity, were higher in SLE mice compared with controls and were lower in SLE mice after 8 weeks of MMF. Mean arterial pressure was elevated in SLE mice compared with controls and lower in SLE mice treated with MMF compared with vehicle‐treated SLE mice. MMF also reduced both renal injury (urinary albumin excretion and glomerulosclerosis) and the infiltration of CD45R+ B cells and CD3+ CD4+ T cells in kidneys from mice with SLE.
Conclusions
These data suggest that MMF selectively depleted CD45R+ B cells and lowered subsequent autoantibody production, furthering the concept that autoantibodies mechanistically contribute to the pathogenesis of hypertension.
Keywords: autoantibodies, hypertension, immunosuppression, lymphocyte, systemic lupus erythematosus
Subject Categories: Hypertension, Inflammation
Introduction
Adaptive immune system dysfunction has been implicated in the pathogenesis of hypertension for several decades. Patients with, for example, essential hypertension or pregnancy‐associated hypertension have been shown to have elevated circulating levels of IgG and IgM,1, 2, 3, 4 and additional studies have shown the presence of autoantibodies specific for host proteins such as the angiotensin II type 1 receptor5 and the α1‐adrenergic receptor,6 among others, in hypertensive patients. These studies suggest a role for abnormal B‐cell activity in the pathogenesis of hypertension in certain patient populations and highlight the potential link between autoimmunity and hypertension.
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune inflammatory disorder that predominantly affects women of childbearing age and is characterized by B and T lymphocyte hyperreactivity and the production of pathogenic autoantibodies, often to nuclear components. These autoantibodies form abnormal immune complexes that deposit in various tissues, including the kidneys, skin, joints, and central nervous system. SLE is associated with prevalent hypertension,7, 8, 9, 10, 11, 12 which contributes to the high risk of cardiovascular disease, which is the major cause of mortality in these patients.13, 14, 15, 16 We recently demonstrated that systemic autoimmunity is a key factor in the development of hypertension in an experimental mouse model of SLE with hypertension (female NZBWF1).17 Nevertheless, the effect of immunosuppression on hypertension in SLE patients remains poorly understood. Mycophenolate mofetil (MMF) is an immunosuppressant that was initially used to prevent organ transplant rejection and has emerged as a frontline agent for both induction18 and maintenance therapy19 in patients with lupus nephritis. MMF inhibits the inducible type II isoform of inosine monophosphate dehydrogenase, which is specifically expressed by activated T and B lymphocytes.20 It effectively lowers blood pressure in some hypertensive animal models, including spontaneously hypertensive rats21 and Dahl SS rats,22, 23 but not others (ie, angiotensin II hypertension24). In human hypertension, a small clinical study of patients with the autoimmune diseases psoriasis and rheumatoid arthritis revealed that MMF treatment effectively lowered arterial blood pressure.25 It is not clear, however, if MMF has similarly beneficial blood pressure effects during SLE. Because of the prevalent hypertension associated with SLE and the widespread use of MMF in this patient population, understanding the impact of MMF on blood pressure is likely to have direct clinical relevance for SLE patients.
Materials and Methods
Animals
Adult (aged 27 weeks) female NZBWF1 (SLE, n=18) and NZW/LacJ (control, n=15) mice (The Jackson Laboratory, Bar Harbor, ME) were used in this study. Mice were maintained on a 12‐hour light/dark cycle in temperature‐controlled rooms with access to chow and water ad libitum. All studies were performed with the approval of the University of Mississippi Medical Center (UMMC) institutional animal care and use committee and in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
MMF Administration
MMF (CellCept Intravenous; Genentech) was dissolved in sterile 5% dextrose. Mice were administered 60 mg/kg per day IP for 8 weeks in a volume of 0.1 mL. Mice not receiving MMF were injected with 0.1 mL 5% dextrose (vehicle).
Blood Pressure
Mean arterial pressure (in mm Hg) was recorded via indwelling carotid artery catheters in freely moving conscious mice, as described previously by our laboratory.17, 26, 27, 28, 29
Preparation of Cells for Flow Cytometry
Blood was collected from the retro‐orbital plexus from MMF‐ or vehicle‐treated animals at 27 week (0 weeks MMF), 31 weeks (4 weeks MMF), and 35 weeks (8 weeks MMF). The blood was centrifuged at 350g to isolate plasma. Erythrocytes were lysed by adding 10× volume of 1X PharmLyse (BD Biosciences). After incubation for 5 minutes at room temperature, blood was centrifuged at 200g for 5 minutes. The pelleted cells were washed in 1X PBS and 2% FCS and centrifuged at 350g for 5 minutes. The purified peripheral blood leukocytes were suspended in 90% FCS and 10% dimethyl sulfoxide and stored at −80°C until use. For isolation of renal immune cells, 1 kidney was homogenized in 5 mL RPMI media containing 200 U/mL DNase and 10 mg/mL collagenase IV using the GentleMACS Octo Dissociator (Miltenyi Biotec) using a user‐defined protocol for mouse kidney. The resulting homogenate was filtered through a 70‐μmol/L cell strainer and washed with 1X PBS containing 2% FCS and 2 mmol/L EDTA. The single‐cell suspension was centrifuged at 300g for 10 minutes. The resulting cell pellet was then resuspended in 1X PBS and 2% FCS. Peripheral blood leukocytes were isolated from the kidney cell suspension using Lymphoprep (Accurate Chemical), according to the manufacturer's instructions.
Flow Cytometric Analyses
Cells were resuspended in 1X PBS, 2% FCS, and 0.9% sodium azide at a concentration of 2×107 cells/mL. Next, 1×106 cells (50 μL) were aliquoted into a flow cytometry tube and incubated with 0.5 μg of anti–mouse CD32/CD16 (FcR block; BD Biosciences) for 5 minutes on ice. Cells were then stained with isotype control antibodies; a T‐cell subset antibody cocktail (BD Biosciences) that contains anti–mouse CD3e‐PE‐Cy7 (clone 145‐2C11), CD4‐PE (clone RM4‐5), and CD8‐APC (clone 53‐6.7); or anti–CD45R‐PE‐Cy7 (clone RA3‐6B2). Cells were incubated on ice for 30 minutes protected from light. Samples were analyzed on a Gallios (Becton Dickinson) flow cytometer at the UMMC flow cytometry core facility. A total of 25 000 events were acquired for each sample. Data were analyzed using Kaluza software (Beckman Coulter).
Autoantibodies
Anti–double‐stranded DNA (anti‐dsDNA) IgG was detected in plasma at 27 and 35 weeks of age (SLE mice) or 36 weeks of age (control mice) using the anti‐dsDNA IgG ELISA (Alpha Diagnostic International) per the manufacturer's instructions and as previously described by our laboratory.17, 26, 28, 29 Isotype‐specific ELISAs were prepared according to the method of Mihara et al.30 Briefly, plates were coated with salmon sperm DNA, incubated with diluted plasma from vehicle‐ and MMF‐treated SLE mice, and probed with horseradish peroxidase–conjugated antibodies specific for each mouse antibody isotype.
Renal Injury
Urinary albumin was monitored weekly by dipstick analysis (Albustix; Siemens). Animals were considered positive for albuminuria at ≥100 mg/dL.17, 26, 27, 29 Urinary albumin excretion rate (in mg/day) was assessed by ELISA (Alpha Diagnostic International) using overnight urine samples collected at the conclusion of the study, as described previously.17, 26, 27, 29 Glomerulosclerosis was assessed using Masson's trichrome, as described previously by our laboratory.17
Statistical Analysis
Data are presented as mean±SEM. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software). A 2‐way ANOVA was used to analyze treatment (MMF versus vehicle) or group (SLE versus control) interactions. For the analysis of MMF‐ and vehicle‐treated SLE and control animals over the course of the study, a 2‐way ANOVA was used to analyze treatment (MMF versus vehicle) or time (27, 31, or 35 weeks of age). One‐way ANOVA was used to analyze individual differences between groups, and Tukey post test for multiple comparisons was used to compare groups. A paired t test was used to analyze anti‐dsDNA IgG levels in SLE mice before and after MMF treatment, and an unpaired t test was used to analyze isotype‐specific anti‐dsDNA levels in SLE mice treated with vehicle or MMF.
Results
Because MMF depletes activated B and T lymphocytes, we analyzed circulating B and T cells at the onset of the treatment (aged 27 weeks) and after 4 weeks (aged 31 weeks) and 8 weeks (aged 35 weeks) of MMF, at the end of the study, using flow cytometry. Figure 1A shows representative histograms from SLE mice treated with vehicle or MMF at the indicated time points, and Figure 1B indicates the percentages of CD45R+ B cells of individual mice in the treatment groups. In the vehicle‐treated SLE mice, there was a progressive increase in the percentage of circulating CD45R+ cells (16.2±1.4%, aged 27 weeks; 19.5±1.4%, aged 31 weeks; 27.4±4.5%, aged 35 weeks). MMF‐treated SLE mice had significantly lower percentages of CD45R+ cells compared with the vehicle‐treated group (19.5±1.3% versus 5.2±1.5%, P<0.05) after 4 weeks of MMF treatment; however, the percentages of CD45R+ cells increased after 8 weeks of treatment (22.8±4.6%). Circulating CD4+ and CD8+ T cells were also analyzed over the 8‐week treatment period. The percentage of CD3+CD4+ T cells decreased equally over the course of the study in both vehicle‐ and MMF‐treated SLE mice (Figure 2A). In addition, no differences in the percentage of CD3+CD8+ T cells were detected between the 2 treatment groups (Figure 2B). No changes were detected in B or T cells in control mice treated with vehicle or MMF (Figure 3).
Figure 1.
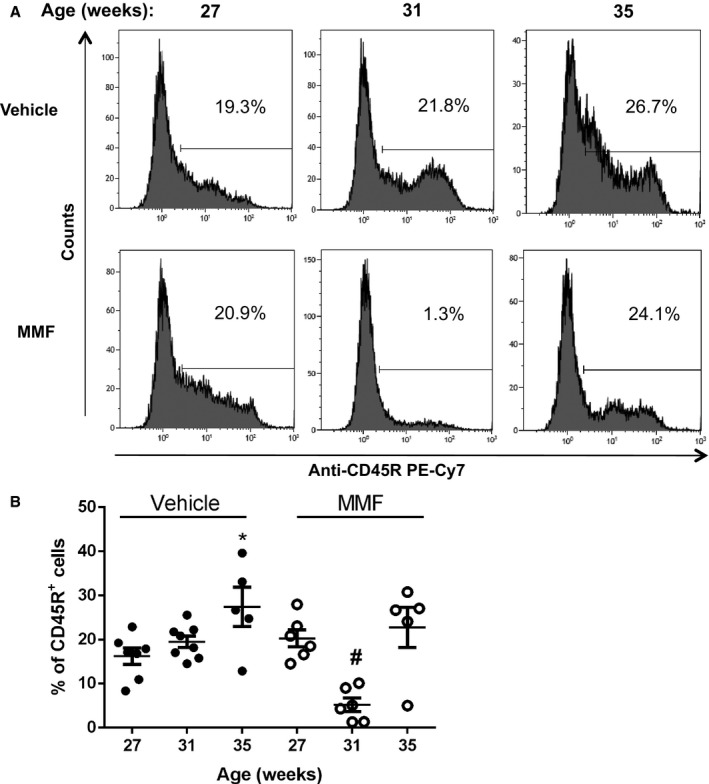
Effect of mycophenolate mofetil (MMF) treatment on circulating B cells in mice with systemic lupus erythematosus (SLE) treated with MMF or vehicle. A, Representative histograms from SLE mice treated with vehicle or MMF. Peripheral blood leukocytes (PBLs) were isolated from mice at 27 weeks (0 weeks of MMF), 31 weeks (4 weeks of MMF), and 35 weeks (8 weeks of MMF) and stained with anti–mouse CD45R phycoerythrin–cyanine 7 (PE‐Cy7). Percentages of positive cells are indicated. B, Percentage of PBLs in vehicle‐ or MMF‐treated mice with staining for anti–mouse CD45R at the indicated time points. The percentage of CD45R+ cells was significantly lower in MMF‐treated animals after 4 weeks. *P<0.05 vs SLE mice treated with vehicle (27 weeks); # P<0.01 vs SLE mice treated with vehicle at 31 and 35 weeks and SLE mice treated with MMF at 27 and 35 weeks.
Figure 2.
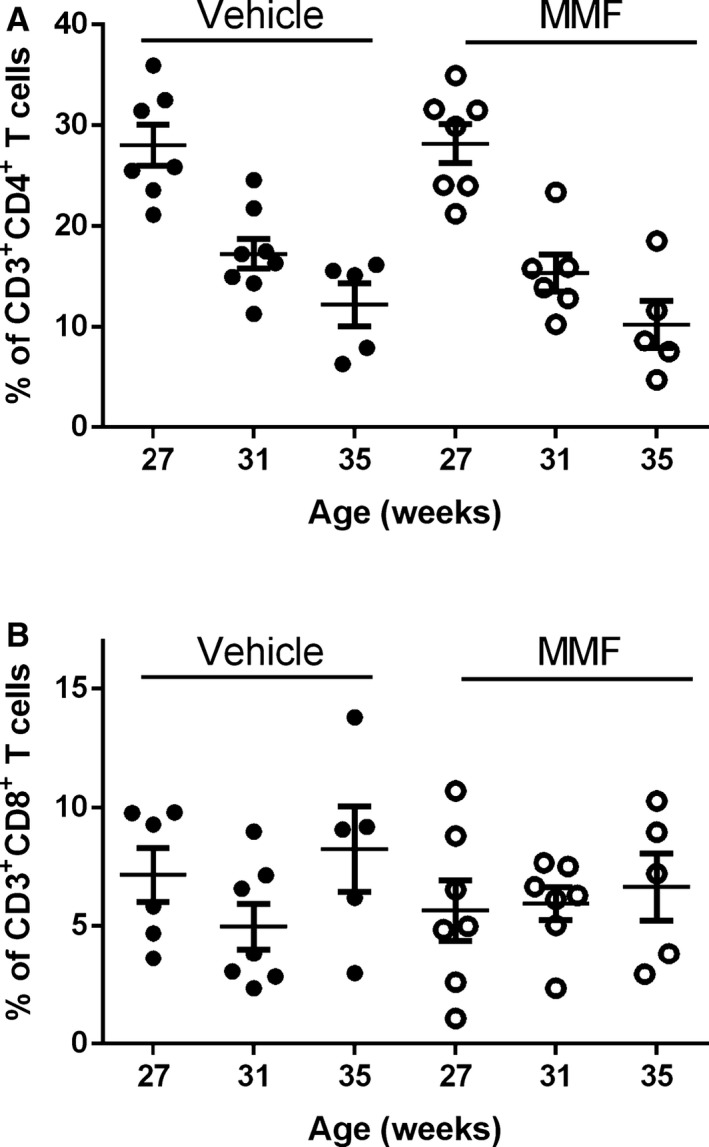
Effect of mycophenolate mofetil (MMF) treatment on circulating T cells in vehicle‐ or MMF‐treated SLE mice. Cells were stained with anti–mouse CD3 phycoerythrin–cyanine 7, anti–mouse CD4 phycoerythrin, and anti–mouse CD8 allophycocyanin. A, Percentage of CD3+ CD4+ T cells at 27 weeks (0 weeks of MMF), 31 weeks (4 weeks of MMF), and 35 weeks (8 weeks of MMF). B, Percentage of CD3+ CD8+ T cells at 27 weeks (0 weeks of MMF), 31 weeks (4 weeks of MMF), and 35 weeks (8 weeks of MMF). No significant differences were seen in relative percentages of T cells at any time points.
Figure 3.
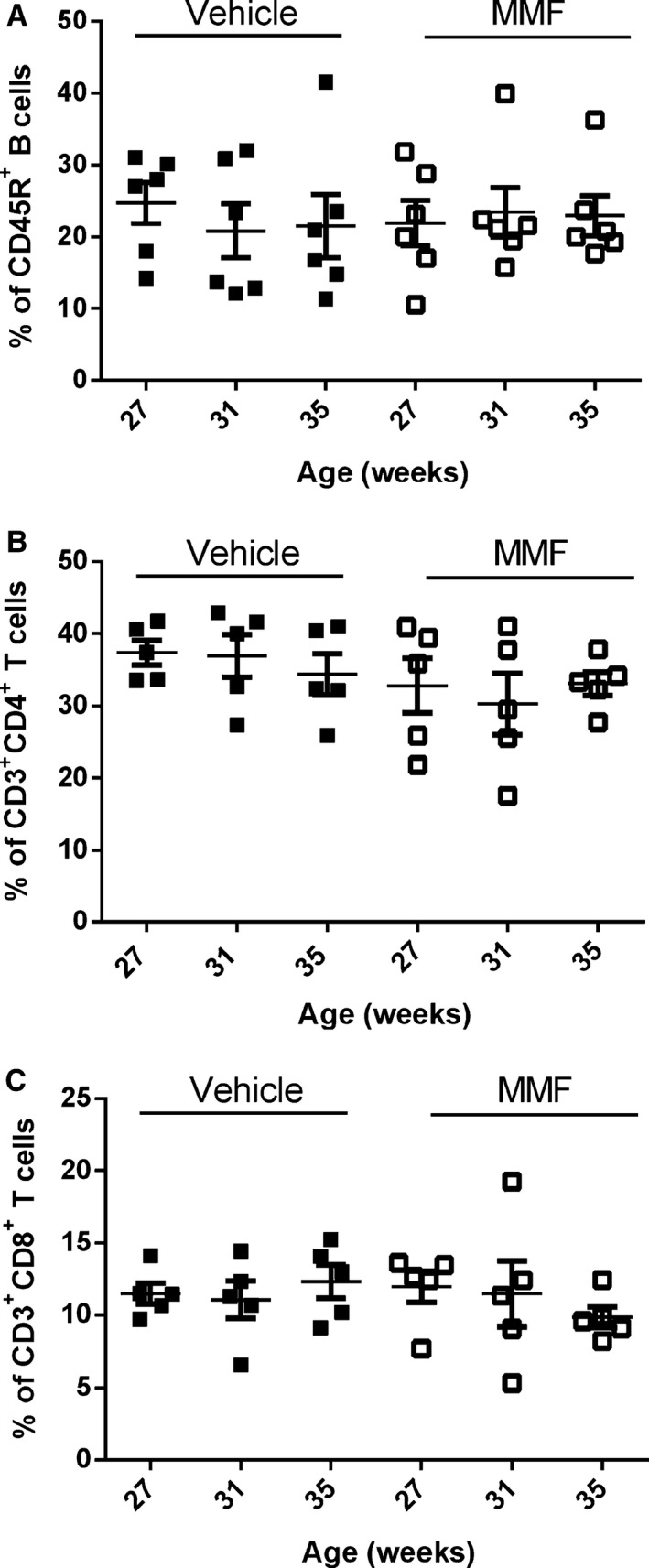
Effect of mycophenolate mofetil (MMF) on circulating B and T cells in vehicle or MMF‐treated control mice. Cells were stained with anti‐mouse CD45R phycoerythrin–cyanine 7 (A) or with anti‐mouse CD3 phycoerythrin–cyanine 7, anti‐mouse CD4 phycoerythrin, and anti‐mouse CD8 allophycocyanin (B and C). A, Percentage of CD45R+ B cells at 27 weeks (0 weeks of MMF), 31 weeks (4 weeks of MMF), and 35 weeks (8 weeks of MMF). B, Percentage of CD3+CD4+ T cells at 27 weeks (0 weeks of MMF), 31 weeks (4 weeks of MMF), and 35 weeks (8 weeks of MMF). C, Percentage of CD3+CD8+ T cells at 27 weeks (0 weeks of MMF), 31 weeks (4 weeks of MMF), and 35 weeks (8 weeks of MMF). No significant differences were seen in relative percentages of T cells at any time points.
Total plasma anti‐dsDNA IgG autoantibody production was increased in SLE mice compared with control (107.7±23.2 versus 769.6±114.9 U/mL, P<0.01) (Figure 4A), in agreement with previous reports by our laboratory.17, 26, 29, 31 MMF‐treated SLE mice had significantly lower autoantibody production compared with vehicle‐treated mice (322.5±90.9 versus 769.6±114.9 U/mL, P<0.01). Anti‐dsDNA autoantibodies were also analyzed at the onset (aged 27 weeks) and conclusion (aged 35 weeks) of the study in SLE mice. Although the production of circulating autoantibodies increased in vehicle‐treated SLE mice by 554% over the course of the study (117.6±24.5 versus 769.6±114.9 U/mL, P<0.0001), there was only a modest, statistically nonsignificant increase (156.2±85.8 versus 322.5±90.9 U/mL, P=0.21) (Figure 4B) in MMF‐treated SLE mice. Circulating anti‐dsDNA IgG of different isotypes were also assessed by ELISA, and treatment with MMF significantly lowered anti‐dsDNA IgG1 autoantibody production (1.3±0.22 versus 0.47±0.19 OD450, P<0.05) and qualitatively lowered production of IgG2a (1.7±0.08 versus 1.3±0.22 OD450, P=0.057) and IgG2b (0.96±0.2 versus 0.42±0.11 OD450, P=0.0501) in SLE mice without affecting IgG3 and IgM anti‐dsDNA autoantibody production (Figure 5).
Figure 4.
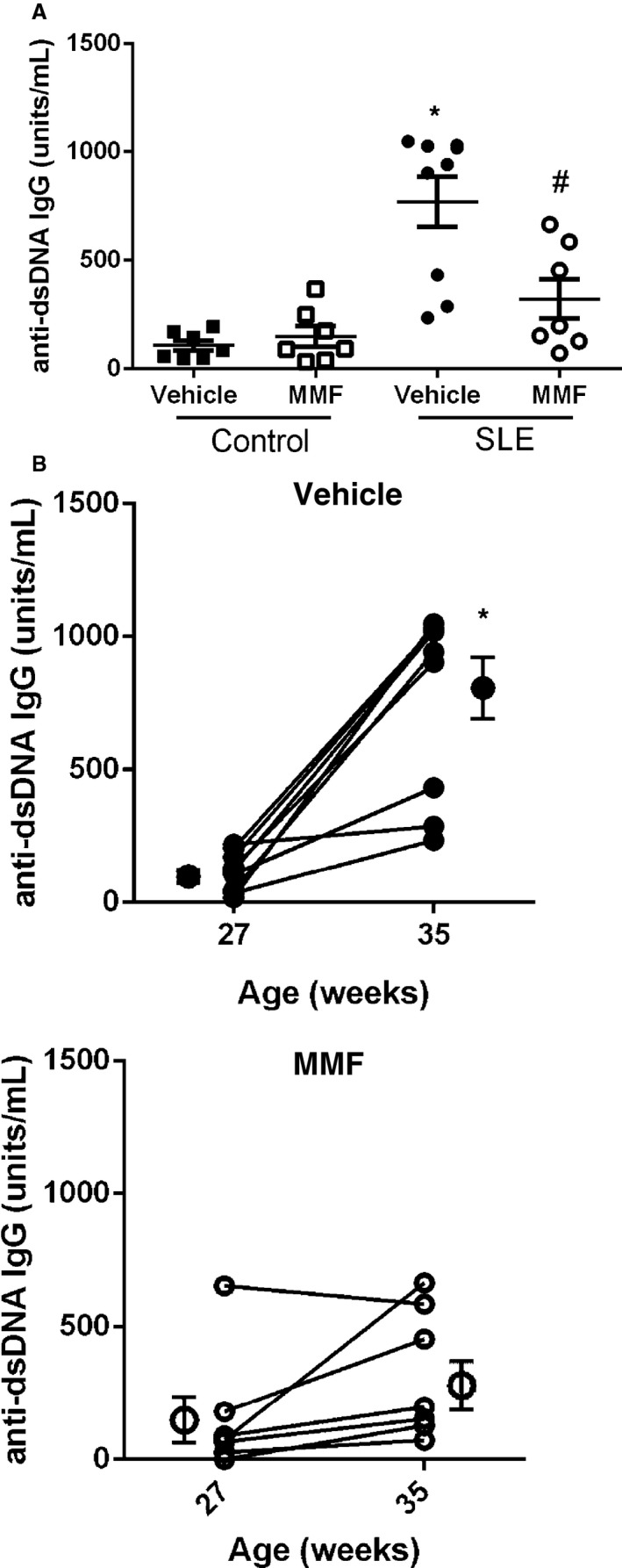
Effect of mycophenolate mofetil (MMF) treatment on anti–double‐stranded DNA (anti‐dsDNA) antibody levels in systemic lupus erythematosus (SLE) and control mice. A, plasma anti‐dsDNA antibodies measured at 35 weeks of age in SLE and control mice. Autoantibodies were higher in vehicle‐treated SLE mice compared with control mice but were lower in SLE mice after 8 weeks of MMF treatment. *P<0.001 vs control mice treated with vehicle and MMF, # P<0.01 vs SLE mice treated with vehicle. B, An increase in anti‐dsDNA autoantibody production was blunted in MMF‐treated SLE mice. Shown are anti‐dsDNA antibody levels before and after the 8‐week treatment in vehicle‐ and MMF‐treated SLE mice. *P<0.01 vs SLE mice treated with MMF (27 weeks).
Figure 5.
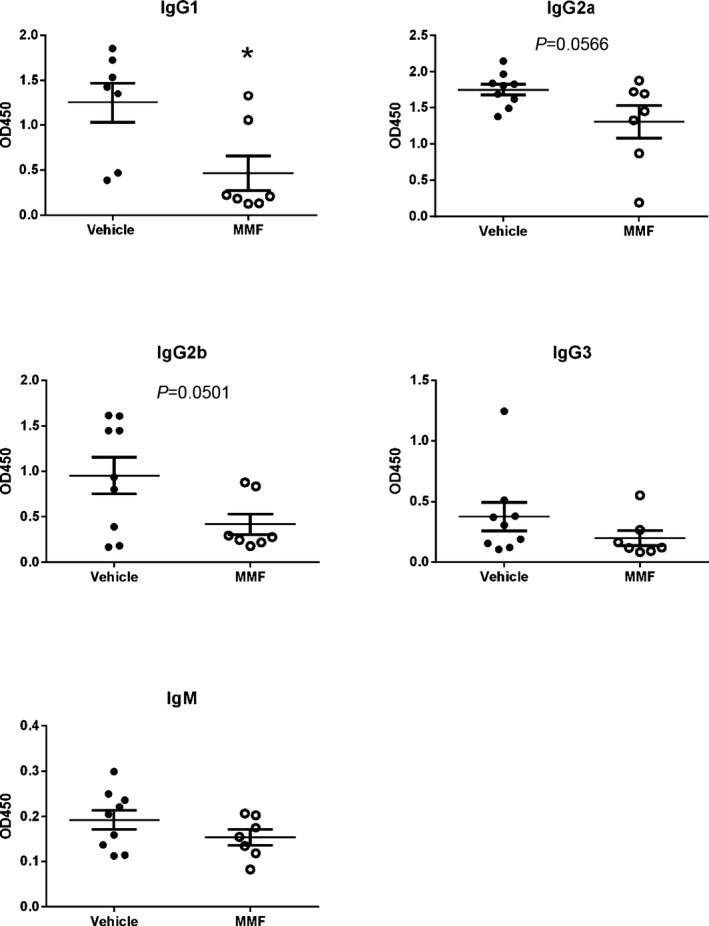
Effect of mycophenolate (MMF) treatment on anti–double‐stranded DNA antibody levels in mice with systemic lupus erythematosus (SLE). *P<0.05 vs vehicle‐treated SLE mice. OD indicates optical density.
To analyze the effect of MMF on hypertension in SLE, mean arterial pressure was measured in conscious, freely moving mice at the conclusion of the study. Vehicle‐treated SLE mice had elevated mean arterial pressure compared with vehicle‐treated control mice, consistent with our previous studies (141±3 versus 120±3 mm Hg, P<0.01) (Figure 6). Blood pressure was significantly lower in SLE mice treated with MMF compared with SLE mice treated with vehicle (120±4 versus 141±3 mm Hg, P<0.01), whereas MMF treatment did not affect blood pressure in control mice (120±3 versus 117±4 mm Hg).
Figure 6.
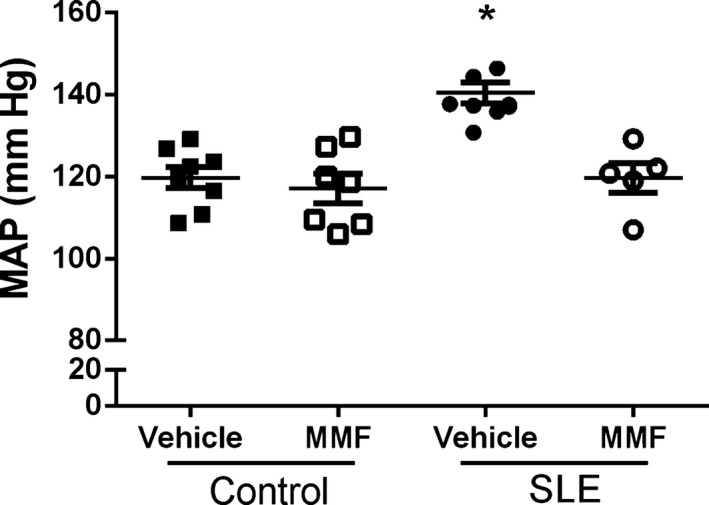
Effect of mycophenolate mofetil (MMF) treatment on mean arterial pressure (MAP) in control and systemic lupus erythematosus (SLE) mice treated with MMF. MAP was significantly higher in SLE compared with control mice. MMF significantly lowered blood pressure in SLE mice but had no effect in control mice. *P<0.01 vs all other groups.
Mice with SLE develop renal injury and excrete large amounts of albumin in their urine. Consistent with previous studies in our laboratory,17, 26, 32, 33 40% of the vehicle‐treated SLE mice developed albuminuria by 35 weeks of age, as measured by dipstick assay, whereas only 1 mouse (15%) treated with MMF developed albuminuria (Figure 7A). Urinary albumin excretion measured at the conclusion of the study was greater in vehicle‐treated SLE mice compared with control mice (3.95±2 versus 0.043±0.02 mg/day). SLE mice treated with MMF had a lower albumin excretion rate (0.27±0.007 mg/day) compared with vehicle‐treated SLE mice (Figure 7B). MMF treatment had no effect on albumin excretion in control mice treated with vehicle (0.043±0.02 mg/day) versus MMF (0.052±0.03 mg/day). In addition to attenuating the development of albuminuria, MMF treatment prevented the development of glomerulosclerosis (Figure 7C). Vehicle‐treated SLE mice had a significantly higher glomerulosclerosis index compared with vehicle‐treated control mice (2.8±0.35 versus 0.14±0.05, P<0.01) (Figure 7D). Glomerular injury was significantly lower in MMF‐treated SLE mice (0.63±0.27, P<0.01 compared with vehicle‐treated SLE mice).
Figure 7.
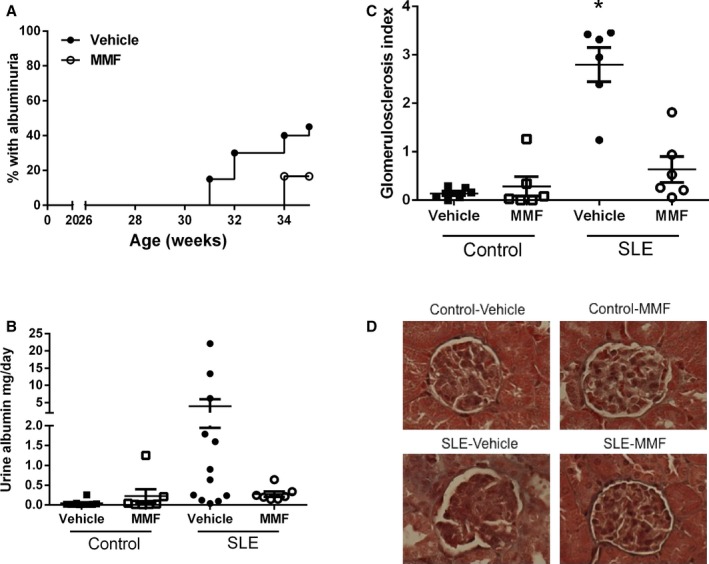
Effect of mycophenolate mofetil (MMF) treatment on urinary albumin excretion and glomerulosclerosis in control and systemic lupus erythematosus (SLE) mice. A, Weekly percentage of SLE mice with positive urinary albumin as measured by dipstick assay. A positive urine test is >100 mg/dL. No control mice developed albuminuria. B, Urine albumin in control mice (aged 36 weeks) and SLE mice (aged 35 weeks) as measured by albumin ELISA. Urine albumin was similar in control mice treated with vehicle or MMF. Albumin was higher in SLE mice than control mice, but MMF treatment lowered albuminuria. C, Glomerulosclerosis index assessed in control and SLE mice administered vehicle or MMF. *P<0.01 vs all other groups. D, Representative pictures of glomerulosclerosis (×40) from paraffin‐embedded kidneys stained with Masson's trichrome.
The accumulation of leukocytes in the kidneys mechanistically contributes to the pathogenesis of hypertension.34, 35, 36, 37 Isolated kidney leukocytes were stained with antibodies specific to B cells (CD45R) and T cells (CD3/CD4/CD8) and subjected to flow cytometric analyses (Figure 8). Vehicle‐treated SLE mice had significantly higher percentages of CD45R+ B cells (23.9±6.8% versus 6.2±1.4%, P<0.05) and CD3+CD4+ T cells (8.58±1.45% versus 4.49±0.82%, P<0.05). There was no significant difference in the percentage of CD3+CD8+ T cells (2.8±0.9% versus 1.8±0.3%, P=0.33) between the 2 groups.
Figure 8.
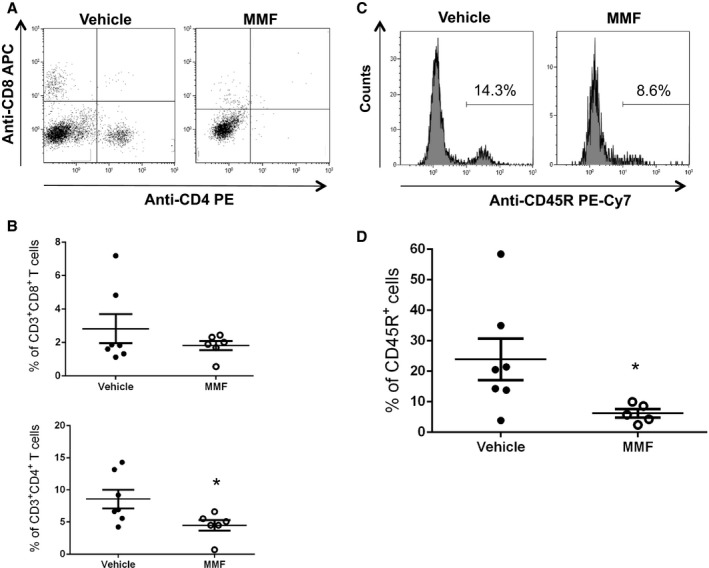
Effect of mycophenolate mofetil (MMF) treatment on renal T and B cells in systemic lupus erythematosus (SLE) mice. A, Representative dot plots from SLE mice treated with MMF and vehicle. Cells were stained with anti–mouse CD3 phycoerythrin–cyanine 7 (PE‐Cy7), anti–mouse CD4 phycoerythrin (PE), and anti–mouse CD8 allophycocyanin (APC). B, Percentage of CD3+ CD4+ T cells and CD3+ CD8+ T cells out of gated leukocytes in vehicle‐ and MMF‐treated SLE mice. *P<0.05 vs vehicle‐treated SLE mice. C, Representative histograms from SLE mice treated with MMF and vehicle. Cells were stained with anti–mouse CD45R PE‐Cy7. D, Percentage of CD45R+ cells among gated lymphocytes in vehicle‐ and MMF‐treated SLE mice. *P<0.05 vs vehicle‐treated SLE mice.
Discussion
Patients with SLE have a high incidence of hypertension, renal injury, and cardiovascular disease. Our laboratory previously showed that impaired renal hemodynamic function,28 peripheral vascular function,31 increased oxidative stress,26 and altered inflammatory cytokine production32, 38 contribute to hypertension in an established female mouse model of SLE. We hypothesize that these reported changes result from the loss of immune tolerance and lead to the production of autoreactive B and T lymphocytes. The present study directly tested whether immunosuppression with the drug MMF, used clinically for the treatment of SLE, would attenuate hypertension in an experimental model of SLE. The major new findings of this study are that MMF treatment (1) depleted circulating B lymphocytes and lowered anti‐dsDNA autoantibody production in SLE mice, independent of any effects on circulating T cells; (2) successfully attenuated the development of SLE‐associated hypertension and renal injury; and (3) reduced lymphocyte infiltration into the kidneys as a likely mechanism contributing to antihypertensive effects.
MMF was initially shown to attenuate lupus nephritis and to prolong life in both MRL/lpr and NZBWF1 mice when administered chronically starting at 8 to 12 weeks of age.39, 40, 41 Dosages of 30 to 200 mg/kg per day were used in these studies, although data conflict concerning the efficacy of MMF for depleting T and B lymphocytes. Two studies reported no change in the relative percentages of T and B lymphocytes in either the peripheral blood or spleen.40, 41 Conversely, another study found that a 7‐week treatment of MRL/lpr mice with MMF (100 mg/kg per day) resulted in a significant decrease in the percentage of circulating CD3+CD4−CD8− T cells,42 which are known to accumulate in the MRL/lpr model.43 Although there were no differences in T cells between vehicle‐ and MMF‐treated mice, there was a decrease in circulating CD3+CD4+ T cells over the course of the study in both groups. This decrease in T cells could be the result of lymphopenia due to SLE disease progression.44, 45 Specifically, CD4+ T cell levels have been shown to be depressed in some patients with SLE.46 In the present study, we demonstrated that after 4 weeks of MMF treatment, SLE mice had almost no circulating CD45R+ B cells, but by 8 weeks of treatment, the efficacy of MMF for depleting B cells was reduced. Despite the reduced ability of MMF to deplete B cells, it is important to note that the production of autoantibodies remained markedly attenuated. This contrasts with the vehicle‐treated mice that displayed a progressive and significant increase in the percentage of both CD45R+ B cells and autoantibodies over the course of the study. The reason for the loss of MMF efficacy in depleting B cells is unclear, although it is possible that the mice became desensitized and that antibody production would have risen if the study had been carried out longer. It is also possible that using higher doses—reportedly well tolerated by SLE mice40, 47—would have resulted in sustained depletion of B cells and could have altered T‐cell percentages. Nevertheless, our results using the selected dose serendipitously depleted circulating B cells and lowered anti‐dsDNA IgG production, independent of changes in T cells. This suggests that autoreactive B cells have an important mechanistic role in the pathogenesis of SLE‐associated hypertension. It may be that the depletion of B cells by MMF early in the study resulted in decreased differentiation into long‐lived plasma cells, which are known to be important producers of autoantibodies in SLE that are not depleted by MMF.48 Fewer autoreactive plasma cells would likely result in sustained decreases in autoantibody production. It should be noted that in patients with SLE, treatment with MMF reduces surrogate markers of B‐cell activation, including circulating plasmablasts and plasma cells during induction therapy.49, 50
The impact of MMF 60 mg/kg per day, used in the present study, on autoantibody production in mice with SLE is consistent with the work of others.39 Ramos et al showed that a lower dose of MMF (30 mg/kg per day) in NZBWF1 mice selectively reduced anti‐dsDNA IgG2a autoantibodies and that a higher dose (100 mg/kg per day) inhibited the production of all anti‐dsDNA autoantibodies, regardless of isotype.47 The findings of the current study showing that 8‐week treatment with MMF significantly lowered anti‐dsDNA IgG1 autoantibody production and qualitatively lowered IgG2a and IgG2b production in SLE mice without affecting IgG3 and IgM anti‐dsDNA autoantibody production further advances the understanding of how MMF affects autoantibody production. The IgG2a and IgG2b isotypes are generally considered the most pathogenic in mice because they can efficiently bind to FcγRIII and FcγRIV, leading to complement activation, whereas IgG1 can bind FcγRIII but cannot fix complement via the classical pathway.51 In general, the role of autoantibodies in the pathogenesis of hypertension is poorly understood in both animal models and humans, although mounting evidence suggests their importance in patients with essential hypertension and pregnancy‐associated hypertension. For example, antibodies specific for the angiotensin type I receptor, α1‐adrenergic receptor, and l‐type voltage gated calcium channels have been identified in patients with essential hypertension,5, 6, 52, 53 and immunoadsorption protocols have been successfully utilized in hypertensive patients with the α1‐adrenergic receptor.6 Autoantibody production is a hallmark characteristic of SLE disease, as ≈95% of SLE patients produce antibodies specific for nuclear components.54 Autoantibodies similar to those found in SLE patients have also been found in patients with essential hypertension.55, 56, 57 In the present study, MMF‐treated SLE mice had lower anti‐dsDNA autoantibody production coupled with attenuated hypertension. Long‐term B‐cell depletion with anti‐CD20 also lowered anti‐dsDNA IgG production and prevented the development of hypertension in SLE mice in a previous study by our laboratory.17 Although these autoantibodies lead to immune complex formation and tissue inflammation, their precise role in the pathogenesis of hypertension remains to be elucidated.
In the present study, SLE mice treated with MMF have less renal injury, as measured by albuminuria and histological assessment of glomerular damage. This is in agreement with previous studies in which MMF treatment delayed onset of renal injury and lowered proteinuria levels.39 Clinically, patients with SLE who were administered MMF have been shown to have normal serum creatinine levels and decreased proteinuria.58, 59, 60 The possibility exists that the prevention of renal injury is the result of the blood pressure–lowering effect of MMF; however, this is unlikely for several reasons. First, other established experimental models (ie, MRL/lpr) develop aggressive renal pathology that causes mortality typically by 20 weeks of age, but they do not develop hypertension.61 Second, studies from our laboratory show that albuminuria does not track with hypertension; for example, early life ovariectomy of NZBWF1 mice ameliorates albuminuria while hypertension persists.62 Importantly, this disconnect between nephritis and the development of hypertension during SLE has also been reported in humans.63, 64
The present study further advances our understanding of the mechanisms by which renal and inflammatory changes, previously reported by us as promoting SLE‐associated hypertension, may be initiated. MMF significantly reduced the number of both CD3+CD4+ T cells and CD45R+ B cells in the kidneys. Inflammatory infiltrates of the kidneys in SLE patients and NZBWF1 mice are predominately composed of CD4+ T cells followed by CD8+ T cells, macrophages, B cells, and plasma cells.65, 66, 67, 68 Inflamed kidneys are often the site of tertiary lymphoid organ formation that support the clonal expansion of B cells in response to T cell aggregates.69 B cells and plasma cells can contribute to both systemic and intrarenal autoantibody production,67, 70 and B cells, T cells, and macrophages contribute to local inflammation and tissue pathology.71, 72 Consequently, a decrease in renal lymphocytes may lower the inflammation that is likely a driving factor in the development of hypertension. A potential mechanism that could lead to the reduction of lymphocytes in the kidneys is decreased immune complex deposition in the glomeruli. Treatment with MMF has been shown to decrease immune complex deposition in SLE mice.40 In addition, MMF can inhibit the glycosylation and expression of adhesion molecules, which decreases the recruitment of lymphocytes into sites of inflammation.20, 73 Data from both experimental and human hypertension indicate that renal immune cell infiltrates and renal injury contribute to the development of hypertension.34, 35, 36, 37
Perspectives
The concept that adaptive immune system dysfunction associates with hypertension is now widely accepted. Both specific activating autoantibodies and circulating antibodies to nuclear antigens correlate with increased blood pressure in patients. In the present study, MMF treatment had an effect on circulating B lymphocytes, anti‐dsDNA autoantibody production, renal injury, and hypertension. Based on the data from this study and previous studies by our laboratory, we believe that autoreactive B cells and pathogenic autoantibodies mechanistically contribute to the pathogenesis of hypertension during SLE. MMF is well established as an option for both induction and maintenance therapy in patients with SLE, and it may have the added benefit of helping to control blood pressure by depleting B cells and lowering autoantibody production. Hypertension affects a significant portion of SLE patients, and current clinical data suggest that patients with SLE have an extremely risk of high cardiovascular disease, making rigorous blood pressure control (ie, <130/80 mm Hg) of paramount importance in this patient population.74
Sources of Funding
Taylor was supported by NIH National Heart, Lung, and Blood Institute (NHLBI) T32HL105324‐05. This work was supported by Veteran's Administration Merit award (BX002604‐01A2) to Ryan and NIH NHLBI awards PO1HL051971, P20GM104357 to UMMC‐Department of Physiology and Biophysics.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005394. DOI: 10.1161/JAHA.116.005394.)
References
- 1. Ebringer A, Doyle AE. Raised serum IgG levels in hypertension. Br Med J. 1970;2:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suryaprabha P, Padma T, Rao UB. Increased serum IgG levels in essential hypertension. Immunol Lett. 1984;8:143–145. [DOI] [PubMed] [Google Scholar]
- 3. Hilme E, Herlitz H, Soderstrom T, Hansson L. Increased secretion of immunoglobulins in malignant hypertension. J Hypertens. 1989;7:91–95. [PubMed] [Google Scholar]
- 4. Gudbrandsson T, Hansson L, Herlitz H, Lindholm L, Nilsson LA. Immunological changes in patients with previous malignant essential hypertension. Lancet. 1981;1:406–408. [DOI] [PubMed] [Google Scholar]
- 5. Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wenzel K, Haase H, Wallukat G, Derer W, Bartel S, Homuth V, Herse F, Hubner N, Schulz H, Janczikowski M, Lindschau C, Schroeder C, Verlohren S, Morano I, Muller DN, Luft FC, Dietz R, Dechend R, Karczewski P. Potential relevance of alpha(1)‐adrenergic receptor autoantibodies in refractory hypertension. PLoS One. 2008;3:e3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Budman DR, Steinberg AD. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med. 1976;136:1003–1007. [PubMed] [Google Scholar]
- 8. Mandell BF. Cardiovascular involvement in systemic lupus erythematosus. Semin Arthritis Rheum. 1987;17:126–141. [DOI] [PubMed] [Google Scholar]
- 9. Selzer F, Sutton‐Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–1082. [DOI] [PubMed] [Google Scholar]
- 10. Al‐Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol. 2003;30:493–496. [PubMed] [Google Scholar]
- 11. Sabio JM, Vargas‐Hitos JA, Navarrete‐Navarrete N, Mediavilla JD, Jimenez‐Jaimez J, Diaz‐Chamorro A, Jimenez‐Alonso J; Grupo Lupus Virgen de las N . Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol. 2011;38:1026–1032. [DOI] [PubMed] [Google Scholar]
- 12. Shaharir SS, Mustafar R, Mohd R, Mohd Said MS, Gafor HA. Persistent hypertension in lupus nephritis and the associated risk factors. Clin Rheumatol. 2015;34:93–97. [DOI] [PubMed] [Google Scholar]
- 13. Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen‐McWilliams L, D'Agostino RB, Kuller LH. Age‐specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. [DOI] [PubMed] [Google Scholar]
- 14. Mody GM, Parag KB, Nathoo BC, Pudifin DJ, Duursma J, Seedat YK. High mortality with systemic lupus erythematosus in hospitalized African blacks. Br J Rheumatol. 1994;33:1151–1153. [DOI] [PubMed] [Google Scholar]
- 15. Abu‐Shakra M, Urowitz MB, Gladman DD, Gough J. Mortality studies in systemic lupus erythematosus. Results from a single center. I. Causes of death. J Rheumatol. 1995;22:1259–1264. [PubMed] [Google Scholar]
- 16. Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, Urowitz M, Fortin PR, Petri M, Barr S, Gordon C, Bae SC, Isenberg D, Zoma A, Aranow C, Dooley MA, Nived O, Sturfelt G, Steinsson K, Alarcon G, Senecal JL, Zummer M, Hanly J, Ensworth S, Pope J, Edworthy S, Rahman A, Sibley J, El‐Gabalawy H, McCarthy T, St Pierre Y, Clarke A, Ramsey‐Goldman R. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–2557. [DOI] [PubMed] [Google Scholar]
- 17. Mathis KW, Wallace K, Flynn ER, Maric‐Bilkan C, LaMarca B, Ryan MJ. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension. 2014;64:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mok CC. Mycophenolate mofetil for lupus nephritis: an update. Expert Rev Clin Immunol. 2015;11:1353–1364. [DOI] [PubMed] [Google Scholar]
- 19. Zhu B, Chen N, Lin Y, Ren H, Zhang W, Wang W, Pan X, Yu H. Mycophenolate mofetil in induction and maintenance therapy of severe lupus nephritis: a meta‐analysis of randomized controlled trials. Nephrol Dial Transplant. 2007;22:1933–1942. [DOI] [PubMed] [Google Scholar]
- 20. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez‐Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera‐Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. [DOI] [PubMed] [Google Scholar]
- 22. Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt‐sensitive rat. Hypertension. 2006;48:149–156. [DOI] [PubMed] [Google Scholar]
- 23. Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD Jr. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt‐sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1018–H1025. [DOI] [PubMed] [Google Scholar]
- 24. Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–F524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herrera J, Ferrebuz A, MacGregor EG, Rodriguez‐Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225. [DOI] [PubMed] [Google Scholar]
- 26. Mathis KW, Venegas‐Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension. 2012;59:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mathis KW, Venegas‐Pont M, Masterson CW, Wasson KL, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt‐sensitive. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1281–R1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venegas‐Pont M, Mathis KW, Iliescu R, Ray WH, Glover PH, Ryan MJ. Blood pressure and renal hemodynamic responses to acute angiotensin II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1286–R1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathis KW, Venegas‐Pont M, Flynn ER, Williams JM, Maric‐Bilkan C, Dwyer TM, Ryan MJ. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol. 2013;305:R711–R719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mihara M, Tan I, Chuzhin Y, Reddy B, Budhai L, Holzer A, Gu Y, Davidson A. CTLA4Ig inhibits T cell‐dependent B‐cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryan MJ, McLemore GR Jr. Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R736–R742. [DOI] [PubMed] [Google Scholar]
- 32. Venegas‐Pont M, Sartori‐Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1282–R1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilbert EL, Mathis KW, Ryan MJ. 17beta‐estradiol protects against the progression of hypertension during adulthood in a mouse model of systemic lupus erythematosus. Hypertension. 2014;63:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson RJ, Rodriguez‐Iturbe B, Nakagawa T, Kang DH, Feig DI, Herrera‐Acosta J. Subtle renal injury is likely a common mechanism for salt‐sensitive essential hypertension. Hypertension. 2005;45:326–330. [DOI] [PubMed] [Google Scholar]
- 35. Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez‐Iturbe B, Vaziri ND, Herrera‐Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt‐sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 2004;286:F606–F616. [DOI] [PubMed] [Google Scholar]
- 37. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Venegas‐Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor‐alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corna D, Morigi M, Facchinetti D, Bertani T, Zoja C, Remuzzi G. Mycophenolate mofetil limits renal damage and prolongs life in murine lupus autoimmune disease. Kidney Int. 1997;51:1583–1589. [DOI] [PubMed] [Google Scholar]
- 40. Van Bruggen MC, Walgreen B, Rijke TP, Berden JH. Attenuation of murine lupus nephritis by mycophenolate mofetil. J Am Soc Nephrol. 1998;9:1407–1415. [DOI] [PubMed] [Google Scholar]
- 41. McMurray RW, Elbourne KB, Lagoo A, Lal S. Mycophenolate mofetil suppresses autoimmunity and mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus. J Rheumatol. 1998;25:2364–2370. [PubMed] [Google Scholar]
- 42. Jonsson CA, Erlandsson M, Svensson L, Molne J, Carlsten H. Mycophenolate mofetil ameliorates perivascular T lymphocyte inflammation and reduces the double‐negative T cell population in SLE‐prone MRLlpr/lpr mice. Cell Immunol. 1999;197:136–144. [DOI] [PubMed] [Google Scholar]
- 43. Reilly CM, Gilkeson GS. Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/lpr mice. Immunol Res. 2002;25:143–153. [DOI] [PubMed] [Google Scholar]
- 44. Hepburn AL, Narat S, Mason JC. The management of peripheral blood cytopenias in systemic lupus erythematosus. Rheumatology (Oxford). 2010;49:2243–2254. [DOI] [PubMed] [Google Scholar]
- 45. Newman K, Owlia MB, El‐Hemaidi I, Akhtari M. Management of immune cytopenias in patients with systemic lupus erythematosus—old and new. Autoimmun Rev. 2013;12:784–791. [DOI] [PubMed] [Google Scholar]
- 46. McInerney MF, Clough JD, Senitzer D, Cathcart MK. Two distinct subsets of patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1988;49:116–132. [DOI] [PubMed] [Google Scholar]
- 47. Ramos MA, Pinera C, Setien MA, Buelta L, de Cos MA, de Francisco AL, Merino R, Arias M. Modulation of autoantibody production by mycophenolate mofetil: effects on the development of SLE in (NZB x NZW)F1 mice. Nephrol Dial Transplant. 2003;18:878–883. [DOI] [PubMed] [Google Scholar]
- 48. Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short‐lived plasmablasts and long‐lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fassbinder T, Saunders U, Mickholz E, Jung E, Becker H, Schluter B, Jacobi AM. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther. 2015;17:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eickenberg S, Mickholz E, Jung E, Nofer JR, Pavenstadt HJ, Jacobi AM. Mycophenolic acid counteracts B cell proliferation and plasmablast formation in patients with systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin Immunopathol. 2006;28:175–184. [DOI] [PubMed] [Google Scholar]
- 52. Luther HP, Homuth V, Wallukat G. Alpha 1‐adrenergic receptor antibodies in patients with primary hypertension. Hypertension. 1997;29:678–682. [DOI] [PubMed] [Google Scholar]
- 53. Zhou ZH, Wang J, Xiao H, Chen ZJ, Wang M, Cheng X, Liao YH. A novel autoantibody in patients with primary hypertension: antibody against L‐type Ca2+ channel. Chin Med J (Engl). 2008;121:1513–1517. [PubMed] [Google Scholar]
- 54. Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus. 2004;13:290–297. [DOI] [PubMed] [Google Scholar]
- 55. Kristensen BO. Autoantibodies in untreated and treated essential hypertension: relationship to histocompatability leucocyte antigen‐B15 and vascular complications. Clin Sci (Lond). 1979;57(suppl 5):287s–290s. [DOI] [PubMed] [Google Scholar]
- 56. Kristensen BO, Andersen PL. Autoantibodies in untreated and treated essential hypertension. I. Acta Med Scand. 1978;203:55–59. [DOI] [PubMed] [Google Scholar]
- 57. Kristensen BO, Andersen PL, Wiik A. Autoantibodies and vascular events in essential hypertension: a five‐year longitudinal study. J Hypertens. 1984;2:19–24. [DOI] [PubMed] [Google Scholar]
- 58. Dooley MA, Cosio FG, Nachman PH, Falkenhain ME, Hogan SL, Falk RJ, Hebert LA. Mycophenolate mofetil therapy in lupus nephritis: clinical observations. J Am Soc Nephrol. 1999;10:833–839. [DOI] [PubMed] [Google Scholar]
- 59. Laskari K, Mavragani CP, Tzioufas AG, Moutsopoulos HM. Mycophenolate mofetil as maintenance therapy for proliferative lupus nephritis: a long‐term observational prospective study. Arthritis Res Ther. 2010;12:R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rezaieyazdi Z, Tavakoli T, Khajehdaluee M, Honarmand S. Efficacy of long‐term maintenance therapy with mycophenolate mofetil in lupus nephritis. Springerplus. 2014;3:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (NZB x NZW)F1, MRL‐lpr, and BXSB mice with lupus nephritis. Am J Pathol. 1984;116:107–114. [PMC free article] [PubMed] [Google Scholar]
- 62. Gilbert EL, Ryan MJ. Impact of early life ovariectomy on blood pressure and body composition in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2014;307:R990–R997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch Intern Med. 1992;152:2082–2088. [PubMed] [Google Scholar]
- 64. Petrin J, Rozman B, Dolenc P, Logar D, Bozic B, Vizjak A, Ferluga D, Jezersek P. The dissociation of arterial hypertension and lupus glomerulonephritis in systemic lupus erythematosus. Blood Press. 1993;2:108–112. [DOI] [PubMed] [Google Scholar]
- 65. Boucher A, Droz D, Adafer E, Noel LH. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. Kidney Int. 1986;29:1043–1049. [DOI] [PubMed] [Google Scholar]
- 66. Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37:100–109. [DOI] [PubMed] [Google Scholar]
- 67. Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur J Immunol. 2001;31:2726–2732. [DOI] [PubMed] [Google Scholar]
- 68. Starke C, Frey S, Wellmann U, Urbonaviciute V, Herrmann M, Amann K, Schett G, Winkler T, Voll RE. High frequency of autoantibody‐secreting cells and long‐lived plasma cells within inflamed kidneys of NZB/W F1 lupus mice. Eur J Immunol. 2011;41:2107–2112. [DOI] [PubMed] [Google Scholar]
- 69. Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR. In situ B cell‐mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Espeli M, Bokers S, Giannico G, Dickinson HA, Bardsley V, Fogo AB, Smith KG. Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol. 2011;22:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, Bottinger EP, Ivashkiv L, Kretzler M, Davidson A. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol. 2011;186:4994–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Katsiari CG, Liossis SN, Sfikakis PP. The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: a reappraisal. Semin Arthritis Rheum. 2010;39:491–503. [DOI] [PubMed] [Google Scholar]
- 73. Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14(suppl 1):s2–s8. [DOI] [PubMed] [Google Scholar]
- 74. Tselios K, Koumaras C, Urowitz MB, Gladman DD. Do current arterial hypertension treatment guidelines apply to systemic lupus erythematosus patients? A critical appraisal. Semin Arthritis Rheum. 2014;43:521–525. [DOI] [PubMed] [Google Scholar]


