Abstract
Background
Outpatient ascertainment of peripheral artery disease (PAD) is rarely considered in the measurement of PAD clinical burden; therefore, the clinical burden of PAD likely has been underestimated while contributing to a decreased awareness of PAD in comparison to other circulatory system disorders.
Methods and Results
The purpose of this study was to estimate the age‐standardized annual period prevalence and incidence of PAD in the outpatient and inpatient settings using data from the Atherosclerosis Risk in Communities (ARIC) study linked with Centers for Medicare and Medicaid Services claims. The majority (>70%) of all PAD encounters occurred in the outpatient setting. The weighted mean age‐standardized prevalence and incidence of outpatient PAD was 11.8% (95% CI 11.5–12.1) and 22.4 per 1000 person‐years (95% CI 20.8–24.0), respectively. Black patients had higher weighted mean age‐standardized prevalence (15.6%; 95% CI 14.6–16.4) compared with white patients (11.4%; 95% CI 11.1–11.7). Black women had the highest weighted mean age‐standardized prevalence (16.9%; 95% CI 16.0–17.8). Black patients also had a higher incidence rate of PAD (31.3 per 1000 person‐years; 95% CI 27.3–35.4) compared with white patients (25.4 per 1000 person‐years; 95% CI 23.5–27.3). PAD prevalence and incidence did not differ by sex alone.
Conclusions
This study provides comprehensive estimates of PAD in the inpatient and outpatient settings where the majority of PAD burden was found. PAD is an important circulatory system disorder similar in prevalence to stroke and coronary heart disease.
Keywords: aging, Atherosclerosis Risk in Communities, claims, epidemiology, Medicare, peripheral artery disease, population science
Subject Categories: Epidemiology, Peripheral Vascular Disease
Introduction
Peripheral artery disease (PAD) is a progressive atherosclerotic disorder that can lead to poor quality of life,1 an increased risk of hospitalization and limb amputation,2 high mortality,3 and high costs of care.4 Early PAD detection in the outpatient setting combined with ambulatory follow‐up care could help slow disease progression and reduce PAD‐related hospitalizations and sequelae.5 However, the extent to which PAD is managed in the outpatient setting is not well documented.
Reported estimates of clinical PAD prevalence and incidence tend to focus on only hospitalized cases.6, 7, 8, 9, 10, 11, 12 Estimates of disease occurrence in both inpatient and outpatient settings could provide a broader, more comprehensive understanding of PAD and could lead to improved resource allocation to prevent PAD‐related complications. Administrative claims data capture comprehensive services across the spectrum of health care settings and provide an opportunity for a more inclusive assessment of PAD burden.
We estimated the age‐standardized annual period prevalence and incidence of PAD in the inpatient and outpatient setting over a 10‐year period (2003–2012) using data from the biracial Atherosclerosis Risk in Communities (ARIC) study cohort13 linked with the Centers for Medicare and Medicaid Services (CMS) claims information for Medicare fee‐for‐service (FFS) beneficiaries aged ≥65 years. To further inform prevention efforts, we examined differences in estimates of annual PAD period prevalence and incidence across strata of age, sex, and race.
Methods
Study Population
The ARIC cohort study
The biracial ARIC cohort, established to examine the etiology of atherosclerosis and its clinical manifestations, includes 15 792 participants (aged 45–64 years at baseline) enrolled between 1987 and 1989. The ARIC cohort was selected by probability sampling from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; the city of Jackson, Mississippi; and the suburbs of Minneapolis, Minnesota.13 ARIC participants enrolled continuously for at least 1 year in Medicare Parts A and B through an FFS plan from 2003 to 2012 were eligible for inclusion. Data were collected on cohort participants at 5 clinic examinations and through annual follow‐up telephone interviews.
Linkage of ARIC cohort data with CMS claims
An interagency agreement between the National Heart, Lung, and Blood Institute and CMS has enabled Medicare claims information to be obtained for the 14 899 ARIC cohort participants who were Medicare eligible between the years 1991 and 2012. Data for ARIC cohort participants were linked with CMS claims data, matching on participants' social security numbers, sex, and date of birth. Of the 14 899 Medicare‐eligible participants, 14 702 ARIC cohort identifiers (98.7%) were matched successfully.
Participant information on enrollment in Medicare FFS was obtained from monthly enrollment indicators for Part A, Part B, and Medicare Advantage buy‐in available through annual CMS Medicare beneficiary summary files. Continuous enrollment periods were created to indicate uninterrupted CMS Medicare FFS coverage, defined as enrollment in CMS Medicare Part A and Part B and lack of enrollment in a Medicare Advantage plan. Participants contributed data to calendar years in which they had uninterrupted FFS coverage. Participants were excluded if they had continuous Medicare Advantage enrollment or gaps in FFS coverage because of (1) missing enrollment information, (2) discontinuation of enrollment, or (3) enrollment in a Medicare Advantage plan at any month in the observation year. Participants aged <65 years and those of a race other than black or white were also excluded (see Table S1). For those with multiple enrollments periods, the longest enrollment period was selected to give the best opportunity to capture relevant claims. The enrollment period selected was the first enrollment period for 10 144 participants (97%). The final analytic sample included 10 481 ARIC participants with 67 492 person‐years of FFS enrollment time.
Demographics and comorbidities
Demographic information on age (at beginning of enrollment year), race, and sex was obtained from annual Medicare beneficiary summary files. Age was categorized as 65 to 74 years of age and ≥75 years of age. The Klabunde adaptation of the Charlson Comorbidity Index was used to identify comorbidity burden using claims from the inpatient and outpatient settings.14, 15 All claims present in each calendar year (prior to a PAD case) were used to calculate an annual Charlson Comorbidity Index score.
Ascertainment of PAD
PAD‐related outpatient office visits, outpatient diagnostic tests, inpatient visits, and procedures were identified from the MedPAR (Medicare Provider Analysis and Review) records and the Carrier and Outpatient claims files using codes from the International Classification of Diseases, Ninth Revision (ICD); Current Procedural Terminology, 4th edition (CPT); Healthcare Common Procedure Coding System (HCPCS); and Federally Qualified Healthcare Center (FQHC) (Table S2). Codes used in the present study were adapted from previous PAD‐related administrative studies.16, 17
Prevalence of PAD
Annual PAD period prevalence was estimated for 2003 to 2012 using information on any PAD encounters in the inpatient and outpatient settings, including both prevalence cases from prior years and new incident cases during the year of observation. Overall mean annual prevalence, weighted to reflect the distribution of cases across all years, was also estimated. The denominator for annual period prevalence estimates included cohort participants alive at the beginning of the year with continuous enrollment in FFS for the entire year of observation or until death. For each year of observation, prevalent inpatient PAD was defined as ≥1 hospitalization with a PAD code in any of the 25 diagnosis or procedure positions; prevalent outpatient PAD was defined as ≥1 claim with PAD diagnosis or procedure codes in any of the 12 diagnosis positions or 6 procedure positions. A sensitivity analysis using ≥2 outpatient claims was conducted to address the possibility of rule‐out diagnoses (Table S3).
Incidence of PAD
A 2‐year look back period was chosen to minimize misclassification of prevalent events as incident events18; therefore, the shortest enrollment window of ARIC participants was >24 months. ARIC participants with a prevalent PAD‐related inpatient or outpatient code occurring any time within 2 years of the year in question were excluded from annual incidence analyses. Annual incidence rates are presented for the years 2005 to 2012. Overall mean incidence, weighted to reflect the distribution of events across all years, was also estimated. The denominator for annual incidence estimates included cohort participants' time at risk in continuous enrollment during the year of observation or until death if it occurred during the year of observation.
Annual inpatient PAD incidence was defined as ≥1 hospitalization with a PAD‐related ICD diagnosis or procedure code during each year of observation. Annual outpatient PAD incidence was defined as ≥2 claims within 12 consecutive months with a PAD‐related ICD, CPT, HCPCS, or FQHC code; the claims had to occur ≥1 day apart, and the incident date was defined as the date of the second claim. If a singular outpatient encounter preceded an inpatient encounter within 365 days, the incident date was the inpatient date of discharge. Singular outpatient encounters occurring with no hospitalizations or outpatient encounters within 365 days were not considered incident PAD. This definition of incidence was chosen to reduce the misclassification of rule‐out diagnoses resulting from a singular encounter in the outpatient setting.19 Each individual contributed between 1 and 12 months to each yearly estimate of incidence. Time contributed to the study for each ARIC participant was converted to and reported in person‐years.
Statistical Analysis
Direct standardization was used to estimate age‐standardized overall and annual prevalence of PAD with 95% CIs from 2003 to 2012. Direct standardization was used to estimate age‐standardized overall and annual incidence of PAD (per 1000 person‐years) with 95% CI from 2005 to 2012. Prevalence estimates were age‐standardized to reflect the age, race, and sex distribution of the 2005 Medicare population aged ≥65 years. Age categories for standardization of prevalence estimates included 65 to 69, 70 to 74, 75 to 79, and ≥80 years of age. Incidence estimates were age‐standardized to reflect the age, race, and sex distribution of the 2005 Medicare population aged ≥67 years, given a 2‐year look‐back period for excluding prevalence cases. Estimates were calculated overall; by health care setting (inpatient versus outpatient setting); and by age, race, sex, and race/sex subgroups. Age categories for incidence estimate standardization included 67 to 69, 70 to 74, 75 to 79, and ≥80 years of age. All analyses were performed using SAS version 9.4 (SAS Institute Inc.). Written informed consent was obtained from participants, and all institutional review boards approved the study.
Results
The 10 481 ARIC cohort members who met eligibility requirements generally reflected the demographic distribution in the original ARIC cohort at baseline. The majority were female (58%) and white (76%), with black men (8%) as the least represented group (Table 1). Mean comorbidity scores were similar across race, sex, and race/sex strata. Mean comorbidity score increased as the cohort aged from 2003 to 2012 (Table 1).
Table 1.
ARIC Fee‐for‐Service Enrollees by Year and Demographic Groups, 2003–2012
| 2003 (n=7293) | 2004 (n=7678) | 2005 (n=7708) | 2006 (n=7372) | 2007 (n=7060) | 2008 (n=6995) | 2009 (n=6504) | 2010 (n=6183) | 2011 (n=5914) | 2012 (n=5546) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, %, y | ||||||||||
| 65–74 | 72 | 69 | 66 | 63 | 60 | 57 | 54 | 49 | 44 | 40 |
| ≥75 | 28 | 31 | 34 | 37 | 40 | 43 | 46 | 51 | 56 | 60 |
| Sex, % | ||||||||||
| Female | 57 | 57 | 58 | 57 | 58 | 58 | 59 | 60 | 60 | 61 |
| Male | 43 | 43 | 42 | 43 | 42 | 42 | 41 | 40 | 40 | 39 |
| Race, % | ||||||||||
| Black | 26 | 26 | 26 | 23 | 21 | 22 | 23 | 24 | 24 | 24 |
| White | 74 | 74 | 74 | 77 | 79 | 78 | 77 | 76 | 76 | 76 |
| Race/sex, % | ||||||||||
| Black woman | 17 | 17 | 18 | 15 | 14 | 14 | 15 | 16 | 16 | 16 |
| Black man | 9 | 9 | 9 | 8 | 7 | 7 | 8 | 8 | 8 | 8 |
| White women | 40 | 40 | 40 | 42 | 44 | 44 | 45 | 44 | 44 | 44 |
| White man | 34 | 34 | 33 | 35 | 35 | 34 | 32 | 32 | 32 | 32 |
| Overall mean comorbidity scorea (SD) | 1.7 (2.2) | 1.8 (2.3) | 1.9 (2.2) | 1.9 (2.2) | 2.1 (2.3) | 2.1 (2.3) | 2.3 (2.5) | 2.4 (2.5) | 2.5 (2.5) | 2.6 (2.6) |
| Overall median comorbidity scorea | 1 (0, 3) | 1 (0, 3) | 1 (0, 3) | 1 (0, 3) | 2 (0, 3) | 2 (0, 3) | 2 (0, 3) | 2 (0, 3) | 2 (0, 4) | 2 (1, 4) |
ARIC indicates atherosclerosis risk in communities.
Klabunde adaptation15 of Charlson comorbidity index.
Age‐Standardized Annual Prevalence and Weighted Mean Annual Prevalence of PAD
Age‐standardized annual and weighted mean annual estimates of the prevalence of PAD across all study years (2003–2012), overall and stratified by health care setting, are provided in Table 2. The weighted mean annual PAD period prevalence was 12.4% (95% CI 12.2–12.8%). Overall age‐standardized prevalence varied modestly from year to year, ranging from 10.3% (95% CI 8.6–12.0%) to 13.5% (95% CI 12.4–14.6%).
Table 2.
Age‐Standardizeda Overall and Annual Prevalence of Peripheral Artery Disease Claims Overall and by Health Care Setting: The ARIC Cohort Study (2003–2012)
| Prevalence, % (95% CI) | |||
|---|---|---|---|
| Overall | Outpatient Setting | Inpatient Setting | |
| 2003 | 10.3 (8.6–12.0) | 9.5 (7.9–11.2) | 1.5 (1.0–1.9) |
| 2004 | 11.2 (10.0–12.4) | 10.4 (9.3–11.6) | 1.8 (1.3–2.3) |
| 2005 | 11.4 (10.5–12.4) | 10.8 (9.8–11.7) | 1.4 (1.1–1.7) |
| 2006 | 12.1 (11.1–13.0) | 11.5 (10.6–12.4) | 1.8 (1.4–2.1) |
| 2007 | 12.3 (11.4–13.2) | 11.5 (10.7–12.4) | 1.4 (1.1–1.7) |
| 2008 | 11.8 (10.9–12.6) | 11.2 (10.4–12.0) | 1.6 (1.3–1.9) |
| 2009 | 12.6 (11.7–13.4) | 12.0 (11.1–12.8) | 1.4 (1.1–1.7) |
| 2010 | 13.2 (12.3–14.1) | 12.7 (11.8–13.5) | 1.4 (1.1–1.7) |
| 2011 | 13.1 (12.2–14.0) | 12.7 (11.7–13.5) | 1.6 (1.3–1.9) |
| 2012 | 13.5 (12.4–14.6) | 12.9 (11.9–14.0) | 1.8 (1.4–2.3) |
| Weighted mean | 12.4 (12.2–12.8) | 11.8 (11.5–12.1) | 1.6 (1.5–1.7) |
ARIC indicates atherosclerosis risk in communities.
Standardized to reflect age distribution of 2005 Medicare population.
Higher annual PAD period prevalence was identified in the outpatient setting compared with the inpatient setting (11.8% versus 1.6%) (Table 2). The majority of all unique PAD claims (>70%) identified were from outpatient settings. Prevalence of outpatient PAD claims ranged across years of observation from 9.5% (95% CI 7.9–11.2%) in 2003 to 12.9% (95% CI 11.9–14.0%) in 2012. By comparison, prevalence of inpatient PAD ranged across years of observation from 1.4% (95% CI 1.1–1.7%) in 2005 to 1.8% (95% CI 1.4–2.3%) in 2012.
Estimates of annual PAD period prevalence were further stratified by demographic groups. Age‐standardized annual PAD period prevalence and mean annual prevalence were consistently higher among those aged ≥75 years compared with those aged 65 to 74 years (Figure 1). Weighted mean annual PAD prevalence among those aged ≥75 years and 65 to 74 years was 16.8% and 8.4%, respectively. From 2003 to 2012, annual PAD prevalence in the age group ≥75 years ranged from 12.5% (95% CI 9.0–15.9%) in 2003 to 18.5% (95% CI 17.1–20.0%) in 2012. Annual PAD prevalence among those aged 65 to 74 years ranged from 8.0% (95% CI 7.2–8.8%) to 9.0% (95% CI 8.0–10.0%) over the same time frame.
Figure 1.
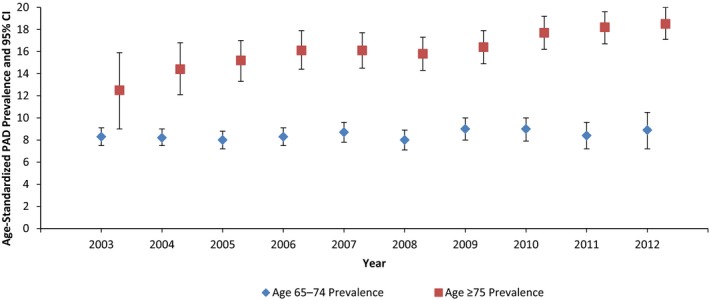
Age‐standardized annual prevalence of peripheral artery disease (PAD) by age group. The ARIC (Atherosclerosis Risk in Communities) study, 2003–2012. Estimates are standardized to the 2005 Medicare population.
Black participants had higher mean annual prevalence of PAD compared with white participants (15.6% versus 11.4%) and had higher annual prevalence of PAD across most years of the observation period (Figure 2). From 2003 to 2012, PAD prevalence among black participants ranged from 13.8% (95% CI 10.4–17.2%) to 17.3% (95% CI 15.0–19.7%), whereas PAD prevalence among white participants ranged from 9.0% (95% CI 7.1–10.9%) to 12.8% (95% CI 11.6–14.0%). Regarding race/sex stratification, black women had the highest weighted mean annual PAD prevalence (16.9%), followed by black men (13.2%), white men (12.1%), and white women (10.9%) (Table S4).
Figure 2.

Age‐standardized annual prevalence (percentage) of peripheral artery disease (PAD) by race group. The ARIC (Atherosclerosis Risk in Communities) study, 2003–2012. Estimates are age‐standardized to 2005 Medicare population.
Age‐standardized prevalence of PAD did not differ by sex alone in any year of observation (2003–2012). Overall, women had higher prevalence of PAD, although confidence intervals overlapped across many years of this study (Table S5).
Age‐Standardized Annual Incidence and Weighted Mean Annual Incidence of PAD
Overall and age‐standardized annual estimates of the incidence of PAD across all observation years (2005–2012), stratified by health care setting, are provided in Table 3. The mean age‐standardized PAD incidence rate across all observation years (2005–2012) was 26.8 per 1000 person‐years (95% CI 25.1–28.6). The age‐standardized incidence of PAD remained relatively consistent across the study period (2005–2012), ranging from 25.6 per 1000 person‐years (95% CI 20.8–30.4) in 2007 to 30.3 per 1000 person‐years (95% CI 24.9–35.7) in 2012 (Table 3).
Table 3.
Age‐Standardizeda Overall and Annual Incidence of Peripheral Artery Disease Claims Overall and by Health Care Setting of Incident Claim: The ARIC Cohort Study (2005–2012)
| Age‐Standardized Rate, Per 1000 Person‐Years (95% CI)a | |||
|---|---|---|---|
| Overall | Outpatient | Inpatient | |
| 2005 | 26.6 (20.7–32.6) | 20.3 (15.1–25.4) | 6.4 (3.4–9.3) |
| 2006 | 25.8 (20.3–31.3) | 20.0 (15.2–24.8) | 5.8 (3.1–8.5) |
| 2007 | 25.6 (20.8–30.4) | 21.5 (17.2–25.9) | 4.0 (2.0–6.1) |
| 2008 | 26.0 (21.0–31.0) | 21.2 (16.7–25.7) | 4.8 (2.7–6.9) |
| 2009 | 25.6 (20.9–30.3) | 20.6 (16.4–24.8) | 5.0 (2.9–7.1) |
| 2010 | 29.3 (24.2–34.4) | 25.9 (21.1–30.7) | 3.4 (1.7–5.0) |
| 2011 | 26.5 (21.7–31.4) | 23.3 (18.8–27.9) | 3.2 (1.5–4.8) |
| 2012 | 30.3 (24.9–35.7) | 26.0 (21.0–30.9) | 4.3 (3.7–5.1) |
| Weighted mean | 26.8 (25.1–28.6) | 22.4 (20.8–24.0) | 4.4 (3.7–5.1) |
ARIC indicates atherosclerosis risk in communities.
Standardized to reflect age distribution of 2005 Medicare population.
The first PAD‐related claim most commonly was found in the outpatient setting (83%), at >5 times the incidence in the inpatient setting (Table 3). Rates of PAD incidence in the outpatient setting per 1000 person‐years ranged from 20.0 (95% CI 15.2–24.8) to 26.0 (95% CI 21.0–30.9). Records of PAD‐related hospitalizations were rare; for the years 2005 to 2012, the annual age‐standardized incidence rates per 1000 person‐years ranged from 3.2 (95% CI 1.5–4.8) in 2011 to 6.4 (95% CI 3.4–9.3) in 2005.
Annual estimates of PAD incidence were stratified by demographic groups. Age‐standardized annual PAD incidence was different by age strata at all years examined except 2009 and 2012 (Figure 3). Incidence of PAD was higher among those aged ≥75 years compared with 65 to 74 years. From 2005 to 2012, estimates of annual PAD incidence per 1000 person‐years among those aged ≥75 years ranged from 31.6 (95% CI 24.1–39.2) in 2009 to 37.2 (95% CI 29.5–45.0) in 2012. Estimates among those aged 65 to 74 years ranged from 16.2 per 1000 person‐years (95% CI 12.3–20.1) to 21.7 per 1000 person‐years (95% CI 14.3–29.1) over the same time period.
Figure 3.

Age‐standardized annual incidence (per 1000 person‐years) of peripheral artery disease (PAD) by age group. The ARIC (Atherosclerosis Risk in Communities) study, 2005–2012. Estimates are age‐standardized to 2005 Medicare population; rates are per 1000 person‐years.
PAD incidence rates were different by race (Figure 4), with higher mean annual (2005–2012) PAD incidence among black participants (31.3 per 1000 person‐years; 95% CI 27.3–35.4) compared with white participants (25.4 per 1000 person‐years; 95% CI 23.5–27.3). Black participants had a higher incidence rate of PAD than white participants across most observation years, although annual differences were attenuated due to low precision resulting from a small sample size among black participants (Figure 4). Incidence rates of PAD among black participants ranged from 28.4 per 1000 person‐years (95% CI 16.4–40.3) to 32.7 per 1000 person‐years (95% CI 21.3–44.1), whereas incidence of PAD among white participants ranged from 23.2 per 1000 person‐years (95% CI 17.1–29.4) to 29.6 per 1000 person‐years (95% CI 23.7–35.6).
Figure 4.
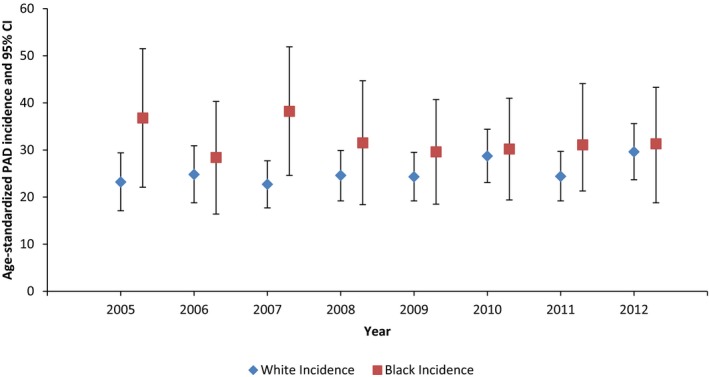
Age‐standardized annual incidence of peripheral artery disease (PAD; per 1000 person‐years), by race groups. The ARIC (Atherosclerosis Risk in Communities) study, 2005–2012. Estimates are age‐standardized to 2005 population; rates are per 1000 person‐years.
The age‐standardized annual incidence of PAD did not differ by sex (Table S6). Mean annual incidence of PAD (2005–2012) was higher among black men and black women (31.8 and 30.9 per 1000 person‐years, respectively) than among white men and white women (25.5 and 25.3 per 1000 person‐years, respectively), although confidence intervals overlapped in many years of observation. Small sample sizes precluded annual assessment of PAD incidence across race/sex groups (Figure S1).
Discussion
We found that the majority of all clinical PAD encounters occurred in the outpatient setting among a biracial, probability‐based sample of 4 US communities, including men and women aged ≥65 years with enrollment in a Medicare FFS program. Studies that focus exclusively on hospitalized events underreport burden and provide a perspective of PAD skewed toward more severe manifestations occurring later in the course of the disease. Black participants had higher prevalence of PAD than white participants, including both men and women. Incidence of PAD was also higher among black participants, although the relatively small proportion of black participants in our study (24%) limited our ability to make inferences in race‐ and race/sex‐ stratified analyses of PAD incidence.
Although sources of administrative claims are increasingly used to study PAD burden, methodological and source population differences make it difficult to compare PAD estimates across studies. In particular, it is well documented that PAD prevalence increases with age16, 20; however, prior claims‐based work did not report age‐adjusted estimates of PAD prevalence, limiting comparisons across populations with differing age groups. A recent study using the MarketScan database, for example, reported higher annual PAD prevalence among Medicare beneficiaries than the present study (14–21% versus 10–14%)16; however, the population in the MarketScan study was older and had more comorbidity. Conversely, a study of a healthier group of managed care enrollees found a lower prevalence of PAD (2%) than what was observed in the present study.2 In the context of these other studies, age‐standardized estimates of PAD prevalence (overall 12.4%) in the present study are within the expected range, given the estimates from younger and older populations.
Estimates of PAD incidence are rare in the literature and, as with prevalence studies, are difficult to compare because of differing study populations and inconsistency in the definition of PAD. When using administrative claims data for the estimation of disease incidence, the use of an appropriate look‐back period is important for the correct identification of index events. Recent analyses suggest that for most chronic diseases, a 2‐year look‐back period is necessary for the exclusion of preexisting conditions.18 Studies that do not include a sufficiently long look‐back period have the potential to reflect prevalent disease that is misclassified as incident (up to 30%).18 Results of the current study, in which we used a 2‐year look‐back period, suggest that 2% to 3% (26.8 per 1000 person‐years) of Medicare beneficiaries had an incident PAD occurrence within any particular observation year (2005–2012). Although our incidence estimates are lower, they are comparable to those in existing studies.16
Prior literature suggests differences in PAD burden by race.21 Black participants were observed to have higher PAD prevalence than white participants in the following studies: NHANES (National Health and Nutrition Examination Survey), SDPS (San Diego Population Study), MESA (Multi‐Ethnic Study of Atherosclerosis), and CHS (Cardiovascular Health Study).6, 7, 22, 23 In the current study, we found that the mean annual prevalence and incidence of PAD was higher among black than white participants, which confirms these prior observations. The current study adds the finding that PAD burden was higher among black participants despite known access to care issue in this population. This study further adds to estimates of PAD burden by providing race/sex analyses. We observed that black women consistently had the highest PAD prevalence, whereas white women had the lowest PAD prevalence across all years of observation (2003–2012) (Table S4). Black men had the highest mean PAD incidence followed by black women (Figure S1). Findings from this study suggest black persons aged ≥65 years have a higher burden of PAD and thus could benefit from prevention efforts targeting individuals well before they become age‐eligible for Medicare.
The American Heart Association (AHA) recently identified sex‐specific estimates of PAD, particularly for women, as a knowledge gap in the literature. The present study answers a challenge from the AHA to produce age‐standardized, sex‐specific estimates.24 Men in this study had nearly identical age‐standardized PAD estimates compared with women overall (mean annual prevalence 12.4% versus 12.5%; mean annual incidence 26.9 versus 26.8 per 1000 person‐years) and at most years of observation. The minimal differences observed in PAD burden by sex were in accordance with the limited literature regarding sex‐specific estimates of PAD prevalence and incidence.10, 20 We acknowledge the potential sex‐based differences in evaluation of PAD and agree with others that these differing practices (1) likely have caused historical underestimation of PAD burden among women and (2) led clinicians to identify male sex as a PAD risk factor.22 Although we are unable to verify this type of sex bias in our study, our data add to a growing body of research suggesting that the burden of PAD is at least similar for women and men.
Finally, despite the known coexistence of PAD with other major circulatory system disorders,25, 26 health professional and public awareness of PAD is low in comparison to the awareness of diseases such as stroke, coronary heart disease, heart failure, and atrial fibrillation.9, 27 Interestingly, PAD prevalence estimates in this study are similar to recent assessments of prevalence of stroke (5–6% in those aged 60–79 years; 14–16% in those aged ≥80 years) and coronary heart disease (10–20% in those aged 60–79 years, 19–32% in those aged ≥80 years).21 Still, although these estimates suggest a significant PAD burden in this population, clinicians often do not evaluate for the presence of PAD. Furthermore, up to 50% of persons with PAD are asymptomatic and might not be actively seeking PAD‐related care.9 Our claims‐based estimates, which capture PAD in a clinical setting and thus would be unlikely to include asymptomatic PAD, are likely an underestimation of the prevalence and incidence of PAD in the Medicare‐aged population.
Strengths and Limitations
The most important strength of this study is the inclusion of outpatient in addition to inpatient clinical encounters in the assessment of prevalent and incident PAD. Prior studies have provided limited information on the burden of PAD stratified by the setting of health care delivery (inpatient versus outpatient). Although a study by Hirsch et al found that inpatient visits represent up to 90% of PAD‐related costs,28 >70% of all PAD claims in the current study were found in the outpatient records. In addition, >80% of all incident PAD events were found to have occurred in the outpatient setting. These estimates are age‐standardized, and look‐back periods for incidence are in accordance with recent recommendations, providing a further strength of this study.
Because this study was based on inpatient and outpatient care among CMS Medicare enrollees in FFS programs, our estimates are not generalizable to Medicare beneficiaries enrolled in Medicare Advantage, who have been reported to be healthier than those in FFS.29 Our estimates reflect cohort survivors, and we did not attempt to quantify PAD prior to enrollment in FFS in 2003. Administrative claims data reflect billing practices; therefore, diagnostic coding found in claims data is not always accurate in relation to documented diagnoses or procedures. Codes selected were not independently validated, which could lead to misclassification of PAD occurrence. Upcoding might increase billing by as much as 15%,30 and illness severity is not readily obtainable from claims data.
Conclusions
Findings from this study suggest that PAD is an important circulatory system disorder similar in prevalence to stroke and coronary heart disease. This study addresses an important gap in the existing literature by providing accurate estimates of PAD in the outpatient setting, where the majority of PAD burden was found. PAD estimates stratified by race corroborated other population‐based studies that reported a higher burden among black compared with white participants; future work should focus on identifying effective prevention of PAD and its sequelae in this group.
Sources of Funding
For C.K. included the National Research Service Award Pre‐Doctoral Traineeship from the National Heart, Lung, and Blood Institute (NHLBI), sponsored by the Cardiovascular Epidemiology Program, University of North Carolina at Chapel Hill (5T32HL007055); a National Research Service Award Pre‐Doctoral Traineeship from the Agency for Healthcare Research and Quality (AHRQ), sponsored by the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill (T32‐HS000032); and a grant from the AHRQ Research Dissertation Program (1R36HS023728‐01). The ARIC study is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Disclosures
None.
Supporting information
Table S1. Exclusion Criteria to Arrive at Final Data Set: The ARIC Study, 2003–2012
Table S2. International Classification of Diseases, Ninth Revision; Current Procedural Terminology, 4th Edition; Healthcare Common Procedure Coding System; and Federally Qualified Healthcare Revenue Center Codes Used to Identify Peripheral Artery Disease in Claims
Table S3. Sensitivity Analysis Comparing Requiring 2 Outpatient Claims Versus 1 Outpatient Claim to Identify Prevalence of Peripheral Artery Disease in the Outpatient Setting: The ARIC Study, 2003–2012
Table S4. *Age‐Standardized Annual Prevalence of Peripheral Artery Disease in the Inpatient and Outpatient Settings by Race/Sex Groups: The ARIC Study, 2003–2012
Table S5. Age‐Standardized Annual Prevalence of Peripheral Artery Disease in the Inpatient and Outpatient Settings by Sex: The ARIC Study, 2003–2012
Table S6. Age‐Standardized Annual Incidence of Peripheral Artery Disease in the Inpatient and Outpatient Settings by Sex: The ARIC Study, 2005–2012
Figure S1. Age‐standardized annual incidence of peripheral artery disease in the inpatient and outpatient setting among 4 race/sex groups: The ARIC (Atherosclerosis Risk in Communities) Study, 2005–2012. Estimates are age‐standardized to 2005 Medicare population rates are per 1000 person‐years.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2017;6:e003796 DOI: 10.1161/JAHA.116.003796.)28468784
References
- 1. McDermott M. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleve Clin J Med. 2006;73:S1–S6. [DOI] [PubMed] [Google Scholar]
- 2. Margolis J, Barron JJ, Grochulski WD. Health care resources and costs for treating peripheral artery disease in a managed care population: results from analysis of administrative claims data. J Manag Care Pharm. 2005;11:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. [DOI] [PubMed] [Google Scholar]
- 4. Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. Transatlantic Inter‐Society Consensus. J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 5. Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. [DOI] [PubMed] [Google Scholar]
- 6. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 7. Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. Ankle‐arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:538–545. [DOI] [PubMed] [Google Scholar]
- 8. Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. [DOI] [PubMed] [Google Scholar]
- 9. Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JC, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. J Am Med Assoc. 2001;286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 10. Murabito JM, Evans JC, D'Agostino RB, Wilson PWF, Kannel WB. Temporal trends in the incidence of intermittent claudication from 1950 to 1999. Am J Epidemiol. 2005;162:430–437. [DOI] [PubMed] [Google Scholar]
- 11. Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–1181. [DOI] [PubMed] [Google Scholar]
- 12. Hooi JD, Kester ADM, Stoffers EJHH, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153:666–672. [DOI] [PubMed] [Google Scholar]
- 13. Investigators TA . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 15. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 16. Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:1–10. [DOI] [PubMed] [Google Scholar]
- 17. Fan J, Arruda‐Olson AM, Leibson CL, Smith C, Liu G, Bailey KR, Kullo IJ. Billing code algorithms to identify cases of peripheral artery disease from administrative data. J Am Med Inform Assoc. 2013;20:e349–e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Griffiths RI, O'Malley CD, Herbert RJ, Danese MD. Misclassification of incident conditions using claims data: impact of varying the period used to exclude pre‐existing disease. BMC Med Res Methodol. 2013;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goyal A, Norton CR, Thomas TN, Davis RL, Butler J, Ashok V, Zhao L, Vaccarino V, Wilson PWF. Predictors of incident heart failure in a large insured population: a one million person‐year follow‐up study. Circ Heart Fail. 2010;3:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaff MR, Cahill KE, Yu AP, Birnbaum HG, Engelhart LM. Clinical outcomes and medical care costs among Medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010;24:577–587. [DOI] [PubMed] [Google Scholar]
- 21. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire VDK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; Subcommittee. obotAHASCaSS . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 22. Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. [DOI] [PubMed] [Google Scholar]
- 23. Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S. The effect of novel cardiovascular risk factors on the ethnic‐specific odds for peripheral arterial disease in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006;48:1190–1197. [DOI] [PubMed] [Google Scholar]
- 24. Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, Hiatt WR, Karas RH, Lovell MB, McDermott MM, Mendes DM, Nussmeier NA, Treat‐Jacobson D. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2012;125:1449–1472. [DOI] [PubMed] [Google Scholar]
- 25. Aronow WS, Ahn C. Prevalence of coexistence of coronary artery disease, peripheral arterial disease, and atherothrombotic brain infarction in men and women <62 years of age. Am J Cardiol. 1994;74:64–65. [DOI] [PubMed] [Google Scholar]
- 26. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PWF. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. J Am Med Assoc. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 27. Hirsch AT, Murphy TP, Lovell MB, Twillman G, Treat‐Jacobson D, Harwood EM, Mohler ER III, Creager MA, Hobson RW II, Robertson RM, Howard WJ, Schroeder P, Criqui MH; Peripheral Arterial Disease C . Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116:2086–2094. [DOI] [PubMed] [Google Scholar]
- 28. Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–215. [DOI] [PubMed] [Google Scholar]
- 29. Landon BE, Zaslavsky AM, Bernard SL, Cioffi MJ, Cleary PD. Comparison of performance of traditional Medicare vs managed care. J Am Med Assoc. 2004;291:1744–1752. [DOI] [PubMed] [Google Scholar]
- 30. Brunt CS. CPT fee differentials and visit upcoding under Medicare Part B. Health Econ. 2011;20:831–841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Exclusion Criteria to Arrive at Final Data Set: The ARIC Study, 2003–2012
Table S2. International Classification of Diseases, Ninth Revision; Current Procedural Terminology, 4th Edition; Healthcare Common Procedure Coding System; and Federally Qualified Healthcare Revenue Center Codes Used to Identify Peripheral Artery Disease in Claims
Table S3. Sensitivity Analysis Comparing Requiring 2 Outpatient Claims Versus 1 Outpatient Claim to Identify Prevalence of Peripheral Artery Disease in the Outpatient Setting: The ARIC Study, 2003–2012
Table S4. *Age‐Standardized Annual Prevalence of Peripheral Artery Disease in the Inpatient and Outpatient Settings by Race/Sex Groups: The ARIC Study, 2003–2012
Table S5. Age‐Standardized Annual Prevalence of Peripheral Artery Disease in the Inpatient and Outpatient Settings by Sex: The ARIC Study, 2003–2012
Table S6. Age‐Standardized Annual Incidence of Peripheral Artery Disease in the Inpatient and Outpatient Settings by Sex: The ARIC Study, 2005–2012
Figure S1. Age‐standardized annual incidence of peripheral artery disease in the inpatient and outpatient setting among 4 race/sex groups: The ARIC (Atherosclerosis Risk in Communities) Study, 2005–2012. Estimates are age‐standardized to 2005 Medicare population rates are per 1000 person‐years.


