Abstract
Background
Out‐of‐hospital cardiac arrest (OHCA) results in significant morbidity and mortality, primarily from neurologic injury. Predicting neurologic outcome early post‐OHCA remains difficult in patients receiving targeted temperature management.
Methods and Results
Retrospective analysis was performed on consecutive OHCA patients receiving targeted temperature management (32–34°C) for 24 hours at a tertiary‐care center from 2008 to 2012 (development cohort, n=122). The primary outcome was favorable neurologic outcome at hospital discharge, defined as cerebral performance category 1 to 2 (poor 3–5). Patient demographics, pre‐OHCA diagnoses, and initial laboratory studies post‐resuscitation were compared between favorable and poor neurologic outcomes with multivariable logistic regression used to develop a simple scoring system (C‐GRApH). The C‐GRApH score ranges 0 to 5 using equally weighted variables: (C): coronary artery disease, known pre‐OHCA; (G): glucose ≥200 mg/dL; (R): rhythm of arrest not ventricular tachycardia/fibrillation; (A): age >45; (pH): arterial pH ≤7.0. A validation cohort (n=344) included subsequent patients from the initial site (n=72) and an external quaternary‐care health system (n=272) from 2012 to 2014. The c‐statistic for predicting neurologic outcome was 0.82 (0.74–0.90, P<0.001) in the development cohort and 0.81 (0.76–0.87, P<0.001) in the validation cohort. When subdivided by C‐GRApH score, similar rates of favorable neurologic outcome were seen in both cohorts, 70% each for low (0–1, n=60), 22% versus 19% for medium (2–3, n=307), and 0% versus 2% for high (4–5, n=99) C‐GRApH scores in the development and validation cohorts, respectively.
Conclusions
C‐GRApH stratifies neurologic outcomes following OHCA in patients receiving targeted temperature management (32–34°C) using objective data available at hospital presentation, identifying patient subsets with disproportionally favorable (C‐GRApH ≤1) and poor (C‐GRApH ≥4) prognoses.
Keywords: heart arrest, hypothermia, prognosis, resuscitation, targeted temperature management
Subject Categories: Sudden Cardiac Death, Cardiopulmonary Arrest, Clinical Studies, Quality and Outcomes
Introduction
The American Heart Association estimates that 535 000 patients suffer cardiac arrest in the United States annually, 60% in out‐of‐hospital settings. Arrest location correlates highly with survival, 10% at hospital discharge after out‐of‐hospital cardiac arrest (OHCA) versus 25% in‐hospital,1 with neurologic injury the leading cause of morbidity and mortality.2 Targeted temperature management (TTM), previously termed therapeutic hypothermia,3 is now the standard of care for comatose patients following cardiac arrest. The American Heart Association's post–cardiac arrest algorithm4 recommends TTM for (class I) ventricular tachycardia/fibrillation (VT/VF) and (class IIb) non‐shockable rhythms, supported by randomized control trial5, 6 and meta‐analysis data, respectively.7, 8, 9
Given poor observed survival, there has been significant interest in predicting outcomes in the OHCA population. Prior to TTM, the “gold standard” for early prediction of neurologic outcomes was the 2006 American Academy of Neurology practice parameter, comprising 6 physical examination and diagnostic test findings on post‐arrest days 1 to 3, each with reported positive predictive value (PPV) of 100% for poor neurologic outcome.10 However, validity in the TTM population has been repeatedly questioned because of decreased individual and composite PPV.11, 12, 13 The current consensus recommendation is to delay prognosis ≥72 hours post‐arrest,14 consistent with a recent study reporting average time to awakening 3.2 days post‐arrest.15
Protracted hospitalization for post‐OHCA patients, the majority of whom will not survive with meaningful neurologic recovery, carries considerable ethical and economic implications.16, 17 Multiple models have previously been developed with the goal of earlier outcome stratification,18, 19, 20, 21 yet all are significantly limited by invalidity in TTM populations and/or weighting toward historically unreliable arrest timing variables (eg, time to return of spontaneous circulation [ROSC]).22 There remains a need for a scoring system that can predict neurologic outcome after OHCA treated with TTM that is also (1) calculated early after hospital presentation; (2) comprised of reliable, objective data (no timing variables); (3) inclusive of all rhythms of arrest; and (4) easily applicable by varied subspecialty providers who routinely participate in post‐OHCA care.
Methods
Development Cohort
The development cohort consisted of prospectively acquired, consecutive adult patients presenting or transferred to a tertiary‐care, rural/suburban US hospital from 2008 to 2012 following OHCA and successful ROSC, in whom TTM was universally initiated as standard clinical care. TTM was managed by cardiac intensive care unit physicians according to a protocol targeting 32°C to 34°C for 24 hours. If TTM targets were not achieved, patients remained in this intention‐to‐treat analysis. Patients were prospectively enrolled in a quality improvement database, and were included irrespective of location of ROSC and TTM initiation, whether pre‐ or posthospital.
Subsequently, institutional review board approval was obtained for retrospective research analysis of the quality improvement database. Informed consent was waived. The primary outcome measure, similar to prior studies, was favorable neurologic outcome at hospital discharge, defined as a Glasgow–Pittsburgh cerebral performance category (CPC) of 1 to 2. In the development cohort, CPC scores were assigned retrospectively using provider notes and physical examinations by a consensus of neurologists who were blinded to the study question. CPC scores ranged from 1 to 5 based on standard definitions (Figure 1).23
Figure 1.
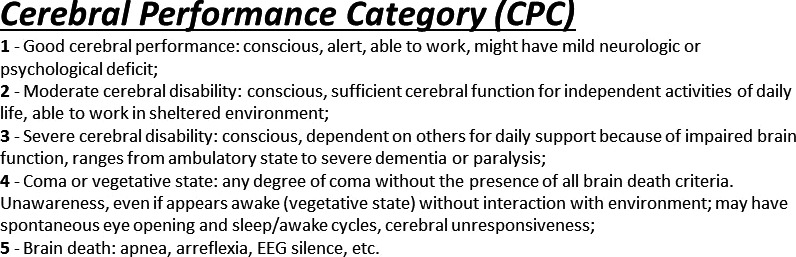
Cerebral performance category definitions. Standard definitions of Glasgow–Pittsburgh cerebral performance category (CPC) with CPC 1 to 2 defined as favorable and 3 to 5 defined as poor neurologic outcome. EEG indicates electroencephalogram.
Patient data including demographics, pre‐arrest medical history, arrest characteristics (witnessed, bystander cardiopulmonary resuscitation [CPR], rhythm of arrest, time to CPR and ROSC), and post‐ROSC laboratory studies obtained at initial hospital presentation were then collected via review of available emergency medical services and prior medical records and compared between favorable (CPC 1–2) and poor (CPC 3–5) neurologic outcome groups. For inter‐hospital transfers, laboratory studies were those from the initial presenting facility. The location where laboratory studies were obtained was determined purely by chronology (first available post‐ROSC), en route by emergency medical services or upon arrival to the emergency department in nearly all cases. Pre‐arrest diagnoses required that conditions be known before the index OHCA event. For example, if a patient suffered OHCA in the setting of acute myocardial infarction as the first manifestation of coronary artery disease (CAD), this would not qualify as pre‐existing CAD in our prediction model.
In order to discriminate favorable versus poor neurologic outcomes, univariable analysis followed by stepwise multivariable logistic regression was then performed, generating a scoring system abbreviated C‐GRApH, to be discussed in further detail in the Statistical Analysis subsection.
Validation Cohort
Subsequently, validation of the C‐GRApH scoring system was performed by applying the model derived in the development cohort to a validation cohort. The validation cohort included 2 subgroups, 1 internal and 1 external. To generate the internal subgroup, the initial institutional review board approval was extended to 2013 to 2014 to enroll new, prospectively collected patients meeting the same inclusion/exclusion criteria, who were categorized (by neurologic outcome) and analyzed in the same fashion using the same data definitions by the same research personnel. To generate the external subgroup, separate institutional review board approval was obtained at a larger quaternary care US health system to retrospectively analyze a similar consecutive, prospectively recorded quality improvement database of OHCA patients treated with TTM from 2012 to 2014 at its main academic center and satellite hospitals. Again, informed consent was waived. Whereas the development cohort and internal subgroup received intravascular TTM, the external subgroup received intravascular or external TTM because of differences in TTM protocols across health system hospitals. CPC assignment in the external subgroup was made prospectively by consulting neurology teams participating in patient care during the index hospitalization.
Statistical Analysis
Model development
Univariable analysis was performed on collected variables in the development cohort with favorable neurologic outcome (CPC 1–2) as the dependent variable. Continuous variables were converted to categorical variables using receiver operating characteristic (ROC) curves to define the maximal sensitivity and specificity of the variables to discriminate between favorable and poor neurologic outcomes. Categorical variables were purposefully used to develop a simple scoring system that could be easily applied by all providers participating in post‐OHCA care. All variables satisfying P<0.05 in univariable analysis were included in a stepwise multivariable logistic regression with bootstrapping to develop the final prediction model: the C‐GRApH scoring system. ROC and Kaplan–Meier survival analysis (censoring data at the time of death or hospital discharge) were then computed for the composite scoring system in the development cohort. To test for the impact of early mortality or withdrawal of care (<72 hours post‐OHCA), the same analysis was repeated, restricting the cohort to only patients surviving ≥72 hours.
Model validation
In the validation cohort, the C‐GRApH score was applied to all patients and ROC was computed. Kaplan–Meier survival analysis was also performed in a similar fashion. As in the development cohort, the same analyses were repeated, censoring patients not surviving ≥72 hours post‐OHCA.
Model calibration
Calibration of the C‐GRApH score was tested in both the development and validation cohorts using the Hosmer–Lemeshow goodness‐of‐fit test. This approach compared observed versus predicted poor neurologic outcomes stratified in deciles of predicted risk. Any ties in predicted risk were broken via software sorting algorithms. A nonsignificant χ2 statistic via Hosmer–Lemeshow indicates good calibration.
All data processing and analysis were performed using IBM SPSS® v23 and R version 3.3.2.
Results
Development Cohort
Patient data for the development cohort (n=122) are summarized in Table 1, subdivided into favorable (CPC 1–2) and poor (CPC 3–5) neurologic outcome. Overall, 34% (n=42) survived to hospital discharge, 27% (n=33) with favorable neurologic outcome. The favorable neurologic outcome group included 27 CPC 1 (82%) and 6 CPC 2 (18%) patients, whereas the poor neurologic outcome group included 7 CPC 3 (8%), 2 CPC 4 (2%), and 80 CPC 5 (90%) patients. For nonsurvivors (CPC 5), mean time from arrest to death was 95±85 hours (range 6–493). Death or withdrawal of care <72 hours post‐OHCA occurred in 43 patients (35%). For patients surviving to hospital discharge (CPC 1–4), mean time from arrest to discharge was 12±7 days. When subdivided by neurologic outcome, patients discharged with poor neurologic outcomes (CPC 3–4) were hospitalized 12 additional days compared with patients discharged with favorable neurologic outcomes (P=0.001). Timing variables (time to CPR/ emergency medical services/ROSC) were not reliably known in 30% of the development cohort.
Table 1.
Baseline Patient Data for Development Cohort (n=122), Compared Between Favorable (n=33) and Poor (n=89) Neurologic Outcome Groups
| Development Cohort (n=122) | Favorable Neurologic Outcome (n=33) | Poor Neurologic Outcome (n=89) | P Value |
|---|---|---|---|
| Age (y) | 53±18 | 62±15 | 0.004a |
| Sex (male) | 73% | 66% | 0.570 |
| Pre‐OHCA coronary artery disease | 21% | 47% | 0.023a |
| Congestive heart failure | 15% | 31% | 0.069 |
| Diabetes mellitus | 18% | 38% | 0.021a |
| Chronic obstructive pulmonary disease | 3% | 21% | 0.052 |
| ≥Stage III chronic kidney disease | 6% | 17% | 0.271 |
| Witnessed arrest | 91% | 83% | 0.260 |
| Ventricular tachycardia/fibrillation | 97% | 56% | <0.001a |
| Bystander CPR | 63% | 62% | 0.605 |
| Time to CPR, min | 3±4 | 4±7 | 0.240 |
| Time to EMS, min | 8±6 | 10±10 | 0.310 |
| Time to ROSC, min | 21±12 | 35±18 | <0.001a |
| Arterial pH | 7.20±0.12 | 7.14±0.16 | 0.021a |
| Lactic acid, mmol/L | 5.6±3.1 | 7.2±3.6 | 0.045a |
| Troponin I, ng/mL | 0.71±2.50 | 2.5±7.95 | 0.227 |
| Blood glucose, mg/dL | 243±86 | 308±125 | 0.008a |
| Creatinine, mg/dL | 1.6±1.8 | 1.7±1.8 | 0.738 |
| White blood cell count, 109/L | 17±10 | 14±7 | 0.231 |
| Hemoglobin, g/dL | 14±2 | 13±2 | 0.060 |
| Early TTM termination | 15% | 24% | 0.282 |
| Time to discharge, d | 10±6 | 21±7 | 0.001a |
CPR indicates cardiopulmonary resuscitation; EMS, emergency medical systems; OHCA, out‐of‐hospital cardiac arrest; ROSC, return of spontaneous circulation; TTM, targeted temperature management.
P<0.05.
Variables satisfying P<0.05 between favorable and poor neurologic outcome in univariable analysis are highlighted in Table 1: age, pre‐arrest CAD, diabetes mellitus, initial VT/VF rhythm of arrest, time to ROSC, and the following initial post‐ROSC laboratory results: arterial pH, blood glucose, and lactic acid. Because of confounding between diabetes mellitus and blood glucose, diabetes mellitus was not included in model development. Lactic acid was not readily available in many patients and was also dropped from the model. Continuous variables were transformed into categorical variables as described above. These included initial blood glucose (≥200 mg/dL), age (>45), and initial arterial pH (≤7.0).
Following stepwise multivariable logistic regression, 5 of the 6 candidate variables remained: CAD, known pre‐arrest; Glucose (blood) ≥200 mg/dL; Rhythm of arrest not VT/VF initially; Age >45; and pH (arterial) ≤7.0. CAD was defined by one of the following: (1) prior history of myocardial infarction; (2) prior coronary revascularization via percutaneous coronary intervention or bypass surgery; or (3) ≥50% stenosis on cardiac catheterization before OHCA. Glucose was the first measurement post‐ROSC, whether by fingerstick, blood gas, or metabolic panel. For rhythm, if the only discriminator available at arrest was an automated external defibrillator, it was considered VT/VF if shockable and non‐VT/VF if nonshockable. If subsequent conversion to VT/VF was observed from an initial non‐VT/VF rhythm, the rhythm was still considered non‐VT/VF. Beta‐coefficients and odds ratios for retained variables in the multivariable model predicting neurologic outcome can be found in Table 2. As preplanned for simplicity of application, each variable was weighted equally (1 point), creating a scoring system ranging from 0 to 5 points and abbreviated C‐GRApH (Figure 2).
Table 2.
Beta‐Coefficients and Odds Ratios for Multivariable Logistic Regression Predicting Neurologic Outcome in the Development Cohort
| Beta‐Coefficient | Odds Ratio | |
|---|---|---|
| CAD, pre‐arrest | 2.16 | 8.67 |
| Glucose ≥200 mg/dL | 0.461 | 1.58 |
| Rhythm non‐VT/VF | 4.16 | 64.1 |
| Age >45 y | 2.22 | 9.21 |
| pH (arterial) ≤7.0 | 21.2 | 1.6 e9 |
CAD indicates coronary artery disease; VT/VF, ventricular tachycardia/fibrillation.
Figure 2.

C‐GRApH scoring system. C‐GRApH is a prediction model for favorable neurologic outcome after out‐of‐hospital cardiac arrest treated with targeted temperature management at 32°C to 34°C. Each variable is equally weighted at 1 point (score range: 0–5) with the following dichotomized variables included: C: coronary artery disease (CAD), known pre‐arrest; G: glucose (blood) ≥200 mg/dL; R: rhythm of arrest not ventricular tachycardia or fibrillation (VT/VF); A: age >45; pH: arterial pH ≤7.0.
The ROC curve for C‐GRApH in the development cohort is shown in Figure 3. The c‐statistic was 0.818 (95% CI, 0.737–0.899, P<0.001). To test the impact of early mortality or withdrawal of care (and thus address the possibility of outcome bias), ROC was repeated after restricting the development cohort to patients surviving ≥72 hours (censored n=79), generating a similar c‐statistic: 0.785 (95% CI, 0.683–0.885, P<0.001). Kaplan–Meier curves for favorable neurologic outcome at hospital discharge stratified by C‐GRApH score are depicted in Figure 4A (log rank χ2 75.0, P<0.001). Favorable neurologic outcomes declined stepwise with increasing C‐GRApH levels: 0: 100% (n=4); 1: 63% (n=16); 2: 36% (n=42); 3: 9% (n=44); 4: 0% (n=15); 5: 0% (n=1). Grouped into low (0–1), medium (2–3), and high (4–5) C‐GRApH scores, rates of favorable neurologic outcome were 70% (n=20), 22% (n=86), and 0% (n=16), respectively. Thus, in the development cohort, the PPV of a low C‐GRApH score (0–1) is 70% for favorable neurologic outcome, whereas the PPV of a high C‐GRApH score (4–5) is 100% for poor neurologic outcome.
Figure 3.
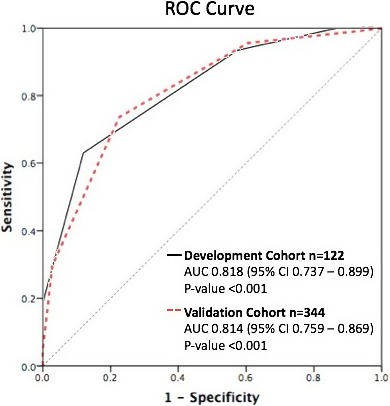
Receiver operating characteristic (ROC) curves for favorable neurologic outcome in development and validation cohorts. ROC curves for predicting favorable neurologic outcome in both the uncensored development (solid black) and validation (dotted red) cohorts, demonstrating similar c‐statistics of 0.82 and 0.81, respectively. AUC indicates area under the curve.
Figure 4.
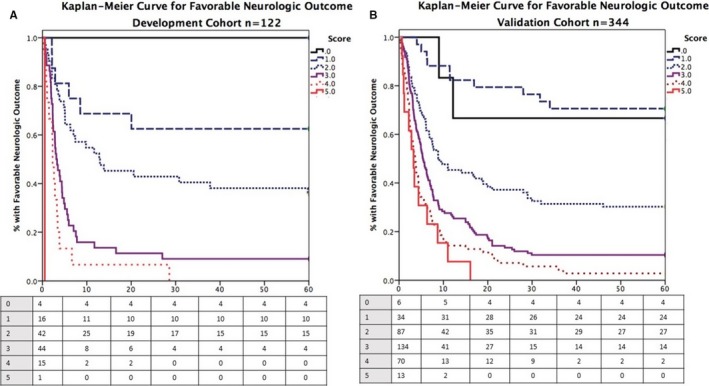
Kaplan–Meier curves of favorable neurologic outcomes at discharge stratified by C‐GRApH score. Kaplan–Meier curves for favorable neurologic outcome at hospital discharge stratified by C‐GRApH score for the development (A) and validation (B) cohorts. Striking similarities exist except at C‐GRApH score 0, a difference attributable to sample size variation between cohorts.
Validation Cohort
The validation cohort was composed of the internal (n=72) and external (n=272) subgroups. Overall, 32% of patients (n=109) survived to hospital discharge, 21% (n=71) with favorable neurologic outcome. The favorable neurologic outcome group included 47 CPC 1 (66%) and 24 CPC 2 (34%) patients, whereas the poor neurologic outcome group included 15 CPC 3 (5%), 24 CPC 4 (9%), and 234 CPC 5 (86%) patients. Mean time from arrest to death was 139±142 hours (range 5–1104). Similar to the development cohort, patients were discharged 5 days later post‐arrest in poor (18±8) versus favorable (13±6) neurologic outcome groups (P=0.003). The validation cohort received 51% intravascular TTM with treatment occurring 58% in coronary care unit settings and 25% in main academic centers as opposed to satellite affiliate hospitals (100% each in development cohort). Favorable neurologic outcome rates did not differ by hospital subtype: 20% in academic (n=87) versus 21% in satellite (n=257) settings (P=0.77), also with similar C‐GRApH scores (2.80±1.11 academic, 2.77±1.04 satellite, P=0.78). Further comparisons of baseline characteristics between the development (n=122) and validation cohorts (n=344) are shown in Table 3. Slightly lower rates of survival and favorable neurologic outcome were observed in the validation cohort, though not statistically significant. Satisfying P<0.05 between the cohorts, there were ∼10% fewer males, witnessed arrests, and deaths/withdrawals <72 hours post‐OHCA in the validation cohort. There were also 29% more patients with non‐VT/VF rhythm of arrest in the validation cohort, almost completely accounting for the 0.4‐point increase in mean C‐GRApH score. Timing variables were not reliably known in >40% of the validation cohort.
Table 3.
Comparison of Patient Data Between Development (n=122) and Validation (n=344) Cohorts
| Development Cohort (n=122) | Validation Cohort (n=344) | P Value | |
|---|---|---|---|
| Survival | 42 (34%) | 110 (32%) | 0.625 |
| Favorable neurologic outcome | 33 (27%) | 71 (21%) | 0.164 |
| Cerebral performance category | 3.8 (1.7) | 4 (1.5) | 0.115 |
| Death/withdrawal <72 h | 43 (35%) | 85 (25%) | 0.034a |
| Witnessed arrest | 104 (85%) | 265 (77%) | 0.038a |
| Bystander CPR | 70 (57%) | 162 (47%) | 0.051 |
| Time to ROSC, min | 30 (17) | 27 (17) | 0.083 |
| Male sex | 83 (68%) | 194 (56%) | 0.025a |
| Coronary artery disease | 49 (40%) | 117 (34%) | 0.226 |
| Glucose, mg/dL | 290 (119) | 268 (127) | 0.089 |
| VT/VF | 82 (67%) | 129 (38%) | <0.001a |
| Age, y | 60 (16) | 62 (15) | 0.110 |
| Arterial pH | 7.17 (0.15) | 7.15 (0.19) | 0.165 |
| C‐GRApH Score | 2.4 (1.0) | 2.8 (1.1) | 0.002a |
Table formatted number (%) for categorical and mean (SD) for numerical variables. CPR indicates cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; VT/VF, ventricular tachycardia/fibrillation.
P<0.05.
The ROC curve for C‐GRApH in the validation cohort is shown in Figure 3. The shape of the curve and the c‐statistic 0.814 (95% CI, 0.759–0.869, P<0.001) are nearly identical to the development cohort. As in the development cohort, a second ROC was computed after censoring patients surviving <72 hours (censored n=258), generating a c‐statistic of 0.789 (95% CI, 0.727–0.851, P<0.001), again similar to the uncensored and censored development cohorts and the uncensored validation cohort. The Kaplan–Meier curve for favorable neurologic outcome at hospital discharge in the validation cohort stratified by C‐GRApH score is shown in Figure 4B, again very similar to the development cohort (log rank χ2 20.7, P<0.001). Favorable neurologic outcome by C‐GRApH score in the validation cohort was as follows: 0: 67% (n=6); 1: 71% (n=34); 2: 31% (n=87); 3: 10% (n=134); 4: 3% (n=70); 5: 0% (n=13). The neurologic outcome stratification is similar to the development cohort, except for lower favorable neurologic outcome at score of 0 (67% versus 100%) and rare favorable neurologic outcome at score of 4 (3% versus 0%), both attributable to the larger sample size in the validation cohort. Again grouped into low (0–1), medium (2–3), and high (4–5) C‐GRApH scores, rates of favorable neurologic outcome in the validation cohort were 70% (n=40), 19% (n=221), and 2% (n=83), respectively. Note these nearly identical figures compared to the development cohort (70% versus 70%, 19% versus 22%, 2% versus 0%), equating to calibration errors of 0% for low, 3% for medium, and 2% for high C‐GRApH scores, respectively. Similarly, in the validation cohort, the PPV of a low C‐GRApH score (0–1) is 70% for favorable neurologic outcome (70% in development), whereas the PPV of a high C‐GRApH score (4–5) is 98% for poor neurologic outcome (100% development).
Model Calibration
The calculated χ2 statistics via the Hosmer–Lemeshow goodness‐of‐fit test were 0.742 (P=0.99) for the development cohort and 4.95 (P=0.76) for the validation cohort. Both tests were strongly nonsignificant, indicating good calibration. Figure 5 displays observed versus predicted in deciles of predicted risk for the development (Figure 5A) and validation (Figure 5B) cohorts, respectively.
Figure 5.
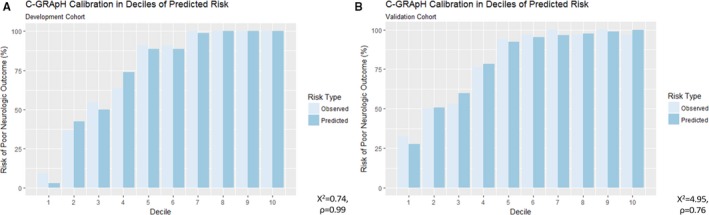
Calibration plots in deciles of predicted risk for the development and validation cohorts. Observed vs predicted risk stratified in deciles of predicted risk for the development (A) and validation (B) cohorts. C‐GRApH calibration was excellent in both cohorts (Hosmer–Lemeshow χ2 0.74 and P=0.99 development, χ2 4.95, P=0.76 validation).
Discussion
Despite utilization of TTM, OHCA remains a highly mortal and morbid event, primarily because of irreversible neurologic injury. OHCA is unexpected and results in physical trauma for patients and emotional trauma for families. OHCA patients may be young or old, previously healthy or with significant pre‐existing comorbidities, and possess varying attitudes toward/planning for end‐of‐life care. These patients are medically and socially complex with each presentation unique, requiring nuanced, individualized post‐OHCA care. The result is often prolonged hospitalization with significant uncertainty regarding timing and confidence of prognosis. With poor expected outcomes in the majority of OHCA patients, prolonged prognostic delays can lead to increased suffering for both patients and families and higher economic costs of continued, often‐futile care.
Multiple prior prediction models have attempted to accurately prognosticate neurologic outcomes early post‐OHCA. The OHCA score was prospective and externally validated from 1999 to 2005 (composite n=340) with primary outcome CPC 1 to 2 at hospital discharge.19 The formula for score calculation is −13×(1 if VT/VF)+6×ln[time to CPR(min)] −1434/Cr(μmol/L)+10×ln(lactate(mmol/L), with higher scores predicting poor neurologic outcome. Despite robust c‐statistics of 0.82 development and 0.88 validation, TTM utilization was markedly higher in the validation cohort (77% versus 11%) and predicted favorable neurologic outcome (P=0.005). In a subsequent study restricted to TTM, the OHCA score did not predict neurologic outcomes.23 The Utstein Osaka Project was similarly prospective and externally validated, enrolling witnessed OHCA from 2005 to 2007 (composite n=1500) with primary outcome CPC 1 to 2 at 1 month.20 Two prediction models were generated: (1) VT/VF: exp(B)/[1+exp(B)], B=−0.02×age—0.109×ROSC(min)+0.677×(1 if prehospital ROSC)+2.442; (2) non‐VT/VF: −0.037×age—0.076×ROSC(min)+1.735×(1 if prehospital ROSC)+1.46×(1 if conversion to VT/VF); higher scores predicted favorable neurologic outcome. Despite a c‐statistic of 0.87, TTM utilization was unknown. The 5‐R score was single‐center, retrospective, and nonvalidated (n=60), enrolling OHCA from 2006 to 2011 with primary outcome CPC 1 to 2 at hospital discharge.21 The 5‐R score ranges 0 to 7: time to CPR <5 minutes (1), absence of re‐arrest (1), intact pupillary light reflex in the emergency department (1), VT/VF (2), time to ROSC <30 minutes (2); higher scores predicted favorable neurologic outcome. Despite c‐statistic 0.95 and PPV 94% for scores ≥5, 5‐R remains unvalidated and utilized a study population healthier than US norms (80% VT/VF, 61% favorable neurologic outcomes), raising concerns about external validity. Goto et al developed a recursive decision‐tree model using sequential branch points (shockable rhythm, age >70, witnessed arrest) classifying neurologic outcome as “good, moderately good, poor, or absolutely poor” with primary outcome CPC 1 to 2 at 1 month in a retrospective cohort from 2005 to 2009 (n=390 000).21 Again, external validity is questionable (4% favorable neurologic outcomes) and TTM utilization was unknown.
C‐GRApH is simple and avoids the aforementioned limitations of previous models. All patients received TTM at 32°C to 34°C for goal 24 hours. Rates of survival and favorable neurologic outcome are consistent with US norms.1 All C‐GRApH components are objective and readily available at hospital presentation via history and commonly ordered laboratory testing (fingerstick/basic metabolic panel, arterial blood gas). No timing variables were retained because of statistical insignificance in multivariable modeling (time to ROSC), but were also unknown in >40%, confirming reliability concerns.
The C‐GRApH score can be rapidly calculated following hospital presentation and provides excellent stratification of eventual neurologic outcome at discharge. When separated into low (0–1), medium (2–3), and high (4–5) C‐GRApH scores, favorable neurologic outcome differs starkly, a finding consistent in both the development and validation cohorts. Favorable neurologic outcome was 70% in both development and validation for low (0–1) C‐GRApH scores, 22% and 19% for development and validation for medium (2–3) C‐GRApH scores, and 0% and 2% for development and validation for high (4–5) C‐GRApH scores. As such, C‐GRApH's prognostic power is most pronounced at its score extremes (0–1 and 4–5), which constitute 34% of the study population. The American Heart Association estimates 10% of OHCA patients survive to hospital discharge,1 not accounting for favorable versus poor neurologic outcome. In our study population, 32% survived to discharge, 22% with favorable neurologic outcomes. These higher observed rates are in line with prior studies and likely reflect censoring of patients not surviving until hospital presentation. Without C‐GRApH, predicted favorable neurologic outcome would be 27% in the development cohort and 21% in the validation cohort. With C‐GRApH, low scores (0–1) have projected favorable neurologic outcome of 70%, and positive reclassification improvements of 43% and 49%, respectively, in development and validation. High scores (4–5) have predicted favorable neurologic outcome of 0% in development and 2% in validation, negative reclassification improvements of 22% and 20%, respectively, with PPV 100% and 98% for poor neurologic outcome. However, for medium scores (2–3), which constitute 66% of the study population, there is marginal (2%) reclassification improvement for neurologic outcome discrimination.
While the application of C‐GRApH in clinical decision‐making was not evaluated in this study, potential uses exist. Early, data‐driven, hopeful prognostication may be emotionally beneficial for families of patients with low C‐GRApH scores. Low C‐GRApH patients may also warrant more aggressive, earlier medical interventions. Conversely, earlier discussions regarding patients' wishes may be merited in patients with high C‐GRApH scores, particularly those with significant comorbidities and/or previously expressed desires to avoid prolonged, intensive end‐of‐life care.
The results of this study suggest that C‐GRApH can be applied to a diverse population of OHCA patients. The development cohort was relatively rural, two‐thirds VT/VF, and treated exclusively in coronary care unit settings with intravascular TTM. The validation cohort was multisite, spanning suburban and urban hospitals, two‐thirds non‐VT/VF, and 50% treated in non–coronary care unit settings using external TTM. Despite some statistical baseline differences between the cohorts, C‐GRApH performed effectively equivalent in the larger, more complex validation cohort (c‐statistic 0.814 validation, 0.818 development with similar qualitative appearance of Kaplan–Meier curves) with excellent model calibration in both cohorts.
C‐GRApH also has limitations. Although patient data were prospectively acquired, the study question was asked ex post facto and the analysis was retrospective in the majority of patients (exception: internal validation subgroup, n=72). Additionally, there is likely some degree of overfitting present in the model. In the development cohort, 6 candidate variables were included for stepwise logistic regression (5 retained), though there were only 33 patients surviving with favorable neurologic outcome; thus, this was a violation of the “rule of 10.” Furthermore, with respect to model overestimation, the patients not surviving ≥72 hours postarrest present some problems. Thirty‐five percent of patients in the development cohort and 25% of patients in the validation cohort died or had care withdrawn before the guideline‐directed 72 hours post‐OHCA. Further broken down, of those 128 total patients, 27 re‐arrested without ROSC and 34 were either declared clinically brain‐dead or had high‐risk computed tomography findings of severe anoxic brain injury. The remaining 67 patients (52% of those not surviving ≥72 hours, 14% of the total study population) experienced withdrawal of care preguidelines by consensus decision of patients' families and provider teams, decisions that were not restricted in the study population. In an attempt to confirm that C‐GRApH is not a self‐fulfilling prophecy, separate analyses of C‐GRApH on the development and validation cohorts excluding patients not surviving ≥72 hours were performed and generated c‐statistics similar to those of the uncensored cohort analyses (0.785 versus 0.818 development, 0.789 versus 0.814 validation). Although reassuring, these adjusted analyses only partially address the early withdrawals/deaths. Because the mean C‐GRApH score <72 hours was 3.2±0.9 versus 2.5±1.0 ≥72 hours (P<0.001), if any number of patients experiencing early withdrawal of care had instead survived with favorable neurologic outcome, the predictive capacity of C‐GRApH would be expected to incrementally worsen. Lastly, though a large study population for TTM in OHCA, C‐GRApH remains constrained by sample size at its score extremes. Although no patients with a score of 5 experienced favorable neurologic outcome, only 14 met this criterion. It would thus be premature to categorize a C‐GRApH score of 5 as a futile treatment group. It should also be noted that poor neurologic outcomes were driven primarily by death (87%) as opposed to discharge with CPC 3 to 4 (13%). This reflects an unfortunate reality of the present epidemiology of OHCA, in line with prior landmark studies.5, 6
Future directions could include additional, fully prospective validation sites, which would add statistical strength to the model and provide larger sample sizes at C‐GRApH score extremes. Whether patients discharged with poor neurologic outcomes (CPC 3–4) subsequently improve to favorable neurologic outcomes (CPC 1–2) at later time intervals (3–12 months) is of interest, but those data are not currently available. Given differences in beta‐coefficients/odds ratios between C‐GRApH variables when predicting neurologic outcomes (Table 2), particularly pH <7.0 (albeit a relatively uncommon occurrence—11% of development and 23% of validation cohorts), a weighted model may be considered to better capture relative variable contribution. Additionally, since the transformation of continuous data (glucose, age, pH) into categorical variables likely reduced the discriminatory capacity of the model, a continuous C‐GRApH may be created (application would require a calculator). Again, the existing model was purposefully unweighted and exclusively categorical for ease of use. Lastly, during the study period, the TTM trial was published, showing noninferiority of TTM at 36°C versus 33°C.24 The validity of C‐GRApH in the 36°C TTM population is unknown.
In summary, the C‐GRApH scoring system successfully stratifies neurologic outcomes following OHCA in patients treated with TTM at 32°C to 34°C, irrespective of the arrest rhythm. C‐GRApH uses objective, reliable data available at hospital presentation and can be easily applied by varied subspecialty providers who routinely participate in post–cardiac arrest care.
Disclosures
None.
Acknowledgments
To my wife, Caitlin, for brainstorming the abbreviation C‐GRApH. To Brian Annex, MD (University of Virginia), Daniel Cantillon, MD, Wael Jaber, MD, and Zoran Popovic, MD, PhD (Cleveland Clinic Foundation) for their steadfast mentoring and assistance with this manuscript.
(J Am Heart Assoc. 2017;6:e003821 DOI: 10.1161/JAHA.116.003821.)28528323
Parts of this work were previously presented in oral format at the American Heart Association Scientific Sessions, November 16–20, 2013 in Dallas, TX and November 7–11, 2015 in Orlando, FL.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. [DOI] [PubMed] [Google Scholar]
- 3. Donnino MW, Andersen LW, Berg KM, Reynolds JC, Nolan JP, Morley PT, Lang E, Cocchi MN, Xanthos T, Callaway CW, Soar J. Temperature management after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative, and Resuscitation. Circulation. 2015;132:2448–2456. [DOI] [PubMed] [Google Scholar]
- 4. Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, Brooks SC, de Caen AR, Donnino MW, Ferrer JM, Kleinman ME, Kronick SL, Lavonas EJ, Link MS, Mancini ME, Morrison LJ, O'Connor RE, Samson RA, Schexnayder S, Singletary EM, Sinz EH, Travers AH, Wyckoff MH, Hazinski MF. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S315–S367. [DOI] [PubMed] [Google Scholar]
- 5. The Hypothermia After Cardiac Arrest Study Group . Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. [DOI] [PubMed] [Google Scholar]
- 6. Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. [DOI] [PubMed] [Google Scholar]
- 7. Lundbye JB, Rai M, Ramu B, Hosseini‐Khalili A, Li D, Slim HB, Bhavnani SP, Nair SU, Kluger J. Therapeutic hypothermia is associated with improved neurologic outcome and survival in cardiac arrest survivors of non‐shockable rhythms. Resuscitation. 2012;83:202–207. [DOI] [PubMed] [Google Scholar]
- 8. Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non‐shockable initial rhythms? A systematic review and meta‐analysis of randomized and non‐randomized studies. Resuscitation. 2012;83:188–196. [DOI] [PubMed] [Google Scholar]
- 9. Sandroni C, Cavallaro F, Antonelli M. Therapeutic hypothermia: is it effective for non‐VF/VT cardiac arrest? Crit Care. 2013;17:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. [DOI] [PubMed] [Google Scholar]
- 11. Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–307. [DOI] [PubMed] [Google Scholar]
- 12. Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, Bell MR, Rabinstein AA. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–914. [DOI] [PubMed] [Google Scholar]
- 13. Samaniego EA, Mlynash M, Caulfield AF, Eyngorn I, Wijman CA. Sedation confounds outcome prediction in cardiac arrest survivors treated with hypothermia. Neurocrit Care. 2011;15:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bbttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez‐Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hook T. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. [DOI] [PubMed] [Google Scholar]
- 15. Grossestreuer AV, Abella BS, Leary M, Perman SM, Fuchs BD, Kolansky DM, Beylin ME, Gaieski DF. Time to awakening and neurologic outcome in therapeutic hypothermia‐treated cardiac arrest patients. Resuscitation. 2013;84:1741–1746. [DOI] [PubMed] [Google Scholar]
- 16. Gray WA, Capone RJ, Most AS. Unsuccessful emergency medical resuscitation—are continued efforts in the emergency department justified? N Engl J Med. 1991;325:1393–1398. [DOI] [PubMed] [Google Scholar]
- 17. Hamel MB, Phillips R, Teno J, Davis RB, Goldman L, Lynn J, Desbiens N, Connors AF II, Tsevat J. Cost effectiveness of aggressive care for patients with non‐traumatic coma. Crit Care Med. 2002;30:1191–1196. [DOI] [PubMed] [Google Scholar]
- 18. Adrie C, Cariou A, Mourvillier B, Laurent I, Dabbane H, Hantala F, Rhaoui A, Thuong M, Monchi M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out‐of‐hospital cardiac arrest: the OHCA score. Eur Heart J. 2006;27:2840–2845. [DOI] [PubMed] [Google Scholar]
- 19. Hayakawa K, Tasaki O, Hamasaki T, Sakai T, Shiozaki T, Nakagawa Y, Ogura H, Kuwagata Y, Kajino K, Iwami T, Nishiuchi T, Hayashi Y, Hiraide A, Sugimoto H, Shimazu T. Prognostic indicators and outcome prediction model for patients with return of spontaneous circulation from cardiopulmonary arrest: the Utstein Osaka Project. Resuscitation. 2011;82:874–880. [DOI] [PubMed] [Google Scholar]
- 20. Okada K, Ohde S, Otani N, Sera T, Mochizuki T, Aoki M, Ishimatsu S. Prediction protocol for neurological outcome for survivors of out‐of‐hospital cardiac arrest treated with targeted temperature management. Resuscitation. 2012;83:734–739. [DOI] [PubMed] [Google Scholar]
- 21. Goto Y, Maeda T, Goto Y. Decision‐tree model for predicting outcomes after out‐of‐hospital cardiac arrest in the emergency department. Crit Care. 2013;17:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oksanen T, Tiainen M, Skrifvars MB, Varpula T, Kuitunen A, Castren M, Pettila V. Predictive power of serum NSE and OHCA score regarding 6‐month neurologic outcome after out‐of‐hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation. 2009;80:165–170. [DOI] [PubMed] [Google Scholar]
- 23. Safar P. Resuscitation after brain ischemia In: Grenvik A, Safar P, eds. Brain Failure and Resuscitation. New York, NY: Churchill Livingstone; 1981:155–184. [Google Scholar]
- 24. Nielsen N, Wettersley J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al‐Subaie N, Boesgaard S, Bro‐Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. [DOI] [PubMed] [Google Scholar]


