Abstract
Background
AHEAD (A: atrial fibrillation; H: hemoglobin; E: elderly; A: abnormal renal parameters; D: diabetes mellitus) score has been related to clinical outcomes of acute heart failure. However, the prognostic value of the AHEAD score in acute heart failure patients with either reduced or preserved left ventricular ejection fraction (HFrEF and HFpEF) remain to be elucidated.
Methods and Results
The study population consisted of 2143 patients (age 77±12 years, 68% men, 38% HFrEF) hospitalized primarily for acute heart failure with a median follow‐up of 23.75 months. The performance of the AHEAD score (atrial fibrillation, hemoglobin <13 mg/dL for men and 12 mg/dL for women, age >70 years, creatinine >130 μmol/L, and diabetes mellitus) was evaluated by Cox's regression analysis for predicting cardiovascular and all‐cause mortality. The mean AHEAD scores were 2.7±1.2 in the total study population, 2.6±1.3 in the HFrEF group, and 2.7±1.1 in the HFpEF group. After accounting for sex, sodium, uric acid, and medications, the AHEAD score remained significantly associated with all‐cause and cardiovascular mortality (hazard ratio and 95% CI: 1.49, 1.38–1.60 and 1.48, 1.33–1.64), respectively. The associations of AHEAD score with mortality remained significant in the subgroups of HFrEF (1.63, 1.47–1.82) and HFpEF (1.34, 1.22–1.48). Moreover, when we calculated a new AHEAD‐U score by considering uric acid (>8.6 mg/dL) in addition to the AHEAD score, the net reclassification was improved by 19.7% and 20.1% for predicting all‐cause and cardiovascular mortality, respectively.
Conclusions
The AHEAD score was useful in predicting long‐term mortality in the Asian acute heart failure cohort with either HFrEF or HFpEF. The new AHEAD‐U score may further improve risk stratification.
Keywords: acute heart failure, ejection fraction, prognosis, uric acid
Subject Categories: Heart Failure
Introduction
Acute heart failure (AHF) is a major and growing health issue in developed countries. It is the leading cause of hospitalization, involving more than 1 million people hospitalized each year in the Unites States, as well as in Europe.1 Given the high rates of in‐hospital mortality2, 3 and postdischarge re‐hospitalization or death,4 a prompt strategy for risk stratification and subsequently tailored therapy would be helpful to improve clinical outcomes. The Seattle Heart Failure Model5 and the Heart Failure Survival Score6 were validated to predict survival and prognosis in patients with heart failure. However, the complexity of these scoring systems has dampened the applications in clinical practice.
Spinar et al7 recently developed the AHEAD (A: atrial fibrillation; H: hemoglobin; E: elderly; A: abnormal renal parameters; D: diabetes mellitus) scoring system as a simple, bedside clinical prognostic model in a multicenter prospective registry of AHF, involving 5846 patients. Based on age and comorbidities, each 1‐point increase of AHEAD score was associated with ≈10% excessive 1‐year mortality. However, whether there are discrepancies or not, the prognostic values of the AHEAD score in different phenotypes of AHF remains to be elucidated.
We therefore investigated the clinical significance of the AHEAD score to predict long‐term prognosis in an Asian AHF cohort comprising AHF subjects with either reduced or preserved left ventricular ejection fraction (HFrEF or HFpEF).
Methods
Study Population
The study population was drawn from the heart failure registry of Taipei Veterans General Hospital (HARVEST registry) that included patients hospitalized for AHF, defined as new‐onset or gradually or rapidly worsening heart failure symptoms and signs requiring urgent therapy. Consecutive AHF patients with New York Heart Association functional class III or IV symptoms, compatible presentations of chest radiograph, and responses to diuretics, were enrolled.8, 9 Subjects with acute coronary syndrome, severe hepatic disease, or severe infection were excluded from this analysis. In a total of 2663 eligible subjects hospitalized primarily for AHF from October 2003 to December 2012, 2143 patients with complete data of hematology, biochemistry, and echocardiogram constituted this study population. The investigation conformed with the principles outlined in the Declaration of Helsinki and was approved by the institutional review board of Taipei Veterans General Hospital. Given the nature of an administrative registry, informed consents were waived.
Definition and Covariates
AHEAD score was calculated by assigning 1 point for each of A: atrial fibrillation, H: hemoglobin <130 g/L for men and 120 g/L for women, E: elderly (age >70 years), A: abnormal renal parameters (creatinine >130 μmol/L), and D: diabetes mellitus.7
Left ventricular ejection fraction (LVEF) was measured by 2‐dimensional‐guided M‐mode echocardiogram.10 Patients with a LVEF of 50% or higher were defined as HFpEF, whereas those with a LVEF of less than 50% were defined as HFrEF. Hemogram, renal function, serum electrolytes, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) were measured immediately upon presentation to the hospital. Uric acid and the lipid profiles were obtained at fasting the next morning. Estimated glomerular filtration rate was then calculated using the modified glomerular filtration rate estimating equation for Chinese patients.11 Comorbidities were identified by medical history and the associated measures during the index hospitalization. Therefore, atrial fibrillation was diagnosed based on past or ad‐hoc ECGs. Diabetes mellitus was confirmed by medical records and a glycosylated hemoglobin ≥6.5% during the index hospitalization. Hypertension was defined according to medical records. Renin–angiotensin system inhibitors were referred to either angiotensin‐converting enzyme inhibitors or angiotensin II receptor antagonists. Given that the commercialized measurement of NT‐proBNP (Roche Diagnostics, Basel, Switzerland) was available only after 2009, some patients had missing NT‐proBNP data in this analysis.
The clinical end point was the occurrence of all‐cause mortality or cardiovascular death. Mortality in the study population was ascertained by the International Classification of Diseases, 9th Revision coding in the National Death Registry with a follow‐up duration of up to 3 years. Cardiovascular death was ascertained based on the International Classification of Diseases, 9th Revision codes of the primary cause of mortality from 390 to 459.15.
Statistical Analysis and Model Performance
Continuous variables are presented as the mean and SD, and categorical variables are presented as numbers and percentages. One‐way ANOVA or Kruskal–Wallis H test, whenever appropriate, was used for comparisons of continuous variables between groups, and the χ2 test was used for categorical variables. Survival analysis according to scores was carried out by the Kaplan–Meier method, and comparisons were made using the log‐rank test. Cox regression was used to examine the associations of AHEAD score with all‐cause mortality and cardiovascular mortality, hazard ratios, and 95% CI of every 1‐point increase in AHEAD score were calculated. Variables, which were independently related to mortality in the multivariate models, were used to compute a new scoring index, in addition to AHEAD. The likelihood ratio χ2 statistic was initially used to test whether the incorporation of new variables into the AHEAD model, consisting of 5 parameters, could give a better fit than the original model. The Akaike information criterion was then used to compare the model fit of prognostic scoring systems. Improvement of prognostic accuracy from the new scoring index was assessed by calculating the net reclassification improvement with category‐free and category‐based methods,12 using the original AHEAD index as the reference. Another cohort of AHF13 was enrolled from January 2013 to September 2015 to validate the new prediction model. We used the Cox proportional hazards model that only included the new scoring index or AHEAD index to estimate the individual risk of mortality. For category‐based net reclassification improvement, subjects were categorized into 3 groups based on predicted probability of deaths, utilizing 2 risk cut‐offs determined by the 25th and 75th percentiles of the distribution of observed 3‐year mortality rates. A 2‐tailed P<0.05 was considered statistically significant. Statistical analyses were performed using the IBM SPSS software version 22.0 (SPSS Inc, Chicago, IL) and SAS software, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Characteristics of Study Population
A total of 2143 patients (age 77±12 years, 68% men, 38% HFrEF) constituted this study, and they were characterized by multiple comorbidities: 60.1% had hypertension, 38.1% had diabetes mellitus, 30.1% had coronary artery disease, and 39.3% had atrial fibrillation. The mean AHEAD scores were 2.7±1.2 in the study population, 2.6±1.3 in HFrEF, and 2.7±1.1 in HFpEF. Along with the increasing AHEAD score, patients were older, had more diabetes mellitus and atrial fibrillation, and had lower hemoglobin, estimated glomerular filtration rate, and total and low‐density lipoprotein cholesterol, but higher creatinine and NT‐proBNP levels (Table 1). LVEF, prevalence of hypertension, and uric acid levels were also significantly different between patients with various AHEAD scores. In addition, subjects with de novo heart failure tended to have lower AHEAD scores. The prescriptions of β‐blockers, mineralocorticoid antagonists, and statins, but not renin‐angiotensin system inhibitors were significantly different among patients with various AHEAD scores.
Table 1.
Baseline Characteristics, Stratified by AHEAD Score
| Variable | AHEAD Score | P Value | |||||
|---|---|---|---|---|---|---|---|
| 0 (n=64) | 1 (n=299) | 2 (n=542) | 3 (n=692) | 4 (n=460) | 5 (n=86) | ||
| Age, y | 54±12 | 68±15 | 77±12 | 79±10 | 81±7 | 80±5 | <0.001 |
| Male sex, n (%) | 47 (39) | 190 (50) | 351 (54) | 474 (69) | 329 (72) | 58 (67) | 0.121 |
| LVEF, % | 44±19 | 51±20 | 56±20 | 56±18 | 56±18 | 52±19 | <0.001 |
| HFrEF, n (%) | 41 (64) | 137 (46) | 186 (34) | 247 (36) | 164 (36) | 42 (49) | <0.001 |
| BMI, kg/m2 | 26±4.4 | 25.5±5.2 | 24±4.8 | 24.3±4.6 | 25.3±4.8 | 25.8±4.3 | 0.002 |
| De novo heart failure, n (%) | 17 (27) | 83 (28) | 112 (21) | 145 (21) | 76 (17) | 12 (14) | 0.003 |
| Comorbidities, n (%) | |||||||
| Hypertension | 25 (62) | 148 (61) | 295 (48) | 450 (65) | 315 (69) | 54 (63) | <0.001 |
| Diabetes mellitus | 0 (0) | 43 (14) | 116 (21) | 254 (37) | 317 (69) | 86 (100) | <0.001 |
| Coronary artery disease | 18 (28) | 94 (31) | 167 (31) | 194 (28) | 147 (32) | 25 (29) | 0.746 |
| Atrial fibrillation | 0 (0) | 41 (14) | 160 (30) | 311 (45) | 245 (53) | 86 (100) | <0.001 |
| COPD | 2 (3) | 38 (13) | 84 (16) | 118 (17) | 77 (17) | 11 (13) | 0.04 |
| Hematology and biochemistry | |||||||
| Hemoglobin, g/dL | 14.7±1.4 | 13.8±1.9 | 12.3±2.2 | 11.2±1.9 | 10.5±1.6 | 10.5±1.3 | <0.001 |
| Creatinine, mg/dL | 1.1±0.2 | 1.2±0.6 | 1.4±1.1 | 2.1±1.6 | 2.5±1.4 | 2.8±1.4 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 82±27 | 72±25 | 64±27 | 47±29 | 33±19 | 26±11 | <0.001 |
| Sodium, mmol/L | 140±3 | 139±4 | 139±5 | 139±5 | 139±5 | 138±7 | 0.246 |
| Uric acid, mg/dL | 9±3 | 8±3 | 8±3 | 9±3 | 9±3 | 8±3 | <0.001 |
| Total cholesterol, mg/dL | 162.5±42.7 | 162.6±48.5 | 153.8±38.7 | 156.3±43.5 | 152±41.8 | 142.7±38.6 | 0.018 |
| LDL cholesterol, mg/dL | 104.9±31.0 | 99.9±36.2 | 93.7±32.5 | 94.6±35.7 | 89.7±33.4 | 80.6±31.0 | <0.001 |
| NT‐proBNP, pg/mLa (n=767) | 3.5±0.5 (n=17) | 3.5±0.7 (n=84) | 3.7±0.6 (n=205) | 3.8±0.6 (n=259) | 3.8±0.5 (n=171) | 3.9±0.5 (n=31) | <0.001 |
| Pharmacological therapy, n (%) | |||||||
| RAS inhibitors | 54 (84) | 247 (83) | 459 (85) | 560 (81) | 393 (85) | 76 (88) | 0.235 |
| β‐Blockers | 48 (75) | 194 (65) | 336 (62) | 409 (59) | 310 (67) | 59 (69) | 0.015 |
| MRAs | 42 (66) | 197 (66) | 322 (59) | 358 (52) | 255 (55) | 48 (56) | 0.001 |
| Statin | 27 (42) | 102 (34) | 195 (36) | 271 (39) | 219 (48) | 36 (42) | 0.002 |
AHEAD indicates A: atrial fibrillation, H: hemoglobin, E: elderly, A: abnormal renal parameters, D: diabetes mellitus; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HFrEF, heart failure with reduced left ventricular ejection fraction; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid antagonists; NT‐proBNP, N‐terminal prohormone brain natriuretic peptide; RAS, renin–angiotensin system.
NT‐proBNP were log‐transformed.
Prognostic Values of AHEAD Score
During a mean follow‐up duration of 22±14 months, 838 patients (39%) died and 375 patients (18%) died of cardiovascular causes. Kaplan–Meier survival curve analysis demonstrated a significantly increased mortality along with an increase in AHEAD score in the total study population and in subjects with either HFrEF or HFpEF over a 3‐year follow‐up period (Figures 1 and 2). In univariate Cox regression analysis, the AHEAD score was associated with an increased risk of all‐cause mortality (hazard ratio and 95% CI: 1.38, 1.30–1.47) and cardiovascular mortality (1.37, 1.25–1.50) in the total study population. The correlations of AHEAD score and mortality remained significant in subjects with either HFrEF or HFpEF (Table 2). After accounting for sex, LVEF, sodium and uric acid levels, comorbidities, and the prescribed medications, AHEAD score was still related to all‐cause (1.49, 1.38–1.60) and cardiovascular mortalities (1.48, 1.33–1.64) in the study population (Table 2, model 1). The independence of AHEAD score in the predictions of clinical outcomes remained significant in subjects with either HFrEF or HFpEF (Table 2, model 1). With further adjustments for NT‐proBNP, the prognostic values of AHEAD score persisted for total and cardiovascular mortality in the study population (Table 2, model 2). However, AHEAD score remained predictive of mortality in patients with HFrEF, but not in HFpEF. When we classified HFrEF with a LVEF of <40% according to 2016 European Society of Cardiology guidelines,9 the results remained unchanged (Table 2).
Figure 1.
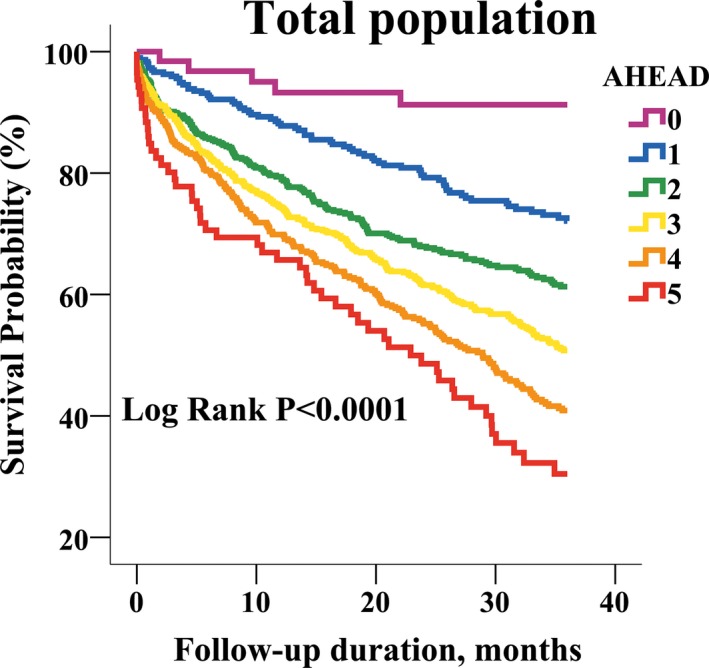
Kaplan–Meier survival curve analysis in the total study population, stratified by AHEAD score.
Figure 2.
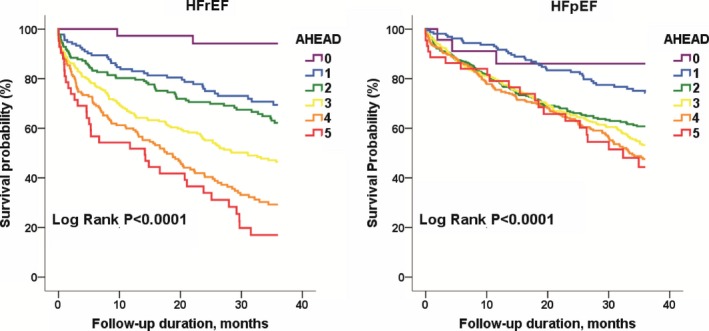
Kaplan–Meier survival curve analysis in heart failure subjects with either reduced (HFrEF, n=817) or preserved ejection fraction (HFpEF, n=1326), stratified by AHEAD score.
Table 2.
Hazard Ratios and 95% CIs of a 1‐Point Increase in AHEAD Score for Long‐Term All‐Cause and Cardiovascular Mortality, Using Univariate and Multivariate Cox Proportional Regression Analysis
| Crude Ratio | Model 1 | Model 2 | |
|---|---|---|---|
| Total study populations | |||
| All‐cause mortality | 1.38 (1.30–1.47) | 1.49 (1.38–1.60) | 1.42 (1.17–1.72) |
| Cardiovascular death | 1.37 (1.25–1.50) | 1.48 (1.33–1.64) | 1.81 (1.36–2.40) |
| HFpEF | |||
| All‐cause mortality | 1.25 (1.16–1.36) | 1.34 (1.22–1.48) | 1.06 (0.83–1.35) |
| Cardiovascular death | 1.23 (1.08–1.40) | 1.34 (1.14–1.59) | 1.20 (0.78–1.85) |
| HFrEF | |||
| All‐cause mortality | 1.55 (1.42–1.69) | 1.63 (1.47–1.82) | 2.18 (1.58–3.01) |
| Cardiovascular death | 1.53 (1.36–1.73) | 1.56 (1.36–1.79) | 2.41 (1.63–3.56) |
| HF with a LVEF <40% | |||
| All‐cause mortality | 1.65 (1.46–1.86) | 1.63 (1.44–1.84) | 2.43 (1.64–3.59) |
| Cardiovascular death | 1.61 (1.38–1.89) | 1.61 (1.37–1.90) | 2.76 (1.65–4.62) |
Model 1: with adjustments for sex, left ventricular ejection fraction, sodium, uric acid, hypertension, use of β‐blockers, mineralocorticoid antagonists, and renin–angiotensin system inhibitors.
Model 2: with adjustments for variables of Model 2 plus N‐terminal prohormone brain natriuretic peptide (n=767).
AHEAD indicates A: atrial fibrillation, H: hemoglobin, E: elderly, A: abnormal renal parameters, D: diabetes mellitus; HFpEF, heart failure with preserved left ventricular ejection fraction; HFrEF, heart failure with reduced left ventricular ejection fraction; LVEF, left ventricular ejection fraction.
Refinement of AHEAD Score
Based on the multivariate Cox proportional hazard models, uric acid was independently related to clinical outcomes in the study population (Table 3). By a cut‐off value of 8.6 mg/dL derived from receiver operating characteristic curve analysis, uric acid was incorporated with the AHEAD score to construct the AHEAD‐U index. Table 4 demonstrates the comparisons of model performance between AHEAD and AHEAD‐U. In short, AHEAD‐U outperformed AHEAD by showing a better model fit according to the likelihood ratio χ2 statistic (8521.58, P=0.0134) and Akaike information criterion (8552.64). Compared with the AHEAD score, AHEAD‐U significantly reclassified more patients into the correct risk categories and achieved a net reclassification improvement of 19.66% (P=0.0002) in all‐cause mortality and 20.08% (P=0.0025) in cardiovascular mortality.
Table 3.
Univariate and Multivariate Cox Proportional Regression Analysis of All‐Cause Mortality in Total Study Population
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| AHEAD Score | 1.38 (1.30–1.47) | <0.01 | 1.49 (1.38–1.60) | <0.01 |
| Male sex, men vs women | 1.09 (0.94–1.26) | 0.26 | 0.97 (0.82–1.16) | 0.74 |
| Hypertensiona | 0.81 (0.71–0.93) | <0.01 | 0.91 (0.77–1.08) | 0.28 |
| LVEF (%) | 0.54 (0.38–0.77) | <0.01 | 0.34 (0.21–0.53) | <0.01 |
| Sodium, mEq/L | 0.97 (0.96–0.99) | <0.01 | 0.98 (0.97–1.00) | 0.06 |
| Uric acid, mg/dL | 1.05 (1.02–1.08) | <0.01 | 1.03 (1.00–1.06) | 0.03 |
| β‐blockersa | 0.65 (0.57–0.75) | <0.01 | 0.68 (0.57–0.82) | <0.01 |
| RAS inhibitorsa | 0.62 (0.52–0.73) | <0.01 | 0.76 (0.61–0.94) | 0.01 |
| MRAsa | 0.75 (0.65–0.86) | <0.01 | 0.84 (0.71–1.00) | 0.05 |
AHEAD indicates A: atrial fibrillation, H: hemoglobin, E: elderly, A: abnormal renal parameters, D: diabetes mellitus; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid antagonists; RAS, renin–angiotensin system.
Yes vs no.
Table 4.
Comparison of Model Performance Between AHEAD and AHEAD‐U Scoring Systems for Predicting 3‐Year All‐Cause and Cardiovascular Mortality
| Model Fit | Reclassification | ||||||
|---|---|---|---|---|---|---|---|
| Likelihood Ratio Statisticsa | AIC | Category‐Free | Category‐Basedb | ||||
| −2 Log L | P Value | NRI | P Value | NRI | P Value | ||
| All‐cause mortality | |||||||
| AHEAD | 8527.69 | 8555.79 | (Reference) | (Reference) | |||
| AHEAD‐U | 8521.58 | 0.0134 | 8552.64 | 0.1966 | 0.0002 | 0.1022 | <0.001 |
| Cardiovascular mortality | |||||||
| AHEAD | 3806.40 | 3845.21 | (Reference) | (Reference) | |||
| AHEAD‐U | 3799.98 | 0.0113 | 3839.05 | 0.2008 | 0.0025 | 0.1025 | <0.001 |
AHEAD indicates A: atrial fibrillation, H: hemoglobin, E: elderly, A: abnormal renal parameters, D: diabetes mellitus; AIC, Akaike information criterion; NRI, net reclassification improvement.
The likelihood ratio test was used to compare the goodness of fit of AHEAD and AHEAD‐U models, with 1 difference in parameters between 2 models.
The risk thresholds of 20% and 45% for all‐cause mortality, and 10% and 23% for cardiovascular mortality were used to classify subjects as low‐, moderate‐, and high‐risk group.
Validation of the AHEAD‐U Score
The baseline characteristics of the validation cohort, comparing to the study population, are demonstrated in Table 5. Although the validation cohort was distinct from the study population, regarding age, left ventricular systolic function, and morbidities, the survival probabilities of the validation population were reduced when every 1‐point increase of the AHEAD‐U score (Figure 3). The AHEAD‐U score was crudely associated with total mortality during a 3‐year follow‐up duration (1.46, 1.21–1.76). With adjustments for age and sex, AHEAD‐U remained related to the outcomes (1.25, 1.01–1.56) of the validation cohort.
Table 5.
Baseline Characteristics of Patients in the HARVEST Registry and the Other Acute Heart Failure Population
| Variable | HARVEST, n=2143 | Validation Cohort, n=175 | P Value |
|---|---|---|---|
| Age, y | 76.7±12.3 | 69.9±15.4 | <0.01 |
| Male sex, n (%) | 1449 (67.6) | 135 (77.1) | <0.01 |
| LVEF, % | 54.9±18.8 | 39.2±15.2 | <0.01 |
| HFrEF, n (%) | 681 (31.8) | 118 (67.4) | <0.01 |
| Comorbidities, n (%) | |||
| Hypertension | 1287 (60.1) | 130 (74.3) | <0.01 |
| Diabetes mellitus | 816 (38.1) | 88 (50.3) | <0.01 |
| Coronary artery disease | 645 (30.1) | 102 (58.3) | <0.01 |
| Atrial fibrillation | 843 (39.3) | 47 (26.9) | <0.01 |
| Hematology and biochemistry | |||
| Hemoglobin, g/dL | 11.8±2.2 | 11.8±2.3 | 0.85 |
| Creatinine, mg/dL | 1.9±1.4 | 1.79±0.96 | 0.20 |
| eGFR, mL/min per 1.73 m2 | 52.0±29.4 | 50.6±27.1 | 0.69 |
| Sodium, mmol/L | 138.7±4.8 | 138.5±4.4 | 0.31 |
| Uric acid, mg/dL | 8.6±3.0 | 9.1±8.7 | 0.86 |
| Total cholesterol, mg/dL | 155.4±42.7 | 156.9±37.6 | 0.26 |
| LDL cholesterol, mg/dL | 94.0±34.4 | 100.3±32.4 | <0.01 |
| NT‐proBNP, pg/mLa | 3.8±0.6 (n=767) | 7.7±1.6 (n=89) | <0.01 |
| Pharmacological therapy, n (%) | |||
| RAS inhibitors | 1789 (83.5) | 125 (71.4) | <0.01 |
| β‐Blockers | 1356 (63.3) | 105 (60.0) | 0.39 |
| MRAs | 1222 (57.0) | 99 (56.6) | 0.91 |
| Statin | 850 (39.7) | 49 (28.0) | <0.01 |
eGFR indicates estimated glomerular filtration rate; HARVEST, heart failure registry of Taipei Veterans General Hospital; HFrEF, heart failure with reduced left ventricular ejection fraction; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid antagonists; NT‐proBNP, N‐terminal prohormone brain natriuretic peptide; RAS, renin‐angiotensin system.
NT‐proBNP were log‐transformed.
Figure 3.
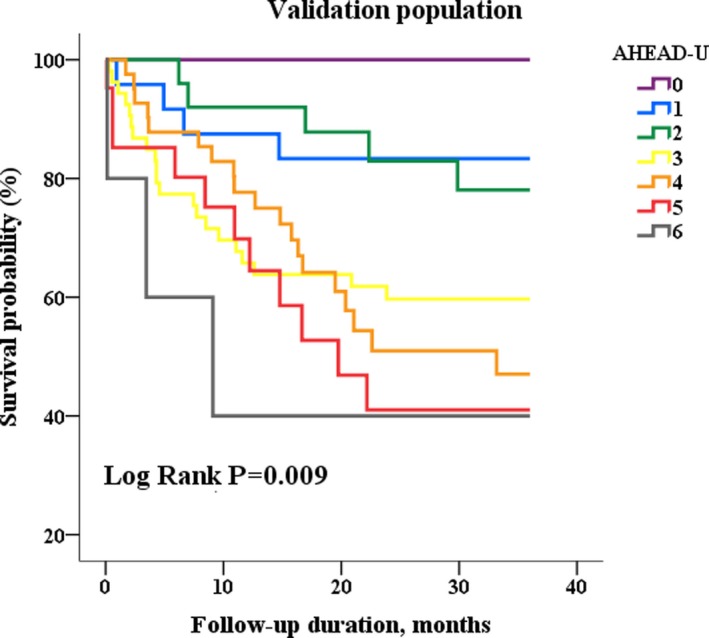
Kaplan–Meier survival curve analysis in the validation cohort, stratified by AHEAD‐U score.
Discussion
The present study independently validated a simple and practical scoring system, obtained from bedside estimation; the AHEAD score was independently associated with the long‐term prognosis in an Asian cohort of AHF. Both in subjects with HFrEF and HFpEF, AHEAD score remained related to mortality consistently. The study may extend the clinical applications of risk‐predicting models in patients with HFpEF, while the established prognostic calculators were constructed based on study populations, in which the majority were HFrEF. Given that uric acid was correlated with clinical outcomes, independent of AHEAD score, AHEAD‐U score was computed by the incorporation with uric acid. AHEAD‐U outperformed AHEAD score in improving the risk classification in patients with AHF.
Risk Classifications in Acute Heart Failure
The use of validated multivariable risk scores to estimate the subsequent risk of mortality in hospitalized patients with AHF has been recommended in the 2013 American College of Cardiology Foundation/American Heart Association Guideline.14 For patients hospitalized with AHF, the ADHERE (Acute Decompensated Heart Failure National Registry) Classification and Regression Tree Model is predictive of in‐hospital mortality.2 The EFFECT (Enhanced Feedback for Effective Cardiac Treatment) Risk Score,15 ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) Risk Model and Discharge score4, and OPTIMIZE HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Risk‐Prediction Nomogram16 are predictive of the longest 1‐year mortality. In comparison with these published models, Spinar et al7 demonstrated that the AHEAD risk scoring system is simpler and easier to obtain, based on comorbidities and bedside estimations. The value of the prognostic model is that of the information related to heart failure prognosis and disease trajectory, which may favorably influence physician‐prescribing behaviors. Unlike CHA2DS2‐Vasc score for atrial fibrillation, the existing HF prediction models are usually too complicated to be popularized. A simple model to predict the mortality of acute heart failure may facilitate the clinical applications at the bedside and finally improve the quality of care.
The study confirmed that the AHEAD risk scoring system could be generalized to an Asian cohort by showing that each 1‐point increment of the AHEAD score was associated with a 38% excessive 3‐year mortality risk. However, given a higher LVEF and the exclusion of patients with acute coronary syndrome, the study population indeed had better survival, compared with that of Spinar et al7 (Table 6).
Table 6.
The Predicted 3‐Year All‐Cause and Cardiovascular Mortality Rates by Sum of Score in AHEAD and AHEAD‐U Indices
| Sum of Score | AHEAD | AHEAD‐U | ||
|---|---|---|---|---|
| Predicted Probability of All‐Cause Death (95% CI), % | Predicted Probability of Cardiovascular Death (95% CI), % | Predicted Probability of All‐Cause Death (95% CI), % | Predicted Probability of Cardiovascular Death (95% CI), % | |
| 0 | 0.16 (0.15–0.18) | 0.08 (0.07–0.09) | 0.16 (0.14–0.18) | 0.08 (0.07–0.08) |
| 1 | 0.22 (0.21–0.23) | 0.11 (0.10–0.11) | 0.20 (0.19–0.21) | 0.10 (0.09–0.10) |
| 2 | 0.26 (0.25–0.27) | 0.13 (0.12–0.14) | 0.24 (0.23–0.26) | 0.12 (0.11–0.13) |
| 3 | 0.33 (0.31–0.34) | 0.17 (0.16–0.18) | 0.29 (0.27–0.30) | 0.15 (0.14–0.15) |
| 4 | 0.40 (0.38–0.42) | 0.21 (0.20–0.22) | 0.37 (0.35–0.39) | 0.19 (0.18–0.20) |
| 5 | 0.47 (0.41–0.53) | 0.26 (0.23–0.30) | 0.42 (0.39–0.45) | 0.23 (0.21–0.25) |
| 6 | ··· | ··· | 0.48 (0.39–0.58) | 0.28 (0.23–0.34) |
AHEAD indicates A: atrial fibrillation, H: hemoglobin, E: elderly, A: abnormal renal parameters, D: diabetes mellitus; AHEAD‐U, indicates A: atrial fibrillation, H: hemoglobin, E: elderly, A: abnormal renal parameters, D: diabetes mellitus, U: Uric acid.
AHEAD Score in Heart Failure With Reduced or Preserved Left Ventricular Systolic Function
While ≈50% of HF patients are HFpEF17 and the mortality rate is similar to those with HFrEF, the neurohormonal activation and pathophysiological heterogeneity indeed are different between the phenotypes of HF.18 Therefore, the prediction model might vary. To our knowledge, this is the first study to describe risk models specifically for hospitalized patients with HFpEF and HFrEF. AHEAD score was independently predictive of mortality in both subjects with HFrEF and HFpEF. However, each increment of AHEAD score was associated with 55% and 25% increasing risks of death in HFrEF and HFpEF, respectively. The results may support a better performance of AHEAD score in patients with HFrEF, and the heterogeneous pathophysiology of HFpEF that subjects with HFpEF have is more strongly associated with noncardiovascular comorbidity and aging.19, 20 In the adjusted Cox regression model, AHEAD was still associated with all‐cause and cardiovascular mortality in subjects with either HFrEF or HFpEF, indicating the AHEAD score was still a prognostic factor in both phenotypic HF. Given the limited sample size (n=494) and events (135 mortalities and 54 cardiovascular deaths, respectively) in the fully adjusted model with NT‐proBNP, we did not have sufficient power to evaluate the prognostic impacts of AHEAD score in the fully adjusted model of HFpEF.
AHEAD‐U Outperformed AHEAD Score in the Prediction of Outcomes
We previously have demonstrated that hyperuricemia was correlated with increased mortality, independent of traditional risk factors and NT‐proBNP in patients hospitalized for AHF with either HFrEF or HFpEF.8 In this study, uric acid remained correlated with all‐cause mortality, independent of AHEAD score and comorbidities. We therefore incorporated hyperuricemia (uric acid >8.6 mg/dL) with the AHEAD score to construct the AHEAD‐U index. The study results showed that the AHEAD‐U index significantly outperformed the AHEAD index by reclassifying 19.7% of subjects appropriately for all‐cause mortality and 20.1% for cardiovascular mortality, which may suggest the AHEAD‐U index is more appropriate for predicting long‐term prognosis in an Asian AHF cohort.
Study Limitations
The study has some limitations. First, since we only enrolled subjects who had undergone echocardiographic examinations in the analysis, selection bias was not avoidable. While the biplane Simpson's method is suggested to evaluate LVEF,21 we used 2‐dimensional‐guided M‐mode measures in this study because the data derived from Simpson's method were not registered in our web‐based system until 2010. However, in a total of 818 patients with available LVEF measured by Simpson's rule, the conclusions remained the same (Table 7). Second, 520 patients have been excluded from this analysis. However, their baseline characteristics regarding age, sex, LVEF, and morbidities, and the rate of mortality or cardiovascular death were similar to the analyzed samples, which suggested the samples were representative of the study population. In addition, there were 1376 missing values of NT‐proBNP mainly (82%) because of the historical constraint that commercialized measurement of NT‐proBNP was available only after 2009. Although there were discrepancies in age, presence of hypertension and atrial fibrillation, hemoglobin, and uric acid levels, the absence of NT‐proBNP values was not associated with mortality (1.14, 0.98–1.33) in Cox proportional hazard model. Meanwhile, the absence of NT‐proBNP values and the mortality rates have a similar trend over the study period. After adjusting for the fixed “year” effect, the absence of NT‐proBNP values also was not associated with mortality in logistic regression analysis. The evidence supported that the absence of NT‐proBNP values was because of the historical constraint and it was not related to the outcomes. Third, considering the clinical feasibility, some other parameters that are reported to correlate with the prognosis of AHF such as active cancer, body mass index, and chronic obstructive pulmonary disease were not included in the present prognostic modeling.2, 22, 23 Fourth, we internally validated the AHEAD‐U score for the long‐term outcomes of AHF patients in a small population. Further studies of clinical practice are needed to confirm the feasibility and generalizability of AHEAD as well as AHEAD‐U scores.
Table 7.
Hazard Ratios and 95% CI of a 1‐Point Increase in AHEAD Score for Long‐Term All‐Cause and Cardiovascular Mortality, Using Univariate and Multivariate Cox Proportional Regression Analysis in Patients with Left Ventricular Ejection Fraction Measured by Simpson's Rule
| Crude Ratio | Model 1 | Model 2 | |
|---|---|---|---|
| Total study populations, n=818 | |||
| All‐cause mortality | 1.28 (1.14–1.44) | 1.46 (1.24–1.72) | 1.26 (1.00–1.58) |
| Cardiovascular death | 1.54 (1.29–1.84) | 1.69 (1.32–2.17) | 1.50 (1.09–2.07) |
| HFrEF, n=328a | |||
| All‐cause mortality | 1.45 (1.19–1.75) | 2.04 (1.49–2.79) | 1.84 (1.29–2.60) |
| Cardiovascular death | 1.73 (1.33–2.26) | 2.28 (1.51–3.45) | 2.04 (1.29–3.22) |
| HFpEF, n=490a | |||
| All‐cause mortality | 1.21 (1.04–1.41) | 1.29 (1.06–1.57) | 0.90 (0.65–1.27) |
| Cardiovascular death | 1.46 (1.13–1.88) | 1.42 (1.02–1.97) | 1.06 (0.62–1.81) |
Model 1: with adjustments for sex, left ventricular ejection fraction, sodium, uric acid, hypertension, use of β‐blockers, mineralocorticoid antagonists, and RAS inhibitors. Model 2: with adjustments for variables of Model 2 plus NT‐proBNP. AHEAD indicates A: atrial fibrillation, H: hemoglobin, E: elderly, A: abnormal renal parameters, D: diabetes mellitus; HFpEF, acute heart failure with preserved ejection fraction; HFrEF, acute heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; RAS, renin–angiotensin system.
LVEF had been calculated by means of Simpson's rule.
Conclusions
In conclusion, the AHEAD score performed well for predicting long‐term mortality in the Asian AHF cohort with either HFrEF or HFpEF. In addition, the AHEAD‐U score may further improve the risk stratification from the AHEAD score.
Sources of Funding
The study was supported by Taipei Veterans General Hospital (V100C‐145, V101C‐092, V102C‐119, V103B‐017, and V104C‐172), WanFang Hospital (105SWF06), Ministry of Science and Technology (MOST 103‐2314‐B‐010‐050‐MY2), and Ministry of Health and Welfare, Taiwan grant (MOHW‐104‐TDU‐B‐211‐113003, MOHW‐105‐TDU‐B‐211‐133017, MOHW106‐TDU‐B‐211‐113001), and the death registry.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004297 DOI: 10.1161/JAHA.116.004297.)28473403
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; on behalf of the American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J. In‐hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57–64. [DOI] [PubMed] [Google Scholar]
- 3. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. [DOI] [PubMed] [Google Scholar]
- 4. O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. [DOI] [PubMed] [Google Scholar]
- 6. Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. [DOI] [PubMed] [Google Scholar]
- 7. Spinar J, Jarkovsky J, Spinarova L, Mebazaa A, Gayat E, Vitovec J, Linhart A, Widimsky P, Miklik R, Zeman K, Belohlavek J, Malek F, Felsoci M, Kettner J, Ostadal P, Cihalik C, Vaclavik J, Taborsky M, Dusek L, Littnerova S, Parenica J. AHEAD score—long‐term risk classification in acute heart failure. Int J Cardiol. 2016;202:21–26. [DOI] [PubMed] [Google Scholar]
- 8. Huang WM, Hsu PF, Cheng HM, Lu DY, Cheng YL, Guo CY, Sung SH, Yu WC, Chen CH. Determinants and prognostic impact of hyperuricemia in hospitalization for acute heart failure. Circ J. 2016;80:404–410. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force M . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 10. Folland ED, Parisi AF, Moynihan PF, Jones DR, Feldman CL, Tow DE. Assessment of left ventricular ejection fraction and volumes by real‐time, two‐dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–766. [DOI] [PubMed] [Google Scholar]
- 11. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 12. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sung SH, Yu WC, Cheng HM, Lee CW, Lin MM, Chuang SY, Chen CH. Excessive wave reflections on admission predict post‐discharge events in patients hospitalized due to acute heart failure. Eur J Heart Fail. 2012;14:1348–1355. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 15. Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. [DOI] [PubMed] [Google Scholar]
- 16. Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O'Connor CM, Felker GM, Hernandez AF. Admission, discharge, or change in B‐type natriuretic peptide and long‐term outcomes: data from organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF) linked to Medicare claims. Circ Heart Fail. 2011;4:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 18. Sanders‐van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner‐La Rocca HP; Investigators T‐C . Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail. 2015;17:1006–1014. [DOI] [PubMed] [Google Scholar]
- 19. Lund LH, Donal E, Oger E, Hage C, Persson H, Haugen‐Lofman I, Ennezat PV, Sportouch‐Dukhan C, Drouet E, Daubert JC, Linde C. Association between cardiovascular vs. non‐cardiovascular co‐morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:992–1001. [DOI] [PubMed] [Google Scholar]
- 20. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; Investigators C, Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 22. Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. [DOI] [PubMed] [Google Scholar]
- 23. Tavazzi L, Senni M, Metra M, Gorini M, Cacciatore G, Chinaglia A, Di Lenarda A, Mortara A, Oliva F, Maggioni AP; Investigators I‐HO . Multicenter prospective observational study on acute and chronic heart failure: one‐year follow‐up results of IN‐HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6:473–481. [DOI] [PubMed] [Google Scholar]


