Abstract
Background
Caffeine in doses <400 mg is typically not considered arrhythmogenic, but little is known about the additional ingredients in energy drinks. We evaluated the ECG and blood pressure (BP) effects of high‐volume energy drink consumption compared with caffeine alone.
Methods and Results
This was a randomized, double‐blind, controlled, crossover study in 18 young, healthy volunteers. Participants consumed either 946 mL (32 ounces) of energy drink or caffeinated control drink, both of which contained 320 mg of caffeine, separated by a 6‐day washout period. ECG, peripheral BP, and central BP measurements were obtained at baseline and 1, 2, 4, 6, and 24 hours post study drink consumption. The time‐matched, baseline‐adjusted changes were compared. The change in corrected QT interval from baseline in the energy drink arm was significantly higher than the caffeine arm at 2 hours (0.44±18.4 ms versus −10.4±14.8 ms, respectively; P=0.02). The QTc changes were not different at other time points. While both the energy drink and caffeine arms raised systolic BP in a similar fashion initially, the systolic BP was significantly higher at 6 hours when compared with the caffeine arm (4.72±4.67 mm Hg versus 0.83±6.09 mm Hg, respectively; P=0.01). Heart rate, diastolic BP, central systolic BP, and central diastolic BP showed no evidence of a difference between groups at any time point. Post energy drink, augmentation index was lower at 6 hours.
Conclusions
The corrected QT interval and systolic BP were significantly higher post high‐volume energy drink consumption when compared with caffeine alone. Larger clinical trials validating these findings and evaluation of noncaffeine ingredients within energy drinks are warranted.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02023723.
Keywords: arrhythmia, blood pressure, electrocardiography, electrophysiology, energy drink
Subject Categories: Arrhythmias, Electrophysiology, Diet and Nutrition, Hypertension
Introduction
There are currently more than 500 energy drink products available on the market purported to boost physical and mental alertness.1 In line with their increased popularity is a coinciding rise in energy drink–associated emergency department visits and deaths, which has led to questions about their true safety profile.2, 3, 4 In a review of energy drink–associated adverse cardiovascular events, abnormal heart rhythms such as atrial and ventricular fibrillation, ST elevation, and QT prolongation have been reported.5 However, the exact relationship between energy drinks and these adverse events has gone primarily unexplained.
Energy drinks usually consist of caffeine plus proprietary energy blends that vary between products. Caffeine has been around for centuries and is generally recognized as safe in doses less than 400 mg by the Food and Drug Administration.6 Caffeine, a natural methylxanthine, acts as a central nervous stimulant in humans by antagonizing adenosine receptors, leading to additional cardiovascular effects such as peripheral vasoconstriction and subsequent increased blood pressure (BP).7, 8 Caffeine alone is not suspected to induce any ECG changes in healthy volunteers at a dose of 400 mg.9 Typically, caffeine toxicity–related adverse events have only been observed in case studies where doses far exceed 400 mg.10, 11
Although the cardiovascular safety profile of caffeine has been relatively well established, there is little published literature on the electrophysiologic and hemodynamic changes with multi‐ingredient energy drinks. We conducted a randomized controlled trial assessing the cardiovascular safety of high‐volume energy drink consumption.
Methods
Study Oversight and Patient Population
The protocol and informed consent forms were approved by the David Grant Medical Center Institutional Review Board (protocol No. FDG20130042H). All participants provided written informed consent.
Participants were recruited via email and flyers between 2013 and 2014 on a US Air Force Base installation. Healthy volunteers between the ages of 18 and 40 years were included. Participants were excluded if they had a current or previous diagnosis of abnormal heart rhythm, a BP >140/90 mm Hg, any comorbid medical conditions, history of substance abuse, renal or hepatic dysfunction, concurrent use of drugs or over‐the‐counter products that may interact with study drinks or affect ECG or BP parameters (excluding oral contraceptives), or were pregnant or lactating.
Study Design
This was a randomized, double‐blind, caffeine‐controlled, crossover study in healthy adults. Using a computer‐generated randomization code, participants were assigned to consume either a 1‐time 32‐ounce (946 mL) dose of a commercially available energy drink (containing 108 g of sugar, vitamin B2, vitamin B3, vitamin B6, and vitamin B12, and a proprietary energy blend of taurine, panax ginseng extract, l‐carnitine, caffeine [320 mg], glucuronolactone, inositol, guarana extract, and maltodextrin) or a matching 32‐ounce (946 mL) control drink containing 320 mg of caffeine, 40 mL of lime juice, and 140 mL of cherry syrup in carbonated water (Figure 1). Other than the caffeine, all added ingredients in the control group were expected to have no impact on any end points and were simply added to match the active drink. The dose was based on the observation that cardiovascular adverse effects typically occur with high consumption of energy drink/caffeine.5 The amount of energy drink participants were asked to consume (2 cans totaling 320 mg caffeine) correlates to the average daily caffeine consumption (300 mg) of the US population.12 Further, nearly 15% of military personnel consume 3 cans a day in the deployed setting, which may predispose them to a higher risk threshold.5, 13 After a minimum 6‐day washout period, participants proceeded to consume the alternate study drink. Participants were required to fast for 12 hours, and abstain from any caffeinated products 48 hours prior to each study day and throughout the 24‐hour follow‐up period. All study drinks were presented in identical containers and were consumed over a 45‐minute period. End points were measured on each study day at baseline (immediately prior to study drink consumption), and 1, 2, 4, 6, and 24 hours post consumption of study drink. Due to the possibility of circadian rhythm changes, the start time for each patient was approximately the same on the 2 study days (maximum difference ≤80 minutes).
Figure 1.
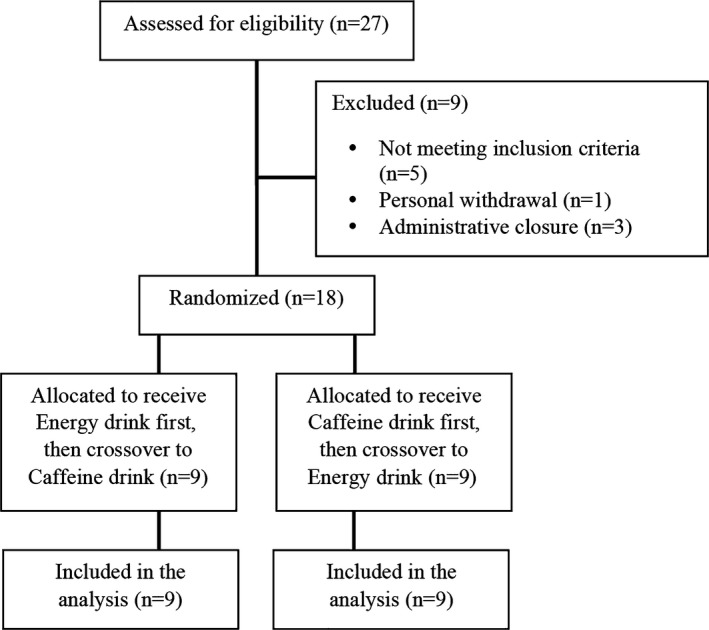
CONSORT flow diagram.
End Points and Assessments
The primary end point was the QTc interval. Secondary end points were uncorrected QT interval, PR interval, QRS duration, heart rate (HR), peripheral systolic BP (pSBP), peripheral diastolic BP (pDBP), central systolic BP (cSBP), central diastolic BP (cDBP), and augmentation index (AI). Participants were also asked to describe any adverse events they were experiencing at each time point.
A 12‐lead ECG (Philips PageWriter Trim III, Philips Medical Systems, Andover, MA) was obtained with the participant in the supine position. The machine was calibrated to a 1‐mV/cm standardization with a paper speed of 25 mm/s. Peripheral BP measurements were obtained in duplicate after a 5‐minute rest using a standard automated vital signs monitor (Masimo SET Vital Sign Monitor; Welch Allyn; Skaneateles Falls, NY). Central BP measurements were obtained using the SphygmoCor PWA system (AtCor Medical Pty Ltd, West Ryde, Australia). SphygmoCor is a validated system that uses applanation tonometry to noninvasively translate a radial pressure waveform taken at the wrist to an aortic pressure waveform. AI was corrected to a HR of 75 beats per minute.
Statistical Analysis
Based on some of our previous data, we expected a change in the energy drink arm of 10 ms and no change in the caffeine control arm. To detect a between‐group difference of 10 ms and assuming an SD of 14 ms (2‐sided α=5% and 80% power), we would need 18 participants for the study. The time‐matched changes from baseline were compared between the energy drink and control arms using the Wilcoxon signed rank test. All data were reported as mean±SD unless otherwise stated. Applanation tonometry requires operator proficiency and only those with an operator index of 70% or greater were included. Intention‐to‐treat analysis using the last‐observation‐carried‐forward methodology was performed to account for the missing values.
Results
Baseline Characteristics
Twelve men and 6 women (n=18) were included, of which 11 identified as white, 3 as Asian, 2 as Hispanic, 1 as black, and 1 undisclosed. Average age, height, and weight were 26.7±4.0 years, 171.9±12.2 cm, and 74.4±15.0 kg, respectively. Nine were regular coffee drinkers (≥1 cup of coffee per day), 5 were occasional drinkers, and 4 reported no coffee consumption. Four reported regular energy drink use (≥1 can per day), 5 occasional energy drink use, and 9 no energy drink use. Data imputation was performed for less than 4% of central BP parameters. At each time point, the change from baseline is reported in Table 1.
Table 1.
Baseline‐Adjusted ECG and BP Parameters
| Drink | 1 Hour | 2 Hours | 4 Hours | 6 Hours | 24 Hours | |
|---|---|---|---|---|---|---|
| QTc, ms | ED | −1.17±20.7 | 0.44±18.4 | 0.83±19.5 | −1.33±17.4 | 1.44±18.3 |
| C | −5.83±17.7 | −10.4±14.8 | −3.67±13.2 | −5.89±15.3 | −4.17±17.4 | |
| P value | 0.38 | 0.02a | 0.35 | 0.61 | 0.47 | |
| QT, ms | ED | −2.44±16.3 | −10.7±20.3 | −3.33±20.7 | −1.78±22.6 | 7.56±27.8 |
| C | −2.44±17.1 | −9.33±18.1 | −2.67±18.5 | −8.67±19.5 | 3.78±23.7 | |
| P value | 0.782 | 0.89 | 0.77 | 0.17 | 0.85 | |
| PR, ms | ED | −1.78±10.47 | −5.11±11.4 | −6.00±15.7 | −6.22±14.7 | 0.67±11.7 |
| C | −3.11±10.2 | −2.67±11.4 | −4.89±10.9 | −4.78±9.43 | 1.77±10.6 | |
| P value | 0.86 | 0.56 | 0.82 | 0.68 | 0.95 | |
| QRS, ms | ED | 6.17±3.33 | 4.22±2.73 | 3.39±4.15 | 3.22±5.01 | 1.94±3.69 |
| C | 6.11±4.91 | 3.28±3.92 | 3.06±2.90 | 1.28±3.82 | 0.78±4.26 | |
| P value | 0.82 | 0.15 | 0.60 | 0.24 | 0.25 | |
| HR, bpm | ED | 0.33±10.5 | 3.39±11.04 | 1.22±11.6 | 0.17±12.3 | −1.50±12.0 |
| C | −1.28±8.41 | −0.61±9.13 | −0.22±8.59 | 0.61±10.1 | −2.28±11.1 | |
| P value | 0.33 | 0.07 | 0.42 | 0.82 | 0.71 | |
| pSBP, mm Hg | ED | 6.00±6.64 | 4.28±6.17 | 3.89±5.4 | 4.72±4.67 | −0.81±4.54 |
| C | 7.08±4.37 | 4.78±4.55 | 2.25±5.45 | 0.83±6.09 | −1.33±6.15 | |
| P value | 0.77 | 0.97 | 0.55 | 0.01a | 1.00 | |
| pDBP, mm Hg | ED | 4.25±4.01 | 1.33±3.87 | 0.28±4.92 | 1.53±3.70 | −0.03±3.76 |
| C | 4.33±4.59 | 2.72±4.32 | 1.39±2.77 | −0.50±5.85 | −1.03±4.65 | |
| P value | 0.75 | 0.37 | 0.36 | 0.27 | 0.40 | |
| cSBP, mm Hg | ED | 4.28±5.48 | 2.89±6.03 | 1.67±5.08 | 2.28±3.54 | −1.44±3.40 |
| C | 3.67±6.11 | 3.06±4.26 | 0.33±5.41 | −0.33±6.27 | −2.00±5.08 | |
| P value | 0.78 | 1.00 | 0.39 | 0.08 | 0.89 | |
| cDBP, mm Hg | ED | 3.89±3.98 | 1.50±3.79 | −0.06±5.00 | 1.22±3.44 | −0.67±3.56 |
| C | 3.78±4.63 | 2.17±4.42 | 1.56±3.33 | −0.56±5.71 | −1.61±4.17 | |
| P value | 0.89 | 0.67 | 0.19 | 0.35 | 0.58 | |
| AI | ED | −2.50±6.48 | −1.61±5.70 | −3.56±5.67 | −3.72±7.61 | −2.61±7.21 |
| C | −0.55±9.39 | 2.78±9.92 | −0.88±9.49 | 1.50±9.85 | 2.33±7.18 | |
| P value | 0.52 | 0.11 | 0.37 | 0.02a | 0.07 |
AI indicates augmentation index, corrected to a heart rate (HR) of 75 beats per minute (bpm); C, caffeine; cDBP, central diastolic blood pressure; cSBP, central systolic blood pressure; ED, energy drink; pDBP, peripheral diastolic blood pressure; pSBP, peripheral systolic blood pressure.
P<0.05.
Baseline QTc interval, uncorrected QT interval, PR interval, QRS duration, and HR were 413±17.3 ms, 405±26.9 ms, 158±21.0 ms, 89.1±9.69 ms, and 63.4±10.5 beats per minute, respectively. Baseline pSBP, pDBP, cSBP, cDBP, and AI were 117±9.89 mm Hg, 72.0±8.56 mm Hg, 103±9.00 mm Hg, 73.4±8.60 mm Hg, and 5.50±10.1, respectively.
ECG Effects
A significant difference in the baseline‐adjusted QTc interval (Figure 2) was evident 2 hours after energy drink consumption when compared with caffeine (0.44±18.4 ms versus −10.4±14.8 ms, respectively; P=0.02). There was no evidence of a statistically significant difference in the baseline‐adjusted HR 2 hours after energy drink consumption when compared with caffeine (3.39±11.04 versus −0.61±9.13, respectively; P=0.07). There was no evidence of time‐matched intragroup differences with QT interval, PR interval, and QRS duration (all P>0.14).
Figure 2.
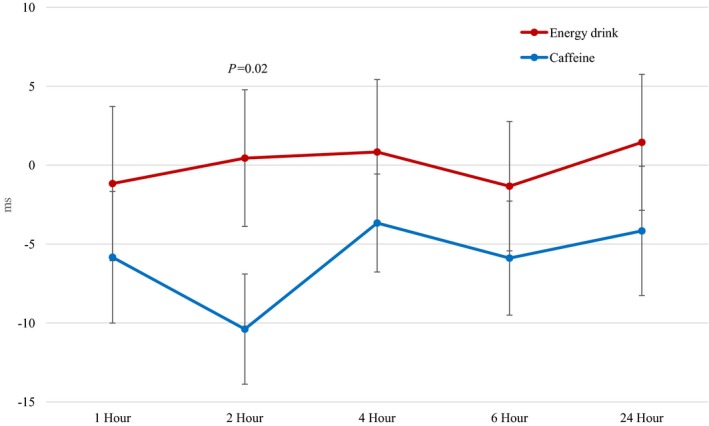
Baseline‐adjusted corrected QT interval with energy drink vs caffeine consumption (0.44±18.4 ms vs −10.4±14.8 ms at 2 hours, respectively; P=0.02). Data are reported as mean±standard error bars.
Blood Pressure Effects
A significant difference in baseline‐adjusted pSBP (Figure 3) was evident 6 hours after energy drink consumption when compared with the caffeine arm (4.72±4.67 mm Hg versus 0.83±6.09 mm Hg, respectively; P=0.01). A significant decrease in baseline‐adjusted AI was evident 6 hours post energy drink when compared with the caffeine arm (−3.72±7.61 versus 1.50±9.85; P=0.02). No evidence of difference was seen with pDBP, cSBP, or cDBP at any time point between the 2 groups (all P>0.07).
Figure 3.
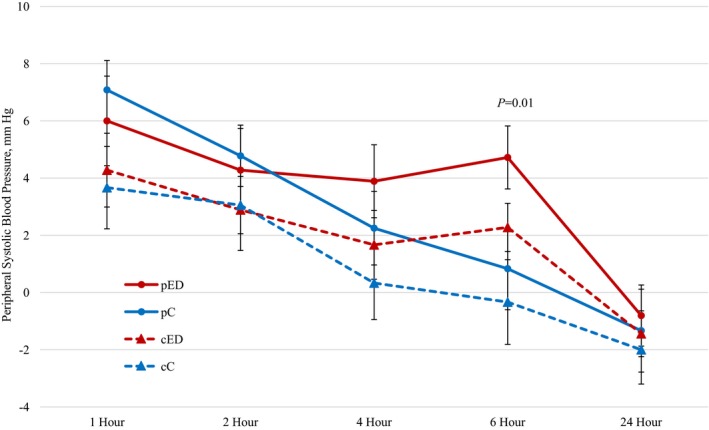
Baseline‐adjusted peripheral systolic blood pressures with energy drink (pED) and caffeine (pC) consumption (4.72±4.67 mm Hg vs 0.83±6.09 mm Hg at 6 hours, respectively; P=0.01). Baseline‐adjusted central systolic blood pressure measurements with energy drink (cED) vs caffeine (cC) consumption. Data are reported as mean±standard error bars.
Adverse Events
Adverse effects were experienced by 15 participants during the energy drink arm and by 13 participants during the caffeine control arm (Table 2). Adverse events included anxiety, difficulty in falling asleep, dizziness, dyspepsia/upset stomach, epistaxis, headache, jitteriness, nausea, palpitations, and shortness of breath. These effects were reported throughout the 24‐hour monitoring period without a discernable pattern. None of the adverse events caused a discontinuation in study participation.
Table 2.
Adverse Events Reported
| Adverse Event | Energy Drink (n=18), No. (%) | Caffeine (n=18), No. (%) |
|---|---|---|
| Any side effect | 15 (83) | 13 (72) |
| Anxiety | 3 (17) | 5 (28) |
| Difficulty in falling asleep | 4 (22) | 2 (11) |
| Dizziness | 3 (17) | 2 (11) |
| Dyspepsia/upset stomach | 4 (22) | 3 (17) |
| Epistaxis | 1 (6) | 1 (6) |
| Headache | 2 (11) | 3 (17) |
| Jitteriness | 8 (50) | 6 (33) |
| Nausea | 2 (11) | 0 (0) |
| Palpitations | 4 (22) | 0 (0) |
| Shortness of breath | 1 (6) | 1 (6) |
Discussion
QTc prolongation is a recognized marker of increased risk for fatal arrhythmias. Prolongation of the QT/QTc interval by more than 60 ms from baseline or a value >500 ms is a marker for life‐threatening arrhythmias. For this reason, several published studies have assessed QT/QTc effects post energy drink consumption. This is one of the first caffeine‐controlled studies that shows significant QTc prolongation of ≈10 ms 2 hours after high‐volume energy drink consumption in young healthy volunteers. It is possible that previous published studies showed a lack of effect due to studying a lower dose of energy drink (250–750 mL), monitoring for an insufficient amount of time (30–240 minutes), or not having a control arm.14, 15, 16, 17, 18, 19 It also appears that the product and dose used in our study was different from the other studies, which might explain our significant findings. Our findings are similar to another study (n=27), which used the same dose as ours and found a significant 6‐ms prolongation in QTc at 2 hours when compared with placebo.20 Further, in a noncontrolled study (n=14) using the same dose (2 cans) as in our study, 57% of participants had a QTc >500 ms post consumption.21 In contrast, a study by Brothers et al22 (n=15), comparing low‐ and moderate‐dose energy drinks, coffee, and water, found no changes in the QTc interval.
The Food and Drug Administration requires a thorough investigation of QT/QTc effects for all new drug entities, with a prolongation over 10 ms prompting regulatory concern.23 Cisapride and other noncardiovascular drugs have been withdrawn from the market because of a 5‐ to 10‐ms QT interval prolongation.24 Ephedra‐containing dietary supplements were also pulled from the market partly because of their association with significant QTc interval prolongation.25 Our findings are concerning since caffeine likely does not affect the QTc interval based on previous studies. Further investigation of other energy drink constituents is necessary.9
Taurine, l‐carnitine, and panax ginseng are some additives found in energy drinks and have conflicting physiologic effects. The preponderance of evidence suggests taurine is more likely an antiarrhythmic than a proarrhythmic.26 A correlation between l‐carnitine deficiency and short QT syndrome has been postulated.27 However, l‐carnitine supplementation is not suspected to result in an overcorrection or prolongation of the QT interval based on published literature. Interestingly, panax ginseng, at a dose of 200 mg in one study, showed a transient prolongation of the QTc interval 2 hours after ingestion, but this appears to be a chance finding and not evident in other studies.20, 28 Due to the fact that multiple ingredients in energy drinks have the ability to alter electrophysiological properties, their sole and concurrent use needs further scrutiny.
The long‐term use of energy drinks also needs particular attention. In one study, consuming a single energy shot (60 mL) twice daily for 7 days conferred no significant ECG changes when compared with placebo.29 Similarly, 500 mL of another energy drink consumed for 7 days showed no ECG‐related changes from baseline.15 However, these results cannot be generalized across all energy drink products. While the degree of BP change seen in our study is generally not concerning in an acute setting, even mild sustained elevations in systolic BP at the population level can increase the risk of adverse cardiovascular outcomes.30, 31
In this study, both the energy drink and caffeine arms exhibited a similar increase in pSBP from baseline for the initial 4 hours post study drink consumption. Caffeine is rapidly absorbed and typically peaks at about 1 hour, explaining the initial BP increase in both study arms.32 This is similar to results from another study by Svatikova et al33 that also suggested activation of the catecholamine pathway. However, the sustained pSBP elevation by ≈4 mm Hg at 6 hours post energy drink consumption suggests that other ingredients may be hemodynamically active. Guarana, another common ingredient in energy drinks, may contain 2% to 15% of its dry weight in caffeine.34 This may or may not explain a delayed BP response when compared with caffeine alone.35 Taurine, in contrast, is thought to have a BP‐lowering effect at higher doses, potentially altering the overall BP response.36 The current literature is lacking regarding the hemodynamic profile of the ingredients contained in energy drinks, making it difficult to ascertain whether it is caffeine interacting with the other ingredients or the other ingredients alone that are driving the BP effects.
Although not statistically significant, a trend in the elevation of cSBP at 6 hours post energy drink consumption was evident. Central BP indices are emerging as superior predictors of cardiovascular risk over peripheral BPs as they better reflect vascular compliance and should be further investigated in future studies.37 The trend towards a higher HR at 2 hours and the lower AI at 6 hours in the energy drink arm are clinically not critical in this acute setting.
Certain populations may consider exercising caution when consuming energy drinks. Those with congenital long QT syndrome are at a predisposed risk for arrhythmias, particularly torsades de pointes.38 More commonly, those with acquired long QT syndrome induced by concomitant use of QT/QTc‐prolonging drugs, hypokalemia, or hypomagnesaemia are at a similar risk.39 Obesity has also been associated with prolongation of the QT interval.40, 41 Interestingly, in one noncontrolled study, the cohort with overweight/obese participants (n=18) showed significant QT prolongation from baseline after consuming 5 mL/kg of an energy drink (340±57 ms versus 357±54 ms, P=0.006).24 The consumption of energy drinks with alcohol is another practice common in some social settings.42 Caution should be exercised as combining alcohol or illicit substances with energy drinks may trigger or exacerbate untoward cardiovascular events.43, 44
Study Limitations
Our results should be interpreted with caution due to several limitations. Importantly, our results only appear to be significant relative to the caffeine group, and the change from baseline post energy drinks is not alarming. Of note, the risk of arrhythmia may be negligible because the QTc difference is transient. Currently, we cannot explain the QTc reduction in the caffeine arm. We did not utilize a true placebo arm in this study, which may be critical for future studies. As such, in another study using a noncaffeinated placebo, a mild reduction in QTc was also evident.20 The hemodynamic changes may be benign if not sustained with chronic use. While caffeine alone is not expected to shorten the QTc interval, this possibility cannot be ruled out. We did not control for food intake post baseline measurement on study days. Food intake has been linked to shortening of the QTc interval, which could possibly explain our discordant findings.45 We also used the machine‐calculated values and did not hand measure the ECG parameters. The latter is considered as the gold standard by some experts. Our results can only be generalized to the time points specified in the study. Consumption of 946 mL (32 ounces) of an energy drink over 45 minutes may not be representative of normal consumption patterns. However, it is important to note that 710‐mL (24 ounces) energy drink cans are readily available in the market and it appears that serious adverse events may be associated with a higher volume of consumption.46 T‐wave peak to T‐wave end interval analysis is emerging as a superior marker of arrhythmic risk and future studies should evaluate not only the QT/QTc interval, but also the transmural dispersion of ventricular repolarization to accurately depict energy drink–related arrhythmic risk.47 Although our sample size mimics some previous published studies, future studies may need to employ a larger sample size to capture differences in sex or dosing.25 Since this was a proof‐of‐concept study, we were not powered for multiple comparisons and, as a result, the reported P values are not adjusted for multiple comparisons.
Conclusions
Our study findings suggest significant prolongation of the QTc interval 2 hours after energy drink consumption when compared with caffeine. Systolic BP remained significantly elevated over the caffeine control at 6 hours post energy drink consumption. Ingredients contained in energy drinks other than caffeine warrant further investigation. Larger clinical trials controlling for the limitations of this study are warranted.
Sources of Funding
Funding was provided by the Clinical Investigations Facility at Travis Air Force Base, CA.
Disclosures
None.
Acknowledgments
The authors acknowledge the support of Ian Riddock, MD, and Bradley Williams, MD. We appreciate the secretarial support of Lori Diaz. The views expressed in this material are those of the authors and do not reflect the official policy or position of the US government, the Department of Defense, the Department of the Air Force, or the University of the Pacific.
(J Am Heart Assoc. 2017;6:e004448 DOI: 10.1161/JAHA.116.004448.)28446495
References
- 1. Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks—a growing problem. Drug Alcohol Depend. 2009;99:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arria AM, O'Brien MC. The “high” risk of energy drinks. JAMA. 2011;305:600–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The DAWN report. Substance Abuse and Mental Services Administration. January 10, 2013. Available at: http://archive.samhsa.gov/data/2k13/DAWN126/sr126-energy-drinks-use.pdf. Accessed November 27, 2015.
- 4. Documents link more deaths to energy drinks. Center for Science in the Public Interest. June 25, 2014. Available at: http://www.cspinet.org/new/201406251.html. Accessed November 28, 2015.
- 5. Goldfarb M, Tellier C, Thanassoulis G. Review of published cases of adverse cardiovascular events after ingestion of energy drinks. Am J Cardiol. 2014;113:168–172. [DOI] [PubMed] [Google Scholar]
- 6. FDA to investigate added caffeine. US Food and Drug Administration Consumer Health Information; 2013. Available at: http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM350740.pdf. Accessed November 27, 2015. [Google Scholar]
- 7. Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–170. [DOI] [PubMed] [Google Scholar]
- 8. Hartley TR, Lovallo WR, Whitsett TL. Cardiovascular effects of caffeine in men and women. Am J Cardiol. 2004;93:1022–1026. [DOI] [PubMed] [Google Scholar]
- 9. Ammar R, Song JC, Kluger J, White CM. Evaluation of electrocardiographic and hemodynamic effects of caffeine with acute dosing in healthy volunteers. Pharmacotherapy. 2001;21:437–442. [DOI] [PubMed] [Google Scholar]
- 10. Poussel M, Kimmoun A, Levy B, Gambier N, Dudek F, Puskarczyk E, Poussel JF, Chenuel B. Fatal cardiac arrhythmia following voluntary caffeine overdose in an amateur body‐builder athlete. Int J Cardiol. 2013;166:e41–e42. [DOI] [PubMed] [Google Scholar]
- 11. Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic Sci Int. 2005;153:67–69. [DOI] [PubMed] [Google Scholar]
- 12. Somogyi LP. Caffeine intake by the US population. August 2010. Available at: http://www.fda.gov/downloads/aboutfda/centersoffices/officeoffoods/cfsan/cfsanfoiaelectronicreadingroom/ucm333191.pdf. Accessed October 26, 2016.
- 13. Toblin RL, Clarke‐Walper K, Kok BC, Sipos ML, Thomas JL. Energy drink consumption and its association with sleep problems among U.S. service members on a combat deployment: Afghanistan, 2010. MMWR. 2012;61:895–898. [PubMed] [Google Scholar]
- 14. Ragsdale FR, Gronli TD, Batool N, Haight N, Mehaffey A, McMahon EC, Nalli TW, Mannello CM, Sell CJ, McCann PJ, Kastello GM, Hooks T, Wilson T. Effect of Red Bull energy drink on cardiovascular and renal function. Amino Acids. 2010;38:1193–1200. [DOI] [PubMed] [Google Scholar]
- 15. Steinke L, Lanfear DE, Dhanapal V, Kalus JS. Effect of “energy drink” consumption on hemodynamic and electrocardiographic parameters in healthy young adults. Ann Pharmacother. 2009;43:596–602. [DOI] [PubMed] [Google Scholar]
- 16. Wiklund U, Karlsson M, Oström M, Messner T. Influence of energy drinks and alcohol on post‐exercise heart rate recovery and heart rate variability. Clin Physiol Funct Imaging. 2009;29:74–80. [DOI] [PubMed] [Google Scholar]
- 17. Elitok A, Öz F, Panc C, Sarıkaya R, Sezikli S, Pala Y, Bugan ÖS, Ateş M, Parıldar H, Ayaz MB, Atıcı A, Oflaz H. Acute effects of Red Bull energy drink on ventricular repolarization in healthy young volunteers: a prospective study. Anatol J Cardiol. 2015;15:919–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alsunni A, Majeed F, Yar T, Alrahim A, Alhawaj AF, Alzaki M. Effects of energy drink consumption on corrected QT interval and heart rate variability in young obese Saudi male university students. Ann Saudi Med. 2015;35:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hajsadeghi S, Mohammadpour F, Manteghi MJ, Kordshakeri K, Tokazebani M, Rahmani E, Hassanzadeh M. Effects of energy drinks on blood pressure, heart rate, and electrocardiographic parameters: an experimental study on healthy young adults. Anatol J Cardiol. 2016;16:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah SA, Occiano A, Nguyen TA, Chan A, Sky JC, Bhattacharyya M, O'Dell KM, Shek A, Nguyen NN. Electrocardiographic and blood pressure effects of energy drinks and Panax ginseng in healthy volunteers: a randomized clinical trial. Int J Cardiol. 2016;218:318–323. [DOI] [PubMed] [Google Scholar]
- 21. Kozik TM, Shah S, Bhattacharyya M, Franklin TT, Connolly TF, Chien W, Charos GS, Pelter MM. Cardiovascular responses to energy drinks in a healthy population: the C‐energy study. Am J Emerg Med. 2016;34:1205–1209. [DOI] [PubMed] [Google Scholar]
- 22. Brothers RM, Christmas KM, Patik JC, Bhella PS. Heart rate, blood pressure and repolarization effects of an energy drink as compared to coffee. Clin Physiol Funct Imaging. 2016. Available at: http://onlinelibrary.wiley.com/doi/10.1111/cpf.12357/full. Accessed March 28, 2017. [DOI] [PubMed] [Google Scholar]
- 23. Guidance for industry E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs. U.S. Department of Health and Human Services; October 2012. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm323656.htm. Accessed November 27, 2015. [Google Scholar]
- 24. Roden DM. Drug‐induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. [DOI] [PubMed] [Google Scholar]
- 25. McBride BF, Karapanos AK, Krudysz A, Kluger J, Coleman CI, White CM. Electrocardiographic and hemodynamic effects of a multicomponent dietary supplement containing ephedra and caffeine: a randomized controlled trial. JAMA. 2004;291:216–221. [DOI] [PubMed] [Google Scholar]
- 26. Schaffer SW, Shimada K, Jong CJ, Ito T, Azuma J, Takahashi K. Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids. 2014;46:1147–1157. [DOI] [PubMed] [Google Scholar]
- 27. Fu L, Huang M, Chen S. Primary carnitine deficiency and cardiomyopathy. Korean Circ J. 2013;43:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caron MF, Hotsko AL, Robertson S, Mandybur L, Kluger J, White CM. Electrocardiographic and hemodynamic effects of Panax ginseng. Ann Pharmacother. 2002;36:758–763. [DOI] [PubMed] [Google Scholar]
- 29. Shah SA, Dargush AE, Potts V, Lee M, Millard‐Hasting BM, Williams B, Lacey CS. Effects of single and multiple energy shots on blood pressure and electrocardiographic parameters. Am J Cardiol. 2016;117:465–468. [DOI] [PubMed] [Google Scholar]
- 30. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 31. Asplund K, Karvanen J, Giampaoli S, Jousilahti P, Niemelä M, Broda G, Cesana G, Dallongeville J, Ducimetriere P, Evans A, Ferrières J, Haas B, Jorgensen T, Tamosiunas A, Vanuzzo D, Wiklund PG, Yarnell J, Kuulasmaa K, Kulathinal S; MORGAM Project . Relative risks for stroke by age, sex, and population based on follow‐up of 18 European populations in the MORGAM Project. Stroke. 2009;40:2319–2326. [DOI] [PubMed] [Google Scholar]
- 32. Liguori A, Hughes JR, Grass JA. Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol Biochem Behav. 1997;58:721–726. [DOI] [PubMed] [Google Scholar]
- 33. Svatikova A, Covassin N, Somers KR, Somers KV, Soucek F, Kara T, Bukartyk J. A randomized trial of cardiovascular responses to energy drink consumption in healthy adults. JAMA. 2015;314:2079–2082. [DOI] [PubMed] [Google Scholar]
- 34. Mclellan TM, Lieberman HR. Do energy drinks contain active components other than caffeine? Nutr Rev. 2012;70:730–744. [DOI] [PubMed] [Google Scholar]
- 35. Meyer K, Ball P. Psychological and cardiovascular effects of guarana and yerbamate: a comparison with coffee. Interam J Psychol. 2004;38:87–94. [Google Scholar]
- 36. Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525–532. [DOI] [PubMed] [Google Scholar]
- 37. Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome: the Strong Heart Study. J Am Coll Cardiol. 2009;54:1730–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. [DOI] [PubMed] [Google Scholar]
- 39. Camm AJ, Janse MJ, Roden DM, Rosen MR, Cinca J, Cobbe SM. Congenital and acquired long QT syndrome. Eur Heart J. 2000;21:1232–1237. [DOI] [PubMed] [Google Scholar]
- 40. Li W, Bai Y, Sun K, Xue H, Wang Y, Song X, Fan X, Song H, Han Y, Hui R. Patients with metabolic syndrome have prolonged corrected QT interval (QTc). Clin Cardiol. 2009;32:E93–E99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramirez AH, Schildcrout JS, Blakemore DL, Masys DR, Pulley JM, Basford MA, Roden DM, Denny JC. Modulators of normal electrocardiographic intervals identified in a large electronic medical record. Heart Rhythm. 2011;8:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malinauskas BM, Aeby VG, Overton RF, Carpenter‐aeby T, Barber‐heidal K. A survey of energy drink consumption patterns among college students. Nutr J. 2007;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Howland J, Rohsenow DJ. Risks of energy drinks mixed with alcohol. JAMA. 2013;309:245–246. [DOI] [PubMed] [Google Scholar]
- 44. Haigney MC, Alam S, Tebo S, Marhefka G, Elkashef A, Kahn R, Chiang CN, Vocci F, Cantilena L. Intravenous cocaine and QT variability. J Cardiovasc Electrophysiol. 2006;17:610–616. [DOI] [PubMed] [Google Scholar]
- 45. Taubel J, Ferber G, Lorch U, Batchvarov V, Savelieva I, Camm AJ. Thorough QT study of the effect of oral moxifloxacin on QTc interval in the fed and fasted state in healthy Japanese and Caucasian subjects. Br J Clin Pharmacol. 2014;77:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marczinski CA. Alcohol mixed with energy drinks: consumption patterns and motivations for use in U.S. college students. Int J Environ Res Public Health. 2011;8:3232–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenthal TM, Stahls PF, Abi Samra FM, Bernard ML, Khatib S1, Polin GM, Xue JQ, Morin DP. T‐peak to T‐end interval for prediction of ventricular tachyarrhythmia and mortality in a primary prevention population with systolic cardiomyopathy. Heart Rhythm. 2015;12:1789–1797. [DOI] [PubMed] [Google Scholar]


