Abstract
Background
Urinary neutrophil gelatinase‐associated lipocalin (U‐NGAL) is an early predictor of acute kidney injury and adverse events in various diseases; however, in acute decompensated heart failure patients, its significance remains poorly understood. This study aimed to investigate the prognostic value of U‐NGAL on the first day of admission for the occurrence of acute kidney injury and long‐term outcomes in acute decompensated heart failure patients.
Methods and Results
We studied 260 acute decompensated heart failure patients admitted to our department between 2011 and 2014 by measuring U‐NGAL in 24‐hour urine samples collected on the first day of admission. Primary end points were all‐cause death, cardiovascular death, and heart failure admission. Patients were divided into 2 groups according to their median U‐NGAL levels (32.5 μg/gCr). The high‐U‐NGAL group had a significantly higher occurrence of acute kidney injury during hospitalization than the low‐U‐NGAL group (P=0.0012). Kaplan‐Meier analysis revealed that the high‐U‐NGAL group exhibited a worse prognosis than the low‐U‐NGAL group in all‐cause death (hazard ratio 2.07; 95%CI 1.38‐3.12, P=0.0004), cardiovascular death (hazard ratio 2.29; 95%CI 1.28‐4.24, P=0.0052), and heart failure admission (hazard ratio 1.77; 95%CI 1.13‐2.77, P=0.0119). The addition of U‐NGAL to the estimated glomerular filtration rate significantly improved the predictive accuracy of all‐cause mortality (P=0.0083).
Conclusions
In acute decompensated heart failure patients, an elevated U‐NGAL level on the first day of admission was related to the development of clinical acute kidney injury and independently associated with poor prognosis.
Keywords: acute heart failure, acute kidney injury, neutrophil gelatinase‐associated lipocalin, outcomes
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
Elevated U‐NGAL on the first day of admission is an early predictor of acute kidney injury and a strong prognostic factor for long‐term outcomes in acute decompensated heart failure patients.
What Are the Clinical Implications?
Heart failure patients with an elevated U‐NGAL on admission have an elevated risk of acute kidney injury and may warrant closer monitoring of blood pressure, urine output, and renal function.
Introduction
Despite marked progress in the long‐term prognosis of patients with chronic heart failure (HF), various problems with HF treatments remain,1, 2 one of which is the treatment strategy for acute heart failure or acutely decompensated heart failure (ADHF). Recent randomized clinical trials that investigated the effect of acute intervention with novel classes of drugs, such as nesiritide, tolvaptan, or a phosphodiesterase III inhibitor, failed to exhibit an improvement in the long‐term prognosis of patients with ADHF.3, 4, 5, 6 To improve outcomes of ADHF, it is essential to elucidate appropriate issues in which cardiologists should intervene in the acute phase, as these affect the long‐term outcomes of ADHF.
Previous reports have demonstrated that acute kidney injury (AKI) during hospitalization is a strong risk factor for long‐term outcomes in ADHF patients.7, 8, 9, 10 To prevent or treat AKI appropriately, prompt diagnosis of AKI itself or stratification of high‐risk patients for AKI is required. However, several days are required to confirm a diagnosis of AKI using serum creatinine and urine volume according to the criteria of the Kidney Disease Improving Global Outcomes Clinical Practice Guidelines for AKI.11
Neutrophil gelatinase‐associated lipocalin (NGAL), a protein of the lipocalin superfamily, is synthesized abundantly in kidney tubules. Its expression is rapidly upregulated by ischemia‐reperfusion injury in renal tubular epithelial cells, and NGAL is released into urine in an experimental model.12 In humans, urinary NGAL (U‐NGAL) has been recognized as a surrogate marker of AKI complicated with various diseases, including sepsis, post–cardiac surgery, and admission to the intensive care unit.13, 14, 15 In particular, a few studies reported an association between the elevation of serum NGAL levels on admission and consequent AKI in patients with chronic heart failure.16, 17, 18 However, in ADHF patients, the predictive value of U‐NGAL for AKI and long‐term outcomes remains poorly understood.
In this context, the present study aimed to investigate whether the U‐NGAL level on the first day of admission predicts AKI during hospitalization and long‐term mortality in patients with ADHF as well as to analyze whether the prognostic value of U‐NGAL is independent of the development of AKI.
Methods
Patient Population
The present study investigated ADHF patients from the NARA‐HF 3 study, which has been described previously.19, 20, 21 The NARA‐HF 3 study recruited 436 consecutive patients following emergency admission to our department for ADHF between April 2011 and December 2014. Diagnosis of HF was based on the criteria of the Framingham study.22 Patients with acute myocardial infarction, acute myocarditis, and acute HF with acute pulmonary embolism were excluded.
Among the patients included, U‐NGAL levels were measured in 260 ADHF patients. Patients were divided into high– and low–U‐NGAL groups according to their median U‐NGAL levels. The present study was approved by the Nara Medical University Institutional Ethics Committee and was performed in accordance with the 1975 Declaration of Helsinki guidelines for clinical research protocols. Informed consent was obtained from all patients.
Outcomes
Primary end points were all‐cause death, cardiovascular death, and HF admission. Cardiovascular death was defined as death due to HF, myocardial infarction, sudden death, stroke, or vascular disease. Vital status and the cause of death were confirmed through patient medical records by clinicians blinded to their U‐NGAL levels. When this information was unavailable in the medical records, blinded clinicians telephoned the patients or their families to collect these data. We also investigated the association between another renal biomarker, cystatin C, and the study outcomes to compare the prognostic power of U‐NGAL with that of cystatin C.
Measurement of U‐NGAL and Cystatin C
For NGAL measurement, 24‐hour urine samples were collected on the first day of admission. U‐NGAL levels were determined with a chemiluminescent immunoassay on the ARCHITECT® platform (Abbott Japan, Tokyo, Japan). The total coefficient of variation for the measurements was <5%.14
Cystatin C was measured with colloidal gold particle‐enhanced colorimetric immunoassay (Nescauto GC Cystatin C, Alfresa Pharma, Osaka, Japan). The total coefficient of variation for the measurements was <10%.
Acute Kidney Injury
The development of AKI was defined as an increase in serum creatinine by 0.3 mg/dL within 48 hours or a 50% increase in serum creatinine from the level on admission during hospitalization, according to the Kidney Disease Improving Global Outcomes Clinical Practice Guidelines for Acute Kidney Injury.11 Urine criteria (0.5 mL/kg per hour for 6 hours) were not utilized for AKI diagnosis because of the potential alterations in urine volume induced by therapeutic medication.
Echocardiography
Ultrasound examinations were performed using the Sonos 7500 system (Philips, Best, The Netherlands) and Acuson Sequoia system (Siemens, Erlangen, Germany). Left ventricular ejection fraction (LVEF) was calculated via the modified Simpson method. LV end‐diastolic diameter, LV end‐systolic diameter, and left atrial diameter were measured via M‐mode echocardiography.
Statistical Analysis
Normally distributed data are presented as means±SD, and nonnormally distributed data as medians and interquartile ranges. Differences between the groups were compared using the chi‐squared test for categorical variables. The Student t‐test (normally distributed data) or Wilcoxon rank‐sum test (nonnormally distributed data) was used for the comparison of continuous variables between the 2 groups. Estimated glomerular filtration rate (eGFR) was calculated according to the published equation for Japanese subjects: 194×serum creatinine−1.094×age−0.287×(0.739 for women).23 Cumulative event‐free rates during follow‐up were assessed using the Kaplan‐Meier method. Univariate and multivariate analyses of event‐free survival were examined using the Cox proportional hazard models. An unadjusted model and 4 models for the adjustment of covariates were utilized: model 1, adjusted for age and sex; model 2, adjusted for all factors in model 1 plus hemoglobin concentration, eGFR, and brain natriuretic peptide (BNP); model 3, adjusted for all factors in model 2 plus LVEF and systolic blood pressure; and model 4, adjusted for all factors in model 3 plus AKI. In the analysis of cystatin C, the eGFR was excluded from the adjusted model. To evaluate the discriminatory ability of eGFR alone (group 1), the combination of eGFR and urine albumin‐to‐creatinine ratio (UACR) (group 2), the combination of eGFR and U‐NGAL (group 3), and the combination of eGFR, UACR, and U‐NGAL (group 4), the c statistic, integrated discrimination improvement, and category‐free net reclassification improvement were calculated at the 2‐year follow‐up in these patients. A P value <0.05 was considered statistically significant. JMP software for Windows version 11 (SAS Institute, Cary, NC) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) were used for all statistical analyses.24
Results
Baseline Characteristics
The mean age of the 260 patients was 74.7 years, and the proportion of men was 59.6% (Table 1). To investigate the impact of U‐NGAL on the prognosis of ADHF, patients were divided into 2 groups according to the median U‐NGAL levels on admission.
Table 1.
Baseline HF Patient Characteristics
| Total | Low U‐NGAL (<32.5 μg/gCr) | High U‐NGAL (≥32.5 μg/gCr) | ||
|---|---|---|---|---|
| N | 260 | 130 | 130 | |
| Demographic | ||||
| Age, y | 74.7±11.3 | 72.8±12.5 | 76.6±9.5 | 0.0182 |
| Sex, male, % | 59.6 | 65.4 | 53.9 | 0.0576 |
| BMI, kg/m² | 24.0±4.4 | 23.8±3.9 | 24.1±4.9 | 0.7471 |
| Causes of HF, % | 0.6712 | |||
| Ischemic heart disease | 35.5 | 37.2 | 33.9 | |
| Dilated cardiomyopathy | 19.7 | 21.7 | 17.7 | |
| Hypertensive heart disease | 3.1 | 1.6 | 4.6 | |
| Valvular heart disease | 18.1 | 17.8 | 18.5 | |
| Medical history, % | ||||
| Previous HF hospitalization | 39.2 | 39.2 | 39.2 | 1.0000 |
| Hypertension | 73.0 | 68.5 | 77.5 | 0.1050 |
| Diabetes mellitus | 41.7 | 34.6 | 48.8 | 0.0206 |
| Atrial fibrillation | 50.4 | 49.6 | 51.2 | 0.8045 |
| Vital sign on admission | ||||
| Heart rate, beats/min | 95.3±25.8 | 96.2±26.5 | 94.3±25.2 | 0.5378 |
| SBP, mm Hg | 144.1±34.5 | 145.1±34.9 | 143.2±34.1 | 0.6024 |
| DBP, mm Hg | 81.8±19.4 | 83.8±19.8 | 79.9±18.8 | 0.1075 |
| Echocardiographic parameters | ||||
| LAD, mm | 47.1±9.8 | 47.9±9.9 | 46.5±9.7 | 0.2075 |
| LVDd, mm | 55.2±10.3 | 57.2±10.7 | 53.3±9.5 | 0.0092 |
| LVDs, mm | 43.4±12.6 | 45.5±13.9 | 41.3±10.8 | 0.0170 |
| LVEF, % | 43.0±17.1 | 42.1±17.8 | 43.9±16.4 | 0.2839 |
| Laboratory data on admission | ||||
| Hemoglobin, g/dL | 11.6±2.4 | 12.2±2.4 | 11.0±2.3 | <0.0001 |
| CRP, mg/dL | 0.5 (0.2‐1.8) | 0.5 (0.2‐1.5) | 0.6 (0.2‐2.0) | 0.5688 |
| BUN, mg/dL | 31.0±19.7 | 27.8±18.1 | 34.2±20.8 | 0.0042 |
| eGFR, mL/min per 1.73 m² | 45.9±24.3 | 52.1±23.7 | 39.7±23.4 | <0.0001 |
| Sodium, mEq/L | 38.6±4.3 | 138.8±3.7 | 138.3±4.9 | 0.6518 |
| BNP, pg/mL | 865 (454‐1614) | 772 (429‐1415) | 997 (509‐1792) | 0.0941 |
| Cystatin C, mg/L | 1.7 (1.2‐2.2) | 1.4 (1.1‐2.0) | 1.8 (1.4‐2.6) | <0.0001 |
| UACR, mg/gCrea | 80.7 (27.1‐218.9) | 54.5 (22.9‐147.4) | 142.5 (41.3‐443.0) | <0.0001 |
| U‐NAG, U/gCre | 11.6 (8.3‐16.9) | 10.5 (6.9‐14.7) | 13.2 (10.2‐18.9) | <0.0001 |
| Medication on admission, % | ||||
| β‐Blockers | 40.8 | 43.8 | 37.7 | 0.3141 |
| ACE‐I/ARBs | 60.6 | 60.8 | 60.5 | 0.9609 |
| Diuretics | 60.8 | 56.9 | 64.6 | 0.2052 |
| Medication at discharge, % | ||||
| β‐Blockers | 72.3 | 72.7 | 71.9 | 0.8952 |
| ACE‐I/ARBs | 88.4 | 92.2 | 84.3 | 0.0531 |
| Diuretics | 85.9 | 86.7 | 85.1 | 0.7191 |
| AKI during hospitalization, % | 35.8 | 26.2 | 45.4 | 0.0012 |
The data are presented as the mean±SD for continuous normally distributed variables, as the median (25th to 75th interquartile range [IQR]) for continuous nonnormally distributed variables, or n (%). ACE‐I indicates angiotensin‐converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; LAD, left atrial diameter; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; UACR, urine albumin‐to‐creatinine ratio; U‐NAG, urine N‐acetyl‐β‐D‐glucosaminidase; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin.
Data on UACR were available for 244 patients.
Table 1 summarizes the characteristics of the ADHF patients according to the median level of U‐NGAL. Compared with patients in the low–U‐NGAL group (<32.5 μg/gCr), patients in the high–U‐NGAL group (≥32.5 μg/gCr) were significantly older; however, the proportion of male/female patients and body mass index were similar in both groups. There were no significant differences in the cause of HF, systolic blood pressure, diastolic blood pressure, LVEF, or plasma BNP levels between the 2 groups. The high–U‐NGAL group exhibited a significantly higher proportion of patients with diabetes mellitus, cystatin C, UACR, urine N‐acetyl‐β‐D‐glucosaminidase, and reduced levels of eGFR compared with the low–U‐NGAL group. There was no significant difference in the proportion of patients treated with β‐blockers, angiotensin‐converting enzyme inhibitors and/or angiotensin II receptor blockers, and diuretics on admission and at discharge between the 2 groups. The high–U‐NGAL group exhibited a significantly increased occurrence of AKI during hospitalization compared with the low–U‐NGAL group (Table 1).
Prognosis and Outcome
During the mean follow‐up period of 18.6 (8.0‐31.1) months, 99 instances of all‐cause death, 47 instances of cardiovascular death, and 80 admissions due to HF occurred. Kaplan‐Meier curves were significantly distinct in the high–U‐NGAL group compared with those in the low–U‐NGAL group for all‐cause death (log rank P=0.0005), cardiovascular death (log rank P=0.0073), and HF admission (log rank P=0.0112) (Figure 1).
Figure 1.
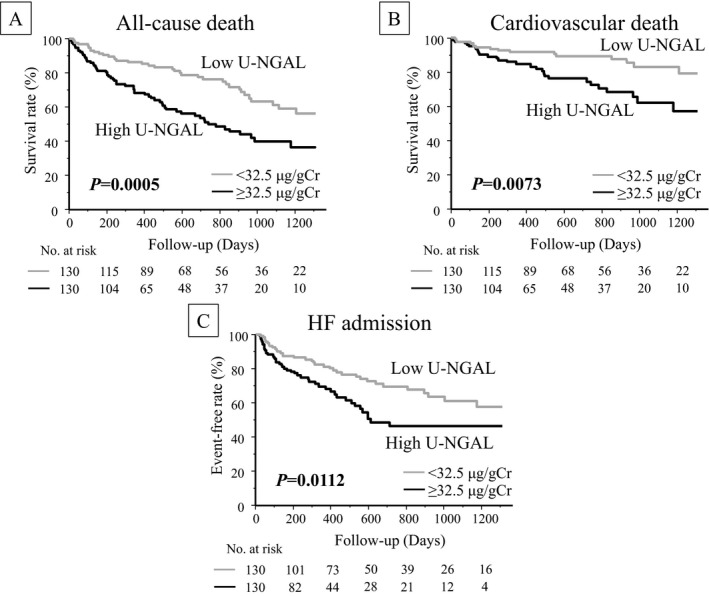
All‐cause death, cardiovascular death, and HF admission. Kaplan‐Meier event‐free survival curves for (A) all‐cause death, (B) cardiovascular death, and (C) HF admission in patients with U‐NGAL levels ≥32.5 μg/gCr (black line, high–U‐NGAL group; n=130) compared with patients with U‐NGAL levels <32.5 μg/gCr (gray line, low–U‐NGAL group; n=130). HF indicates heart failure; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin.
Table 2 shows unadjusted and adjusted hazard ratio (HR) of U‐NGAL for outcomes in the 2 groups. Elevated levels of U‐NGAL were significantly associated with a higher rate of all‐cause death (Cox regression analysis HR 2.07; 95%CI 1.38‐3.12, P=0.0004), cardiovascular death (Cox regression analysis HR 2.29; 95%CI 1.28‐4.24, P=0.0052), and HF admission (Cox regression analysis HR 1.77; 95%CI 1.13‐2.77, P=0.0119). Following adjustment for covariates including age, sex, hemoglobin concentration, eGFR, BNP, systolic blood pressure, and LVEF (model 3), a high U‐NGAL level remained as an independent predictor of all‐cause mortality and HF admission (Cox regression analysis HR 1.77; 95%CI 1.17‐2.72, P=0.0073; and Cox regression analysis HR 1.69; 95%CI 1.05‐2.72, P=0.0293, respectively). Following adjustment for AKI (model 4), these findings attenuated but remained significant (Table 2).
Table 2.
Cox Regression Analysis of U‐NGAL for Adverse Outcomes
| All‐Cause Death | Cardiovascular Death | HF Admission | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <32.5 μg/gCr | ≥32.5 μg/gCr | P‐Value | <32.5 μg/gCr | ≥32.5 μg/gCr | P‐Value | <32.5 μg/gCr | ≥32.5 μg/gCr | P‐Value | |
| Unadjusted HR | 1 | 2.07 (1.38‐3.12) | 0.0004 | 1 | 2.29 (1.28‐4.24) | 0.0052 | 1 | 1.77 (1.13‐2.77) | 0.0119 |
| Adjusted HR (model 1) | 1 | 1.90 (1.27‐2.88) | 0.0018 | 1 | 2.02 (1.12‐3.75) | 0.0188 | 1 | 1.77 (1.13‐2.80) | 0.0124 |
| Adjusted HR (model 2) | 1 | 1.71 (1.13‐2.62) | 0.0115 | 1 | 1.75 (0.95‐3.31) | 0.0711 | 1 | 1.66 (1.04‐2.67) | 0.0333 |
| Adjusted HR (model 3) | 1 | 1.77 (1.17‐2.72) | 0.0073 | 1 | 1.86 (1.01‐3.52) | 0.0466 | 1 | 1.69 (1.05‐2.72) | 0.0293 |
| Adjusted HR (model 4) | 1 | 1.60 (1.05‐2.46) | 0.0303 | 1 | 1.66 (0.89‐3.16) | 0.1109 | 1 | 1.62 (1.07‐2.62) | 0.0471 |
Model 1, adjusted for age, sex; model 2, adjusted for age, sex, hemoglobin, eGFR, BNP; model 3, adjusted for age, sex, hemoglobin, eGFR, BNP, SBP, LVEF; model 4, adjusted for age, sex, hemoglobin, eGFR, BNP, SBP, LVEF, AKI. AKI indicates acute kidney injury; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; LVEF, left ventricle ejection fraction; SBP, systolic blood pressure; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin.
We also investigated the relationship between cystatin C and the study outcomes to compare the predictive value of U‐NGAL with that of cystatin C. As shown in Table 3, above‐ versus below‐median levels of cystatin C were significantly associated with all‐cause death (Cox regression analysis HR 2.27; 95%CI 1.48‐3.56, P=0.0001), cardiovascular death (Cox regression analysis HR 2.76; 95%CI 1.49‐5.44, P=0.0010), and HF admission (Cox regression analysis HR 2.63; 95%CI 1.65‐4.26, P<0.0001) in the unadjusted model. However, a significant association of cystatin C with all‐cause death (Cox regression analysis HR 1.51; 95%CI 0.94‐2.46, P=0.0876) and cardiovascular death (Cox regression analysis HR 1.69; 95%CI 0.85‐3.53, P=0.1357) was not observed in the fully adjusted model.
Table 3.
Cox Regression Analysis of Cystatin C for Adverse Outcomes
| All‐Cause Death | Cardiovascular Death | HF Admission | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <1.66 mg/L | ≥1.66 mg/L | P‐Value | <1.66 mg/L | ≥1.66 mg/L | P‐Value | <1.66 mg/L | ≥1.66 mg/L | P‐Value | |
| Unadjusted HR | 1 | 2.27 (1.48‐3.56) | 0.0001 | 1 | 2.76 (1.49‐5.44) | 0.0010 | 1 | 2.63 (1.65‐4.26) | <0.0001 |
| Adjusted HR (model 1) | 1 | 1.89 (1.22‐2.99) | 0.0039 | 1 | 2.31 (1.23‐4.60) | 0.0084 | 1 | 2.42 (1.51‐3.95) | 0.0002 |
| Adjusted HR (model 2) | 1 | 1.70 (1.07‐2.74) | 0.0233 | 1 | 1.97 (1.01‐4.05) | 0.0466 | 1 | 2.40 (1.46‐4.02) | 0.0005 |
| Adjusted HR (model 3) | 1 | 1.51 (0.94‐2.46) | 0.0876 | 1 | 1.69 (0.85‐3.53) | 0.1357 | 1 | 2.37 (1.44‐3.99) | 0.0006 |
| Adjusted HR (model 4) | 1 | 1.26 (0.76‐2.09) | 0.3706 | 1 | 1.40 (0.68‐2.99) | 0.3708 | 1 | 2.17 (1.29‐3.71) | 0.0036 |
Model 1, adjusted for age, sex; model 2, adjusted for age, sex, hemoglobin, BNP; model 3, adjusted for age, sex, hemoglobin, BNP, SBP, LVEF; model 4, adjusted for age, sex, hemoglobin, BNP, SBP, LVEF, AKI. AKI indicates acute kidney injury; BNP, brain natriuretic peptide; HF, heart failure; HR, hazard ratio; LVEF, left ventricle ejection fraction; SBP, systolic blood pressure.
Combination Use of Renal Biomarkers
As shown in Table 4, the predictive values of all‐cause mortality were compared among group 1 (eGFR alone), group 2 (combination of eGFR and UACR), group 3 (combination of eGFR and U‐NGAL), and group 4 (combination of eGFR, UACR, and U‐NGAL). C statistics analysis demonstrated that U‐NGAL significantly improved the discriminatory ability of all‐cause mortality compared with group 1 and group 2 (group 3 versus group 1, from 0.620 to 0.678, P=0.0083; and group 4 versus group 2, from 0.624 to 0.706, P=0.0059). U‐NGAL also improved the integrated discrimination improvement (group 3 versus group 1, 0.045, 95%CI 0.012‐0.078, P=0.0080; and group 4 versus group 2, 0.079, 95%CI 0.036‐0.121, P=0.0003) and category‐free net reclassification improvement (group 3 versus group 1, 0.295, 95%CI 0.018‐0.572, P=0.0372; and group 4 versus group 2, 0.390, 95%CI 0.113‐0.666, P=0.0057).
Table 4.
Improvement in Discriminatory Ability for All‐Cause Mortality
| Biomarker | c Statistics | P‐Value | IDI | P‐Value | NRI | P‐Value |
|---|---|---|---|---|---|---|
| All‐cause mortality | ||||||
| Group 1 (eGFR) | 0.620 (0.535‐0.704) | |||||
| Group 2 (eGFR+UACRa) | 0.624 (0.540‐0.709) | |||||
| Group 3 (eGFR+U‐NGAL) | 0.678 (0.596‐0.760) | |||||
| Group 4 (eGFR+UACRa+U‐NGAL) | 0.706 (0.628‐0.784) | |||||
| Group 3 vs Group 1 | 0.0083 | 0.045 (0.012‐0.078) | 0.0080 | 0.295 (0.018‐0.572) | 0.0372 | |
| Group 4 vs Group 2 | 0.0059 | 0.079 (0.036‐0.121) | 0.0003 | 0.390 (0.113‐0.666) | 0.0057 | |
Group 1, eGFR alone; group 2, combination of eGFR and UACR; Group 3, combination of eGFR and U‐NGAL; Group 4, combination of eGFR and UACR and U‐NGAL. eGFR indicates estimated glomerular filtration rate; IDI, integrated discrimination improvement; NRI, category‐free net reclassification improvement; UACR, urine albumin‐to‐creatinine ratio; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin.
Data on UACR were available for 244 patients.
Subgroup Analyses
Figure 2 presents subgroup analyses of all‐cause death with baseline characteristics. With the exception of 2 subgroups (age ≥75 years and LVEF ≥50%), a high U‐NGAL level (≥32.5 μg/gCr) was the strong risk factor for poor prognosis. Heterogeneity was detected in the LVEF subgroup.
Figure 2.
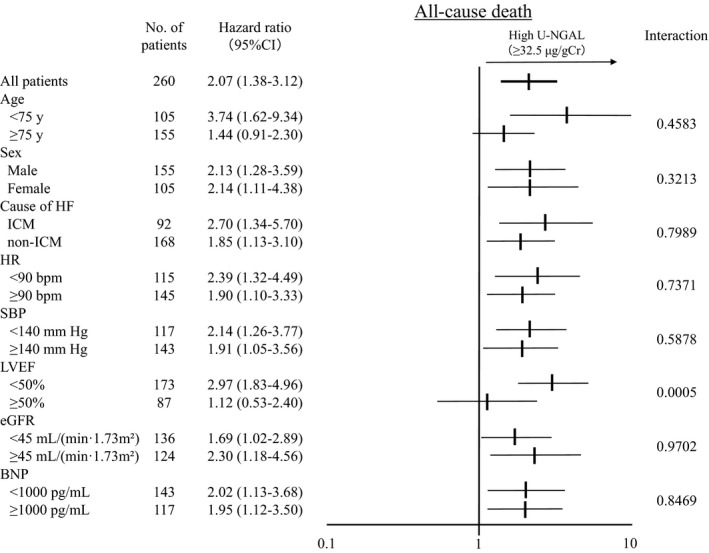
Subgroup analyses of all‐cause death by baseline characteristics. Hazard ratios for 8 predefined subgroups. Horizontal bars represent 95%CIs. P values are for the tests of subgroup heterogeneity (tests of interactions). BNP indicates brain natriuretic peptide; bpm, beats per minute; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; ICM, ischemic cardiomyopathy; LVEF, left ventricle ejection fraction; SBP, systolic blood pressure; U‐NGAL, urinary neutrophil gelatinase‐associated lipocalin.
A high U‐NGAL level predicted adverse events in HF with reduced LVEF (HFrEF; <50%) patients but not in HF with preserved LVEF (HFpEF; ≥50%) patients, with a significant interaction between the HFrEF and HFpEF subgroups (P=0.0005).
Discussion
The present study was the first to demonstrate an association between U‐NGAL on the first day of admission and all‐cause mortality and HF admission in patients with ADHF. The HRs of U‐NGAL were attenuated in the fully adjusted model; however, U‐NGAL remained independently associated with all‐cause mortality and HF admission. Subgroup analyses revealed that this association was observed in HFrEF patients but not HFpEF patients. Furthermore, the addition of U‐NGAL on the first day of admission into eGFR significantly improved the discrimination ability of all‐cause mortality.
NGAL is a 25‐kDa lipocalin‐superfamily glycoprotein that is expressed by neutrophil and epithelial cells in various human tissues. NGAL levels rise rapidly after nephrotoxic injury and predict AKI in various patient groups, such as patients admitted to the intensive care unit and patients after cardiopulmonary bypass surgery.13, 14, 15 Previous studies have demonstrated the association of serum and urine NGAL with AKI and with adverse events in chronic HF patients16, 17, 18; however, few studies have investigated the impact of NGAL on AKI among patients with ADHF.25, 26, 27 Aghel et al measured serum NGAL levels in 91 ADHF patients and reported that an elevated serum NGAL level on admission is associated with the development of worsening renal function (defined as creatinine level increase ≥0.3 mg/dL).26 In this study NGAL levels were measured by urine samples in a larger number of patients, and worsening renal function was defined by AKI criteria. Although these differences were present, as is the case with previous studies, U‐NGAL levels on the first day of admission were demonstrated to be a strong predictor of AKI in the present study. Because current AKI criteria based on creatinine lead to delays in diagnosis, U‐NGAL may be highly beneficial for cardiologists to stratify high‐risk ADHF patients for AKI on the first day of admission.
Alvelos et al reported that the serum NGAL level was an independent predictor of the 3‐month risk of death, and Palazzuoli et al also reported that the serum NGAL level was associated with 6‐month postdischarge outcomes.28, 29 However, these previous studies did not assess urine samples or the association between U‐NGAL and long‐term outcomes. In the present study, U‐NGAL levels were demonstrated to be associated with long‐term outcomes and improved diagnostic accuracy for the prediction of adverse events on the first day of admission. To the best of our knowledge, this is the first report to reveal that U‐NGAL on the first day of admission is a strong prognostic factor for long‐term outcomes in patients with ADHF.
In the present study high U‐NGAL levels were significantly associated with increased development of AKI and long‐term adverse outcomes. Moreover, high U‐NGAL on the first day of admission was independently associated with long‐term adverse outcomes; this association remained after adjustment for the occurrence of AKI during hospitalization in patients with ADHF. It remains unclear why U‐NGAL on the first day of admission is associated with both acute and chronic events. In this study ~50% of the deaths were not associated with cardiovascular events, including death due to infectious diseases (such as pneumonia and sepsis), and the high–U‐NGAL group had a worse prognosis than the low–U‐NGAL group in non‐cardiovascular deaths (HR 1.89; 95%CI 1.09‐3.33, P=0.0235). NGAL is released from injured tubular cells after various damaging stimuli and is expressed by stimulation of endotoxin and inflammatory cytokines, such as IL‐1β, during bacterial infection. Taken together, these findings indicate that there may be distinct mechanisms relating the association of NGAL to acute and chronic events, respectively. In ADHF patients eGFR is measured on the first day of admission because renal impairment is common and is strongly associated with poor outcome.30, 31 In this study the addition of U‐NGAL on the first day of admission to the eGFR significantly improved the discrimination ability of all‐cause mortality. This suggests that the management strategy of ADHF patients becomes better with the combined use of eGFR and U‐NGAL levels on the first day of admission.
In the present study U‐NGAL is a more powerful predictor of mortality than cystatin C. Similarly, a comparative analysis between NGAL and cystatin C has been previously studied: van Deursen et al reported that plasma NGAL is a stronger predictor of mortality than cystatin C in patients with mild or moderate heart failure.32 Palazzuoli et al reported that serum NGAL has a higher predictive value of the composite end point with cardiac death and hospital readmission in heart failure than BNP and cystatin C.29 Various other renal biomarkers predicting AKI as well as NGAL have been examined,33, 34, 35 but further investigations are necessary to elucidate which biomarkers, including NGAL, are most suitable to predict AKI and AKI‐related worsening of heart failure prognosis.
In the subgroup analyses, a high U‐NGAL level predicted adverse events in HFrEF patients but not in HFpEF patients. Between the HFrEF and HFpEF groups, the distribution of U‐NGAL levels remained similar, and the median U‐NGAL levels exhibited no significant differences (32.3 μg/gCr in the HFrEF group and 35.7 μg/gCr in the HFpEF group, P=0.35). No significant differences in baseline renal function were detected between the 2 groups (45.3±23.9 mL/min per 1.73 m² in the HFrEF group and 47.2±25.2 mL/min per 1.73 m² in the HFpEF group, P=0.54). It remains unclear why there was clinical heterogeneity of the prognostic power of U‐NGAL between the HFrEF and HFpEF subgroups. However, high U‐NGAL levels are associated with the occurrence of AKI in both HFrEF and HFpEF subgroups. One possible explanation is the relatively small number of HFpEF patients in this study. Thus, further examinations in a larger number of patients are required to elucidate the mechanisms associated with U‐NGAL and HFpEF.
Limitations
The present study had several limitations. First, this was a single‐center study involving a relatively small number of ADHF patients. Second, this study was a retrospective analysis of prospectively collected data. Third, residual potential confounders could exist even with adjustment. Fourth, this study was performed in Japan and only included Japanese patients; therefore, Western populations were not assessed.
Conclusions
An elevated U‐NGAL level on the first day of admission is strongly and independently associated with the long‐term prognosis in ADHF patients. A combination of the measurement of U‐NGAL and eGFR versus eGFR alone significantly improved the predictive accuracy of all‐cause mortality. These findings indicate that ADHF patients with elevated U‐NGAL require careful management with close monitoring of blood pressure and urine output and appropriate doses of diuretics in order to avoid the development of AKI.
Disclosures
Yoshihiko Saito has received honoraria from Otsuka Pharmaceutical, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Daiichi Sankyo, MSD, and Novartis Pharma; has received subsidies or donations from MSD, Daiichi Sankyo, Bayer Holding, Baxter, Otsuka Pharmaceutical, Kyowa Hakko Kirin, Dainippon Sumitomo Pharma, Astellas Pharma, Takeda Pharmaceutical, Ono Pharmaceutical, Teijin Pharma, Mitsubishi Tanabe Pharma, Eisai, ZERIA Pharmaceutical, Nihon Medi‐Physics, Chugai Pharmaceutical, Genzyme Japan, Medtronic, and Pfizer Japan; and has been endowed department funding by commercial entities from MSD. The other authors have no financial conflicts of interest to disclose.
Acknowledgments
The authors would like to acknowledge Yoko Wada, Yuki Kamada, and Ikuyo Yoshida for their support in the data collection process. Abbott Japan performed the testing of the ARCHITECT Urine NGAL assay.
(J Am Heart Assoc. 2017;6:e004582 DOI: 10.1161/JAHA.116.004582.)28522674
References
- 1. Pang PS, Komajda M, Gheorghiade M. The current and future management of acute heart failure syndromes. Eur Heart J. 2010;31:784–793. [DOI] [PubMed] [Google Scholar]
- 2. Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993‐2006. JAMA. 2010;303:2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 4. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 5. Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. [DOI] [PubMed] [Google Scholar]
- 6. Cuffe MS, Califf RM, Adams KF Jr, Benza R, Bourge R, Colucci WS, Massie BM, O'Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M; Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) Investigators . Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. [DOI] [PubMed] [Google Scholar]
- 7. Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K. Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J. 2013;77:687–696. [DOI] [PubMed] [Google Scholar]
- 8. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
- 9. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. [DOI] [PubMed] [Google Scholar]
- 10. Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. [DOI] [PubMed] [Google Scholar]
- 11. Kidney disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(Suppl 1):138. [Google Scholar]
- 12. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase‐associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. [DOI] [PubMed] [Google Scholar]
- 13. Herget‐Rosenthal S. One step forward in the early detection of acute renal failure. Lancet. 2005;365:1205–1206. [DOI] [PubMed] [Google Scholar]
- 14. Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase‐associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damman K, Masson S, Hillege HL, Voors AA, van Veldhuisen DJ, Rossignol P, Proietti G, Barbuzzi S, Nicolosi GL, Tavazzi L, Maggioni AP, Latini R. Tubular damage and worsening renal function in chronic heart failure. JACC Heart Fail. 2013;1:417–424. [DOI] [PubMed] [Google Scholar]
- 17. Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;10:997–1000. [DOI] [PubMed] [Google Scholar]
- 18. Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, Bonventre JV, Voors AA, Hillege HL. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ueda T, Kawakami R, Sugawara Y, Okada S, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Worsening of renal function during 1 year after hospital discharge is a strong and independent predictor of all‐cause mortality in acute decompensated heart failure. J Am Heart Assoc. 2014;3:e110074 DOI: 10.1161/JAHA.114.001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueda T, Kawakami R, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Plasma renin activity is a strong and independent prognostic indicator in patients with acute decompensated heart failure treated with renin‐angiotensin system inhibitors. Circ J. 2015;79:1307–1314. [DOI] [PubMed] [Google Scholar]
- 21. Nakada Y, Kawakami R, Nakano T, Takitsume A, Nakagawa H, Ueda T, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Okura H, Saito Y. Sex differences in clinical characteristics and long‐term outcome in acute decompensated heart failure patients with preserved and reduced ejection fraction. Am J Physiol Heart Circ Physiol. 2016;310:H813–H820. [DOI] [PubMed] [Google Scholar]
- 22. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators Developing the Japanese Equation for Estimated GFR . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 24. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, Clopton P, van Veldhuisen DJ. Prognostic utility of plasma neutrophil gelatinase‐associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B‐type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase‐associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail. 2010;16:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soyler C, Tanriover MD, Ascioglu S, Aksu NM, Arici M. Urine neutrophil gelatinase‐associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail. 2015;37:772–776. [DOI] [PubMed] [Google Scholar]
- 28. Alvelos M, Lourenço P, Dias C, Amorim M, Rema J, Leite AB, Guimarães JT, Almeida P, Bettencourt P. Prognostic value of neutrophil gelatinase‐associated lipocalin in acute heart failure. Int J Cardiol. 2013;165:51–55. [DOI] [PubMed] [Google Scholar]
- 29. Palazzuoli A, Ruocco G, Pellegrini M, De Gori C, Del Castillo G, Franci B, Nuti R, Ronco C. Comparison of neutrophil gelatinase‐associated lipocalin versus B‐type natriuretic peptide and cystatin C to predict early acute kidney injury and outcome in patients with acute heart failure. Am J Cardiol. 2015;116:104–111. [DOI] [PubMed] [Google Scholar]
- 30. Hamaguchi S, Tsuchihashi‐Makaya M, Kinugawa S, Yokota T, Ide T, Takeshita A, Tsutsui H; JCARE‐CARD Investigators . Chronic kidney disease as an independent risk for long‐term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE‐CARD). Circ J. 2009;73:1442–1447. [DOI] [PubMed] [Google Scholar]
- 31. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J;ADHERE Scientific Advisory Committee and Investigators . High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. [DOI] [PubMed] [Google Scholar]
- 32. van Deursen VM, Damman K, Voors AA, van der Wal MH, Jaarsma T, van Veldhuisen DJ, Hillege HL. Prognostic value of plasma neutrophil gelatinase‐associated lipocalin for mortality in patients with heart failure. Circ Heart Fail. 2014;7:35–42. [DOI] [PubMed] [Google Scholar]
- 33. Liu S, Che M, Xue S, Xie B, Zhu M, Lu R, Zhang W, Qian J, Yan Y. Urinary L‐FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers. 2013;18:95–101. [DOI] [PubMed] [Google Scholar]
- 34. McEvoy JW, Chen Y, Halushka MK, Christenson E, Ballantyne CM, Blumenthal RS, Christenson RH, Selvin E. Galectin‐3 and risk of heart failure and death in blacks and whites. J Am Heart Assoc. 2016;5:e003079 DOI: 10.1161/JAHA.115.003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsubara J, Sugiyama S, Nozaki T, Akiyama E, Matsuzawa Y, Kurokawa H, Maeda H, Fujisue K, Sugamura K, Yamamoto E, Matsui K, Jinnouchi H, Ogawa H. Incremental prognostic significance of the elevated levels of pentraxin 3 in patients with heart failure with normal left ventricular ejection fraction. J Am Heart Assoc. 2014;3:e000928 DOI: 10.1161/JAHA.114.000928. [DOI] [PMC free article] [PubMed] [Google Scholar]


