Abstract
Background
Congestive hepatopathy is a recognized complication of Fontan physiology. Data regarding the incidence of hepatopathy and risk factors are lacking.
Methods and Results
Liver biopsies and cardiac catherizations were performed as part of an evaluation offered to all patients ≥10 years after Fontan. Quantitative determination of hepatic fibrosis was performed using Sirius red staining with automated calculation of collagen deposition per slide (%CD). Biopsies from included subjects were compared to stained specimens from controls without known fibrotic liver disease. Patient characteristics, echocardiographic findings, and hemodynamic measures were evaluated as potential risk factors. The cohort consisted of 67 patients (31 female) at mean age of 17.3±4.5 years and mean time from Fontan of 14.9±4.5 years. Right ventricular morphology was present in 37 subjects. Median %CD by Sirius red staining was 21.6% (range 8.7% to 49.4%) compared to 2.6% (range 2.2% to 3.0%) in controls. There was a significant correlation between time from Fontan and degree of Sirius red staining (r=0.33, P<0.01). Serum liver enzymes and platelet count did not correlate with %CD. The median inferior vena cava pressure was 13 mm Hg (range 6‐24 mm Hg) and did not correlate with %CD. There was no difference in %CD based on ventricular morphology or severity of atrioventricular valve insufficiency.
Conclusions
In this cohort of predominantly asymptomatic children and adolescents electively evaluated after a Fontan operation, all exhibited evidence for hepatic fibrosis as measured by collagen deposition in the liver. Time from Fontan was the only factor significantly associated with collagen deposition. These findings demonstrate that liver fibrosis is an inherent feature of Fontan physiology and that the degree of fibrosis increases over time.
Keywords: fibrosis, Fontan procedure
Subject Categories: Congenital Heart Disease, Heart Failure, Fibrosis
Introduction
The Fontan operation creates a stable circulation that allows for the survival of children with heterogeneous forms of single‐ventricle congenital heart disease. By channeling systemic venous return directly to the pulmonary circulation, it reduces intracardiac mixing and thus simultaneously raises arterial oxygen saturation and reduces volume load on the single ventricle.1, 2 However, the price of this form of palliation is a unique form of indolent heart failure characterized by chronic systemic venous hypertension and low cardiac output.3 Excellent early surgical results and decades of experience in creating survivors are well demonstrated,4, 5, 6 but there is a growing recognition of the deleterious impact of this circulation on a number of extracardiac organ systems.7, 8, 9, 10, 11, 12, 13
The liver is particularly vulnerable to alterations in cardiac output and venous pressure, given its unique blood supply and the egress of hepatic venous effluent directly into the Fontan circuit, and there is evidence of both immediate and progressive pathologic change in the livers of patients after Fontan.14, 15, 16 Prospective serial evaluation of the liver is thus of clinical importance, but accurately characterizing the status of the liver in a Fontan circulation has proven to be extremely challenging. Biochemical markers of liver function do not correlate with the degree of fibrosis, and imaging studies used for other forms of liver disease may not be applicable to the pathophysiology associated with the Fontan circulation.17, 18 Liver biopsy is the traditional gold standard for the detection of fibrosis, but this can be limited by the heterogeneity of the biopsy cores as well as by the subjective interpretation of the reviewing pathologist. Nevertheless, in the absence of a current quantifiable alternative, liver biopsy remains the most reliable means for assessment.
Given the clinical importance of understanding the degree of liver fibrosis in an individual patient, we gathered a multidisciplinary group of specialists in 2012 to create an institutional consensus on how to systematically manage this problem.17 Because of the absence of any previously accepted clinical regimen, the group recommended performance of a liver biopsy with cardiac catheterization assessment of hemodynamics as part of systematic routine protocolized evaluation to be undertaken in all of our Fontan patients at ≈10 years following Fontan completion.17 For the last 5 years this has been part of the standard outpatient follow‐up at our institution. In order to overcome the potential bias of qualitative liver biopsy grading and in order to best characterize the unique nature of combined portal and centrilobular fibrosis typically seen in Fontan‐associated hepatopathy,19 we utilized a novel method of comprehensive quantification of liver fibrosis using Sirius red staining.20 This technique allows for automated quantitative detection of collagen deposition, is shown to be superior to trichrome staining for quantitative image analysis assessment, and correlates well with markers of liver fibrosis.21
In this study we review the results of liver biopsies using quantitative image analysis of Sirius red staining in a cross‐sectional cohort of children and adolescents who underwent liver biopsy as part of the greater comprehensive assessment of their Fontan circulation. The goals of this study are (1) to assess the prevalence and degree of liver fibrosis after Fontan operation using this unique quantitative technique and (2) to investigate associations between potential risk factors and the degree of fibrosis as characterized by percentage collagen deposition.
Methods
This is a retrospective cross‐sectional review of data collected prospectively on all patients who underwent comprehensive clinical assessment including cardiac catheterization and liver biopsy at The Children's Hospital of Philadelphia between 2009 through 2014. This evaluation is offered as part of routine clinical care to all patients evaluated through the Single Ventricle Survivorship Program at ≈10 years following Fontan and to additional patients based on clinical concern or provider preference. Patients are referred to this program via internal referral from local cardiologists or via external referral. Those who were referred from outside of our institution were either self‐referrals or physician referrals. The population evaluated in the clinic is comprised of both “well patients,” referred for routine screening, and patients referred based on the presence of an underlying Fontan complication. All patients who were ≈10 years out from Fontan surgery were offered the comprehensive evaluation regardless of the reason for referral. The data analysis was approved by the Institutional Review Board for the Protection of Human Subjects (CHOP IRB 12‐009791), and a waiver of informed consent was granted.
Liver biopsies were obtained via ultrasound‐guided percutaneous needle biopsy in 1 or 2 cores. Protocol included obtaining a hemoglobin level 6 to 12 hours after biopsy with comparison to the prebiopsy level and overnight observation in our postprocedure recovery unit. Percutaneous biopsy was chosen over a transvenous approach in order to obtain a more representative sample of liver tissue and eliminate sampling bias in which more severe fibrosis may, theoretically, be found surrounding the hepatic veins. Quantitative determination of hepatic fibrosis was performed using Sirius red staining for collagen with automated calculation of percentage positive staining per slide. Slides were stained with Sirius red and digitally scanned using Aperio Scanscope CS‐O (Leica Biosystems, Vista, CA). Whole‐slide image analysis with the Color Deconvolution V9 algorithm was used to calculate quantitative collagen deposition by automated detection of the percentage of area containing Sirius red staining (Leica Biosystems, Vista, CA). For subjects with biopsy specimens on multiple slides, the percentage of Sirius red staining was averaged across all slides. Liver explant tissue from 4 pediatric age patients with metabolic liver disease without cause for hepatic fibrosis was stained with Sirius red and analyzed by the same technique as part of internal laboratory controls. High‐grade fibrosis was defined as collagen deposition >30% based on correlations between collagen deposition and fibrosis grading scales.20
Cardiac catheterizations were undertaken as part of clinical care. All subjects were required to be in a fasting state for 8 hours prior to the catheterization, as per institutional policy. The use of light sedation, deep sedation, or general anesthesia was at the discretion of the catheterization team. Original pressure tracings and data from cardiac catheterization were reviewed and measurements were repeated by a single reviewer (M.O.) to assure consistency of data interpretation for all study subjects. Percutaneous liver biopsy was performed on the same day (in 42/67, 63% of patients), within 3 months (in 60/67, 90% of patients), or within 6 months (in 67/67, 100% of patients) of cardiac catheterization.
Patient‐specific data and blood laboratory data were collected by review of the electronic medical record. Echocardiographic data were obtained from review of the echocardiogram most proximal to the date of the liver biopsy. If cardiac magnetic resonance imaging (MRI) was performed as part of the overall hemodynamic evaluation, those data were abstracted from the report and verified by a cardiologist with specialized training in congenital cardiac MRI (M.F.). Laboratory data were mostly obtained at the time of liver biopsy but, if not available, were included if obtained any time within 6 months of the procedure. Patient‐specific data included age at Fontan, age at liver biopsy, ventricular morphology, and Fontan‐associated complications. Echocardiographic data included qualitative description of ventricular function and atrioventricular valve regurgitation. Cardiac MRI data included cardiac output and systemic‐to‐pulmonary arterial collateral flow burden, using methods previously described.22 Blood testing included aspartate aminotransferase, alanine aminotransferase, total protein, albumin, alkaline phosphatase, γ‐glutamyl transferase, total bilirubin, brain natriuretic peptide, complete blood count, prothrombin time, and international normalized ratio. Patients on warfarin were instructed to withhold the drug for 5 to 7 days prior to liver biopsy procedure.
Statistical Analysis
Patient‐specific characteristics, echocardiographic findings, and hemodynamic measures were summarized using standard descriptive statistics. Normally distributed variables were reported as mean±standard deviation, and skewed variables were reported as median with range. The relationship between patient‐specific characteristics and hemodynamics versus percentage collagen deposition were evaluated using Pearson correlation for normally distributed and Spearman correlation for non–normally distributed variables. Clinically significant differences in patient characteristics and hemodynamics were identified based on review of the data, and differences in collagen deposition based on dichotomous subgroup analysis were assessed using the Student t test. A cut point of 18 years after Fontan was chosen to try to differentiate those in the adult congenital cohort from those in the pediatric cohort. All other risk factors were dichotomized according to important clinical distinctions. Statistical significance was established a priori at a 2‐tailed α<0.05. All analyses were performed using STATA v10 (Stata Corp, College Station, TX).
Results
Liver biopsies with hemodynamic data were obtained in 67 subjects. All liver biopsies were performed safely without intraprocedural complications. Ten patients (15%) exhibited a modest decline in postprocedural hemoglobin level or complained of excessive pain at site of biopsy and received follow‐up abdominal ultrasound with findings reported as normal or a small collection of fluid in 9. One patient had a small perihepatic hematoma that resolved without intervention. For the overall group, the mean drop in hemoglobin concentration from before catheterization and biopsy to after catheterization and biopsy was 1.0 g/dL versus 1.3 g/dL in those who underwent ultrasound.
The clinical characteristics of the cohort are summarized in Table 1. The mean age at biopsy was 17.3 years at a mean of 14.9 years from time of Fontan surgery. The majority of subjects underwent an intra‐atrial lateral tunnel type of Fontan, and most were of single right ventricle morphology. A diagnosis of plastic bronchitis or protein‐losing enteropathy was either current or historically present in 28% of subjects. Our cohort had generally preserved ventricular function on echocardiography; 92% were graded as having normal or mildly diminished function, and 62% had no more than mild atrioventricular valve regurgitation. The median pressure in the inferior vena cava was 13 mm Hg (range 6‐24 mm Hg). In the subset of patients who had data available from cardiac MRI (n=34, 51%), the mean cardiac index was 2.43±0.54 L/(min·m2), and the mean aortopulmonary collateral flow burden as a percentage of total aortic flow was 23.5±11%.
Table 1.
Patient Characteristics and Hemodynamic Data
| Characteristic | |
|---|---|
| Age at biopsy, mean±SDa | 17.3±4.5 years |
| Time from Fontan, mean±SDa | 14.9±4.5 years |
| Sex (female)a | 46% |
| Fontan connection typea | |
| Intra‐atrial lateral tunnel | 60% |
| Extracardiac conduit | 36% |
| Other | 4% |
| Patent fenestrationa | 39% |
| Ventricular morphologya | |
| Right ventricular morphology | 55% |
| Left or mixed ventricular morphology | 45% |
| Qualitative AV valve insufficiencya | |
| Mild or less | 62% |
| More than mild | 38% |
| Qualitative ventricular functiona | |
| Normal | 77% |
| Mildly diminished | 15% |
| Worse than mildly diminished | 8% |
| Catheter‐derived hemodynamics (median; range)a | |
| Inferior vena cava pressure | 13 mm Hg; 6 to 24 mm Hg |
| End‐diastolic pressure | 9 mm Hg; 3 to 16 mm Hg |
| Fontan complications, %a | |
| Protein‐losing enteropathy | 19% |
| Plastic bronchitis | 9% |
| Sirius red (CD±SD) | 23.8±9.5% |
| MRI‐derived hemodynamics, mean±SDb | |
| Cardiac index | 2.43±0.54 L/min per m2 |
| Systemic‐pulmonary arterial collateral flow (% of aortic flow) | 23.5±11% |
AV indicates atrioventricular; CD, collagen deposition.
N=67.
N=34.
In all patients the median percentage collagen deposition as measured by quantitative automated Sirius red staining was 21.6% (range 8.7% to 49.4%; Figure 1). The median percentage collagen deposition for the nonfibrotic controls was 2.6±0.3% (range 2.2% to 3.0%). High‐grade fibrosis was present in 23% of patients (Figure 2).
Figure 1.
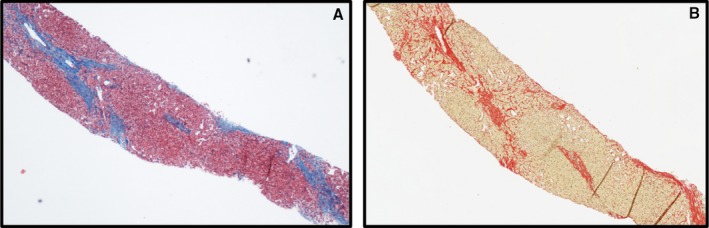
A, A liver biopsy prepared using hematoxylin and eosin staining. The blue color represents fibrotic changes within the liver core. B, A similar liver core prepared using Sirius red. In this image the fibrotic segments are red and are measured quantitatively using automated detection.
Figure 2.
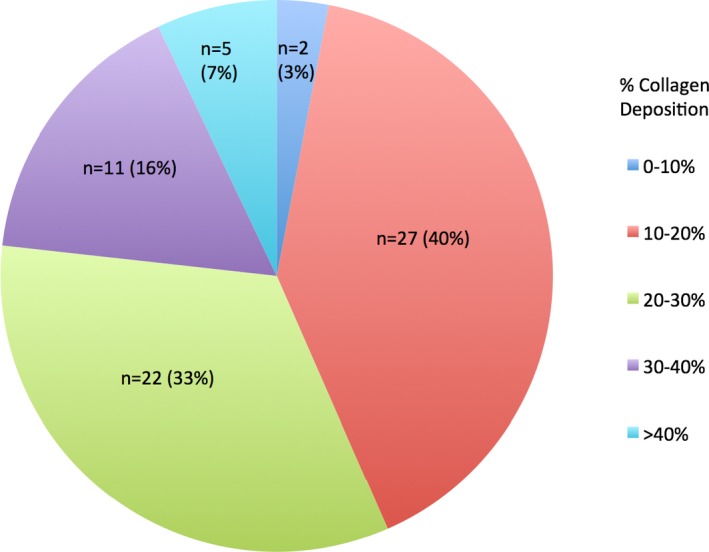
Distribution of percentage collagen distribution among the 67 included liver cores.
Of the potential risk factors evaluated, only age and time from Fontan correlated with the degree of collagen deposition (Table 2, Figure 3). None of the biochemical markers of hepatic injury or hepatic synthetic function was significantly associated with collagen deposition. Ventricular function, atrioventricular valve regurgitation, and direct measurements of Fontan hemodynamics were not associated with collagen deposition. The presence of a Fontan‐associated complication was likewise not associated with the degree of collagen deposition.
Table 2.
Correlations Between Percentage Collagen Deposition and Patient Age, Laboratory Values, and Hemodynamic Measures
| Laboratory Test/Assessment (n) | r | P Value |
|---|---|---|
| Age (67) | 0.27 | 0.03 |
| Time from Fontan (67) | 0.33 | <0.01 |
| Brain‐type natriuretic peptide (61) | −0.15 | 0.26 |
| γ‐Glutamyl transferase (64) | 0.21 | 0.09 |
| Prothrombin time (63) | 0.15 | 0.25 |
| Platelet count (67) | −0.04 | 0.75 |
| Inferior vena cava pressure (67) | 0.003 | 0.98 |
| End‐diastolic pressure (61) | −0.045 | 0.73 |
| Cardiac index (34) | 0.28 | 0.11 |
| Collateral flow as % of total aortic flow (32) | −0.31 | 0.10 |
Figure 3.
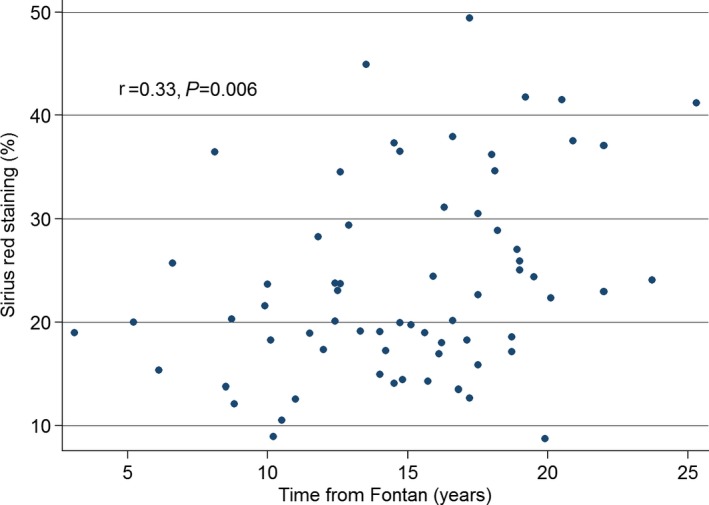
Scatter plot of Sirius red staining vs time from Fontan.
The findings were similar when potential risk factors were evaluated in a dichotomous manner (Table 3). Time from Fontan >18 years was associated with a higher degree of collagen deposition as compared with ≤18 years (28.2% versus 22.3%; P=0.03), but there were no differences in liver collagen deposition when evaluating by ventricular morphology, degree of atrioventricular valve insufficiency, ventricular function, serum brain‐type natriuretic peptide level, measured pressure in the inferior vena cava, MRI‐derived cardiac index, or aortopulmonary collateral flow burden.
Table 3.
Differences in Collagen Deposition Based on Dichotomous Evaluation of Risk Factors
| Risk Factor (n) | Percentage Collagen Deposition | P Value |
|---|---|---|
| Time from Fontan >18 years (50) | 28.2±9.5 | 0.03 |
| Time from Fontan ≤18 years (17) | 22.3±9.1 | |
| Right ventricular morphology (37) | 22.6±9.3 | 0.27 |
| Left or mixed ventricular morphology (30) | 25.2±9.7 | |
| Mild or less AVVR (41) | 24.0±9.5 | 0.86 |
| More than mild AVVR (25) | 23.6±9.7 | |
| Normal ventricular function (50) | 24.1±9.7 | 0.84 |
| Mild ventricular dysfunction or worse (15) | 23.5±9.4 | |
| No diagnosis of PLE or PB (50) | 23.7±9.6 | 0.91 |
| Diagnosis of PLE or PB (17) | 24.0±9.2 | |
| BNP ≤100 pg/mL (56) | 23.8±8.8 | 0.39 |
| BNP >100 pg/mL (5) | 20.3±3.6 | |
| IVC pressure <15 mm Hg (46) | 24.1±9.7 | 0.73 |
| IVC pressure ≥15 mm Hg (21) | 23.2±9.2 |
AVVR indicates atrioventricular valve regurgitation; BNP, B‐type natriuretic peptide; IVC, inferior vena cava; PB, plastic bronchitis; PLE, protein‐losing enteropathy.
Discussion
The end‐organ consequences of the Fontan circulation are of growing concern, with increasing attention focused toward the deleterious effects on the liver. In this study we present the results of our systematic clinical evaluation of a large cross section of children and adolescents with a Fontan circulation. Patients came through a screening program, were generally considered to be doing quite well, and were in a good functional state, yet all demonstrated variable degrees of liver fibrosis. With quantitative image analysis of Sirius red–stained biopsies, a methodology that provides an objective automated measure of comprehensive tissue fibrosis, nearly 1 out of 4 patients was classified as having high‐grade fibrosis (23% with >30% collagen deposition). Also notable is the finding of a clear correlation between time from Fontan operation or age at biopsy and quantitative degree of fibrosis. We found no other patient‐specific or hemodynamic predictors of liver fibrosis among the variables reviewed. It is noteworthy that liver fibrosis was not different in those with protein‐losing enteropathy or plastic bronchitis, suggesting that degree of fibrosis is independent of these particular Fontan‐associated complications.
Hepatic fibrosis following a Fontan operation is well documented.10, 11, 16, 19, 23 Although similarities exist to the pathophysiology of congestive hepatopathy seen in adults with right‐sided heart failure, Fontan‐associated liver hepatopathy is more complex and likely of multifactorial etiology. A series of insults from birth onward may contribute in an additive manner. Liver damage is possible at the time of neonatal presentation where hemodynamic instability can result in altered hepatic perfusion. Further liver injury is possible at the time of interval cardiac surgeries. Moderate cyanosis is present during the first 2 years of life and is accepted as part of the process of staged palliation until the Fontan operation; however, the potential negative effects of early age cyanosis on the liver are unclear. Following a Fontan operation, systemic venous pressure is increased 3‐ to 4‐fold that of normal. Because the portal circulatory system must ultimately drain into the hepatic and systemic venous systems, portal venous pressures are obligatorily elevated as well. Elevated venous pressure throughout the hepatic vascular tree leads to mechanical transduction of hepatic stellate cells that transform into activated collagen‐depositing myofibroblasts, resulting in tissue fibrosis and liver scarring in both a centrilobular and portal venous pattern.15, 19, 24
Evidence for changes in the liver after a Fontan operation is abundant. In the immediate postoperative period there is an increase in liver size and a mild elevation in enzymes associated with hepatic cell injury.16 Over the long term, more substantial changes in hepatic architecture have been demonstrated by various imaging modalities, and increased liver stiffness has been measured by both ultrasound and magnetic resonance elastography.25, 26, 27 Fibrotic changes to the liver parenchyma have been noted on autopsy series and on other reviews of biopsy data.19, 28 Clinically important hepatic failure within the time frame of the first to second decade after Fontan is uncommon. However, the potential for development of cirrhosis, which will progress to end‐stage liver disease, is clear. Perhaps most worrisome have been reports of malignant transformation of hepatic fibrosis to hepatocellular carcinoma in relatively young patients.23, 29
Conventional pathology grading systems for liver fibrosis (eg, METAVIR) are either qualitative or semiquantitative at best, often prone to subjective interpretation bias, and have been developed primarily for use in conditions of chronic hepatitis. Fontan‐associated liver disease manifests as a combination of both centrilobular as well as portal fibrosis with very different pathophysiological origins than that of chronic hepatitis. We are of the opinion that Fontan‐associated liver disease does not lend itself to adequate characterization through these conventional grading systems alone. An objective quantitative measure of “total” tissue fibrosis such as percentage collagen deposition through Sirius red staining is a more precise means of characterizing comprehensive changes within the liver and is therefore a better tool to use when exploring for associated clinical variables and possible etiologies. The use of such a quantitative characterization of liver fibrosis in our study strengthens the finding of an association between time from Fontan and degree of liver scarring.
Although we demonstrate an association between hepatic fibrosis and age/time from Fontan, there is considerable interpatient variability suggesting that the duration of insult associated with Fontan physiology is not the only factor associated with hepatic changes. There are a number of unmeasured factors that could contribute. Potential injury prior to Fontan operation is an important factor. Genetic variation is likely in the response of hepatocytes to the dual stressors of elevated central venous pressure and relatively diminished cardiac output. Although increased central venous pressure is common to all patients with a Fontan circulation, subtleties in the systemic venous pathway geometry and variability in flow dynamics through the Fontan circuit may play a role. Impedance to egress from the hepatic venous system may be related to specific flow characteristics downstream within the Fontan connection, unique to each individual. There are data to suggest that a fluid bolus at the time of catheterization may acutely increase Fontan pressure, and further study evaluating this relationship using computational flow dynamics and power loss analysis in combination with quantitative characterization of liver fibrosis may offer additional insights.30
Our community is just beginning to explore in earnest the presence of liver fibrosis after Fontan operation, and the nature and rate of possible progression of this phenomenon are unknown. Our cross‐sectional study demonstrates an association with time from Fontan; however, longitudinal serial study will determine the rate of progression for individual patients. Clinical surveillance protocols must be developed. Understanding the degree of liver fibrosis within the context of overall Fontan hemodynamics is helpful when considering medical, catheter‐based, or perhaps further surgical interventions. Although there are no medications currently proven for the treatment of Fontan‐associated complications, diuretics and pulmonary vasodilators are of theoretical but unproven merit. Such agents might be considered for those in whom an unloading of the Fontan circulation is desired. Utilization of antifibrotic strategies through mechanisms such as aldosterone inhibition, alteration of the renin‐angiotensin system, or other mechanisms may be useful.31, 32, 33 Spironolactone may be an ideal candidate agent to offer benefit both through its potential antifibrotic properties as well as its salutary circulatory effects, and may be worthy of consideration for future study.
Although the importance of ongoing hepatic surveillance to help inform patient‐specific care is clear, the specific modality to apply in order to monitor and accurately inform on hepatic health is less certain. We have had no serious complications in this series, but liver biopsy is invasive and is not without risks. A noninvasive method of hepatic evaluation that correlates with biopsy results would have significant advantages. The absence of any reliable biomarker for the determination of liver fibrosis in the Fontan population suggests that direct assessment of hepatic architecture might still be the most reliable method of evaluation. Development of a reliable noninvasive tool for surveillance of changes in hepatic parenchyma is imperative in order to determine rates of progression as well as to gauge efficacy of therapeutic or preventative strategies. Encouraging preliminary data suggest that magnetic resonance elastography assessment of liver tissue stiffness may be such a tool, though it has yet to be compared to liver biopsy in a large series of patients.27
Limitations
This was a retrospective study in which clinical results were evaluated from a select group referred for comprehensive hemodynamic evaluation. We used Sirius red staining to try to limit subjective variability in pathological interpretation, but there is still known significant variation within individual cores and between cores in a single patient. Because only clinically indicated laboratory studies were obtained, it is possible that there are unmeasured variables that may be associated with the degree of fibrosis. Given the nature of the Single Ventricle Survivorship Clinic, there was an overrepresentation of patients with a history of Fontan‐associated complications including protein‐losing enteropathy and plastic bronchitis. In addition, as referral to the clinic is at the discretion of the primary cardiologist, it is possible that there is a referral bias toward those subjects with more concerning findings on routine pediatric or cardiac evaluations. Finally, our ability to identify associations was inherently limited by the size of our cohort. Future multicenter collaborative efforts would be helpful to generate a larger sample size and to demonstrate more subtle associations.
Conclusion
Liver fibrosis is universal after the Fontan operation. In this cross section of patients there were no identified patient‐specific or hemodynamic risk factors for more advanced fibrosis. Duration of exposure to the Fontan circulation is a risk factor for fibrotic progression, but the correlation is modest, and there are likely many other unmeasured risk factors. The absence of a noninvasive method for the determination of the degree of fibrosis argues for ongoing surveillance of the liver parenchyma to allow for the most appropriate patient‐specific care plan. Efforts to understand the potential value of newer noninvasive techniques of hepatic evaluation as well as the potential role of medications to reduce the rate of fibrotic progression would be of significant value. For developing future studies to assess the utility and validity of noninvasive tools to gauge liver fibrosis, we suggest the use of quantitative image analysis of Sirius red percentage collagen staining as an optimal means to characterize the comprehensive state of liver fibrosis present in this unique population.
Sources of Funding
This work is supported by the Robert and Dolores Harrington Endowed Chair for Pediatric Cardiology Research at The Children's Hospital of Philadelphia.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004809 DOI: 10.1161/JAHA.116.004809.)28446492
References
- 1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66:613–621. [PubMed] [Google Scholar]
- 3. Gewillig M, Goldberg DJ. Failure of the Fontan circulation. Heart Fail Clin. 2014;10:105–116. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch JC, Goldberg C, Bove EL, Salehian S, Lee T, Ohye RG, Devaney EJ. Fontan operation in the current era: a 15‐year single institution experience. Ann Surg. 2008;248:402–410. [DOI] [PubMed] [Google Scholar]
- 5. Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O'Leary PW, Driscoll DJ, Cetta F. 40‐year follow‐up after the Fontan operation: long‐term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66:1700–1710. [DOI] [PubMed] [Google Scholar]
- 6. Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, Rush CH, Gaynor JW, Goldberg DJ. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol. 2012;60:1018–1025. [DOI] [PubMed] [Google Scholar]
- 7. Avitabile CM, Goldberg DJ, Zemel BS, Brodsky JL, Dodds K, Hayden‐Rush C, Whitehead KK, Goldmuntz E, Rychik J, Leonard MB. Deficits in bone density and structure in children and young adults following Fontan palliation. Bone. 2015;77:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avitabile CM, Leonard MB, Zemel BS, Brodsky JL, Lee D, Dodds K, Hayden‐Rush C, Whitehead KK, Goldmuntz E, Paridon SM, Rychik J, Goldberg DJ. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart. 2014;100:1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma S, Ruebner RL, Furth SL, Dodds KM, Rychik J, Goldberg DJ. Assessment of kidney function in survivors following Fontan palliation. Congenit Heart Dis. 2016;11:630–636. [DOI] [PubMed] [Google Scholar]
- 10. Wu FM, Jonas MM, Opotowsky AR, Harmon A, Raza R, Ukomadu C, Landzberg MJ, Singh MN, Valente AM, Egidy Assenza G, Perez‐Atayde AR. Portal and centrilobular hepatic fibrosis in Fontan circulation and clinical outcomes. J Heart Lung Transplant. 2015;34:883–891. [DOI] [PubMed] [Google Scholar]
- 11. Pundi K, Pundi KN, Kamath PS, Cetta F, Li Z, Poterucha JT, Driscoll DJ, Johnson JN. Liver disease in patients after the Fontan operation. Am J Cardiol. 2016;117:456–460. [DOI] [PubMed] [Google Scholar]
- 12. Rychik J, Goldberg D, Rand E, Semeao E, Russo P, Dori Y, Dodds K. End‐organ consequences of the Fontan operation: liver fibrosis, protein‐losing enteropathy and plastic bronchitis. Cardiol Young. 2013;23:831–840. [DOI] [PubMed] [Google Scholar]
- 13. Rychik J. The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19:37–43. [DOI] [PubMed] [Google Scholar]
- 14. Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward‐Sadler H, Haw M, Cope R, Salmon AP, Sivaprakasam MC, Kendall T, Keeton BR, Iredale JP, Veldtman GR. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kendall TJ, Stedman B, Hacking N, Haw M, Vettukattill JJ, Salmon AP, Cope R, Sheron N, Millward‐Sadler H, Veldtman GR, Iredale JP. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol. 2008;61:504–508. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz MC, Glatz AC, Daniels K, Goldberg DJ, Rand E, Epelman MS, Cohen MS. Hepatic abnormalities are present before and early after the Fontan operation. Ann Thorac Surg. 2015;100:2298–2304. [DOI] [PubMed] [Google Scholar]
- 17. Rychik J, Veldtman G, Rand E, Russo P, Rome JJ, Krok K, Goldberg DJ, Cahill AM, Wells RG. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guha IN, Bokhandi S, Ahmad Z, Sheron N, Cope R, Marshall C, Veldtman G. Structural and functional uncoupling of liver performance in the Fontan circulation. Int J Cardiol. 2013;164:77–81. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz MC, Sullivan LM, Glatz AC, Rand E, Russo P, Goldberg DJ, Rome JJ, Cohen MS. Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatr Cardiol. 2013;34:135–142. [DOI] [PubMed] [Google Scholar]
- 20. Surrey LF, Russo P, Rychik J, Goldberg DJ, Dodds K, O'Byrne ML, Glatz AC, Rand EB, Lin HC. Prevalence and characterization of fibrosis in surveillance liver biopsies of patients with Fontan circulation. Hum Pathol. 2016;57:106–115. [DOI] [PubMed] [Google Scholar]
- 21. Huang Y, de Boer WB, Adams LA, MacQuillan G, Rossi E, Rigby P, Raftopoulos SC, Bulsara M, Jeffrey GP. Image analysis of liver collagen using Sirius red is more accurate and correlates better with serum fibrosis markers than trichrome. Liver Int. 2013;33:1249–1256. [DOI] [PubMed] [Google Scholar]
- 22. Whitehead KK, Gillespie MJ, Harris MA, Fogel MA, Rome JJ. Noninvasive quantification of systemic‐to‐pulmonary collateral flow: a major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging. 2009;2:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Josephus Jitta D, Wagenaar LJ, Mulder BJ, Guichelaar M, Bouman D, van Melle JP. Three cases of hepatocellular carcinoma in Fontan patients: review of the literature and suggestions for hepatic screening. Int J Cardiol. 2016;206:21–26. [DOI] [PubMed] [Google Scholar]
- 24. Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoo BW, Choi JY, Eun LY, Park HK, Park YH, Kim SU. Congestive hepatopathy after Fontan operation and related factors assessed by transient elastography. J Thorac Cardiovasc Surg. 2014;148:1498–1505. [DOI] [PubMed] [Google Scholar]
- 26. Serai SD, Wallihan DB, Venkatesh SK, Ehman RL, Campbell KM, Sticka J, Marino BS, Podberesky DJ. Magnetic resonance elastography of the liver in patients status‐post Fontan procedure: feasibility and preliminary results. Congenit Heart Dis. 2014;9:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poterucha JT, Johnson JN, Qureshi MY, O'Leary PW, Kamath PS, Lennon RJ, Bonnichsen CR, Young PM, Venkatesh SK, Ehman RL, Gupta S, Smyrk TC, Dearani JA, Warnes CA, Cetta F. Magnetic resonance elastography: a novel technique for the detection of hepatic fibrosis and hepatocellular carcinoma after the Fontan operation. Mayo Clin Proc. 2015;90:882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Evans WN, Winn BJ, Yumiaco NS, Galindo A, Rothman A, Acherman RJ, Restrepo H. Transvenous hepatic biopsy in stable Fontan patients undergoing cardiac catheterization. Pediatr Cardiol. 2014;35:1273–1278. [DOI] [PubMed] [Google Scholar]
- 29. Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med. 2013;368:1756–1757. [DOI] [PubMed] [Google Scholar]
- 30. Averin K, Hirsch R, Seckeler MD, Whiteside W, Beekman RH III, Goldstein BH. Diagnosis of occult diastolic dysfunction late after the Fontan procedure using a rapid volume expansion technique. Heart. 2016;102:1109–1114. [DOI] [PubMed] [Google Scholar]
- 31. Kim G, Kim J, Lim YL, Kim MY, Baik SK. Renin‐angiotensin system inhibitors and fibrosis in chronic liver disease: a systematic review. Hepatol Int. 2016;10:819–828. [DOI] [PubMed] [Google Scholar]
- 32. Queisser N, Happ K, Link S, Jahn D, Zimnol A, Geier A, Schupp N. Aldosterone induces fibrosis, oxidative stress and DNA damage in livers of male rats independent of blood pressure changes. Toxicol Appl Pharmacol. 2014;280:399–407. [DOI] [PubMed] [Google Scholar]
- 33. Wang S, Zhang Z, Zhu X, Wu H, Gao H, Yang C. Effect of aldosterone and its antagonist on the expression of PAI‐1 and TGF‐β1 in rat hepatic stellate cells. Int J Clin Exp Med. 2014;7:4677–4685. [PMC free article] [PubMed] [Google Scholar]


