Abstract
Background
Mean amplitude of glycemic excursion (MAGE) is commonly used to gauge the degree of glucose level fluctuations. MAGE plays a significant role in vascular endothelial dysfunction and cardiovascular events in patients with diabetes mellitus (DM), but its significance is not clear in non‐DM patients. Thus, we examined the impact of MAGE and vascular endothelial dysfunction on clinical outcomes in non‐DM patients with coronary artery disease.
Methods and Results
We followed non‐DM patients (n=65) for 12 months who underwent percutaneous coronary intervention and assessed the relationship among MAGE, reactive hyperemia index (RHI) measured by reactive hyperemia peripheral arterial tonometry as endothelial function, and cardiovascular events. Cardiovascular events analyzed were cardiovascular death, myocardial infarction, unstable angina, and revascularizations. Compared with patients with MAGE <65 mg/dL (normal glycemic excursions), the group with MAGE ≥65 mg/dL (high glycemic excursions) had significantly higher high‐sensitivity C‐reactive protein (0.10±0.11 mg/dL versus 0.18±0.13 mg/dL, P=0.006) and lower RHI (0.64±0.21 versus 0.51±0.22, P=0.035). The multivariable analysis identified high MAGE and low RHI (≤0.56) as risk factors associated with cardiovascular events (hazard ratio, 5.6; 95% RI, 1.72–18.4 [P=0.004] versus hazard ratio, 4.5; 95% RI, 1.37–14.9 [P=0.013]). When the prognosis was classified by combination with MAGE and RHI, the incidence of cardiovascular events was 46.7% (high MAGE+low RHI), 26.7% (high MAGE+high RHI), 20.0% (low MAGE+low RHI), and 6.6% (low MAGE+high RHI) in descending order (P=0.014). Receiver operating characteristic curve analysis revealed that MAGE, RHI, and MAGE+RHI were each associated with cardiovascular events (area under the curve 0.780, 0.727, and 0.796, respectively).
Conclusions
MAGE was associated with cardiovascular events in non‐DM patients with coronary artery disease. Furthermore, the combination with MAGE and RHI was useful for further subdivision of the risk of cardiovascular events.
Keywords: cardiovascular events, mean amplitude of glycemic excursions, reactive hyperemia index
Subject Categories: Quality and Outcomes
Introduction
It has been reported that intensive blood glucose control decreases the risk of microvascular complications, but not macrovascular disease, in patients with type 2 diabetes mellitus (DM).1, 2, 3 Investigators have highlighted the importance of blood glucose monitoring because it not only reduces glycated hemoglobin, prevents hypoglycemia, and reduces postprandial hyperglycemia, but also narrows the range of glycemic excursions.4, 5 By increasing advanced glycosylation end product levels and protein kinase C activation and attenuating superoxide dismutase glycosylation, hyperglycemia increases oxidative stress, exacerbates insulin resistance, causes chronic inflammation, and impedes vascular endothelial function, ultimately increasing the likelihood of cardiovascular events.6, 7 In contrast, recent reports have stated that methylglyoxal (generated by the breakdown of glucose into pyruvic acid in the glycolytic pathway) is a causative agent of oxidative stress, and its accumulation in the blood is more related to glycemic excursions than hyperglycemia. Furthermore, glycemic excursions are known to lead to endothelial impairments by inducing inflammatory cytokines and increasing oxidative stress.8, 9
Even basic science experiments with the cultures of human umbilical vein endothelial cells have demonstrated that cellular apoptosis is accelerated to a greater degree by repetitive glycemic fluctuations than by continuous hyperglycemia. In addition, in cultured human umbilical vein endothelial cells, exposure to glycemic excursions has been reported to cause greater increases in protein kinase C activity, an exacerbating factor of oxidative stress, resulting in higher levels of oxidative stress markers, 8‐hydroxy‐2′‐deoxyguanosine, and nitrotyrosine.10, 11 These findings suggest that large glycemic excursions may be more closely linked to endothelial dysfunction and cardiovascular events than that of hyperglycemia.
Continuous glucose monitoring (CGM) is used clinically to evaluate glycemic excursions. It has been covered by medical insurance in Japan since 2010 and is generally used to evaluate the glycemic variability of diabetic patients. CGM devices typically measure blood glucose levels at 5‐minute intervals and record up to 288 readings per day. The mean amplitude of glycemic excursion (MAGE) is the mean of blood glucose values exceeding one SD from the 24‐hour mean blood glucose and is used as an index of glycemic variability.4, 5 According to the scientific literature, the value of MAGE in patients without DM are nearly 30 to 40 mg/dL,12, 13, 14 and the cutoff value of MAGE for cardiovascular events are considered nearly 60 to 70 mg/dL.15, 16, 17
In patients with type 2 DM, CGM‐based MAGE has been significantly correlated with urinary 8‐iso‐prostaglandin F2a levels, Gensini score, and reactive hyperemia index (RHI).18, 19, 20, 21 These 3 clinical variables are an oxidative stress marker, a vascular endothelial dysfunction index, and a coronary stenosis index, respectively. Moreover, the association between acute hyperglycemia and endothelial dysfunction have been suggested in not only DM patients but also non‐DM persons.22, 23
CGM‐based MAGE is reportedly an independent risk factor for coronary stenosis. Furthermore, studies that evaluated MAGE in postmyocardial infarction (MI) patients demonstrated that MI patients with high MAGE values had a significantly higher incidence of secondary cardiac events.15, 16, 17 While prior reports have examined the relationship between MAGE and primary and secondary cardiovascular events in diabetic patients, few studies have been conducted in nondiabetic patients.
As previously mentioned, RHI is significantly correlated with MAGE and each one is an independent risk factor associated with cardiovascular events. Therefore, we considered that the combination with MAGE and RHI was useful for further subdivision of the risk of cardiovascular events.
Thus, we examined not only the impact of MAGE on clinical outcomes in non‐DM patients with coronary artery disease but also the prognosis of the patients classified by combination with MAGE and RHI.
Methods
Study Population
We recruited patients with stable angina during their hospitalizations for elective percutaneous coronary intervention (PCI) at Kumamoto University Hospital from May 2013 to April 2014. We excluded patients with heart failure with reduced left ventricular ejection fraction, hemodialysis, malignant diseases, and collagen disease and patients who did not give consent. In addition, patients with elevated white blood cell counts (>9000) and/or serum high‐sensitivity C‐reactive protein (>0.5 mg/dL) were excluded to avoid the potentially confounding effects of occult infection or other systemic inflammatory diseases on high‐sensitivity C‐reactive protein levels.
Patients were considered hypertensive if their blood pressure was >140/90 mm Hg. Chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min·1.73 m2. Dyslipidemia was defined as low‐density lipoprotein >140 mg/dL, high‐density lipoprotein <40 mg/dL, or triglycerides >150 mg/dL. Impaired glucose tolerance was defined as a fasting plasma glucose concentration <126 mg/dL combined with an elevated 2‐hour plasma glucose concentration (≥140 and <200 mg/dL) after a 75‐g glucose load in an oral glucose tolerance test (75‐g OGTT). Patients were assigned the diagnosis of DM if they had an elevated 2‐hour plasma glucose concentration (≥200 mg/dL) after a 75‐g OGTT, an fasting plasma glucose concentration ≥126 mg/dL, a glycated hemoglobin score ≥6.5%, physician‐diagnosed DM, and/or the use of a diabetic medication. CGM, 75‐g OGTT, and reactive hyperemia peripheral arterial tonometry (RH‐PAT) were performed on each different day before undergoing elective PCI after admission.
Patients were followed up prospectively for about 12 months. During the follow‐up period, cardiovascular events were registered, including cardiovascular death, nonfatal myocardial infarction, unstable angina, and revascularization. Cardiovascular events were ascertained from a review of medical records and confirmed by direct contact with the patients, their families, and physicians. For patients who had >2 cardiovascular events, only the first event was considered in the analysis.
The study complied with the Declaration of Helsinki, and informed consent was obtained from all patients.
CGM and MAGE
All patients enrolled in this study were equipped with CGM (iPro2; Medtronic, Minneapolis, MN) and monitored for 48 consecutive hours before undergoing elective PCI. All patients received optimal meals (25–28 kcal/kg of ideal body weight; 60% carbohydrates, 15–20% protein, and 20–25% fat) during CGM. A CGM sensor was inserted into the subcutaneous abdominal fat tissue and calibrated according to the standard Medtronic iPro2 (Medtronic) operating guidelines. While wearing the CGM, the patients checked their blood glucose levels with a self‐monitoring blood glucose device (Medisafe Mini; Terumo, Tokyo, Japan) at least 4 times per day. The patients then entered their self‐monitored blood glucose data and the time of each meal into the CGM. After the patients were monitored for 48 hours, the recorded CGM data were downloaded onto a personal computer and the patient's glucose profile and glucose excursion parameters were analyzed using the iPro2 software. The MAGE value that was reported for each patient was derived from the intermediate 24 hours of each patient's recording to avoid bias due to the insertion and removal of the CGM or insufficient stability of the monitoring system. The MAGE was calculated by measuring the arithmetic mean of the differences between consecutive peaks and nadirs, provided that the differences were >1 SD of the mean glucose value. We used the value of 65 mg/dL to divide patients into low and high MAGE groups because it has been reported that the cutoff value of MAGE for cardiovascular events is 60 to 70 mg/dL.15, 16, 17
RH‐PAT and RH‐PAT Index
RH‐PAT measurements were analyzed with a computerized automated algorithm to reduce intraobserver and interobserver variability (Endo‐PAT2000 software, version 3.0.4; Itamar Medical Ltd, Caesarea, Israel). The RH‐PAT ratio was calculated using the ratio of the average PAT signal amplitude during a 1‐minute interval, starting 1.5 minutes after cuff deflation (where control arm=A and the occluded arm=C), divided by the average PAT signal amplitude 2.5 minutes before cuff inflation (baseline) (where the control arm=B and the occluded arm=D) and the RH‐PAT ratio=(C/D)/(A/B). Because RH‐PAT ratio results have a skewed distribution, we used the Ln RH‐PAT ratio and the RHI for analyses. The RHI was derived from the following equation: RHI=Ln {[RH‐PAT ratio]×[0.226×Ln (baseline)−0.2]}.24, 25 The Ln RH‐PAT ratio and baseline pulse amplitude were retrospectively analyzed using Endo‐PAT2000 software (version 3.4.4); however, reanalysis was impossible in 2 patients for unknown reasons. Peripheral endothelial function as assessed by the RHI was validated by the coronary artery response to acetylcholine, which is the gold standard coronary endothelial functional measurement.26, 27 Previous studies have demonstrated that RH‐PAT technology has excellent reproducibility.28, 29, 30, 31, 32 It was reported that intraobserver and interobserver variability coefficients for RH‐PAT measurements were 16.1% and 22.6%33 and the reproducibility was confirmed by some studies.29, 34 In this study, intraobserver and interobserver variability coefficients for RH‐PAT measurements was 22.1%. We used the median value of the RHI (0.56) to divide patients into low and high RHI groups.
Statistical Analysis
All statistical analyses were performed using SPSS version 22.0 (SPSS Inc, Chicago, IL) and STATA version 11 (StataCorp, College Station, TX). The data are presented as frequencies and percentages for categorical variables and mean±SD for continuous variables, unless otherwise indicated. Differences between the 2 groups were assessed using χ2 and unpaired t tests. The Kaplan–Meier survival curve analysis was used to represent the proportional risk of cardiovascular events based on the MAGE values, and the log‐rank test was performed to assess differences between high levels and low levels of those variables. To ascertain the independent contribution of clinical variables to subsequent cardiovascular events, a multivariable regression analysis using Cox hazard model analysis and stepwise backward method was made. We also examined whether the addition of MAGE improved the discriminatory power of the model of RHI by using receiver operating characteristic (ROC) analysis for cardiovascular events. ROC analysis was performed by logistic model analysis and adjusted by hypertension. We used the bootstrap method to estimate ROC curves and area under the curve (AUC) values. P values <0.05 were regarded as statistically significant.
In sample size, we planned cardiovascular event rates in the high MAGE group as 35% and that in the low MAGE group as 5.0%.15, 16 The power ratio was 0.8 and alpha was 0.05. We needed at least 25 patients with high MAGE and 32 patients with low MAGE in sampling analysis.
Results
From May 2013 to April 2014, 190 patients underwent elective PCI. From these patients, we excluded those with heart failure with reduced left ventricular ejection fraction, hemodialysis, malignant disease, collagen disease, others (without agreement, with infection or other inflammatory diseases), formerly diagnosed DM, and newly diagnosed DM by 75‐g OGTT. After filtering, 65 patients who underwent elective PCI were enrolled in this study. All patients followed up for 12 months (Figure 1). After the CGM reports of the non‐DM patients were analyzed, 26 patients comprised the high MAGE (≥65 mg/dL) group and 39 patients comprised the low MAGE (<65 mg/dL) group.
Figure 1.
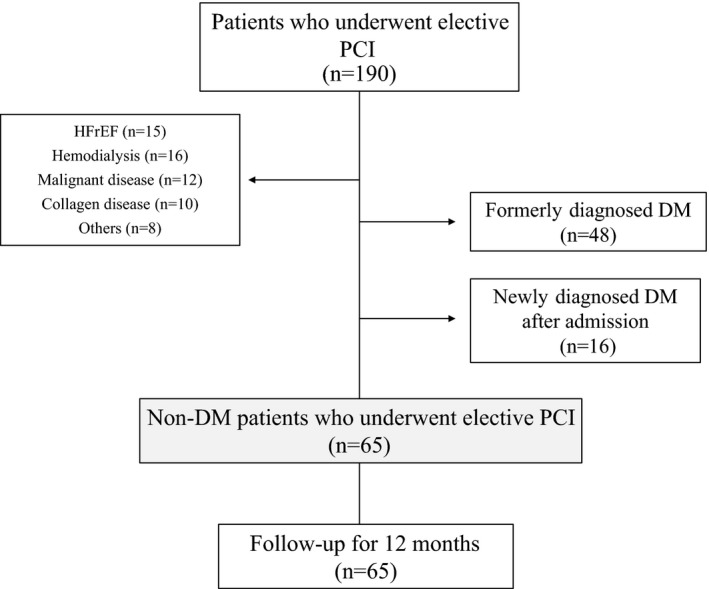
Study protocol flow chart. DM indicates diabetes mellitus; HFrEF, heart failure with reduced left ventricular ejection fraction; PCI, percutaneous coronary intervention.
When the clinical characteristics of the high MAGE and low MAGE groups were compared, hypertension was significantly more prevalent in the high MAGE group than in the low MAGE group (88% versus 67%, P=0.044).
When the glucose parameters were analyzed, the levels of the 1‐hour glucose tolerance test, the mean of the 24‐hour glucose level, and the MAGE were significantly higher in the high MAGE group than the low MAGE group (188.5±36.4 mg/dL versus 164.0±35.3 mg/dL [P=0.010], 127.0±17.91 mg/dL versus 114.4±12.98 mg/dL [P=0.002], and 85.7±20.6 mg/dL versus 39.3±11.5 mg/dL [P<0.001], respectively). The levels of the 2‐hour glucose tolerance test and the insulinogenic index showed a trend toward differences between the 2 groups (160.5±34.2 mg/dL versus 140.2±38.1 mg/dL [P=0.056] and 0.42±0.15 versus 0.67±0.13 [P=0.070], respectively), but these results were not statistically significant. There were no significant differences between the 2 groups with regard to their medical therapies (Table 1).
Table 1.
Clinical Characteristics
| MAGE <65 mg/dL n=39 | MAGE ≥65 mg/dL n=26 | P Value | |
|---|---|---|---|
| Age, mean (SD), y | 70.9 (9.8) | 71.8 (10.3) | 0.729 |
| Men, No. (%) | 19 (70) | 12 (57) | 0.342 |
| Body mass index, mean (SD), kg/m2 | 23.5 (2.82) | 23.8 (4.92) | 0.779 |
| Abdominal circumference, mean (SD), cm | 78.6 (29.7) | 83.2 (32.2) | 0.383 |
| Current smoking, No. (%) | 7 (18) | 4 (17) | 0.860 |
| Hypertension, No. (%) | 26 (67) | 22 (88) | 0.044 |
| Dyslipidemia, No. (%) | 32 (82) | 20 (80) | 0.837 |
| Chronic kidney disease, No. (%) | 11 (28) | 10 (38) | 0.386 |
| Previous MI, No. (%) | 6 (15) | 5 (20) | 0.633 |
| Previous OCI, No. (%) | 5 (13) | 5 (20) | 0.440 |
| PAD, No. (%) | 1 (3) | 1 (4) | 0.730 |
| LDL cholesterol, mean (SD), mg/dL | 96.5 (26.4) | 91.7 (26.7) | 0.496 |
| BNP, mean (SD), pg/mL | 76.1 (64.0) | 68.5 (60.6) | 0.202 |
| LVEF, mean (SD), % | 57.6 (10.6) | 62.2 (6.19) | 0.103 |
| IGT, No. (%) | 18 (46.2) | 14 (53.8) | 0.443 |
| Glycated hemoglobin, mean (SD), % | 5.95 (0.39) | 6.03 (0.37) | 0.387 |
| Fasting glucose level, mean (SD), mg/dL | 99.8 (13.6) | 104 (17.1) | 0.279 |
| 1‐h glucose level, mean (SD), mg/dL | 164.0 (35.3) | 188.5 (36.4) | 0.010 |
| 2‐h glucose level, mean (SD), mg/dL | 140.2 (38.1) | 160.5 (34.2) | 0.056 |
| IRI (pre), mean (SD), μU/mL | 6.77 (3.64) | 7.59 (5.76) | 0.594 |
| IRI (30 min), mean (SD), μU/mL | 46.5 (14.2) | 41.1 (22.4) | 0.465 |
| Insulinogenic index, mean (SD) | 0.67 (0.13) | 0.42 (0.15) | 0.070 |
| HOMA‐β, mean (SD) | 73.2 (29.4) | 72.1 (15.8) | 0.945 |
| HOMA‐IR, mean (SD) | 1.58 (0.86) | 1.81 (1.50) | 0.457 |
| Mean 24‐h glucose level, mean (SD), mg/dL | 114.4 (12.98) | 127.0 (17.91) | 0.002 |
| MAGE, mean (SD), mg/dL | 39.3±11.5 | 85.7±20.6 | <0.001 |
| Aspirin, No. (%) | 34 (87) | 22 (85) | 0.769 |
| Clopidogrel, No. (%) | 30 (77) | 18 (69) | 0.489 |
| ARB or ACEI, No. (%) | 25 (64) | 15 (58) | 0.603 |
| CCB, No. (%) | 20 (51) | 15 (57) | 0.612 |
| β‐Blocker, No. (%) | 26 (67) | 18 (69) | 0.829 |
| Statins, No. (%) | 37 (95) | 24 (93) | 0.792 |
Background between mean amplitude of glycemic excursion (MAGE) <65 and MAGE ≥65 mg/dL. Data are expressed as mean (SD), median values (25th to 75th percentile range), or number (percentage). Insulinogenic index: {IRI (30 minutes)–IRI (pre)}/{glucose level (30 minutes)–fasting glucose level}; HOMA‐β: (IRI×360)/(fasting glucose level‐63); HOMA‐IR: (IRI×fasting glucose level)/405. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CCB, calcium channel blocker; HOMA, Homeostatic Model Assessment; IGT, impaired glucose tolerance; IR, insulin resistance; IRI, immunoreactive insulin; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MAGE, mean amplitude of glycemic excursion; MI, myocardial infarction; OCI, old cerebral infarction; PAD, peripheral arterial disease.
The high‐sensitivity C‐reactive protein levels were significantly higher in the high MAGE group than the low MAGE group (0.18±0.13 mg/dL versus 0.10±0.11 mg/dL, P=0.006) (Figure 2A), and the RHI levels were significantly lower in the high MAGE group than the low MAGE group (0.51±0.22 mg/dL versus 0.64±0.21 mg/dL, P=0.035) (Figure 2B).
Figure 2.
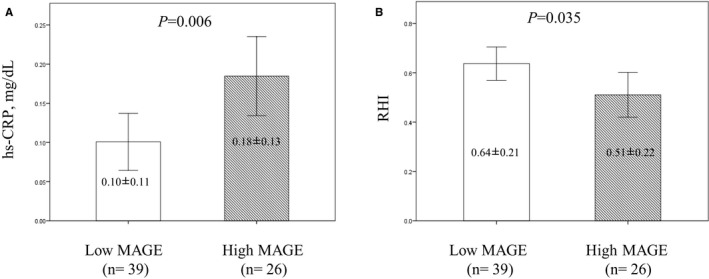
High‐sensitivity C‐reactive protein (hs‐CRP) and reactive hyperemia index (RHI) between the patients with low mean amplitude of glycemic excursion (MAGE) and high MAGE. A, There was a significantly higher hs‐CRP value in the high MAGE group than in the low MAGE group. B, There was a significantly lower mean RHI value in the high MAGE group than the low MAGE group. Data are expressed as mean±SD.
The data of 65 non‐DM patients were available for analyzing cardiovascular events. During the follow‐up period, no patients dropped out. The median follow‐up period was 272 days. The cardiovascular event rate was significantly higher in the high MAGE group than the low MAGE group (26.4% versus 11.8%, P=0.038). There was a trend toward more de novo PCIs in the high MAGE group than the low MAGE group (15.3% versus 5.1%, P=0.078) (Figure 3A, Table 2), but this finding was not statistically significant.
Figure 3.
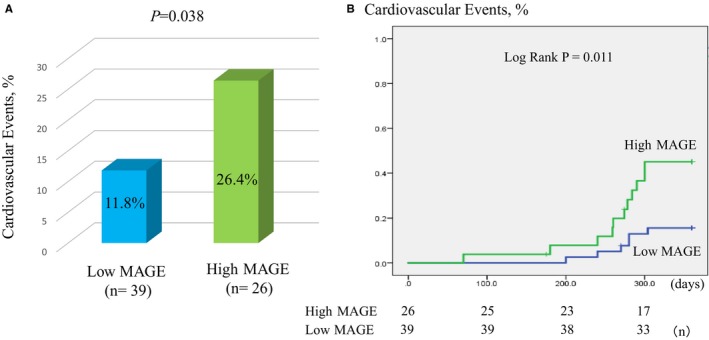
Comparison between the high mean amplitude of glycemic excursion (MAGE) group and the low MAGE group for cardiovascular events. A, There was a significantly higher incidence of cardiovascular events in the high MAGE group than the low MAGE group. B, Kaplan–Meier analysis for cardiovascular events in high‐risk patients based on a MAGE value of 65 mg/dL.
Table 2.
Clinical Outcomes
| MAGE <65 mg/dL n=39 | MAGE ≥65 mg/dL n=26 | P Value | |
|---|---|---|---|
| Cardiovascular events | 6 (11.8) | 9 (26.4) | 0.038 |
| Cardiovascular death | 0 (0) | 0 (0) | 1.000 |
| Myocardial infarction | 0 (0) | 2 (7.7) | 0.217 |
| Unstable angina | 1 (2.6) | 1 (3.8) | 0.778 |
| De novo PCI | 3 (5.1) | 5 (15.3) | 0.078 |
| ISR | 3 (7.7) | 4 (11.5) | 0.327 |
Cardiovascular events between mean amplitude of glycemic excursion (MAGE) <65 mg/dL and MAGE ≥65 mg/dL. Data are expressed as number (percentage). ISR indicates in‐stent restenosis; de novo percutaneous coronary intervention (PCI), PCI for new lesion stenosis (without stenting).
Kaplan–Meier survival curve analysis demonstrated a significantly higher risk of cardiovascular events in the high MAGE group than the low MAGE group (log‐rank P=0.011) (Figure 3B).
Multivariable analysis revealed that hypertension, low RHI (RHI ≤0.56), and high MAGE (MAGE ≥65 mg/dL) were risk factors associated with cardiovascular events (hazard ratio, 3.27; 95% CI, 1.13–9.43 [P=0.028]; hazard ratio, 4.53; 95% CI, 1.37–14.9 [P=0.013]; hazard ratio, 5.63; 95% CI, 1.72–18.4 [P=0.004], respectively) (Table 3).
Table 3.
Multivariable Regression (Stepwise Backward Method) for Cardiovascular Events
| Univariable Regression | Multivariable Regression | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age ≥70 y | 1.01 | 0.95–1.07 | 0.773 | ··· | ··· | ··· |
| Male sex | 1.17 | 0.45–9.74 | 0.342 | ··· | ··· | ··· |
| BMI ≥25 | 0.89 | 0.31–2.57 | 0.834 | ··· | ··· | ··· |
| Current smoking | 1.02 | 0.22–4.75 | 0.975 | ··· | ··· | ··· |
| Dyslipidemia | 1.72 | 0.37–7.99 | 0.488 | ··· | ··· | ··· |
| Hypertension | 1.35 | 0.29–6.25 | 0.258 | 3.27 | 1.13–9.43 | 0.028 |
| CKD | 1.19 | 0.42–3.38 | 0.741 | ··· | ··· | ··· |
| IGT | 2.24 | 0.85–5.92 | 0.201 | 2.72 | 0.97–7.58 | 0.075 |
| RHI ≤0.56 | 3.84 | 1.25–11.8 | 0.019 | 4.53 | 1.37–14.9 | 0.013 |
| MAGE ≥65 mg/dL | 3.44 | 1.27–9.34 | 0.015 | 5.63 | 1.72–18.4 | 0.004 |
BMI indicates body mass index; CKD, chronic kidney disease; HR, hazard ratio; IGT, impaired glucose tolerance; MAGE, mean amplitude of glycemic excursion; RHI, reactive hyperemia index.
The RHI value was used to stratify patients into a high‐level vascular endothelial dysfunction group (RHI ≤0.56) and a normal group (RHI >0.56). When the RHI stratification was analyzed in conjunction with the high MAGE (≥65 mg/dL) and low MAGE (<65 mg/dL) groupings (the P value of the interaction between MAGE and RHI was 0.462), the incidence of cardiovascular events was 46.7% (high MAGE+low RHI group), 26.7% (high MAGE+high RHI group), 20.0% (low MAGE+low RHI group), and 6.6% (low MAGE+high RHI group) in descending order (P=0.014) (Figure 4A).
Figure 4.
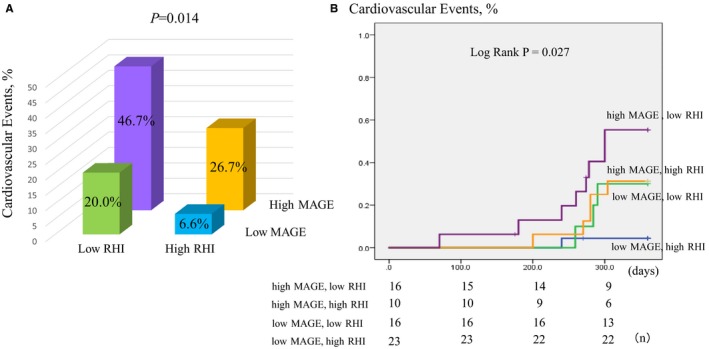
Comparison among the 4 groups by combination with mean amplitude of glycemic excursion (MAGE) and reactive hyperemia index (RHI) for cardiovascular events. A, There was a significantly higher incidence of cardiovascular events in the high MAGE and low RHI groups than the other groups. B, Kaplan–Meier analysis for the cardiovascular events based on the combination with a MAGE value of 65 mg/dL and median RHI value of 0.56.
Kaplan–Meier survival curve analysis also revealed a significantly higher risk of cardiovascular events in the low RHI+high MAGE group (log‐rank P=0.027). Among the 4 pairings, we found that there were no significant differences between the high MAGE+high RHI and the low MAGE+low RHI group (log‐rank P=0.937), and there was a trend toward differences between the low MAGE+high RHI and high MAGE+high RHI groups (log‐rank P=0.081) (Figure 4B).
The ROC curve (logistic model analysis and adjusted by hypertension) demonstrated that each MAGE, RHI, and MAGE+RHI was associated with cardiovascular events (AUC, 0.780; 95% CI, 0.661–0.899 [P=0.004]; AUC, 0.727; 95% CI, 0.566–0.888 [P=0.027]; AUC, 0.796; 95% CI, 0.682–0.910 [P=0.017], respectively). Although not significantly different between the RHI and MAGE+RHI groups (0.727 versus 0.796, P=0.259), the MAGE+RHI group had a higher AUC area than RHI alone (Figure 5A and 5B).
Figure 5.
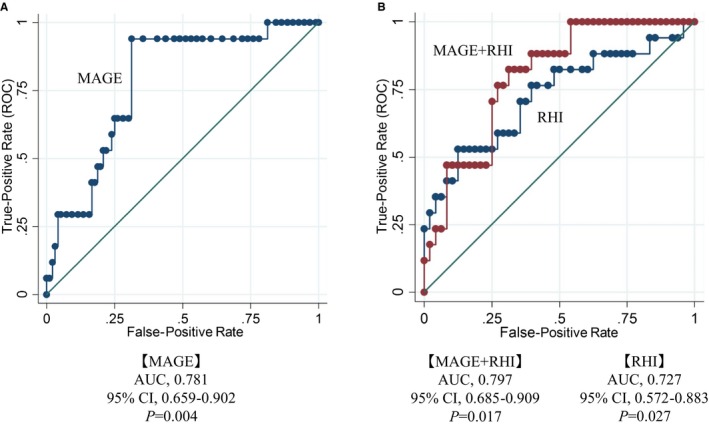
Receiver operating characteristic (ROC) curve for cardiovascular events in mean amplitude of glycemic excursion (MAGE), reactive hyperemia index (RHI), and MAGE+RHI. A, MAGE was a risk factor associated with cardiovascular events. B, Each RHI and MAGE+RHI was a risk factor associated with cardiovascular events. Dates were adjusted by hypertension. AUC indicates area under the curve.
Discussion
In our study, we showed that in nondiabetic post PCI patients, MAGE was correlated with chronic inflammation and impairments of vascular endothelial function, and high MAGE was an independent risk factor associated with cardiovascular events.
The results of this study indicate that non‐DM patients with high MAGE scores have evidence of low initial insulinogenic index. It is postulated that low initial insulinogenic index can result in significant fluctuations in blood glucose levels, even in non‐DM patients. This effect is thought to be associated with the significantly higher levels of glucose detected with 75‐g OGTT, particularly during the first hour. In addition, there were significantly more patients with hypertension in this high MAGE group. Patients with hypertension will have the insulin resistance and lead to high glycemic excursions. Hypertension may be an early predictor of high MAGE in non‐DM patients.
In studies that have employed optical coherence tomography to assess the coronary artery at the time of a myocardial infarction, investigators determined that plaque ruptures were significantly more frequent in patients with high MAGE values, even in non‐DM patients.35 Moreover, reports from prior studies have noted that ≈50% of myocardial infarctions originate from changes in an existing pathology where treatment with a stent was deemed unnecessary.36 Furthermore, a recent study suggested that high MAGE measured early after the onset of a first‐episode acute coronary syndrome was correlated with thinner fibrous cap measurements and a higher prevalence of thin‐cap fibroatheroma at the nonculprit plaque in the nonculprit vessel.37 The findings by these investigators may support our results that cardiovascular events, especially in de novo PCI, were frequently higher in the high MAGE group than that in the low MAGE group.
We not only showed the impact of MAGE on clinical outcomes in non‐DM patients with coronary artery disease but also that the 4 pairings (combination with MAGE and RHI) were useful for further subdivision of the risk of cardiovascular events. Among the 4 pairings, there was a trend toward differences between the low MAGE+high RHI and high MAGE+high RHI groups. That may mean even if the patients have high RHI values (ie, those who maintained a certain degree of vascular endothelial function), the patients with high MAGE values (ie, large glycemic excursions) may have higher risk for cardiovascular events. Moreover, there were no significant differences between the low MAGE+low RHI and high MAGE+high RHI groups. This may mean that even if the patients have high RHI values, the patients with high MAGE values may have a similar risk as patients with low RHI values. Thus, it is possible that our findings suggest that glycemic excursions were not merely a cause of vascular endothelial dysfunction but also an early risk factor associated with such dysfunction, and that they could be used to associate future deterioration of the vascular endothelium.
No significant differences between RHI and MAGE+RHI were seen in the ROC curve, but MAGE+RHI was associated with higher AUC area than RHI alone. Investigation with a larger sample size may elucidate the synergistic effect of MAGE+RHI rather than RHI alone associated with cardiovascular events.
Clinical Implications
In patients with DM and those with impaired glucose tolerance, alpha glucosidase inhibitors, pioglitazone, and dipeptidyl peptidase 4 inhibitors have previously been reported to improve glycemic excursions, reduce inflammation, and ameliorate coronary arteriolar thickening.38, 39, 40 Both DM and non‐DM patients who exhibit postprandial hyperglycemia have been reported to have exacerbated insulin resistance and decreased glucagon‐like peptide‐1 levels.41 These adverse effects on glucose handling and endothelial function can potentially be ameliorated by glycemic variability–reducing drugs, such as dipeptidyl peptidase 4 inhibitors, when taken by nondiabetic patients with high MAGE.42
Limitations and Strengths
The limitations of this study include the small sample size and single‐center design and that cardiovascular events were not adjudicated. As a method to measure MAGE, the CGM system employed in this study was expensive, invasive, and time‐consuming. We hope that a simple and inexpensive biomarker for measuring glycemic excursions will be available in the future.
The study's strengths include the fact that it was a prospective follow‐up study of non‐DM patients identified by 2‐hour glucose tolerance test, and all of the patients had measurements of MAGE obtained by CGM and RHI determined by RH‐PAT. At the hospital where the study was conducted, patients are typically seen for post‐PCI follow‐up 1, 3, 9, and 12 months following discharge. Furthermore, all of the patients were provided with nutrition education prior to discharge in an attempt to raise their awareness of blood glucose monitoring.
Family history of DM is one of the main findings of early‐phase endothelial function and cardiovascular events, especially in patients with pre‐DM.43 However, we did not have complete data on family history of DM in this study.
Finally, ranolazine can be used in patients with stable angina44 and can improve insulin resistance45 and influence glycemic control.46 Taking ranolazine is a confounding factor; however, ranolazine is not for off‐label use in Japan and there were no patients taking ranolazine in this study.
Conclusions
MAGE was associated with cardiovascular events in non‐DM patients with coronary artery disease. Furthermore, the combination of MAGE and RHI was useful for further subdivision of the risk of cardiovascular events.
Sources of Funding
This study was supported in part by grants‐in‐aid for the Japan Heart Foundation, Tokyo, and the National Cerebral and Cardiovascular Center Foundation, Osaka, Japan.
Disclosures
None.
Acknowledgments
We wish to thank medical secretaries S. Iwasita, M. Oda, R. Usui, and S. Ogata (Kumamoto University) for research and data collection.
(J Am Heart Assoc. 2017;6:e004841 DOI: 10.1161/JAHA.116.004841.)28446494
References
- 1. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33), UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2. Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;2008:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;2008:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real‐time continuous glucose sensor a randomized controlled trial. Diabetes Care. 2006;29:44–50. [DOI] [PubMed] [Google Scholar]
- 5. Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemoglobin: a pilot study. Diabetes Res Clin Pract. 1999;46:183–190. [DOI] [PubMed] [Google Scholar]
- 6. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans role of oxidative stress. Circulation. 2002;106:2067–2072. [DOI] [PubMed] [Google Scholar]
- 7. Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163:1306–1316. [DOI] [PubMed] [Google Scholar]
- 8. Ogawa S, Kobori H, Ohashi N, Urushihara M, Nishiyama A, Mori T, Ishizuka T, Nako K, Ito S. Angiotensin II type 1 receptor blockers reduce urinary angiotensinogen excretion and the levels of urinary markers of oxidative stress and inflammation in patients with type 2 diabetic nephropathy. Biomark Insights. 2009;4:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogawa S, Nakayama K, Nakayama M, Mori T, Matsushima M, Okamura M, Senda M, Nako K, Miyata T, Ito S. Methylglyoxal is a predictor in type 2 diabetic patients of intima‐media thickening and elevation of blood pressure. Hypertension. 2010;56:471–476. [DOI] [PubMed] [Google Scholar]
- 10. Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924–E930. [DOI] [PubMed] [Google Scholar]
- 11. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells the role of protein kinase C and NAD(P)H‐oxidase activation. Diabetes. 2003;52:2795–2804. [DOI] [PubMed] [Google Scholar]
- 12. Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. [DOI] [PubMed] [Google Scholar]
- 13. Zhou J, Li H, Ran X, Yang W, Li Q, Peng Y, Li Y, Gao X, Luan X, Wang W. Establishment of normal reference ranges for glycemic variability in Chinese subjects using continuous glucose monitoring. Med Sci Monit. 2010;17:CR9–CR13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su G, Mi SH, Li Z, Tao H, Yang HX, Zheng H. Prognostic value of early in‐hospital glycemic excursion in elderly patients with acute myocardial infarction. Cardiovasc Diabetol. 2013;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, Zhou Y, Tian L. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36:1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang JW, He LJ, Cao SJ, Yang Q, Yang SW, Zhou YJ. Effect of glycemic variability on short term prognosis in acute myocardial infarction subjects undergoing primary percutaneous coronary interventions. Diabetol Metab Syndr. 2014;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Xu X, Jiao X, Wu J, Zhou S, Lv X. The effects of glucose fluctuation on the severity of coronary artery disease in type 2 diabetes mellitus. J Diabetes Res. 2013;2013:576916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, Zhou Y, Ma C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow‐mediated endothelium‐dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. [DOI] [PubMed] [Google Scholar]
- 23. Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium‐dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. [DOI] [PubMed] [Google Scholar]
- 24. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 25. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]
- 27. Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 28. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S. The assessment of endothelial function from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. [DOI] [PubMed] [Google Scholar]
- 30. Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, de Ferranti SD. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–905. [DOI] [PubMed] [Google Scholar]
- 31. Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–1786. [DOI] [PubMed] [Google Scholar]
- 32. Komura N, Tsujita K, Yamanaga K, Sakamoto K, Miyazaki T, Tabata N, Ishii M, Akasaka T, Arima Y, Ono T. Impaired peripheral endothelial function assessed by digital reactive hyperemia peripheral arterial tonometry and risk of in‐stent restenosis. Circulation. 2014;130:A11845–A11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. JCS Joint Working Group . Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–2801. [DOI] [PubMed] [Google Scholar]
- 34. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 35. Teraguchi I, Imanishi T, Ozaki Y, Tanimoto T, Ueyama M, Orii M, Shiono Y, Shimamura K, Ishibashi K, Yamano T. Acute‐phase glucose fluctuation is negatively correlated with myocardial salvage after acute myocardial infarction. Circ J. 2014;78:170–179. [DOI] [PubMed] [Google Scholar]
- 36. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 37. Gohbara M, Hibi K, Mitsuhashi T, Maejima N, Iwahashi N, Kataoka S, Akiyama E, Tsukahara K, Kosuge M, Ebina T. Glycemic variability on continuous glucose monitoring system correlates with non‐culprit vessel coronary plaque vulnerability in patients with first‐episode acute coronary syndrome‐optical coherence tomography study. Circ J. 2016;80:202–210. [DOI] [PubMed] [Google Scholar]
- 38. Hanefeld M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc Diabetol. 2007;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, Mawatari K, Yasukawa H, Kaida H, Ishibashi M. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes: a prospective, randomized, comparator‐controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging. 2011;4:1110–1118. [DOI] [PubMed] [Google Scholar]
- 40. Kaku K, Kadowaki T, Terauchi Y, Okamoto T, Sato A, Okuyama K, Arjona Ferreira J, Goldstein B. Sitagliptin improves glycaemic excursion after a meal or after an oral glucose load in Japanese subjects with impaired glucose tolerance. Diabetes Obes Metab. 2015;17:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–E206. [DOI] [PubMed] [Google Scholar]
- 42. Imai C, Harazaki T, Inoue S, Mochizuki K, Goda T. Treatment with DPP‐4I anagliptin or α‐GI miglitol reduces IGT development and the expression of CVD risk factors in OLETF rats. J Nutr Sci Vitaminol. 2015;61:313–321. [DOI] [PubMed] [Google Scholar]
- 43. Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F, Dentamaro I, Gentile F, Gesualdo M, Maiello M, Modesti PA. Endothelial function in pre‐diabetes, diabetes and diabetic cardiomyopathy: a review. J Diabetes Metab. 2014;5:364. [Google Scholar]
- 44. Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, Ben‐Yehuda O, Katz A, Jones PG, Olmsted A, Belardinelli L, Chaitman BR. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol. 2013;61:2038–2045. [DOI] [PubMed] [Google Scholar]
- 45. Eckel RH, Henry RR, Yue P, Dhalla A, Wong P, Jochelson P, Belardinelli L, Skyler JS. Effect of ranolazine monotherapy on glycemic control in subjects with type 2 diabetes. Diabetes Care. 2015;38:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caminiti G, Fossati C, Battaglia D, Massaro R, Rosano G, Volterrani M. Ranolazine improves insulin resistance in non‐diabetic patients with coronary heart disease. A pilot study. Int J Cardiol. 2016;219:127–129. [DOI] [PubMed] [Google Scholar]


