Abstract
Background
Atrial fibrillation (AF) is the most common cardiac dysrhythmia associated with significant morbidity and mortality. Several small studies have reported that low serum total testosterone (TT) levels were associated with a higher incidence of AF. In contrast, it is also reported that anabolic steroid use is associated with an increase in the risk of AF. To date, no study has explored the effect of testosterone normalization on new incidence of AF after testosterone replacement therapy (TRT) in patients with low testosterone.
Methods and Results
Using data from the Veterans Administrations Corporate Data Warehouse, we identified a national cohort of 76 639 veterans with low TT levels and divided them into 3 groups. Group 1 had TRT resulting in normalization of TT levels (normalized TRT), group 2 had TRT without normalization of TT levels (nonnormalized TRT), and group 3 did not receive TRT (no TRT). Propensity score–weighted stabilized inverse probability of treatment weighting Cox proportional hazard methods were used for analysis of the data from these groups to determine the association between post‐TRT levels of TT and the incidence of AF. Group 1 (40 856 patients, median age 66 years) had significantly lower risk of AF than group 2 (23 939 patients, median age 65 years; hazard ratio 0.90, 95% CI 0.81–0.99, P=0.0255) and group 3 (11 853 patients, median age 67 years; hazard ratio 0.79, 95% CI 0.70–0.89, P=0.0001). There was no statistical difference between groups 2 and 3 (hazard ratio 0.89, 95% CI 0.78– 1.0009, P=0.0675) in incidence of AF.
Conclusions
These novel results suggest that normalization of TT levels after TRT is associated with a significant decrease in the incidence of AF.
Keywords: atrial fibrillation, testosterone, testosterone replacement therapy
Subject Categories: Arrhythmias
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide, with significant morbidity, mortality, and financial burden.1, 2, 3 In the United States, 2.7 to 6.1 million people had AF in 2010, and that number is expected to double by 2050.4, 5 The prevalence and incidence of AF is known to increase with age and is higher in men than in women.6 Although the underlying mechanisms of this sex difference are still unclear, 1 preclinical and several small clinical studies have suggested that testosterone deficiency may play a role in the development of AF.7, 8, 9 It was also reported that anabolic steroid use was associated with an increased risk of AF.10, 11
Serum levels of testosterone decline with age.12 Hypogonadism is estimated to be present in 38% of men aged >45 years.13 However, the effect of testosterone replacement therapy (TRT) on cardiovascular health is a topic of ongoing debate.14, 15 Our group and others have reported that normalization of testosterone levels after TRT is associated with a significant decrease in all‐cause mortality, myocardial infarction, and stroke.16 In addition, we found that TRT did not increase the incidence of deep venous thrombosis or pulmonary embolism.17
To date, no studies have investigated the effect of testosterone‐level normalization on incidence of new AF in men after TRT. Patients receiving TRT are older, and advanced age increases the risk of AF; therefore, novel information on the effect of testosterone levels and TRT on the incidence of AF would be clinically significant. In this study, we investigated the incidence of AF in hypogonadal men with documented low testosterone levels. We compared the incidence of AF among patients who did not receive any TRT, those who received TRT that resulted in normalization of total testosterone (TT), and those who received TRT but that did not result in normal TT levels. This allowed us to evaluate the impact of nontreatment as well as the impact of adequate replacement therapy on AF incidence in this specific population. We hypothesized that normalization of TT levels after TRT would be associated with a significant decrease in the incidence of AF.
Methods
Data Source
The US Veterans Health Administration (VHA) provides care to veterans at >1400 establishments across the United States. Clinical data from these establishments are archived in the Corporate Data Warehouse and made available for research through the Veterans Administration Informatics and Computing Infrastructure. Information regarding data quality can be found at the US Department of Veterans Affairs (VA) Information Resource Center (http://www.virec.research.va.gov). We conducted a retrospective cohort study of male veterans who received medical care through the VHA during the period December 1999 to May 2014. The institutional review board of the Kansas City VA Medical Center approved the study. Because this study used retrospective database research, the requirement for informed consent was waived.
Ascertainment of TT Level
We observed wide variability in the units used to express testosterone test results obtained from a large number of laboratories in the VA health system over the long follow‐up period involved in this study. In addition, reported TT levels lacked uniform normal laboratory reference ranges. Thus, TT was considered low when the reported value was less than the lower limit of the normal laboratory reference range of the test result. We used this method rather than a discrete cutoff value because we found that reference ranges and reporting units varied with assays used at different facilities across the VA.18, 19 Even in the same hospital, the assay used changed over time. A lack of standardization for testosterone levels and other tests using stoichiometric measurements further adds to differences in the presentation of test results.20, 21 Consequently, we classified each test result as low or normal TT based on the respective normal laboratory reference range reported with the test result, as this was the most accurate method to minimize the effect of use of multiple assays.
Exposure Variable
We determined the use of TRT from patient medication prescription records. Patients who received any form of TRT (injection, gel, or patch) were considered to as treated. Untreated patients did not receive any form of TRT. Those who received TRT were categorized as normalized treated or nonnormalized treated depending on whether their TT levels improved above the normal laboratory reference range while on treatment or remained low.
Outcome Variable
Primary outcome measure was the incidence of AF (International Classification of Diseases, Ninth Revision [ICD‐9] code 427.31).
Confounding Factors
Confounding measures were patient demographics; comorbidity, such as diabetes mellitus, hypertension, chronic obstructive pulmonary disease, obstructive sleep apnea, congestive heart failure, peripheral vascular disease, coronary artery disease, hyperthyroidism, rheumatic valve disease, nonrheumatic valve disease, structural heart disease, and cardiomyopathy; baseline body mass index; low‐density lipoprotein; and use of aspirin, β‐blockers, and statins. The ICD‐9 Clinical Modification diagnosis codes were used to capture the coexisting conditions.
Study Population
Figure 1 presents the patient‐selection process.
Figure 1.
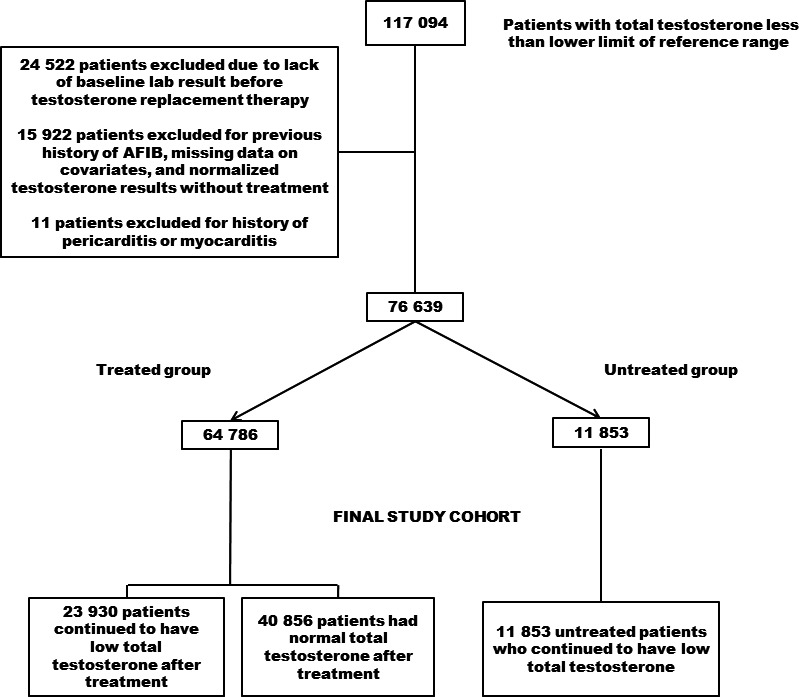
Methodology and patient‐selection process, with inclusion and exclusion criteria to obtain the final cohort. AFib indicates atrial fibrillation.
Inclusion criteria
We included patients whose TT levels in the first test were lower than the respective normal laboratory reference range.
Exclusion criteria
We excluded (1) women, (2) patients who received TRT before the first results showing low TT, (3) those who had AF before the first day of this study, (4) those with normalized TT levels on second testing without evidence of TRT, and (5) those with history of pericarditis and myocarditis.
Statistical Analysis
To enhance the robustness of our analysis and to ensure that the groups being compared were well matched, we utilized propensity score–weighted stabilized inverse probability of treatment weights (IPTW). Use of IPTW allowed us to keep the largest number of participants in the study after matching while using the propensity scores to achieve a balance between each pair of subgroups studied. Using this method, participants are weighted by the inverse probability of their treatment status. We preferred a stabilized IPTW approach to regular IPTW or propensity score matching because stabilized IPTW enhances inclusion of the largest number of patients in the analysis, whereas propensity score matching may result in a decreased sample size. In addition, stabilized IPTW significantly corrects for the instability in estimated treatment weights that potentially results from the use of regular IPTW for participants with very low probability of treatment.22 Furthermore, propensity scores were used to correct for potential systematic differences between treated and untreated participants. Each participant's propensity scores for receiving TRT were computed and adjusted for the covariates in a logistic regression analysis. The covariates included were age, body mass index, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, obstructive sleep apnea, congestive heart failure, peripheral vascular disease, coronary artery disease, hyperthyroidism, rheumatic valve disease, nonrheumatic valve disease, structural heart disease, cardiomyopathy, low‐density lipoprotein, and use of aspirin, β‐blockers, and statins.
We computed the incidence of AF in each subgroup. The χ2 test and Student t test were used to compare normally distributed baseline characteristics of patients. Nonparametric tests were used for nonnormally distributed variables. Univariate and multivariable Cox proportional hazards regression analyses were conducted to assess the differences between groups. Continuous variables were reported as mean and standard deviation, categorical variables were reported as percentages. We also applied the stabilized IPTW to obtain Kaplan–Meier survival curves and to compare event‐free survival time between groups, along with log‐rank P value. Patients who did not develop AF episode were censored at the end date of the observation period. Patients who died were censored at the time of death. This enabled us to capture the time contributed to the study by all enrolled patients. We assumed that censoring was random and unrelated to the likelihood of developing AF; therefore, patient failure times or time to incident AF would be the same as if these participants were actually observed for the study period. SAS Enterprise Guide 7.1 supported on SAS 9.4 (SAS Institute) was used for statistical analyses, with TRT as a time‐varying exposure variable. The study hypothesis was tested at a 2‐sided level of significance with P<0.05.
Results
Cohort Description
We identified 117 094 patients with low TT. Of these, we excluded 24 522 patients who did not have baseline TT results before initial TRT. We also excluded 15 922 patients who had previous history of AF, those who had missing data on the baseline covariates, and those whose achieved normalized TT without any record of treatment, and 11 patients were excluded for history of pericarditis and/or myocarditis. The final cohort for the study comprised 76 639 participants who were later divided into 40 856 normalized‐treated and 23 930 nonnormalized‐treated, and 11 853 untreated patients.
We excluded those with normalized TT without any record of treatment because we could not rule out the possibility of non‐VA prescriptions as a reason for this finding. To prevent misclassification bias, these participants with spuriously normalized testosterone levels were excluded.
Baseline Characteristics of Study Participants
Table 1 presents the baseline variables for the unmatched and stabilized IPTW‐matched cohorts. Median ages at enrollment were 66.0, 65.0, and 67.0 years for groups 1, 2, and 3, respectively. The mean body mass index (kg/m2) at enrollment was 33.1 (SD 6.6), 33.7 (SD 6.9), and 32.9 (SD 6.8) for groups 1, 2, and 3, respectively. Mean follow‐up time was 6.0±3.1 years for the normalized TRT group, 4.4±2.9 years for the nonnormalized TRT group, and 4.5±2.9 years for the untreated group.
Table 1.
Baseline Characteristics of All Patients in the Study Before and After Propensity Matching
| Baseline Characteristics of Cohorts | P Values for Unmatched and IPTW‐Matched Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normalized Treated (n=40 856) | Nonnormalized Treated (n=23 930) | Untreated (n=11 853) | 1 P Valuea | 1 P Valueb | 1 P Valuec | 2 P Valuea | 2 P Valueb | 2 P Valuec | |
| Age ≥50 y, n (%) | 36 590 (89.6) | 21 260 (88.8) | 10 687 (90.2) | 0.0044 | 0.0567 | 0.0001 | 0.9910 | 0.9910 | 0.9958 |
| Age, median (y) | 66.0 | 65.0 | 67.0 | ||||||
| Body mass index ≥30 | 27 034 (66.2) | 16 614 (69.4) | 7583 (64.0) | <0.0001 | <0.0001 | <0.0001 | 0.9306 | 0.9306 | 0.9498 |
| Body mass index, mean (SD) | 33.1 (6.6) | 33.7 (6.9) | 32.9 (6.8) | ||||||
| Follow‐up time (y), mean (SD) | 6.0 (3.1) | 4.4 (2.9) | 4.5 (2.9) | ||||||
| Hypertension, n (%) | 7252 (17.8) | 4931 (20.6) | 2108 (17.8) | <0.0001 | 0.9313 | <0.0001 | 0.9406 | 0.9406 | 0.9403 |
| Diabetes mellitus, n (%) | 12 666 (31.0) | 8787 (36.7) | 3886 (32.8) | <0.0001 | 0.0002 | <0.0001 | 0.9925 | 0.9925 | 0.9260 |
| Chronic obstructive pulmonary disease, n (%) | 489 (1.2) | 427 (1.8) | 188 (1.6) | <0.0001 | 0.0009 | 0.1744 | 0.9478 | 0.9478 | 0.9121 |
| Obstructive sleep apnea, n (%) | 761 (1.9) | 671 (2.8) | 259 (2.2) | <0.0001 | 0.0249 | 0.0005 | 0.9983 | 0.9983 | 0.8923 |
| Congestive heart failure, n (%) | 643 (1.6) | 589 (2.5) | 288 (2.4) | <0.0001 | <0.0001 | 0.8557 | 0.9937 | 0.9937 | 0.8371 |
| Peripheral vascular disease, n (%) | 354 (0.9) | 291 (1.2) | 146 (1.2) | <0.0001 | 0.0003 | 0.8987 | 0.9851 | 0.9851 | 0.8857 |
| Coronary artery disease | 2258 (5.5) | 1695 (7.1) | 697 (5.9) | <0.0001 | 0.1406 | <0.0001 | 0.9646 | 0.9646 | 0.9668 |
| Depression, n (%) | 3477 (8.5) | 2187 (9.1) | 780 (6.6) | 0.0062 | <0.0001 | <0.0001 | 0.9594 | 0.9594 | 0.8799 |
| Cardiomyopathy, n (%) | 840 (2.1) | 587 (2.5) | 327 (2.8) | 0.0009 | <0.0001 | 0.0844 | 0.9903 | 0.9903 | 0.9613 |
| Rheumatic valve disease, n (%) | 484 (1.2) | 380 (1.6) | 181 (1.5) | <0.0001 | 0.0033 | 0.6624 | 0.9913 | 0.9913 | 0.8648 |
| Nonrheumatic valve disease, n (%) | 1427 (3.5) | 938 (3.9) | 577 (4.9) | 0.0052 | <0.0001 | <0.0001 | 0.9814 | 0.9814 | 0.8922 |
| Structural heart disease, n (%) | 758 (1.9) | 531 (2.2) | 266 (2.2) | 0.0014 | 0.0069 | 0.8792 | 0.9525 | 0.9525 | 0.9111 |
| Bacterial endocarditis, n (%) | 47 (0.1) | 47 (0.1) | 17 (0.1) | 0.9433 | 0.4346 | 0.5069 | 0.9533 | 0.9533 | 0.9734 |
| Hyperthyroidism, n (%) | 559 (1.4) | 273 (1.1) | 180 (1.5) | 0.0131 | 0.2202 | 0.0026 | 0.9451 | 0.9451 | 0.9662 |
| LDL >100 mg/dL, n (%) | 21 082 (51.6) | 11 458 (47.9) | 5778 (48.8) | <0.0001 | <0.0001 | 0.1229 | 0.9476 | 0.9476 | 0.8929 |
| Concomitant therapy | |||||||||
| Antiplatelet agents, n (%) | 11 907 (29.1) | 7535 (31.5) | 3550 (30.0) | <0.0001 | 0.0895 | 0.0031 | 0.9708 | 0.9708 | 0.9679 |
| β‐Blockers, n (%) | 15 336 (37.5) | 9999 (41.8) | 4579 (38.6) | <0.0001 | 0.0304 | <0.0001 | 0.9765 | 0.9765 | 0.9549 |
| Statins, n (%) | 24 352 (59.6) | 15 141 (63.2) | 7134 (60.2) | <0.0001 | 0.2547 | <0.0001 | 0.9619 | 0.9619 | 0.9592 |
1 P indicates unmatched P value; 2 P, matched P value; IPTW, inverse probability of treatment weights; LDL, low‐density lipoprotein.
Normalized treated vs nonnormalized treated.
Normalized treated vs untreated.
Nonnormalized treated vs untreated.
Relationship Between TRT and AF
The incidence of AF was as follows: group 1 (normalized TRT), 497 per 100 000 person years; group 2 (nonnormalized TRT), 626 per 100 000 person‐years; group 3 (no TRT), 697 per 100 000 person‐years. Table 2 presents the results of Cox proportional hazards regression analysis. Group 1 had significantly lower risk of AF than group 2 (hazard ratio 0.90, 95% CI 0.81–0.99, P=0.0255) and group 3 (hazard ratio 0.79, 95% CI 0.70–0.89, P=0.0001). There was no statistically significant decrease in AF risk between groups 2 and 3 (hazard ratio 0.89, 95% CI 0.78–0.1.009, P=0.0675).
Table 2.
The Risk of Incident AF Was Significantly Lower in the Normalized Treated Group Compared With the Nonnormalized Treated and Untreated Groups
| Hazard Ratios for Atrial Fibrillation | |||
|---|---|---|---|
| HR | 95% CI | P Value | |
| Normalized treated vs untreated (reference) | |||
|
Univariate n=40 856 vs n=11 853 |
0.713 | 0.635–0.801 | <0.0001 |
|
Propensity matched (stabilized IPTW) n=40 857 vs n=11 853 |
0.792 | 0.702–0.893 | 0.0001 |
| Normalized treated vs nonnormalized treated (reference) | |||
|
Univariate n=40 856 vs n=23 930 |
0.800 | 0.727–0.880 | <0.0001 |
|
Propensity matched (stabilized IPTW) n=40 859 vs n=23 928 |
0.896 | 0.813–0.987 | 0.0255 |
| Nonnormalized treated vs untreated (reference) | |||
|
Univariate n=23 930 vs n=11 853 |
0.895 | 0.788–1.017 | 0.0881 |
|
Propensity matched (stabilized IPTW) n=23 930 vs n=11 855 |
0.888 | 0.782–1.009 | 0.0675 |
The risk was also lower in non‐normalized treated group compared to the untreated group but lacks statistical significance. AF indicates atrial fibrillation; HR, hazard ratio; IPTW, inverse probability of treatment weights.
The Kaplan–Meier curves (Figure 2A and 2B) showed that the normalized‐TRT group was associated with significantly higher probability of AF‐free survival compared with the no‐TRT group (log‐rank P=0.0001) (Figure 2A) or the nonnormalized‐TRT group (log‐rank P=0.03) (Figure 2B). There was no significant difference regarding AF‐free survival probability between the nonnormalized‐ and no‐TRT groups (Figure 2C).
Figure 2.
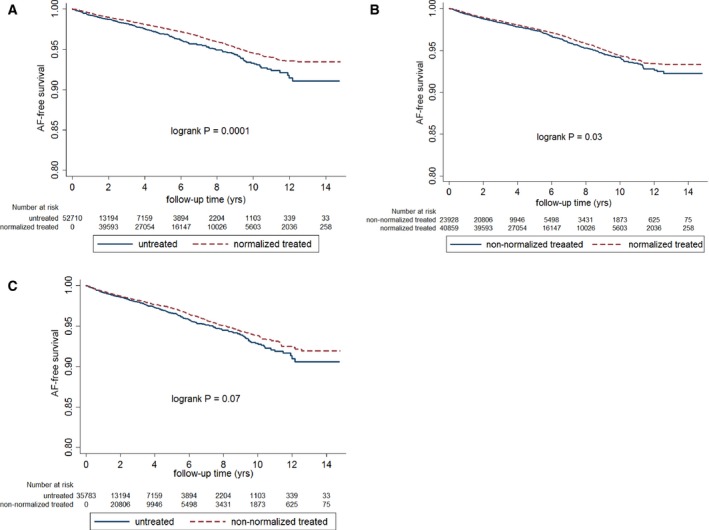
A, Kaplan–Meier curve showing atrial fibrillation–free (AF‐free) survival probability between normalized treated and untreated participants. Normalized treated patients had higher AF‐free survival probability than untreated participants. B, Kaplan–Meier curve showing AF‐free survival probability between normalized and nonnormalized treated patients. Normalized treated patients had higher AF‐free survival probability than the nonnormalized treated patients. C, Kaplan–Meier curve showing AF‐free survival probability between nonnormalized treated and untreated participants. There was no statistically significant difference regarding AF‐free survival probability between nonnormalized treated and no‐TRT groups.
Discussion
This study highlights that normalization of TT levels by TRT is associated with a significantly lower incidence of AF in men with low initial TT levels and without prior history of AF compared with a matched group of participants who did not receive TRT. In addition, the incidence of AF was higher in participants who failed to achieve normal TT levels after TRT (likely due to inadequate replacement dose or noncompliance) compared with those with normalization of TT levels following TRT. Our retrospective study describes the largest cohort of such patients and the longest follow‐up of TRT to date.
AF is the most common cardiac arrhythmia encountered in clinical practice.3 Significant sex‐ and age‐based differences exist in the prevalence of AF. Men have a 1.5‐fold higher risk of developing AF compared with women after adjustment for several confounding factors.23 In addition, age‐specific differences in the prevalence of AF were more pronounced in men compared with women.24, 25 Increase in the incidence of AF in men appears to coincide with a decrease in serum testosterone levels related to aging.26 In a preclinical study, Tsuneda et al used orchiectomized male Sprague‐Dawley rats to show that electrical stimulus caused a significant increase in repetitive atrial responses without changes in other electrophysiological properties. They also found that increased atrial responses were abolished by administration of testosterone. The authors proposed that deficiency of testosterone was associated with increased atrial arrhythmogenicity and AF.8 It is also important to note that Tsai et al showed that in aged rabbits, testosterone replacement increased pulmonary vein and left atrium arrhythmogenesis by enhancing adrenergic activity.27
The mechanisms by which normal levels of testosterone might reduce risk of AF development remain to be established and are likely to be multifactorial. It has been proposed that calcium leakage caused by phosphorylation of ryanodine receptor type 2 from the sarcoplasmic reticulum plays a pivotal role in pathogenesis and progression of AF.28 Tsuneda et al, in their orchiectomized male rat model, showed that the difference in atrial response was associated with increased ryanodine receptor type 2 and sodium‐calcium exchange, which were attenuated by administration of testosterone. This study suggests an antiarrhythmogenic property for testosterone.8 In recent years, inflammation has been implicated in the pathogenesis of AF.29, 30, 31, 32, 33 Inflammatory markers such tumor necrosis factor α, interleukin 6, interleukin 2, and C‐reactive protein were all found to be elevated in patients with AF.34, 35, 36, 37, 38, 39, 40 Although data are not consistent, several authors have reported decreased levels of tumor necrosis factor α, interleukin 6, C‐reactive protein, and other proinflammatory cytokines after TRT.41, 42, 43, 44, 45 Consequently, the anti‐inflammatory property of testosterone is a potential mechanism for the observed reduction in the risk of AF. However, additional basic science as well as clinical studies will be needed to appropriately define the mechanisms of testosterone‐induced reduction in the risk of AF.
The first clinical study that suggested an association between low testosterone and AF was a single‐center study of 58 male participants with lone AF. Participants were age and sex matched. The mean age of the participants was 46.1±9.7 for lone AF and 45.2±8.6 years for control. This study found that mean testosterone levels were significantly lower in participants with lone AF.7 Recently, a study included 1251 participants (aged 68.0±8.2 years) and assessed the 10‐year risk of AF by utilizing multivariable‐adjusted hazard models.46 Participants were stratified into ages 55 to 69 years (n=786), 70 to 79 years (n=351), and ≥80 years (n=114). The authors found that the risk of AF increased by 1.3‐ and 3.5‐fold in men aged 55 to 69 years and ≥80 years, respectively, with every 1‐SD decrease in TT levels. They also observed a trend of increased AF in men aged 70 to 79 years with decreased testosterone, but that was statistically not significant (hazard ratio 1.14, 95% CI 0.91–1.44).
Compared with the studies discussed above, the present study included 76 639 participants with a median age of 65 to 66 years. Those with low initial TT were followed for 4 to 6 years, with or without TRT treatment. We found that the incidence of AF was significantly higher among participants with low TT levels who received no treatment compared with those who achieved normal TT levels after TRT. These results, in conjunction with previous studies, strengthen the hypothesis that testosterone deficiency may potentiate the risk of developing AF. Stroke is a major disabling and sometimes fatal complication of AF. Our previous study,16 which showed a significant decrease in the incidence of stroke with testosterone normalization after TRT, strengthens the current findings.
Although studies7, 46 have demonstrated association between low testosterone and an increased risk of AF, additional studies will be needed to evaluate the strength of the association between hypogonadism and AF compared with the strength of other established risk factors. It is possible that low endogenous testosterone is associated with these risk factors and may not be a part of the causal pathway. The TOM (Testosterone in Older Men) trial, which was designed to determine the effects of TRT on lower extremity strength and physical function in older men with limitations in mobility and low serum levels of TT or free T, found 3 AF episodes in the testosterone group compared with none in the placebo group.15 In a recent study, Snyder et al reported 11 hospitalizations for arrhythmias in the testosterone group compared with only 7 in the placebo group.47 In addition, 2 case reports suggest a risk of AF by anabolic steroid use or abuse.10, 11
It is worth mentioning that the US Food and Drug Administration recently approved changes to the labeling instructions on testosterone products that provides warning regarding the abuse potential of testosterone and the serious adverse outcomes, especially those related to cardiovascular and mental health, that have been reported in association with testosterone/anabolic androgenic steroid abuse.48
Study Limitations
As an observational study, certain limitations are inherent to the design. Unmeasured confounding or hidden biases might be present. Another limitation of our study is the lack of randomization. Our database does not permit us to ascertain the time of the day when TT levels were drawn. Although blood samples are normally collected during morning hours in the VA health care system, some patients may have had their samples drawn later in the day, resulting in potential underestimation of TT levels. Another limitation of observational studies is the validity of inclusion and exclusion parameters. However, these criteria were determined using ICD‐9 codes, and the VA cohort ICD‐9 codes have been validated for determining outcomes.14 Despite this, we recognize that in some patients in our study, AF episodes may have been missed either because patients did not recognize having an AF episode or an AF episode spontaneously stopped before a patient sought medical help.
Conclusion
To the best of our knowledge, this study is the first and the largest to evaluate the association between TRT and the incidence of AF. Results from our study show that low TT levels are associated with increased incidence of AF. The largest and significant decrease in the incidence of AF was observed in the group with normalized TT levels following TRT. Future prospective randomized controlled trials are needed to validate these results.
Disclosures
None. The contents of this article are those of authors and do not necessarily reflect the position and policy of the Department of Veterans Affairs or the United States Government.
(J Am Heart Assoc. 2017;6:e004880 DOI: 10.1161/JAHA.116.004880.)28487389
References
- 1. Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J, Hong Y; American Heart Association Statistics C and Stroke Statistics S . Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. [DOI] [PubMed] [Google Scholar]
- 4. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice G . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:1–76. [DOI] [PubMed] [Google Scholar]
- 5. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 6. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam Study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 7. Lai J, Zhou D, Xia S, Shang Y, Want L, Zheng L, Zhu J. Reduced testosterone levels in males with lone atrial fibrillation. Clin Cardiol. 2009;32:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsuneda T, Yamashita T, Kato T, Sekiguchi A, Sagara K, Sawada H, Aizawa T, Fu LT, Fujiki A, Inoue H. Deficiency of testosterone associates with the substrate of atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. 2009;20:1055–1060. [DOI] [PubMed] [Google Scholar]
- 9. Liu T, Shehata M, Li G, Wang X. Androgens and atrial fibrillation: friends or foes? Int J Cardiol. 2010;145:365–367. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan ML, Martinez CM, Gallagher EJ. Atrial fibrillation and anabolic steroids. J Emerg Med. 1999;17:851–857. [DOI] [PubMed] [Google Scholar]
- 11. Lau DH, Stiles MK, John B, Shashidhar, Young GD, Sanders P. Atrial fibrillation and anabolic steroid abuse. Int J Cardiol. 2007;117:86–87. [DOI] [PubMed] [Google Scholar]
- 12. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of A . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. [DOI] [PubMed] [Google Scholar]
- 13. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vigen R, O'Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. [DOI] [PubMed] [Google Scholar]
- 15. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede‐Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Ambrose JA, Barua RS. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–2715. [DOI] [PubMed] [Google Scholar]
- 17. Sharma R, Oni OA, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Barua RS, Gupta K. Association between testosterone replacement therapy and the incidence of deep vein thrombosis and pulmonary embolism: a retrospective cohort study of the Veterans Administration database. Chest. 2016;150:563–571. [DOI] [PubMed] [Google Scholar]
- 18. Lazarou S, Reyes‐Vallejo L, Morgentaler A. Wide variability in laboratory reference values for serum testosterone. J Sex Med. 2006;3:1085–1089. [DOI] [PubMed] [Google Scholar]
- 19. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography‐tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. [DOI] [PubMed] [Google Scholar]
- 21. Vesper HW, Botelho JC. Standardization of testosterone measurements in humans. J Steroid Biochem Mol Biol. 2010;121:513–519. [DOI] [PubMed] [Google Scholar]
- 22. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 23. Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D'Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J. 1996;131:790–795. [DOI] [PubMed] [Google Scholar]
- 24. Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin. 2009;27:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127:e15–e16. [DOI] [PubMed] [Google Scholar]
- 26. Tenover JS. Androgen administration to aging men. Endocrinol Metab Clin North Am. 1994;23:877–892. [PubMed] [Google Scholar]
- 27. Tsai WC, Lee TI, Chen YC, Kao YH, Lu YY, Lin YK, Chen SA, Chen YJ. Testosterone replacement increases aged pulmonary vein and left atrium arrhythmogenesis with enhanced adrenergic activity. Int J Cardiol. 2014;176:110–118. [DOI] [PubMed] [Google Scholar]
- 28. Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. [DOI] [PubMed] [Google Scholar]
- 29. Korantzopoulos P, Kolettis TM, Kountouris E, Siogas K, Goudevenos JA. Variation of inflammatory indexes after electrical cardioversion of persistent atrial fibrillation. Is there an association with early recurrence rates? Int J Clin Pract. 2005;59:881–885. [DOI] [PubMed] [Google Scholar]
- 30. Qu YC, Du YM, Wu SL, Chen QX, Wu HL, Zhou SF. Activated nuclear factor‐kappaB and increased tumor necrosis factor‐alpha in atrial tissue of atrial fibrillation. Scand Cardiovasc J. 2009;43:292–297. [DOI] [PubMed] [Google Scholar]
- 31. Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157:243–252. [DOI] [PubMed] [Google Scholar]
- 33. Joseph P, Ishai A, MacNabb M, Abdelbaky A, Lavender ZR, Ruskin J, Nahrendorf M, Tawakol A. Atrial fibrillation is associated with hematopoietic tissue activation and arterial inflammation. Int J Cardiovasc Imaging. 2016;32:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wazni O, Martin DO, Marrouche NF, Shaaraoui M, Chung MK, Almahameed S, Schweikert RA, Saliba WI, Natale A. C reactive protein concentration and recurrence of atrial fibrillation after electrical cardioversion. Heart. 2005;91:1303–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe E, Arakawa T, Uchiyama T, Kodama I, Hishida H. High‐sensitivity C‐reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol. 2006;108:346–353. [DOI] [PubMed] [Google Scholar]
- 36. Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin‐6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henningsen KM, Therkelsen SK, Bruunsgaard H, Krabbe KS, Pedersen BK, Svendsen JH. Prognostic impact of hs‐CRP and IL‐6 in patients with persistent atrial fibrillation treated with electrical cardioversion. Scand J Clin Lab Invest. 2009;69:425–432. [DOI] [PubMed] [Google Scholar]
- 38. Hak L, Mysliwska J, Wieckiewicz J, Szyndler K, Siebert J, Rogowski J. Interleukin‐2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). J Interferon Cytokine Res. 2009;29:327–332. [DOI] [PubMed] [Google Scholar]
- 39. Rizos I, Tsiodras S, Rigopoulos AG, Dragomanovits S, Kalogeropoulos AS, Papathanasiou S, Sakadakis EA, Kremastinos DT. Interleukin‐2 serum levels variations in recent onset atrial fibrillation are related with cardioversion outcome. Cytokine. 2007;40:157–164. [DOI] [PubMed] [Google Scholar]
- 40. Cabrera‐Bueno F, Medina‐Palomo C, Ruiz‐Salas A, Flores A, Rodriguez‐Losada N, Barrera A, Jimenez‐Navarro M, Alzueta J. Serum levels of interleukin‐2 predict the recurrence of atrial fibrillation after pulmonary vein ablation. Cytokine. 2015;73:74–78. [DOI] [PubMed] [Google Scholar]
- 41. Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross‐sectional study. PLoS One. 2013;8:e61466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C‐reactive protein levels in men. Clin Endocrinol. 2010;72:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. [DOI] [PubMed] [Google Scholar]
- 44. Nettleship JE, Pugh PJ, Channer KS, Jones T, Jones RD. Inverse relationship between serum levels of interleukin‐1beta and testosterone in men with stable coronary artery disease. Horm Metab Res. 2007;39:366–371. [DOI] [PubMed] [Google Scholar]
- 45. Ruige JB, Bekaert M, Lapauw B, Fiers T, Lehr S, Hartwig S, Herzfeld de Wiza D, Schiller M, Passlack W, Van Nieuwenhove Y, Pattyn P, Cuvelier C, Taes YE, Sell H, Eckel J, Kaufman JM, Ouwens DM. Sex steroid‐induced changes in circulating monocyte chemoattractant protein‐1 levels may contribute to metabolic dysfunction in obese men. J Clin Endocrinol Metab. 2012;97:E1187–E1191. [DOI] [PubMed] [Google Scholar]
- 46. Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, Vasan RS, Benjamin EJ, Coviello AD. Association of sex hormones, aging, and atrial fibrillation in men: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2014;7:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens‐Shields AJ, Cauley JA, Gill TM, Barrett‐Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER III, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS; Testosterone Trials I . Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. FDA approves new changes to testosterone labeling regarding the risks associated with abuse and dependence of testosterone and other anabolic androgenic steroids (AAS). Available at: http://www.fda.gov/Drugs/DrugSafety/ucm526206.htm. Accessed April 19, 2017.


