Abstract
Background
We aimed to investigate the regulatory effects of hydrogen sulfide (H2S) on carotid sinus baroreceptor sensitivity and its mechanisms.
Methods and Results
Male Wistar‐Kyoto rats and spontaneously hypertensive rats (SHRs) were used in the experiment and were given an H2S donor or a cystathionine‐β‐synthase inhibitor, hydroxylamine, for 8 weeks. Systolic blood pressure and the cystathionine‐β‐synthase/H2S pathway in carotid sinus were detected. Carotid sinus baroreceptor sensitivity and the functional curve of the carotid baroreceptor were analyzed using the isolated carotid sinus perfusion technique. Effects of H2S on transient receptor potential cation channel subfamily V member 1 (TRPV1) expression and S‐sulfhydration were detected. In SHRs, systolic blood pressure was markedly increased, but the cystathionine‐β‐synthase/H2S pathway in the carotid sinus was downregulated in comparison to that of Wistar‐Kyoto rats. Carotid sinus baroreceptor sensitivity in SHRs was reduced, demonstrated by the right and upward shift of the functional curve of the carotid baroreceptor. Meanwhile, the downregulation of TRPV1 protein was demonstrated in the carotid sinus; however, H2S reduced systolic blood pressure but enhanced carotid sinus baroreceptor sensitivity in SHRs, along with TRPV1 upregulation in the carotid sinus. In contrast, hydroxylamine significantly increased the systolic blood pressure of Wistar‐Kyoto rats, along with decreased carotid sinus baroreceptor sensitivity and reduced TRPV1 protein expression in the carotid sinus. Furthermore, H2S‐induced enhancement of carotid sinus baroreceptor sensitivity of SHRs could be amplified by capsaicin but reduced by capsazepine. Moreover, H2S facilitated S‐sulfhydration of TRPV1 protein in the carotid sinus of SHRs and Wistar‐Kyoto rats.
Conclusions
H2S regulated blood pressure via an increase in TRPV1 protein expression and its activity to enhance carotid sinus baroreceptor sensitivity.
Keywords: carotid sinus baroreceptor, hydrogen sulfide, hypertension, transient receptor potential cation channel subfamily V member 1
Subject Categories: Hypertension, Vascular Biology, High Blood Pressure
Introduction
Hypertension is a common chronic disease and a major risk factor for cardiovascular diseases. It can cause heart failure, myocardial infarction, and organ injuries, such as renal, cerebral and myocardial damage, which can significantly harm a person's quality of life. Carotid sinus baroreflex is one of the most important neural feedback mechanisms to regulate and maintain stable blood pressure. A study suggested that in patients with hypertension, baroreflex sensitivity (BRS) was decreased significantly, which was associated with the thickness of the baroreceptor vascular wall.1 Hypertension, hypotension and orthostatic tachycardia may occur in patients with bilateral carotid sinus resection as a result of trauma or local tumor surgery, whereas syncope led by hypotension occurs in patients with hypersensitivity of the carotid sinus.2, 3 Thus, the carotid sinus plays important physiological and pathophysiological roles in the regulation of blood pressure.
The carotid sinus baroreceptor is an important part of baroreflex and is dominant in baroreceptors and blood pressure regulation. It can sense the mechanical stretch stimulation of blood vessel walls directly, and its sensitivity is regulated by many factors, mainly from circulation or secreted by cells in the carotid sinus. Prostacyclin, for example, which is released from endothelial cells, can enhance carotid sinus baroreceptor sensitivity by acting on nerve endings instead of changing the vascular tension. In sensory neurons, nitric oxide can be generated and can inhibit carotid sinus baroreceptor sensitivity, although the mechanisms remain unclear.4
Gaseous signal molecules with the characteristics of small molecular weight, continuous production and rapid dispersion, can freely pass through a variety of biological membranes. Hydrogen sulfide (H2S) was discovered as the third gaseous molecule after nitric oxide and carbon monoxide. H2S is generated from the cysteine metabolic pathway catalyzed by cystathionine‐β‐synthase (CBS), cystathionine γ‐lyase (CSE), and 3‐mercaptopyruvate sulfurtransferase, and it has extensive biological effects.5, 6, 7, 8, 9 Our group found that gaseous molecules have important roles in regulating local vascular function.10, 11 Downregulation of the endogenous H2S system is an important mechanism in spontaneously hypertensive rats (SHRs).12, 13 Previous studies have indicated that H2S was involved in short‐term and long‐term blood pressure regulation, under both physiological and pathological conditions.12, 13, 14, 15, 16
A study revealed that an H2S donor facilitated carotid sinus baroreceptor activity in normal rats, and the baroreflex functional curve indicated that carotid sinus baroreceptor sensitivity was enhanced by H2S. The effect was concentration dependent,17 which suggested that H2S might be involved in regulation of carotid sinus baroreceptor sensitivity. This gives rise to a series of questions that have yet to be answered: During the development of hypertension, does carotid sinus baroreceptor sensitivity change? Is H2S involved in regulating sinus baroreceptor sensitivity? What is the possible specific mechanism?
It is accepted that endogenous regulatory factors can regulate the baroreceptor by affecting receptor nerve terminals directly targeting voltage‐dependent ion channels, such as transient receptor potential cation channel subfamily V member 1 (TRPV1), transient receptor potential cation channel subfamily C member 5 (TRPC5), and acid‐sensing ion channel 2 (ASIC2).4, 18, 19 TRPV1, the first member of the transient receptor potential family to be discovered, is distributed mainly in sensory nerve endings and mediates thermal effect and pain afferent.20 TRPV1 distribution was found in aortic baroreceptors, and TRPV1 participated in the perception, conduction, and regulation of mechanical stimulation.21 Studies suggested that TRPV1 mediated H2S‐induced neurogenic inflammation22 and promoted the release of inhibitory neuropeptide.23, 24 Nevertheless, it is unclear whether H2S regulates carotid sinus baroreceptor sensitivity by activating TRPV1, and the mechanism by which H2S affects TRPV1 is unknown.
This study aimed to explore whether H2S regulated carotid sinus baroreceptor sensitivity through TRPV1 in the development of hypertension and to examine the possible molecular mechanisms.
Methods
Animals
Animal experiment
Four‐week‐old male Wistar‐Kyoto (WKY) rats and SHRs were used as experimental animal models. Each type of rats was divided randomly into 3 groups (8 rats in each group): control (0.9% normal saline 4 mL/kg, intraperitoneal injection every day for 8 weeks), hydroxylamine (HA; HA is an inhibitor of CBS; 12.5 mg/kg,25 intraperitoneal injection every day for 8 weeks), and NaHS (90 μmol/kg, intraperitoneal injection every day for 8 weeks).
Isolated carotid sinus perfusion experiment
Overall, 56 WKY rats and 56 SHRs (male, weight 300±10 g) were used in the experiment. SHRs and WKY rats were randomly divided into 7 groups (in total, 14 groups with 8 rats in each group): control (perfusate: Krebs‐Henseleit [K‐H] solution for 50 minutes), NaHS (perfusate: K‐H solution for 20 minutes, then change to K‐H solution containing 90 μmol/L NaHS for 30 minutes), HA (perfusate: K‐H solution for 20 minutes, then K‐H solution containing 12.5 mg/L HA for 30 minutes), capsaicin (perfusate: K‐H solution for 20 minutes, then K‐H solution containing capsaicin 1.0 mg/L for 30 minutes), capsazepine (perfusate: K‐H solution for 20 minutes, then K‐H solution containing capsazepine 15 mg/L for 30 minutes), capsaicin plus NaHS (perfusate: K‐H solution containing capsaicin 1.0 mg/L perfused for 20 minutes, then changed to K‐H solution containing NaHS 90 μmol/L for 30 minutes), and capsazepine plus NaHS (perfusate: K‐H solution containing capsazepine 15 mg/L perfused for 20 minutes, then changed to K‐H solution containing NaHS 90 μmol/L for 30 minutes).
Incubation of carotid sinus
In total, 24 WKY rats (male, weight 300±10 g) were randomly divided into 3 groups (8 rats in each group): control (carotid sinus tissues were incubated with 95% O2 to 5% CO2 K‐H solution for 6 hours), HA (carotid sinus tissues were incubated with 95% O2 to 5% CO2 K‐H solution containing HA 12.5 mg/L for 6 hours), and NaHS (carotid sinus tissues were incubated with 95% O2 to 5% CO2 K‐H solution containing NaHS 90 μmol/L for 6 hours).
All experimental animals were purchased from Beijing Vital River Laboratory Animal Technology Co, Ltd (license number SCXK 2012‐0001). All rats were raised in the Animal Center of Peking University First Hospital. During the experiment, all rats were given normal diets at 25°C constant temperature with a circadian rhythm of 12‐hour dark/light. All experimental operations strictly abided by laboratory animal welfare and operational guidelines and were approved by the experimental ethics committee of Peking University First Hospital.
Measurement of Hemodynamic Parameters in Rats
Rats were anesthetized with 5% urethane (5 mL/kg) by intraperitoneal injection and fixed with the left carotid artery separated. One end of a polyethylene catheter was inserted into the left carotid artery, whereas the other end was connected to a pressure transducer (PT‐100; Chengdu Technology & Market Co Ltd) and biological experimental system (BL‐420S; Chengdu Technology & Market Co Ltd). In this way, the systolic blood pressure (SBP) of rats was recorded.
Detection of H2S Concentration in Plasma
Arterial blood was taken and centrifuged at 3000g at 4°C for 20 minutes to get plasma. H2S concentration was detected by using the sensitive sulfur electrode (PXS‐270; Shanghai Leici). Standard solution, prepared by Na2S, and plasma were mixed with equal volume of antioxidants. A sulfur‐sensitive electrode and the reference electrode were immersed at the same time. Results were recorded when the reading was stabilized. H2S concentration in plasma was calculated according to the standard curve.26
Detection of Carotid Sinus Baroreceptor Sensitivity by Isolated Carotid Sinus Perfusion Technique
The rats were anesthetized with 25% urethane (5 mL/kg) by intraperitoneal injection. Carotid sinus area of rats was fully exposed with endotracheal intubation. Under the microscope, the bilateral aortic nerves, cervical sympathetic nerves, recurrent laryngeal nerves, and right carotid sinus nerve were isolated and cut off. The left carotid sinus was isolated, and the internal carotid artery, superior thyroid artery, ascending pharyngeal artery, and occipital artery were ligated, leaving the left common carotid artery as the inflow tract and the left external carotid artery as the outflow tract. The inflow tract was connected to the pressure transducer, which monitored the intrasinus pressure (ISP) in the perfusion process. The distal end of the left femoral artery was ligated, and the proximal end was inserted by a polyethylene catheter, which was connected to a pressure transducer to record mean arterial pressure (MAP). A biological experimental system (BL‐420S) was used for recording ISP and MAP. The functional curve of the carotid baroreceptor (FCCB) was made with ISP as abscissa and MAP as ordinate. The following parameters were detected: threshold pressure (TP; the ISP value when MAP dropped 5.025 mm Hg reflectively), equilibrium pressure (EP; the pressure value when MAP is equal to ISP), saturation pressure (SP; the ISP value when MAP showed no further decrease with the increase of ISP), operating range (OR; calculated as SP minus TP), peak slope (PS; maximum slope of the FCCB), and reflex decrease (RD; the maximum decrease of MAP). When the carotid sinus baroreceptor sensitivity was enhanced, the FCCB moved to the left and downward; in contrast, with the reduction of carotid sinus baroreceptor sensitivity, the FCCB moved to the right and upward.17
Detection of TRPV1 mRNA Level by Quantitative Real‐Time Polymerase Chain Reaction
TRPV1 mRNA level in incubated carotid sinus tissue was detected by quantitative real‐time polymerase chain reaction (PCR). Trizol reagent was used to extract total RNA. Oligodt primer, 2.5 mmol/L dNTP, and M‐MLV reverse transcriptase were used for reverse transcription. Primers and probes were synthesized by Sangon Biotech Company: for TRPV1, forward, 5′‐TGTTTGTGGACAGCTACAGTGAGA‐3′, reverse, 5′‐AGTACCACAGACACCAGCATGAA‐3′, Taqman probe, 5′‐ ACTTTTCTTTGTACAGTCGCT‐3′; for β‐actin, forward, 5′‐ACCCGCGAGTACAACCTTCTT‐3′, reverse, 5′‐TATCGTCATCCATGGCGAACT‐3′, Taqman probe, 5′‐CCTCCGTCGCCGGTCCACAC‐3′. The PCR mixture contained 2.5 μL of 10× PCR buffer, 2 μL of 2.5 mmol/L each dNTP, 2.5 U Taq DNA polymerase, 0.5 μL of 6‐carboxy‐X‐rhodamine, 7.5 pmol of each forward and reverse primer, 5 pmol Taq Man probe, and 2 μL of cDNA template or standard DNA in a total volume of 25 μL. Samples and standard DNA were detected in duplicate. Quantitative real‐time PCR was performed on an ABI PRISM 7300 instrument (Applied Biosystems), and the condition was set to predenaturing at 95°C for 5 minutes, and then 95°C for 15 seconds and 60°C for 1 minute for 40 cycles.27
Detection of CBS, TRPV1, CSE, ASIC2, and TRPC5 Protein Expression by Western Blotting
Carotid sinus and common carotid artery samples were homogenized in 1× tissue lysate and centrifuged at 13 000g for 20 minutes at 4°C to get supernatant. Protein content was determined according to the Bradford method, with bovine serum albumin used as a standard. Protein samples were mixed with 2× loading buffer containing 5% β‐mercaptoethanol and boiled for 5 minutes. Equal amounts of protein were added to the polyacrylamide gel for electrophoresis and transferred onto a nitrocellulose membrane. Nonspecific binding was blocked by incubation in milk for 1 hour at room temperature. Primary antibodies CBS, TRPV1, CSE, ASIC2, TRPC5, GAPDH, and β‐actin were added and incubated at 4°C overnight. After wash with Tris‐buffered saline with 1% Tween, secondary antibodies were added and incubated at room temperature for 1 hour. A chemiluminescence detection kit and AlphaImager gel imaging system (ProteinSimple) were used to enhance immunoreactions and to analyze the density of protein bands, respectively.
Detection of S‐Sulfhydration Modification Level of TRPV1
After 50‐minute‐perfusion experiment, the carotid sinuses of rats were homogenized in 1× tissue lysate and centrifuged at 13 000g at 4°C for 20 minutes. The supernatant was taken and divided into 2 parts: one part for total protein and another part for S‐sulfhydryl protein extraction. Next, 2.5% SDS and 20 mmol/L S‐methyl methanethiosulfonate were added and incubated with gentle shaking at 50°C for 20 minutes. Acetone, precooled at −20°C, was added to the mixture with volume ratio of 4:1, precipitated at −20°C for 2 hours, and centrifuged at 12 000g at 4°C for 10 minutes to get precipitate. After being resuspended in 1 mL 1× tissue lysate and incubated with Z‐Link iodoacetyl‐PEG2 biotin, avoiding light at 4°C for 12 hours, Ultra Link Immobilized NeutrAvidin (Thermo Scientific) was added and incubated with shaking at 4°C for 4 hours. The precipitate was retrieved with centrifugation and washed with PBS (0.01 mmol/L). The samples were mixed with loading buffer without β‐mercaptoethanol and centrifuged at 5000g for 10 minutes to get supernatant containing S‐sulfhydryl protein. Finally, western blotting was used to detect the S‐sulfhydration modification level of TRPV1.
Statistical Analysis
SPSS17.0 software was used for statistical analysis. All results are expressed as mean±SE. For normally distributed data, comparisons among groups were analyzed by 1‐way ANOVA. When the overall difference showed significance, Bonferroni analysis was performed for comparison of the difference between the 2 groups. For nonnormally distributed data, the Kruskal–Wallis rank sum test was used for multiple comparisons. For outcomes measured serially of FCCB sensitivity, repeated‐measures analysis was used to test for overall differences before the tests performed at each ISP level. P<0.05 was considered statistically significant.
Results
The Endogenous CBS/H2S Pathway Was Downregulated in Carotid Sinus of SHRs
To detect the changes in endogenous H2S concentration in rats, we used a sensitive sulfur electrode and western blotting to detect H2S concentration in plasma and CBS protein expressions in the carotid sinus and the common carotid artery. Compared with WKY rats, SBP in SHRs was increased (Figure 1A), H2S concentration in the plasma of SHRs decreased significantly (Figure 1B), CBS expression in carotid sinus was clearly reduced (Figure 1C), and no significant changes in the common carotid artery were noted (Figure 1D).
Figure 1.
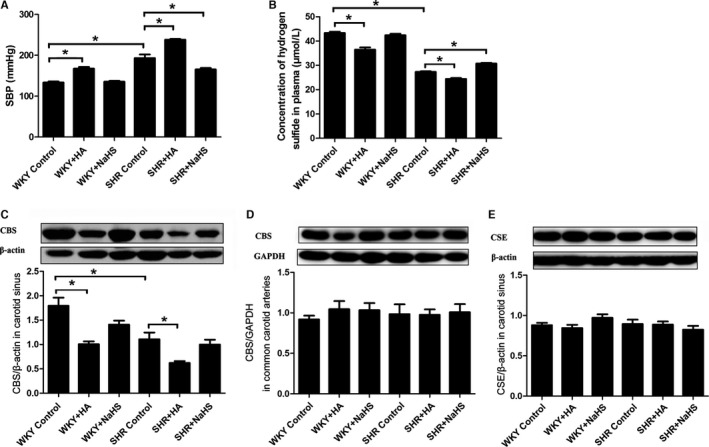
Systolic blood pressure, plasma H2S concentration, and CBS and CSE protein expressions in SHRs and WKY rats. A, SBP in rats. B, H2S concentration in plasma. C, CBS protein expression in carotid sinus. D, CBS protein expression in common carotid arteries. E, CSE protein expression in carotid sinus. Mean±SE, n=8. *P<0.05. CBS indicates cystathionine‐β‐synthase; CSE, cystathionine γ‐lyase; HA, hydroxylamine; SBP, systolic blood pressure; SHR, spontaneously hypertensive rat; WKY, Wistar‐Kyoto rat.
H2S Reduced Blood Pressure of Hypertensive Rats
We used H2S donor NaHS or CBS inhibitor HA to increase or decrease H2S content in rats. In SHRs, with H2S supplement, H2S concentration in plasma increased and SBP decreased significantly, with no change in CBS protein expression in the carotid sinus. After the use of HA, CBS protein expression was significantly reduced in the carotid sinus of SHRs, which led to a decrease in H2S concentration in plasma and a further increase in SBP (Figure 1).
After giving H2S donor NaHS, H2S concentration in plasma, the expression of CBS in the carotid sinus, and SBP of WKY rats did not change. In contrast, with the administration of HA, CBS expression in the carotid sinus decreased, followed by a reduction in H2S concentration in plasma, which finally resulted in an increase in SBP in WKY rats. However, CBS protein expression in common carotid arteries did not differ among different groups, nor did CSE protein expression in carotid sinus tissues (Figure 1D and 1E).
Carotid Sinus Baroreceptor Sensitivity Was Reduced in Hypertensive Rats
We used the isolated carotid sinus perfusion technique to detect carotid sinus baroreceptor sensitivity. The FCCB was plotted with ISP and MAP recorded and related parameters including TP, EP, SP, PS, OR and RD that were subsequently analyzed. Results showed that in comparison to WKY rats, carotid sinus baroreceptor sensitivity in SHRs was reduced. This was demonstrated by the fact that the FCCB moved to the right and upward (Figure 2) and TP and EP were increased, whereas OR, RD, and PS were decreased significantly (Table 1).
Figure 2.
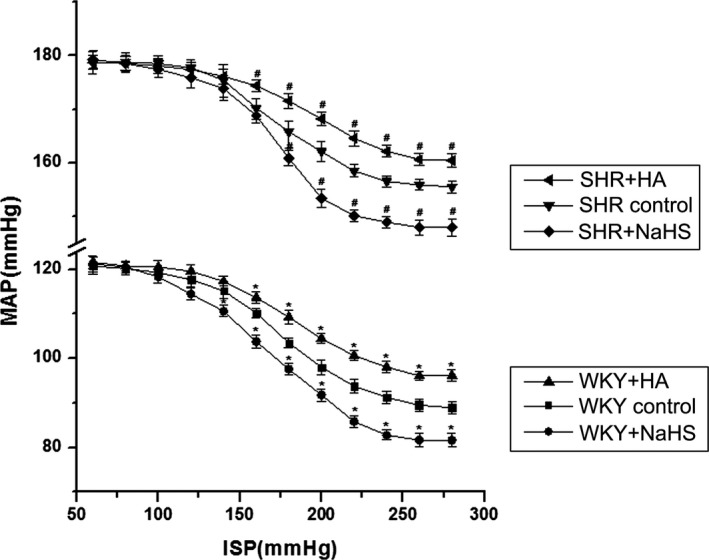
Effects of H2S on carotid sinus baroreceptor sensitivity in rats (mean±SE, n=8). When the carotid sinus baroreceptor sensitivity was enhanced, the FCCB moved to the left and downward; in contrast, with the reduction of carotid sinus baroreceptor sensitivity, the FCCB moved to the right and upward. *P<0.05 vs WKY control group, # P<0.05 vs SHR control group. FCCB indicates functional curve of carotid baroreceptor; HA, hydroxylamine; ISP, intrasinus pressure; MAP, mean arterial pressure; SHR, spontaneously hypertensive rat; WKY, Wistar‐Kyoto rats.
Table 1.
Effects of H2S on the Functional Parameters of Carotid Sinus Baroreceptor in Rats
| Groups | TP (mm Hg) | EP (mm Hg) | SP (mm Hg) | OR (mm Hg) | PS (mm Hg/mm Hg) | RD (mm Hg) |
|---|---|---|---|---|---|---|
| WKY control | 134.90±1.77 | 118.19±0.22 | 234.57±1.07 | 99.67±1.08 | 0.28±0.01 | 31.78±0.42 |
| WKY+NaHS | 115.11±0.86a | 115.39±0.47a | 221.64±1.00a | 106.53±0.92a | 0.30±0.01 | 39.57±0.15a |
| WKY+HA | 143.33±1.51a | 119.69±0.25a | 238.86±1.42 | 95.53±1.26a | 0.22±0.01a | 25.41±0.66a |
| SHR control | 148.66±1.84a | 168.66±0.86a | 224.55±1.25a | 75.89±1.99a | 0.22±0.01a | 23.47±0.65a |
| SHR+NaHS | 136.45±1.01b | 165.94±0.40b | 217.56±0.47b | 81.11±0.92 | 0.33±0.01b | 31.16±0.53b |
| SHR+HA | 163.80±1.42b | 172.33±0.48b | 230.87±1.30b | 67.07±1.83b | 0.16±0.01b | 18.32±0.20b |
EP indicates equilibrium pressure; HA, hydroxylamine; K‐H, Krebs‐Henseleit; OR, operating range; PS, peak slope; RD, reflex decrease; SHR, spontaneously hypertensive rat; SP, saturation pressure; TP, threshold pressure; WKY, Wistar‐Kyoto rat.
P<0.05 vs WKY control.
P<0.05 vs SHR control.
H2S Facilitated Carotid Sinus Baroreflex of Rats
In the carotid sinus perfused rat model, we intervened with the H2S content of carotid sinus by adding NaHS or HA into the perfusate. With the supplement of H2S, carotid sinus baroreceptor sensitivity in SHRs was enhanced; the FCCB shifted to the left and downward (Figure 2), TP, EP and SP were reduced, whereas PS and RD were increased significantly (Table 1). After inhibiting the generation of H2S, carotid sinus baroreceptor sensitivity in SHRs was reduced; the FCCB shifted to the right and upward (Figure 2), TP, EP and SP were increased, whereas OR, RD, and PS were decreased significantly (Table 1).
After administration of NaHS, carotid sinus baroreceptor sensitivity in WKY rats was enhanced; the FCCB shifted to the left and downward (Figure 2), TP, EP and SP decreased noticeably, whereas OR and RD were increased (Table 1). With the inhibition of H2S generation, carotid sinus baroreceptor sensitivity in WKY rats was reduced; the FCCB shifted to the right and upward, TP and EP were increased noticeably, whereas OR, RD, and PS were clearly decreased (Table 1).
TRPV1 Was Involved in H2S‐induced Enhancement of Carotid Sinus Baroreflex
To test whether TRPV1 was involved in H2S‐induced enhancement of carotid sinus baroreflex, we added TRPV1 agonist capsaicin or antagonist capsazepine into perfusate and incubated with low‐speed perfusion for 20 minutes before it was perfused with K‐H solution containing NaHS. Results showed that with the activation of TRPV1, carotid sinus baroreceptor sensitivity in SHRs was further enhanced; the FCCB moved to the left and downward (Figure 3), TP, EP, and SP were decreased, whereas OR, RD, and PS were increased significantly (Table 2). After we inhibited TRPV1 activity, carotid sinus baroreceptor sensitivity in SHRs was inhibited; the FCCB moved to the right and upward (Figure 3), TP, EP, and SP were increased, whereas OR, RD, and PS were decreased significantly (Table 2).
Figure 3.
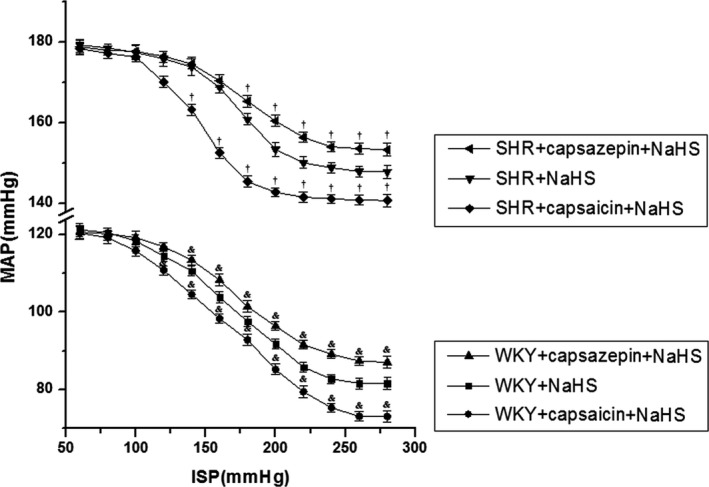
TRPV1 (transient receptor potential cation channel subfamily V member 1) mediated the regulation of H2S in carotid sinus baroreceptor sensitivity (mean±SE, n=8). The functional curve of the carotid baroreceptor shifted to the left and downward with carotid sinus baroreceptor sensitivity being enhanced; however, it shifted to the right and upward when carotid sinus baroreceptor sensitivity was reduced. *P<0.05 vs WKY+NaHS group, † P<0.05 vs SHR+NaHS group. ISP indicates intrasinus pressure; MAP, mean arterial pressure; SHR, spontaneously hypertensive rat; WKY, Wistar‐Kyoto rat.
Table 2.
The Role of TRPV1 in the Regulation of Carotid Sinus Baroreceptor by H2S
| Groups | TP (mm Hg) | EP (mm Hg) | SP (mm Hg) | OR (mm Hg) | PS (mm Hg/mm Hg) | RD (mm Hg) |
|---|---|---|---|---|---|---|
| WKY+NaHS | 115.11±0.86 | 115.39±0.47 | 221.64±1.00 | 106.53±0.92 | 0.30±0.01 | 39.57±0.15 |
| WKY+capsaicin+NaHS | 100.04±0.76a | 112.70±0.44a | 212.32±0.70a | 112.28±0.89a | 0.32±0.01 | 47.28±0.64a |
| WKY+capsazepine+NaHS | 126.64±1.66a | 117.50±0.26a | 227.56±1.05a | 100.92±1.53a | 0.28±0.01a | 33.51±0.38a |
| SHR+NaHS | 136.45±1.01 | 165.94±0.40 | 217.56±0.47 | 81.11±0.92 | 0.33±0.01 | 31.16±0.53 |
| SHR+capsaicin+NaHS | 110.55±0.54b | 155.82±0.45b | 195.88±0.78b | 85.33±0.93b | 0.40±0.01b | 37.66±0.18b |
| SHR+capsazepine+NaHS | 147.52±0.69b | 168.74±0.30b | 220.98±0.88b | 73.46±0.98b | 0.23±0.01b | 25.83±0.27b |
EP indicates equilibrium pressure; K‐H, Krebs‐Henseleit; OR, operating range; PS, peak slope; RD, reflex decrease; SHR, spontaneously hypertensive rat; SP, saturation pressure; TP, threshold pressure; WKY, Wistar‐Kyoto rat.
P<0.05 vs WKY+NaHS.
P<0.05 vs SHR+NaHS.
With activation of TRPV1, carotid sinus baroreceptor sensitivity in WKY rats was further enhanced; the FCCB moved to the left and downward (Figure 3), TP, EP, and SP were decreased, whereas OR and RD were increased significantly (Table 2). After being pretreated with capsazepine, carotid sinus baroreceptor sensitivity in WKY rats was reduced; the FCCB moved to the right and upward (Figure 3), TP, EP, and SP were increased, whereas OR, RD, and PS were decreased significantly (Table 2).
Carotid sinus baroreceptor sensitivity in WKY rats and SHRs was enhanced by capsaicin, and the FCCB moved to the left and downward. Alternatively, with the administration of capsazepine, the sensitivity of carotid sinus baroreceptor in WKY rats and SHRs was weakened, and the FCCB shifted to the right and upward (Figure 4).
Figure 4.
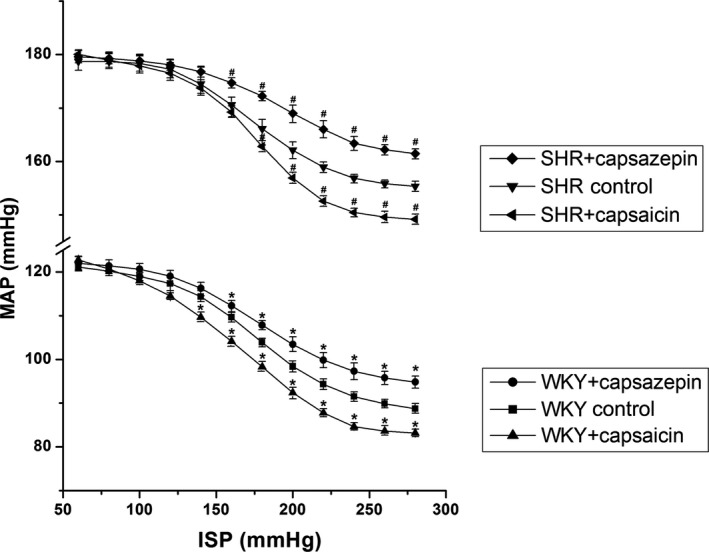
The regulatory effects of TRPV1 (transient receptor potential cation channel subfamily V member 1) on carotid sinus baroreceptor sensitivity (mean±SE, n=8). The functional curve of carotid baroreceptor shifted to the left and downward with carotid sinus baroreceptor sensitivity enhancement. Otherwise, it moved to the right and upward when carotid sinus baroreceptor sensitivity was reduced. *P<0.05 vs WKY control group, # P<0.05 vs SHR control group. ISP indicates intrasinus pressure; MAP, mean arterial pressure; SHR, spontaneously hypertensive rat; WKY, Wistar‐Kyoto rat.
TRPV1 mRNA Level Was Decreased by Inhibition of H2S Generation
Real‐time PCR was used to detect TRPV1 mRNA levels in carotid sinus tissues of WKY rats after incubation with K‐H solution containing CBS inhibitor or H2S donor. TRPV1 mRNA level in carotid sinus was decreased after treatment with HA but did not change after treatment with the H2S donor (Figure 5A).
Figure 5.
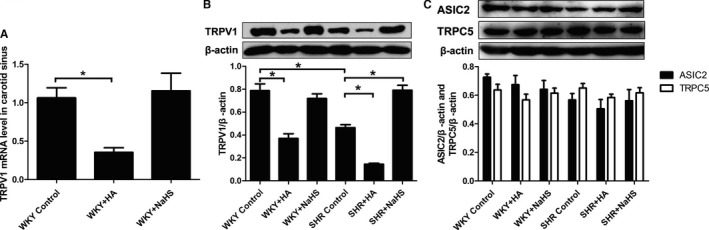
The TRPV1 mRNA level and protein expressions of TRPV1, ASIC2, and TRPC5 in the carotid sinus of rats. A, TRPV1 mRNA level in the carotid sinus after 6‐hour‐incubation experiment. B, Protein expression of TRPV1 in the carotid sinus of rats after 8‐week‐experiment. C, Protein expressions of ASIC2 and TRPC5 in the carotid sinus of rats after 8‐week‐experiment. Mean±SE, n=8. *P<0.05. ASIC2 indicates acid‐sensing ion channel 2; HA, hydroxylamine; SHR, spontaneously hypertensive rats; TRPC5, transient receptor potential channel subfamily C member 5; TRPV1, transient receptor potential cation channel subfamily V member 1; WKY, Wistar‐Kyoto rats.
H2S Increased TRPV1 Protein Expression in Carotid Sinus
Protein expression of TRPV1 in the carotid sinus of SHRs and WKY rats was detected by western blotting after 8 weeks of experiment. Compared with WKY rats, TRPV1 protein expression in the carotid sinus of SHRs was reduced significantly. With the supplement of H2S, the expression of TRPV1 was increased noticeably; in contrast, TRPV1 protein expression was decreased significantly with the inhibition of H2S generation (Figure 5B).
With the administration of NaHS, protein expression of TRPV1 in the carotid sinus of WKY rats was unchanged; however, with the inhibition of H2S production, the expression of TRPV1 in the carotid sinus was reduced significantly in WKY rats (Figure 5B).
Considering that ASIC2 and TRPC5 were important constituents contributing to aortic baroreflex, we detected the change in expression of ASIC2 and TRPC5 in carotid sinus. Results showed that there was no difference in expression of ASIC2 and TRPC5 in carotid sinus tissues among the 6 groups (Figure 5C).
H2S Induced S‐Sulfhydration of TRPV1 in the Carotid Sinus
To explore the mechanism for TRPV1 activation induced by H2S, we examined TRPV1 S‐sulfhydration in perfused carotid sinus of rats. The results showed that with the supplement of H2S, TRPV1 S‐sulfhydration was increased significantly in SHRs. Similarly, with the administration of NaHS, TRPV1 S‐sulfhydration was increased significantly in WKY rats (Figure 6).
Figure 6.
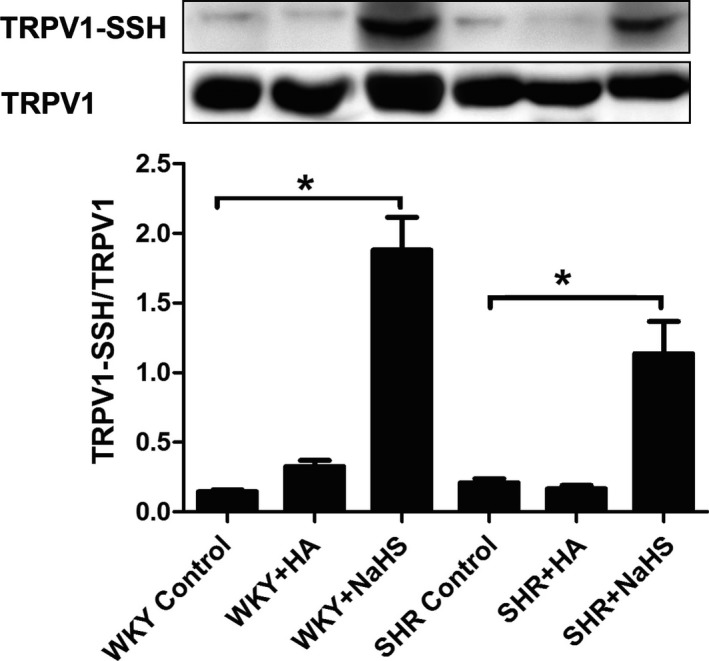
H2S induced S‐sulfhydration of TRPV1 in perfused carotid sinus of SHR and WKY. Mean±SE, n=8. *P<0.05. HA indicates hydroxylamine; SHR, spontaneously hypertensive rats; SSH, S‐sulfhydration; TRPV1, transient receptor potential cation channel subfamily V member 1; WKY, Wistar‐Kyoto rats.
Discussion
As the third gaseous molecule, H2S can be generated endogenously in the cardiovascular system and plays an important protective role in the regulation of blood pressure. H2S regulates blood pressure in multiple ways: relaxing vessels,11 inhibiting smooth muscle cell proliferation,28 alleviating vascular remodeling29 and so on. In the present study, we explored the effect of endogenous CBS/H2S in the carotid sinus in the development of hypertension and its possible mechanism. The data showed that the endogenous CBS/H2S pathway in the carotid sinus of SHRs was downregulated; however, CBS protein expression in the common carotid artery was not decreased. The different change might result from the fact that carotid sinus is a specialized structure of carotid artery. Unlike the common carotid artery, the carotid sinus is a major baroreception site in humans and most mammals and consists of many nerve endings expressing CBS. It was reported that CBS expression in the central nervous system in SHRs was downregulated compared with WKY rats.30 Consequently, we assumed that the downregulation of CBS expression in the carotid sinus of SHRs observed in our present study might be associated with the downregulation of CBS expression in the nerve terminals that are predominantly distributed in the carotid sinus. In addition, there was no difference in CSE expression in the carotid sinus between SHRs and WKY rats. The results suggested that an abnormal carotid sinus baroreceptor reflex in hypertension might be associated with the CBS/H2S pathway in the carotid sinus. With the supplement of H2S donor, the concentration of H2S in the plasma of SHRs was elevated and resulted in a decrease in blood pressure. After inhibition of endogenous H2S generation, the blood pressure of SHRs was further increased. In the same way, after inhibiting endogenous H2S generation of WKY rats, their blood pressure was raised. The results suggested that H2S played an important defensive role in the process of hypertension. The findings were consistent with previous studies12 that laid the foundation for further research on the mechanisms by which H2S regulates blood pressure.
Previous studies indicated that H2S participated in neuromodulation of arterial blood pressure.31 Carotid sinus baroreflex is the main route for neuromodulation of arterial blood pressure and can play an important role in hypertension development.32, 33, 34, 35 Carotid sinus baroreceptor sensitivity can be regulated by a variety of substances that come from the circulation or are released by cells in the carotid sinus region.4 Produced by vascular endothelial cells and neurons, H2S acts as a regulatory factor of autocrine and paracrine; however, how H2S participates in the regulation of the carotid sinus baroreceptor remains unclear. Previous studies have shown that H2S could facilitate carotid sinus baroreflex in a dose‐dependent manner under physiological condition17; however, during the development of hypertension, it has not been clear whether carotid sinus baroreceptor sensitivity changes and whether H2S is involved in regulating sinus baroreceptor sensitivity. In our present study, carotid sinus baroreceptor sensitivity in hypertensive rats was decreased compared with that of normal rats and is associated with the downregulation of the endogenous CBS/H2S pathway in the carotid sinus. When we supplemented the H2S donor NaHS to the perfusate, carotid sinus baroreceptor sensitivity in hypertensive rats was significantly increased, whereas if given the CBS inhibitor HA, carotid sinus baroreceptor sensitivity was evidently decreased. The results suggested that during the development of hypertension, the endogenous CBS/H2S pathway played an important role in the regulation of carotid sinus BRS.
As an important member of the transient receptor potential family, TRPV1 plays an important role in thermal effect and introduction of pain.20, 36, 37, 38, 39 TRPV1, however, was reported to be expressed in the aortic arch and participated in the regulation of aortic baroreflex.21 In the present study, we found that TRPV1 was expressed in the carotid sinus and significantly reduced in SHRs. Moreover, the agonist or antagonist of TRPV1 could enhance or inhibit carotid sinus BRS, suggesting that carotid sinus TRPV1 played roles in blood pressure regulation. Furthermore, we found that the H2S donor could enhance TRPV1 expression, whereas HA could suppress it in carotid sinus tissue of SHRs. To explore whether TRPV1 mediated the effect of H2S on carotid sinus BRS, we observed the impact of TRPV1 antagonist capsazepine and its agonist capsaicin on H2S effect. The data showed that TRPV1 antagonist blunted the effect of H2S on carotid sinus baroreflex. In contrast, the TRPV1 agonist capsaicin enhanced the effect of H2S on carotid sinus baroreflex. These results suggested that TRPV1 mediated the regulation of carotid sinus BRS by H2S.
The thiol group is the important functional group of proteins, and the ‐S bond of H2S can combine with the thiol (‐SH) bond of cysteine residues to form persulfide (‐SSH). Previous studies have shown that H2S could make a series of reactions with sulfhydryl of cysteine residues on the protein to adjust the structure and function of the protein.40, 41, 42, 43 TRPV1 could be activated by H2O2, 4‐hydroxynonenal, or nitric oxide via posttranslational modification of cysteine‐free sulfhydryl groups in TRPV1.44, 45 We found that after giving H2S donor NaHS, S‐sulfhydration of TRPV1 was increased significantly in the carotid sinus of SHRs and WKY rats. The results provided a possible clue to examine the molecular mechanism by which H2S activated TRPV1.
ASIC2 and TRPC5 were important ion channels related to the regulation of aortic baroreceptor, and their defects or dysfunction would lead to the development of hypertension.18, 19 In our study, we found that protein expressions of ASIC2 and TRPC5 in the carotid sinus were not significantly different among different groups, indicating that these 2 ion channels might not be the target of CBS/H2S to regulate carotid sinus baroreflex.
In conclusion, our main findings were (1) that in the SHRs with high blood pressure, endogenous H2S and CBS in the carotid sinus were related to hypertension and (2) that carotid sinus TRPV1 might be a target of H2S in the regulation of carotid sinus baroreceptor sensitivity; H2S increased TRPV1 protein expression and activity to enhance carotid sinus baroreceptor sensitivity. This study revealed the molecular mechanism by which H2S regulated carotid sinus baroreceptor reflex sensitivity, which could have great potential for providing a new therapeutic strategy for the treatment of hypertension.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81500325 and 81100181), the Major Basic Research Development Program of China (Nos. 2013CB933801) and National Youth Top‐Notch Talent Support Program.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004971 DOI: 10.1161/JAHA.116.004971.)28512115
References
- 1. Lábrová R, Honzíková N, Maderová E, Vysocanová P, Nováková Z, Závodná E, Fiser B, Semrád B. Age‐dependent relationship between the carotid intima‐media thickness, baroreflex sensitivity, and the inter‐beat interval in normotensive and hypertensive subjects. Physiol Res. 2005;54:593–600. [PubMed] [Google Scholar]
- 2. Ketch T, Biaggioni I, Robertson RM, Robertson D. Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation. 2002;105:2518–2523. [DOI] [PubMed] [Google Scholar]
- 3. Freitas J. Carotid sinus syndrome. Management and approach. Rev Port Cardiol. 2004;23:903–910. [PubMed] [Google Scholar]
- 4. Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann N Y Acad Sci. 2001;940:1–19. [DOI] [PubMed] [Google Scholar]
- 5. Chen CQ, Xin H, Zhu YZ. Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol Sin. 2007;28:1709–1716. [DOI] [PubMed] [Google Scholar]
- 6. Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2010;41:113–121. [DOI] [PubMed] [Google Scholar]
- 7. Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload‐induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006;40:119–130. [DOI] [PubMed] [Google Scholar]
- 9. Xie ZZ, Yang L, Bian JS. Hydrogen sulfide and cellular redox homeostasis. Oxid Med Cell Longev. 2016;2016:6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao Y, Chen S, Liu X, Zhang Q, Ai Y, Wang Y, Jin H, Tang C, Du J. Flow‐mediated vasodilation and endothelium function in children with postural orthostatic tachycardia syndrome. Am J Cardiol. 2010;106:378–382. [DOI] [PubMed] [Google Scholar]
- 11. Sun Y, Huang Y, Zhang R, Chen Q, Chen J, Zong Y, Liu J, Feng S, Liu AD, Holmberg L, Liu D, Tang C, Du J, Jin H. Hydrogen sulfide upregulates KATP channel expression in vascular smooth muscle cells of spontaneously hypertensive rats. J Mol Med. 2015;93:439–455. [DOI] [PubMed] [Google Scholar]
- 12. Yan H, Du JB, Tang CS. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. [DOI] [PubMed] [Google Scholar]
- 13. Li L, Liu D, Bu D, Chen S, Wu J, Tang C, Du J, Jin H. Brg1‐dependent epigenetic control of vascular smooth muscle cell proliferation by hydrogen sulfide. Biochim Biophys Acta. 2013;1833:1347–1355. [DOI] [PubMed] [Google Scholar]
- 14. Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1879–1885. [DOI] [PubMed] [Google Scholar]
- 15. Zhang QY, Du JB, Shi L, Zhang CY, Yan H, Tang CS. Interaction between endogenous nitric oxide and hydrogen sulfide in pathogenesis of hypoxic pulmonary hypertension. Beijing Da Xue Xue Bao. 2004;36:52–56. [PubMed] [Google Scholar]
- 16. Jin HF, Sun Y, Liang JM, Tang CS, Du JB. Hypotensive effects of hydrogen sulfide via attenuating vascular inflammation in spontaneously hypertensive rats. Chin J Cardiol. 2008;36:541–545. [PubMed] [Google Scholar]
- 17. Xiao L, Wu YM, Wang R, Liu YX, Wang FW, He RR. Hydrogen sulfide facilitates carotid sinus baroreceptor activity in anesthetized male rats. Chin Med J. 2007;120:1343–1347. [PubMed] [Google Scholar]
- 18. Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lau OC, Shen B, Wong CO, Tjong YW, Lo CY, Wang HC, Huang Y, Yung WH, Chen YC, Fung ML, Rudd JA, Yao X. TRPC5 channels participate in pressure‐sensing in aortic baroreceptors. Nat Commun. 2016;7:11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. [DOI] [PubMed] [Google Scholar]
- 21. Sun H, Li DP, Chen SR, Hittelman WN, Pan HL. Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J Pharmacol Exp Ther. 2009;331:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ang SF, Moochhala SM, Bhatia M. Hydrogen sulfide promotes transient receptor potential vanilloid 1‐mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med. 2010;38:619–628. [DOI] [PubMed] [Google Scholar]
- 23. Fernandes VS, Ribeiro AS, Martínez P, López‐Oliva ME, Barahona MV, Orensanz LM, Martínez‐Sáenz A, Recio P, Benedito S, Bustamante S, García‐Sacristán A, Prieto D, Hernández M. Hydrogen sulfide plays a key role in the inhibitory neurotransmission to the pig intravesical ureter. PLoS ONE. 2014;9:e113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu W, Li J, Gong L, Xu X, Han T, Ye Y, Che T, Luo Y, Li J, Zhan R, Yao W, Liu K, Cui S, Liu C. H2S modulates duodenal motility in male rats via activating TRPV1 and KATP channels. Br J Pharmacol. 2014;171:1534–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han Y, Qin J, Chang X, Yang Z, Tang X, Du J. Hydrogen sulfide may improve the hippocampal damage induced by recurrent febrile seizures in rats. Biochem Biophys Res Commun. 2005;327:431–436. [DOI] [PubMed] [Google Scholar]
- 26. Li W, Du JB, Jin HF. Effects of hydrogen sulfide donor on production of adrenomedullin and atrial natriuretic peptide in rats with atherosclerosis. Chin J Contemp Pediatr. 2015;17:1119–1123. [PubMed] [Google Scholar]
- 27. Jin HF, Du SX, Zhao X, Wei HL, Wang YF, Liang YF, Tang CS, Du JB. Effects of endogenous sulfur dioxide on monocrotaline‐induced pulmonary hypertension in rats. Acta Pharmacol Sin. 2008;29:1157–1166. [DOI] [PubMed] [Google Scholar]
- 28. Yang G, Wu L, Wang R. Pro‐apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. [DOI] [PubMed] [Google Scholar]
- 29. Jin HF, Cong BL, Zhao B, Zhang CY, Liu XM, Zhou WJ, Shi Y, Tang CS, Du JB. Effects of hydrogen sulfide on hypoxic pulmonary vascular structural remodeling. Life Sci. 2006;78:1299–1309. [DOI] [PubMed] [Google Scholar]
- 30. Duan XC, Liu SY, Guo R, Xiao L, Xue HM, Guo Q, Jin S, Wu YM. Cystathionine‐β‐synthase gene transfer into rostral ventrolateral medulla exacerbates hypertension via nitric oxide in spontaneously hypertensive rat. Am J Hypertens. 2015;28:1106–1113. [DOI] [PubMed] [Google Scholar]
- 31. Yokoyama T, Nakamuta N, Kusakabe T, Yamamoto Y. Vesicular glutamate transporter 2‐immunoreactive afferent nerve terminals in the carotid body of the rat. Cell Tissue Res. 2014;358:271–275. [DOI] [PubMed] [Google Scholar]
- 32. Katayama PL, Castania JA, Dias DP, Patel KP, Fazan R Jr, Salgado HC. Role of chemoreceptor activation in hemodynamic responses to electrical stimulation of the carotid sinus in conscious rats. Hypertension. 2015;66:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Victor RG. Carotid baroreflex activation therapy for resistant hypertension. Nat Rev Cardiol. 2015;12:451–463. [DOI] [PubMed] [Google Scholar]
- 34. Heusser K, Tank J, Brinkmann J. Acute response to unilateral unipolar electrical carotid sinus stimulation in patients with resistant arterial hypertension. Hypertension. 2016;67:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doumas M, Faselis C, Kokkinos P, Anyfanti P, Tsioufis C, Papademetriou V. Carotid baroreceptor stimulation: a promising approach for the management of resistant hypertension and heart failure. Curr Vasc Pharmacol. 2014;12:30–37. [DOI] [PubMed] [Google Scholar]
- 36. Takayama Y, Uta D, Furue H, Tominaga M. Pain‐enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci USA. 2015;112:5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Assas BM, Wakid MH, Zakai HA, Miyan JA, Pennock JL. Transient receptor potential vanilloid 1 expression and function in splenic dendritic cells: a potrntial role in immune homeostasis. Immunology. 2016;147:292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Louise G, Stefan J, Henri D. Somatostatin 4 receptor activation modulates TRPV1 currents in dorsal root ganglion neurons. Neurosci Lett. 2014;573:35–39. [DOI] [PubMed] [Google Scholar]
- 39. Alawi KM, Aubdool AA, Liang L, Wilde E, Vepa A, Psefteli MP, Brain SD, Keeble JE. The sympathetic nervous system is controlled by transient receptor potential vanilloid 1 in the regulation of body temperature. FASEB J. 2015;29:4285–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohno K, Okuda K, Uehara T. Endogenous S‐sulfhydration of PTEN helps protect against modification by nitric oxide. Biochem Biophys Res Commun. 2015;456:245–249. [DOI] [PubMed] [Google Scholar]
- 41. Chen PH, Fu YS, Wang YM, Yang KH, Wang DL, Huang B. Hydrogen sulfide increases nitric oxide production and subsequent S‐nitrosylation in endothelial cells. Sci World J. 2014;2014:480387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu C, Kavalier A, Lukyanov E, Gross SS. S‐sulfhydration/desulfhydration and S‐nitrosylation/denitrosylation: a common paradigm for gasotransmitter signaling by H2S and NO. Methods. 2013;62:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo C, Liang F, Masood WS, Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s‐sulfhydration, MAPK dependent anti‐apoptosis and NF‐κB dependent anti‐inflammation pathway. Eur J Pharmacol. 2014;725:70–78. [DOI] [PubMed] [Google Scholar]
- 44. Shimizu S, Takahashi N, Mori Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). Handlb Exp Pharmacol. 2014;223:767–794. [DOI] [PubMed] [Google Scholar]
- 45. DelloStritto DJ, Sinharoy P, Connell PJ, Fahmy JN, Cappelli HC, Thodeti CK, Geldenhuys WJ, Damron DS, Bratz IN. 4‐Hydroxynonenal dependent alteration of TRPV1‐mediated coronary microvascular signaling. Free Radic Biol Med. 2016;101:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


