Abstract
Background
Whether outcomes differ between sexes following treatment with pacemakers (PM), implantable cardioverter defibrillators, and cardiac resynchronization therapy (CRT) devices is unclear.
Methods and Results
Consecutive US patients with newly implanted PM, implantable cardioverter defibrillators, and CRT devices from a large remote monitoring database between 2008 and 2011 were included in this observational cohort study. Sex‐specific all‐cause survival postimplant was compared within each device type using a multivariable Cox proportional hazards model, stratified on age and adjusted for remote monitoring utilization and ZIP‐based socioeconomic variables. A total of 269 471 patients were assessed over a median 2.9 [interquartile range, 2.2, 3.6] years. Unadjusted mortality rates (MR; deaths/100 000 patient‐years) were similar between women versus men receiving PMs (n=115 076, 55% male; MR 4193 versus MR 4256, respectively; adjusted hazard ratio, 0.87; 95% CI, 0.84–0.90; P<0.001) and implantable cardioverter defibrillators (n=85 014, 74% male; MR 4417 versus MR 4479, respectively; adjusted hazard ratio, 0.98; 95% CI, 0.93–1.02; P=0.244). In contrast, survival was superior in women receiving CRT defibrillators (n=61 475, 72% male; MR 5270 versus male MR 7175; adjusted hazard ratio, 0.73; 95% CI, 0.70–0.76; P<0.001) and also CRT pacemakers (n=7906, 57% male; MR 5383 versus male MR 7625, adjusted hazard ratio, 0.69; 95% CI, 0.61–0.78; P<0.001). This relative difference increased with time. These results were unaffected by age or remote monitoring utilization.
Conclusions
Women accounted for less than 30% of high‐voltage implants and fewer than half of low‐voltage implants in a large, nation‐wide cohort. Survival for women and men receiving implantable cardioverter defibrillators and PMs was similar, but dramatically greater for women receiving both defibrillator‐ and PM‐based CRT.
Keywords: cardiac resynchronization therapy, implantable cardioverter defibrillators, pacemaker, sex, sex‐specific
Subject Categories: Electrophysiology, Heart Failure, Catheter Ablation and Implantable Cardioverter-Defibrillator, Mortality/Survival
Clinical Perspectives
What is New?
In a large, nation‐wide parallel cohort study of patients receiving remote monitoring enabled cardiac implantable electronic devices, postimplant survival in women compared to men after pacemakers and implantable cardioverter defibrillators was similar, but superior following resynchronization therapy (whether pacemaker or defibrillator based).
The results contradict the notion that women derive less benefit from implantable device therapy.
What are the Clinical Implications?
Although cardiac electronic device therapy is used less frequently in women, sex should not be a barrier during candidate selection.
Mechanisms underlying better response to cardiac resynchronization therapy in women require elucidation.
Introduction
There is increasing recognition of sex as a modulator of disease risk and response to pharmacotherapy,1 but less so with treatment with cardiac implantable electronic devices (CIEDs). Whether women gain similar benefits to men implanted with pacemakers (PM), implantable cardioverter defibrillators (ICD), and cardiac resynchronization therapy (CRT) pacemakers (CRT‐P) or defibrillators (CRT‐D) is unclear. In women, compared with men, ICDs have been reported to have less (or no) efficacy, with increased risk of complications.2, 3 Conversely, CRT‐Ds may have enhanced efficacy in women according to some, but not all, reports.4 Patients implanted with PMs and CRT‐Ps are not well studied, and are not included in national registries, for example, the National Cardiovascular Data Registry (NCDR). Lack of sex‐specific evidence potentially affects clinical decision making, but also has regulatory implications given that CIEDs are class III devices subject to the most stringent US Food and Drug Agency review.5
Characterization of sex‐specific outcomes post‐CIED implant demands analysis of large data sets, but this is limited by the significant underrepresentation of women in randomized, clinical trials, leading to persistent uncertainties. Hence, there is a need to gather “real‐world” evidence to provide necessary insights.6 Here, we leveraged a nation‐wide remote monitoring database, collecting longitudinal follow‐up data in a large cohort of CIED patients receiving the range of CIEDs. We evaluated sex‐based differences in frequency of implants and subsequent all‐cause survival within each CIED device type. Furthermore, we determined whether sex‐specific outcomes were modulated by age, remote monitoring (RM) utilization, and socioeconomic status.
Methods
Study Design and Patient Selection
This retrospective, national, observational cohort study evaluated all consecutive patients implanted with market‐released St. Jude Medical, Inc (Sylmar, CA) ICDs and CRT‐Ds between October 2008 and November 2011, and PMs and CRT‐Ps between October 2009 and November 2011. To allow for adjustment of RM utilization, which is associated with significant changes in outcome, only patients implanted with CIEDs capable of automatic radiofrequency‐enabled RM were included (Figure 1). Among these, patients enrolled in a clinical trial or with follow‐up duration <90 days were excluded. Included patients were followed until death, device replacement, or device removal through November 2013. Survival was assessed for male and female patients within each device type group.
Figure 1.
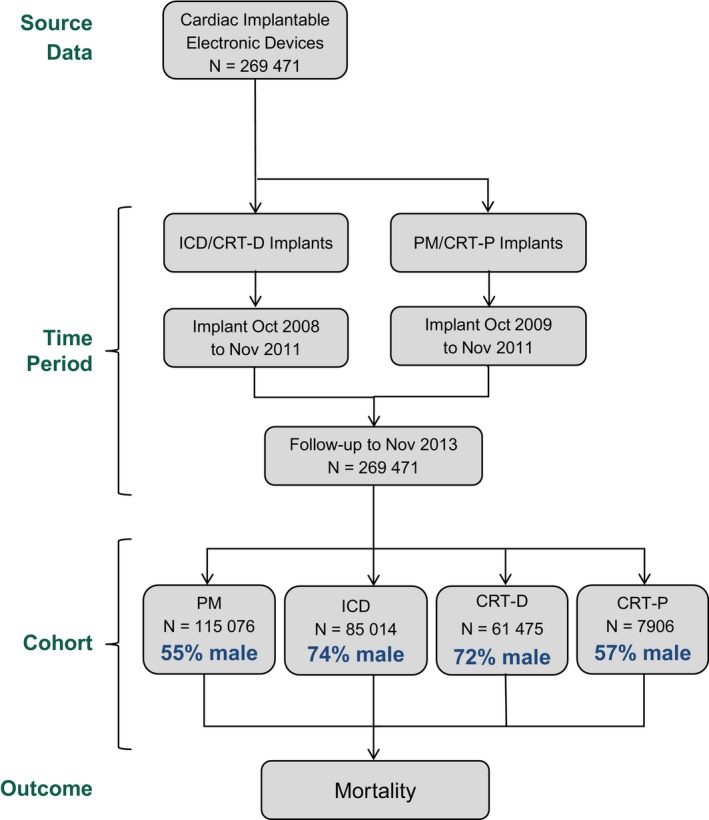
Study design. This study included CIEDs capable of automatic radiofrequency (RF)‐enabled remote monitoring. RF‐enabled CIEDs comprised 89.2% of all patients implanted with St. Jude Medical, Inc CIEDs between 2009 and 2013, with similar proportions between sexes (86.4% female; 90.9% male). The distribution of CIED device types studied here from a single manufacturer was similar to nation‐wide profiles (including age, sex distribution) observed in other nation‐wide databases inclusive of all manufacturers.7 CIEDs indicates cardiac implantable electronic devices; CRT‐D, cardiac resynchronization therapy with defibrillation capability; CRT‐P, cardiac resynchronization therapy with pacing capability; ICD, implantable cardioverter defibrillator; PM, pacemaker.
Data Acquisition
Study data were obtained from 4 sources: device implant registration at St. Jude Medical, Inc, the Merlin.net™ RM system, the 2012 American Community Survey of the US Census, and the US Social Security Death Index Master File. Sex, age at implant, patient ZIP code, and state of residence at implant, date of implant, device model number, and follow‐up duration were ascertained using manufacturer device tracking data. De‐identified data from weekly Merlin.net™ maintenance transmissions were linked to implant registration data to determine RM status. The date of death was determined from the Social Security Death Index, a database of internal records from the US Social Security Administration Death Master File, with all death records through November 30, 2013. The Social Security Death Index is a validated tool for research that contains records of >94 million deceased individuals in the United States. It maintains high accuracy in determination of mortality status, with increased sensitivity in patients >65 years.8 Death reports made directly to the device manufacturer's US tracking system by healthcare providers or family members through November 30, 2013 were added to the analysis, accounting for <1% of all deaths. Demographic data on 11 socioeconomic variables were gathered from the 2012 American Community Survey by individual ZIP code tabulation area. The American Community Survey ZIP code tabulation area–based data were then linked to individual patient ZIP codes for the following data in percent of population in ZIP code tabulation area, except as noted: 4‐year college degree; median income ($); below poverty level; Supplemental Nutrition Assistance Program recipient; landline telephone service; employment status; labor force participation rate; civilian status; healthcare insurance; race (white, black, American Indian, Asian, 2 races, other); and total urban/rural classification. The urban percentage for a region was computed as the ratio of urban to total population counts.
Statistical Analysis
The primary end point for this study was all‐cause mortality. Unadjusted mortality rates and the mortality rate ratio (MRR) of female/male patients were determined from patient deaths and the computed follow‐up duration within each group. All‐cause survival was measured by the Kaplan–Meier method and was compared between female and male CIED recipients using a multivariable Cox proportional hazards model stratified on age. Covariates incorporated into the model included RM utilization and a ZIP‐based propensity score computed from the American Community Survey census variables using a boosted logistic regression. Patients with at least 1 transmission were classified as “RM Any,” whereas those who never transmitted during the study period were classed “No RM”. The adjusted Cox proportional hazards ratio (HR) and 95% CI were determined. Follow‐up duration was calculated for each patient as the time from device implant until device explant/replacement, death, or end of study surveillance.
To evaluate sex‐based differences in mortality across a range of ages, patients within each device type were binned into 6 groups based on their age at the time of implant: 35 to 44, 45 to 54, 55 to 64, 65 to 74, 75 to 84, and ≥85 years. Because of the small number of patients under the age of 35 years, they were excluded from the age stratification analysis. The unadjusted mortality rate for male and female patients within each age group was plotted with the 95% CI. The absolute number of male and female patients within each age range bin was also plotted for all 4 device types.
Geographical Analysis
To assess the sex‐specific distribution of CIED implants across the United States, patients were binned into groups based on device type and state of residence. The proportion of male patients in each state was calculated and plotted on a 40‐step color scale between 10% and 90%. States with <50% male were represented by a blue color gradient, whereas those with ≥50% male were plotted on an orange gradient. Any states with no or missing data were plotted in gray.
All statistical analyses were performed with Revolution R Open 3.2.1. Patient demographics were assessed as mean and SD, median and interquartile range, or count and proportion. The P value for means comparison was Student's t test, for median was Wilcoxon rank‐sum, and for counts was chi‐square. Because statistical significance is commonly obtained for benign differences in large cohort studies, comparisons that were not clinically relevant are described as “similar” in the text.
Results
Study Population
The study cohort consisted of 269 471 patients (mean age, 71.0±13.5 years) implanted with CIEDs capable of automatic wireless RM during the study period with a median follow‐up of 2.9 [interquartile range, 2.2, 3.6] years (Figure 1; Table 1). Across all device types, 47% of patients utilized RM. Missing ZIP code data accounted for <0.1% (1694) of patients, in whom missing values were imputed from the median value for the state of residence. The CIED distribution included 115 076 (43%) patients implanted with a PM, 85 014 (32%) with an ICD, 61 475 (23%) with a CRT‐D, and 7906 (3%) with a CRT‐P. Mean ages of patients implanted with PMs (75.9±11.8 years) and CRT‐Ps (76.1±12.4 years) was higher than with ICDs (64.4±14.1 years) and CRT‐Ds (70.1±11.6 years). Among defibrillators, the proportion of single‐chamber/dual‐chamber/resynchronization devices was 21%/37%/42%, respectively. Collectively, these characteristics are similar to those noted in national databases inclusive of all manufacturers' devices.7, 9, 10
Table 1.
Patient Demographics and Characteristics
| All | Malea | Femalea | P Value | |
|---|---|---|---|---|
| N | 269 471 | 174 553 (64.8) | 94 918 (35.2) | <0.001 |
| Follow‐up, y | 2.9 [2.2, 3.6] | 2.9 [2.2, 3.6] | 2.9 [2.3, 3.5] | <0.001 |
| Age, y | 71.0±13.5 | 70.3±13.2 | 72.2±14.0 | <0.001 |
| Remotely monitored | 127 706 (47.4) | 82 450 (47.2) | 45 256 (47.7) | 0.189 |
| Device type | ||||
| PM | 115 076 (42.7) | 62 750 (35.9) | 52 326 (55.1) | <0.001 |
| ICD | 85 014 (31.5) | 62 934 (36.1) | 22 080 (23.3) | <0.001 |
| CRT‐D | 61 475 (22.8) | 44 345 (25.4) | 17 130 (18.0) | <0.001 |
| CRT‐P | 7906 (2.9) | 4524 (2.6) | 3382 (3.6) | <0.001 |
| ZIP code–linked datab | ||||
| Have telephone | 97.5±2.3 | 97.5±2.3 | 97.4±2.3 | <0.001 |
| Median income | 54.6±21.8 | 55.2±22.1 | 53.5±21.0 | <0.001 |
| Below poverty line | 14.1±8.4 | 13.8±8.3 | 14.5±8.5 | <0.001 |
| Receive SNAP | 1.1±1.1 | 1.1±1.1 | 1.2±1.1 | 0.002 |
| Bachelor's degree | 26.2±15.1 | 26.5±15.3 | 25.6±14.8 | <0.001 |
| Race: white | 76.7±21.6 | 77.3±21.1 | 75.5±22.4 | <0.001 |
| Race: black | 12.6±18.9 | 12.0±18.2 | 13.7±19.9 | <0.001 |
| Race: American Indian | 0.7±3.2 | 0.7±3.2 | 0.7±3.1 | 0.758 |
| Race: Asian | 3.8±7.0 | 3.8±6.9 | 3.8±7.1 | 0.777 |
| Race: other | 3.7±6.4 | 3.7±6.3 | 3.8±6.6 | <0.001 |
| Race: 2 races | 2.4±2.2 | 2.4±2.3 | 2.4±2.2 | 0.001 |
| Uninsured | 14.6±7.5 | 14.4±7.4 | 14.8±7.5 | <0.001 |
| Civilian | 62.3±8.9 | 62.2±9.0 | 62.4±8.6 | <0.001 |
| Unemployed | 9.7±4.4 | 9.7±4.4 | 9.9±4.5 | <0.001 |
| Not in labor force | 37.4±8.9 | 37.5±9.1 | 37.3±8.6 | <0.001 |
| Urban residence | 76.3±33.4 | 76.0±33.7 | 77.0±32.9 | <0.001 |
Values presented as mean±SD, median [interquartile range], or N (%). CRT‐D indicates cardiac resynchronization therapy with defibrillation capability; CRT‐P, cardiac resynchronization therapy with pacing capability; ICD, implantable cardioverter defibrillator; PM, pacemaker; SNAP, Supplemental Nutrition Assistance Program.
For some parameters, comparison between male and female patients yields differences that are very small in magnitude and clinically insignificant, but statistically significant. This is attributed to the large number of patients in each group, for whom even a small difference between largely similar populations becomes statistically significant. This pattern persisted across all device types.
All parameters in this section were measured as % in ZIP code except median income, which was thousands of dollars in ZIP code.
Sex Distribution
Overall, 174 553 (65%) of patients in the study cohort were male, but the sex distribution across device types was highly skewed (Figure 2A). The proportion of male patients implanted with ICDs and CRT‐Ds was 74% and 72%, and with PMs and CRT‐Ps was 55% and 57%, respectively. Across all device types, the mean age, follow‐up duration, and proportion of patients utilizing RM were similar between male and female patients. However, patients receiving high‐voltage devices compared with pacemakers not only were younger, but also included a much higher proportion of men.
Figure 2.
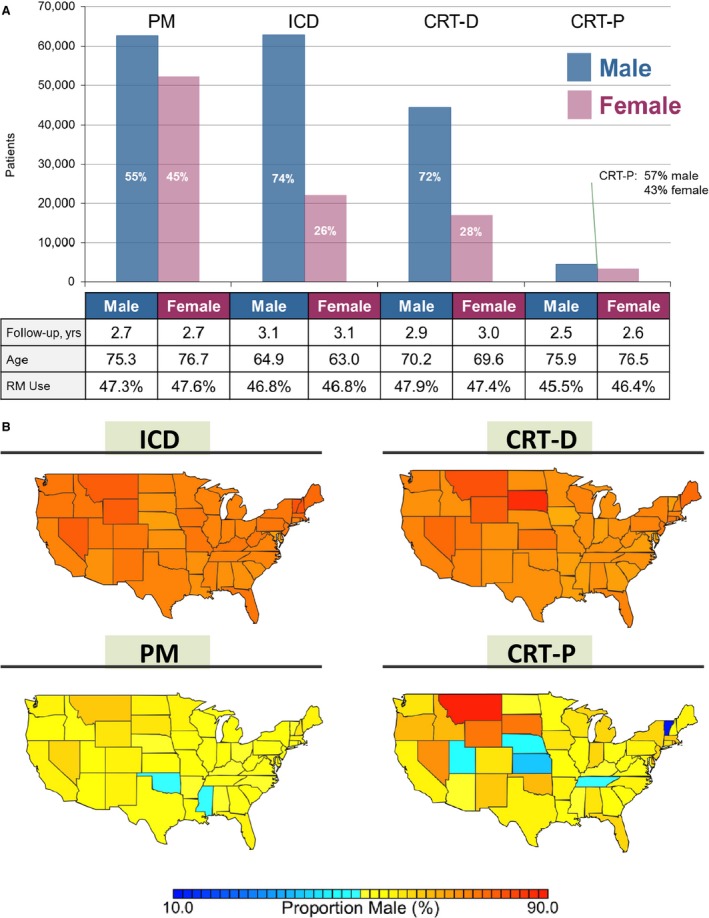
Distribution of male and female patients with cardiac implantable electronic devices by (A) device type and (B) geographically across the United States. CRT‐D indicates cardiac resynchronization therapy with defibrillation capability; CRT‐P, cardiac resynchronization therapy with pacing capability; ICD, implantable cardioverter defibrillator; PM, pacemaker; RM, remote monitoring.
There was moderate geographical heterogeneity in the proportion of male versus female patients implanted with CIEDs across the United States (Figure 2B). The relatively low proportion of female patients receiving ICD and CRT‐D therapy was consistent across every state (Hawaii and Alaska not shown). The small number of total patients implanted with CRT‐P likely contributes to the apparent variability in the proportion of male patients in certain states, such as Montana and Vermont.
Mortality and Survival Results
Survival was similar between female and male patients implanted with PMs and ICDs. In patients implanted with PMs, the unadjusted MRR was 0.99 (95% CI, 0.95–1.02) and the adjusted HR was 0.87 ([95% CI, 0.84–0.90]; P<0.001; Figure 3). Outcomes were similar between sexes for single‐ and dual‐chamber PMs. For female versus male ICD patients, the unadjusted MRR was 0.99 (95% CI, 0.95–1.03) and the adjusted HR was 0.98 ([95% CI, 0.93–1.02]; P=0.244; Figure 4). Results were similar for ICD cohorts dichotomized into single‐ and dual‐chamber devices. In the CRT‐D cohort, 11 877 of 61 475 (19%) patients had died during a median follow‐up of 2.9 [interquartile range, 2.2, 3.6] years (similar to rates reported from the NCDR11). However, female CRT patients implanted with CRT‐D and CRT‐P devices had improved survival compared with male CRT patients in the 4 to 5 years postimplant (CRT‐D adjusted HR, 0.73 [95% CI, 0.70–0.76]; P<0.001; CRT‐P adjusted HR, 0.69 [95% CI, 0.61–0.78]; P<0.001; Figure 5). The unadjusted MRR for CRT‐D was 0.73 (95% CI, 0.70–0.77) and for CRT‐P was 0.71 (95% CI, 0.63–0.79; Table 2). Across all age ranges, the unadjusted mortality rates were similar between male and female patients implanted with PMs and ICDs. However, for patients implanted with CRT‐D and CRT‐P devices, the unadjusted survival curves begin to diverge between ages 45 to 54 years and ages 65 to 74 years, respectively (Figure 6). When directly compared, overall 3‐year survival in CRT‐D was inferior to CRT‐P cohorts (adjusted HR=0.77 [95% CI 0.72–0.81]; P<0.001) with similar differences between sexes (male, N=48 869: HR=0.82 [95% CI 0.77–0.89]; P<0.001; female, N=20 512: HR=0.78 [95% CI, 0.70–0.87]; P<0.001). The extensively overlapping 95% CIs for each group (overall, male, and female) indicate that the association with device type was not modulated by sex. Sex‐specific survival results were consistent across patients with all CIED device types with and without RM (Figure 7).
Figure 3.
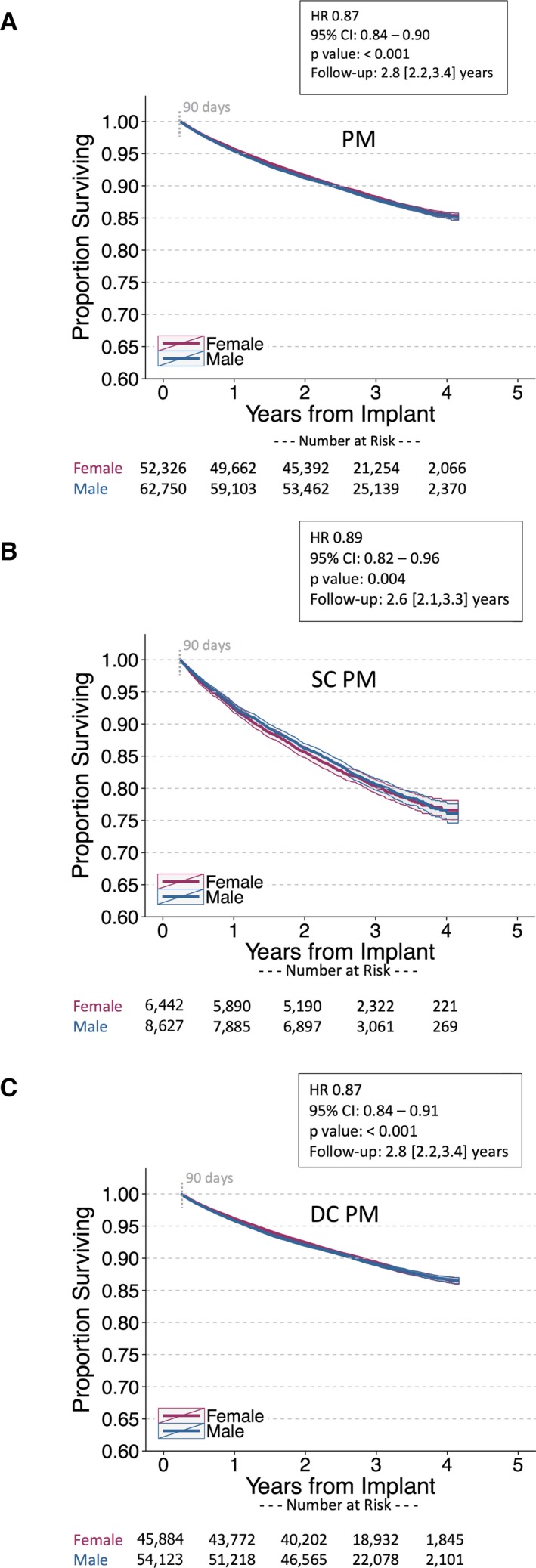
Postimplant survival in female vs male patients implanted with pacemakers (PM). Unadjusted Kaplan–Meier survival curves for (A) all PM patients (B) single‐chamber (SC) PM patients, and (C) dual‐chamber (DC) PM patients over 4 years of follow‐up. Patients receiving SC pacemakers were slightly older than those receiving DC pacemakers (79.2±10.3 vs 75.4 ± 11.9 years, respectively). Cox proportional hazards models are stratified on age and adjusted for remote monitoring utilization and ZIP code–linked covariates. Postimplant survival was slightly better for female pacemaker patients (it is common to obtain statistical significance for benign differences with large cohorts, as in this instance). HR indicates hazard ratio. Follow‐up duration reported as median [interquartile range].
Figure 4.
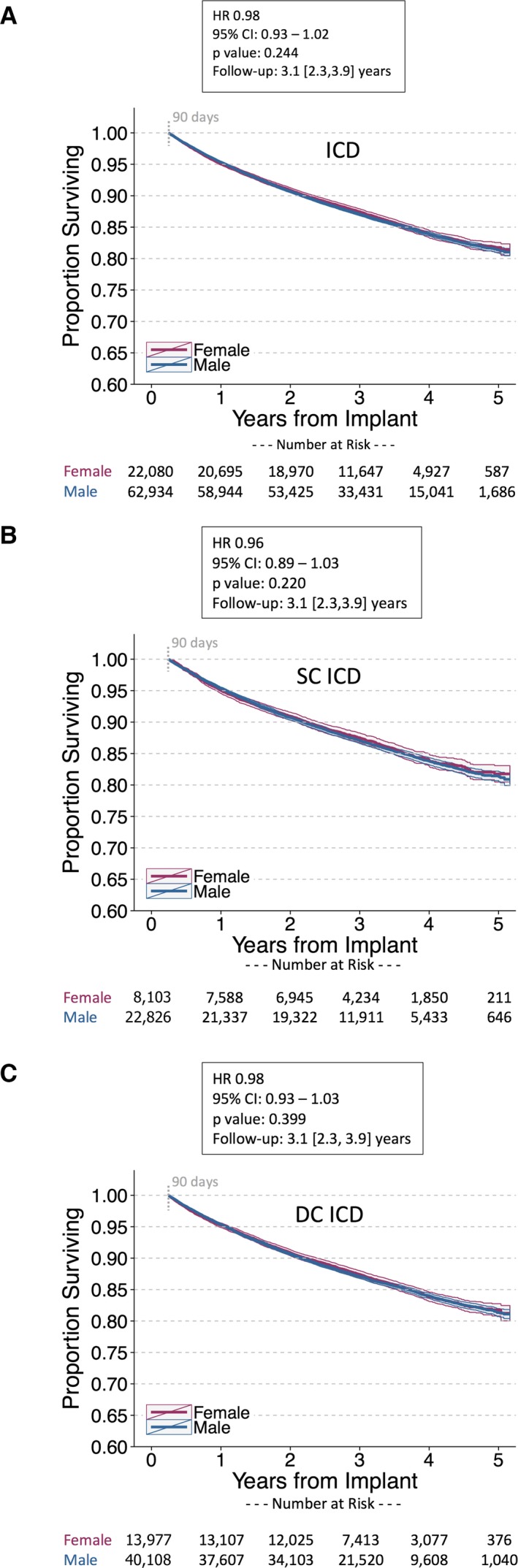
Postimplant survival in female vs male patients implanted with implantable cardioverter defibrillators (ICD). Unadjusted Kaplan–Meier survival curves for (A) all ICD patients, (B) single‐chamber (SC) ICD patients, and (C) dual‐chamber (DC) ICD patients. Patients receiving SC ICDs were slightly younger than those receiving DC ICDs (62.2±14.9 vs 65.7±13.4 years, respectively). Cox proportional hazards models are stratified on age and adjusted for remote monitoring utilization and ZIP code–linked covariates. Adjusted mortality is similar between female and male patients implanted with ICDs. HR indicates hazard ratio. Follow‐up duration reported as median [interquartile range].
Figure 5.
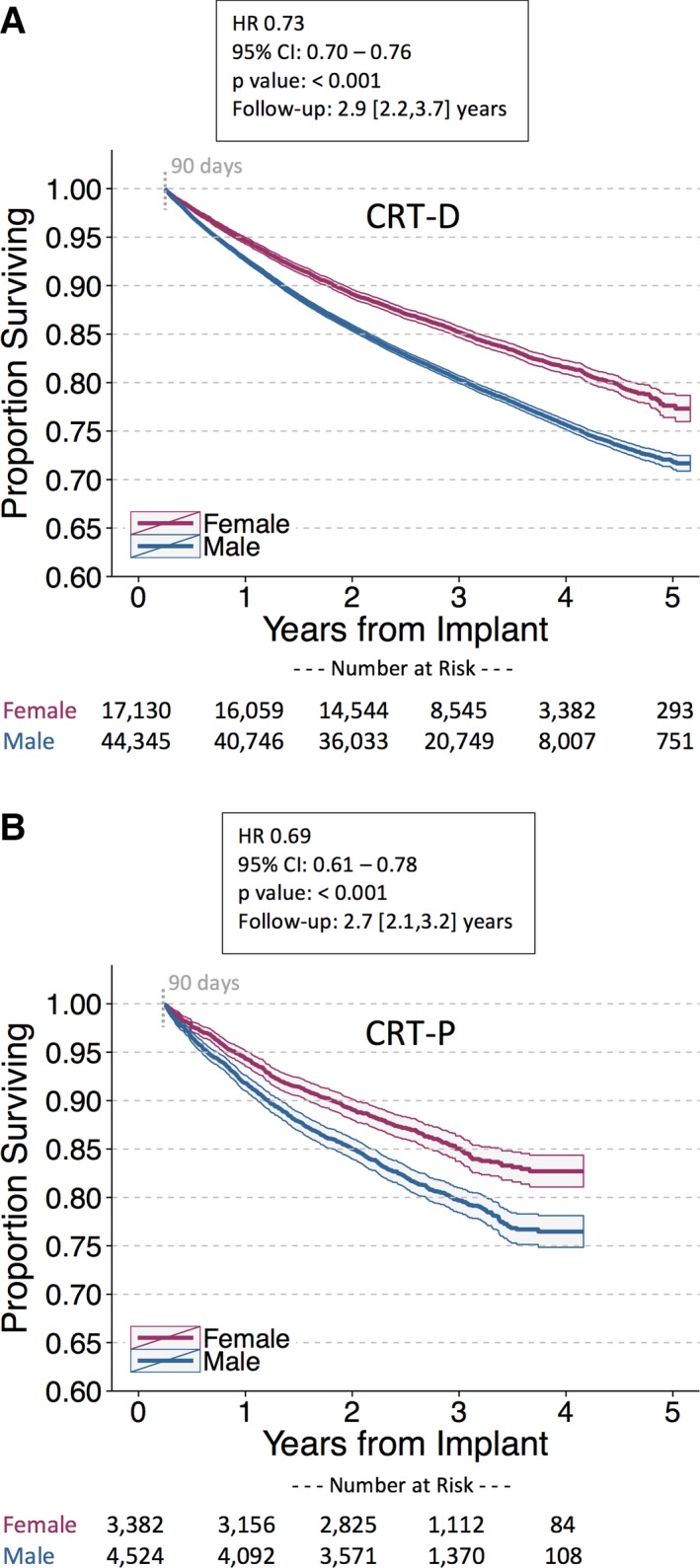
Postimplant survival in female vs male patients implanted with cardiac resynchronization therapy (CRT) devices. Unadjusted Kaplan–Meier survival curves for (A) CRT with defibrillation capability (CRT‐D); (B) CRT with pacing capability (CRT‐P). Female patients implanted with high‐voltage and low‐voltage CRT devices are associated with improved survival compared with male CRT patients. CRT‐D indicates cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; HR, hazard ratio. Follow‐up duration reported as median [interquartile range].
Table 2.
Male and Female Unadjusted Mortality Rates Across Each Device Type
| Device | N | Deaths (%) | Male (%) | Mortality Rates (Per 100 000 Patient‐Years) | ||
|---|---|---|---|---|---|---|
| Male [95% CI] | Female [95% CI] | MRR [95% CI] | ||||
| All | 269 471 | 38 130 (14.1%) | 64.8 | 5160 [5098–5224] | 4492 [4412–4572] | 0.87 [0.85–0.89] |
| PM | 115 076 | 13 256 (11.5%) | 54.5 | 4256 [4159–4355] | 4193 [4089–4301] | 0.99 [0.95–1.02] |
| ICD | 85 014 | 11 652 (13.7%) | 74.0 | 4479 [4386–4574] | 4417 [4262–4579] | 0.99 [0.95–1.03] |
| CRT‐D | 61 475 | 11 877 (19.3%) | 72.1 | 7175 [7030–7324] | 5270 [5075–5473] | 0.73 [0.70–0.77] |
| CRT‐P | 7906 | 1345 (17.0%) | 57.2 | 7625 [7135–8150] | 5383 [4922–5887] | 0.71 [0.63–0.79] |
Data presented as N (%) or median [interquartile range]. CRT‐D indicates cardiac resynchronization therapy with defibrillation capability; CRT‐P, cardic resynchronization therapy with pacing capability; ICD, implantable cardioverter defibrillator; MRR, mortality rate ratio (female/male); PM, pacemaker.
Figure 6.
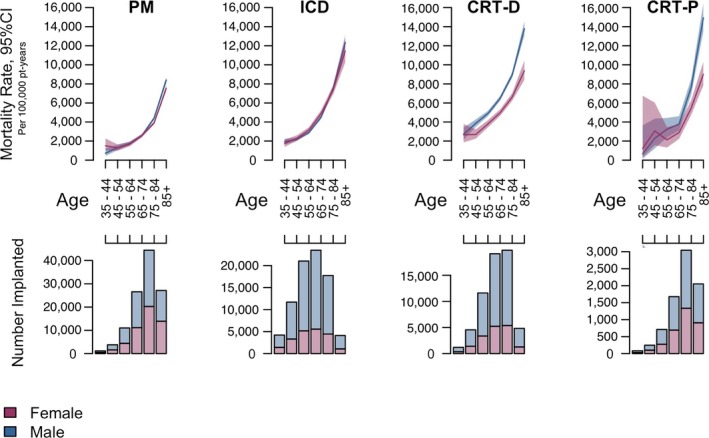
Mortality rates across various age ranges in each device type. Unadjusted mortality rates for female and male patients in each device type, binned into 6 discrete age groups (top). Number of female and male patients implanted within each age group (bottom). Plots show that patients with ICD and pacemaker implants do not exhibit mortality rate difference based on sex, whereas female patients with CRT‐D and CRT‐P clearly have lower mortality rates. Younger age bins have fewer patients (with wide CIs), and so this difference is not clear, but starting at 45 years for CRT‐D and 65 to 75 years for CRT‐P, there is a significant mortality difference favoring female patients. CRT‐D indicates cardiac resynchronization therapy with defibrillation capability; CRT‐P, cardiac resynchronization therapy with pacing capability; ICD, implantable cardioverter defibrillator; PM, pacemaker.
Figure 7.
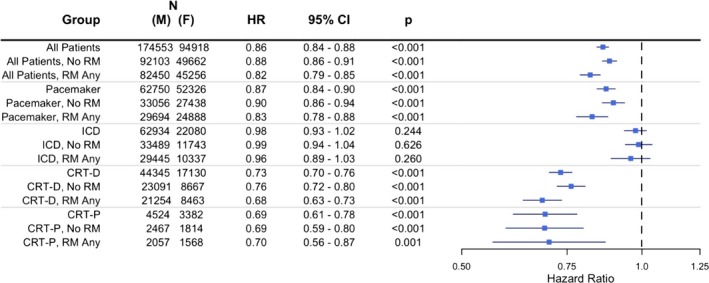
Sex‐specific Cox proportional hazards model results for female vs male postimplant survival, stratified on remote monitoring (RM) utilization. CRT‐D indicates cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; HR, hazard ratio; ICD, implantable cardioverter defibrillator.
Discussion
This is the first longitudinal, nation‐wide evaluation of patients receiving CIED implants of all device types to compare sex‐specific implant rates and patient survival in parallel cohorts, and the effect(s) of important covariates, such as age and RM utilization, in contemporary practice. Across low‐voltage and high‐voltage devices, more men than women received CIEDs. The implant imbalance was comparatively slight for pacemakers (including CRT‐P), but the incidence of ICD (single‐ or dual‐chamber) and CRT‐D implantation was 3‐fold greater in men than women (Figure 2A). Survival in women compared with men after PM and ICD implant was similar, but superior following resynchronization therapy, whether CRT‐D or CRT‐P. These effects were independent of age, RM utilization, and ZIP‐based socioeconomics. In particular, although RM is a strong modulator of survival following CIED implant, our sex‐specific survival results were consistent across patients with and without RM (Figure 7).
We present the first survival data for large patient cohorts receiving pacemakers, which accounted for the majority of implanted CIEDs. Most patients received dual‐chamber units, in whom survival was best (Figure 3). Sex‐specific differences in survival following treatment with both single‐ or dual‐chamber pacemakers were slight if any (MRR=0.99 [Table 2] with superimposed Kaplan–Meier curves [Figure 3]), but considerable in the population treated with CRT‐Ps (see below).
The proportion of single‐chamber/dual‐chamber/CRT defibrillators in our cohort (21%/37%/42%) was virtually identical to those reported (21%/38%/41%, respectively) in the NCDR over the same time period.9 Among patients treated with ICDs, there was no difference in overall survival between those receiving single‐ versus dual‐chamber devices. Women did not fare worse with ICD therapy compared with men. This is an important result, given that traditionally the risk‐benefit ratio has been perceived to be less favorable in women, which may generate a reluctance to use this therapy. This may explain the lesser proportion of overall implants. Another contributory factor may be simply that fewer women meet indications for ICD therapy. Compared with men, women with heart failure more often have preserved ejection fraction and are thus ineligible for guideline‐based defibrillator implantation. Nevertheless, even appropriate female candidates with systolic dysfunction are less likely to receive ICDs for primary or secondary indications.12, 13, 14, 15 However, this cannot be determined from the current data set. Procedural complications (acute and chronic) may be higher in women, discouraging ICD use.3, 16, 17 Some previous reports indicate attenuated ICD benefit, although survival data are scant.3, 18, 19 Meta‐analyses of clinical trial data have yielded conflicting conclusions.2, 20 Propensity‐matched cohorts from registries (on a smaller scale) report similar risks and benefits between men and women.21 This conclusion is supported by our long‐term outcomes from an inclusive nation‐wide cohort of over 85 000 ICD patients demonstrating no evidence of an interaction between sex and ICD benefit with respect to survival.
In contrast to pacemaker and ICD therapy, sex‐specific differences in survival with CRT were striking, irrespective of device platform (ie, CRT‐D or CRT‐P). Although women constituted only 28% of CRT‐D implants (Figure 2A), a proportion similar to national registries,9 female advantage manifested early and with a progressive divergence of survival curves, indicating that relative differences increase with time (Figure 5). Previous reports of sex‐specific CRT response are mixed, likely attributed to small female representation.4 Some randomized, controlled trials or meta‐analyses indicated no effect,22, 23, 24 whereas others suggest a greater CRT benefit in women.17, 25 Registry results are similarly conflicting.26, 27 However, results from an NCDR analysis of a smaller set of nonconsecutive patients drawn from the same epoch as ours with similar overall demographics (age 70±11 years, 68% male, 17% mortality over 2.9 years) reported that CRT‐D therapy in women was associated with an 18% lower mortality risk than in men.11 We discovered a relative risk reduction of 27% in women in the current study, which is consonant with a meta‐analysis of US‐based randomized trials comprising 27 547 individuals.25 Hence, large data set inquiry has yielded more consistent results.
Our data for CRT‐P are unique. Generally, this population of patients is not well characterized, even in large, observational registries data. The NCDR does not register these devices. In the National Implant Sample, CRT‐P accounted for 14% of implanted resynchronization devices in 2009, with 58% male proportion and age 75 years (ie, demographics identical to our cohort).10 However, this registry lacks postimplant follow‐up data. Among randomized trials, the CARE‐HF (Cardiac Resynchronization in Heart Failure) trial did not show sex‐specific effects with CRT‐P, but was likely underpowered for this conclusion, given that only 215 female patients were enrolled.23 Here, we show that post‐CRT‐P implant survival was better among women. Comparison to our CRT‐D cohort is instructive. The sex imbalance for CRT‐D implantation rates was not matched with CRT‐P, suggesting that it is not simply the higher risk of procedural complications among females receiving resynchronization therapy17 that accounts for reduced utilization of defibrillator therapy. Overall, postimplant survival was better in patients treated with CRT‐P, but effect of sex was similar. This is notable given that CRT‐P and CRT‐D recipients differ significantly in US practice. CRT‐P patients were more likely to be older, have atrial fibrillation, complete atrioventricular block, and have greater accompanying comorbidities in the National Implant Sample, and were reported to have better superior left ventricular function in the ADVANCE CRT Registry.10, 28 That survival advantage in women receiving CRT‐P was of similar extent to CRT‐D, despite these baseline differences, may point to a primary sex‐specific difference in CRT effect.
The reasons for superior CRT outcome in women cannot be elucidated from this registry. Possibilities are: systematic differences in patient selection and treatment differing to guidelines, presence of confounding factors encountered in real‐world practice, but not measured in randomized trials or accounted for in guidelines, or a fundamental sex‐specific difference in efficacy of therapy itself. In other reports, sex‐specific CRT effect manifests even among patients matched for characteristics, such as nonischemic cardiomyopathy, left bundle branch block and QRS duration.29 Possibly, the value of the selection criterion of left bundle branch block is different between sexes, as noted for other diagnostic criteria in cardiology.1 Left ventricular size has recently been shown to be one such modulator, not accounted for in previous studies.30 These potential modulators demand prospective investigation, given the magnitude and importance of effect demonstrated.
Strengths and Limitations
Our large cohort study of consecutive patients fulfills an important need described by the US Food and Drug Administration to gather real‐world evidence, especially when randomized trial data are limited and generazibility to clinical practice questionable.31 Given that any trial aiming to randomize on a sex‐specific basis is likely to be unethical, large, observational data are particularly important to resolve sex‐specific treatment (but not comparative efficacy) effects in device patients.32 Available registries differ in important aspects. For instance, the NCDR collects data from Medicare patients (ie, age >65 years, excluding younger patients) receiving ICDs for primary prophylactic indications, but not pacemakers. In contrast, the National Implant Sample includes patients from all payers, but only those coded for inpatient hospitalization, and lacks some critical clinical data such as left ventricular ejection fraction. Neither registry performs longitudinal assessment, although subgroups may be linked to the Social Security Death Index for mortality studies (as performed here). The NCDR and National Implant Sample do not collect information on RM utilization, which we report here occurring at rates similar to those indicated in national claims databases.7 Here, we present a large, nation‐wide cohort, which, though restricted to a single manufacturer's remote monitoring database, nevertheless mirrored the mix of device types, sex and age distribution, and overall survival post CIED‐implant observed in other national registries. Our cohorts are likely to share the detailed patient characteristics, comorbidities and CIED indications described in those other registries, although this cannot be confirmed directly from our remote monitoring database.
Our analysis of parallel CIED cohorts has the advantage of permitting sex‐specific comparisons across different CIED types, for example, ICD versus CRT‐D (differences when CRT was added to a common defibrillator platform) and CRT‐D versus CRT‐P (reported for the first time). This also permits examination of the potential impact of the longer estimated life span of women on CRT outcome. We observed no clinical difference in all‐cause survival for men versus women implanted with PMs and ICDs, whether single‐ or dual‐chamber, despite similar mean ages of patients implanted with low‐voltage devices (PM: 75.9 years and CRT‐P: 76.1 years) and high‐voltage devices (ICD: 64.4 years and CRT‐D: 70.1 years). Importantly, superior outcome manifest only in those women implanted with CRT‐P or CRT‐D devices, suggesting a mechanism that is independent of the intrinsic difference in life span between sexes. Furthermore, sex‐specific survival difference with CRT was observed at a younger age than may be expected if life span was driving the results (Figure 6). For example, in the subset of CRT‐D patients aged 45 to 54 years, the unadjusted mortality rate for females and males is 2689 and 3857 per 100 000 patient‐years, respectively (P<0.001). In contrast, unadjusted mortality rates are similar for men and women implanted with PMs and ICDs across all age ranges.
The lack of control groups precludes determination of absolute treatment effects, for example, ICDs may have different therapeutic efficacy in men versus women despite identity of postimplant survival curves (Figure 4). Although level of RM utilization correlates strongly with survival post‐CIED implant,33, 34 and may reflect patient and physician adherence to excellent care, this did not affect sex‐specific survival. Geographical differences exist in overall utilization rates of ICD and CRT therapies,10, 15 but no regional sex‐specific differences were noted in state‐based implant rates across each device type in our nation‐wide analysis. Socioeconomic level may affect general ICD outcome in heart failure patients.15, 35 We used residential ZIP code–based classification of socioeconomic status, which reflects the aggregate characteristics of its residents and prevailing healthy and unhealthy habits, inclusive of environmental attributes (eg, availability and access to healthcare resources), that may, in turn, have a direct or indirect impact on its residents' health. Such neighborhood effects might not be completely mediated or moderated by individual socioeconomic, behavioral, or biological risk factors.36 Notably, our sex‐specific results remained unaffected when accounting for these potential effects.
Conclusion
The deficit of sex‐specific data for cardiovascular devices, which have potentially differing safety and effectiveness profiles in men versus women, has clinical and regulatory implications.1, 5 Our results contradict the notion that women derive less benefit from CIED therapy. Rather, compared with men, survival in women was similar post‐ICD and PM implant, but dramatically greater following resynchronization therapy with both CRT‐D and CRT‐P. These data point to significant modulators (or “confounders”) of CIED efficacy as applied in contemporary practice, as opposed to randomized trials. These need to be identified in future prospective studies. Our observations may help to resolve barriers in candidate selection to lessen disparate care as a function of sex in patients eligible for device therapy.37
Sources of Funding
This research was funded by St. Jude Medical, Inc.
Disclosures
Varma has received consulting fees/honoraria from St. Jude Medical, Inc, Boston Scientific, Sorin, Biotronik, and Medtronic. Piccini has received consulting fees/honoraria from Medtronic and research grants from Boston Scientific. Prillinger receives salary from St. Jude Medical, Inc, and holds public stock in Medtronic and St. Jude Medical, Inc. Snell has received consulting fees from St. Jude Medical, Inc, and holds public stock in St. Jude Medical, Inc, Medtronic, and Boston Scientific. Dalal receives salary from St. Jude Medical, Inc. Mittal has received consulting fees/honoraria from St. Jude Medical, Inc, Medtronic, and Boston Scientific.
(J Am Heart Assoc. 2017;6:e005031 DOI: 10.1161/JAHA.116.005031.)28490521
This work was presented as an abstract at the Late Breaking Trials session of Europace Congress, June 19, 2015, in Milan, Italy.
References
- 1. Legato MJ, Johnson PA, Manson JE. Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA. 2016;316:1865–1866. [DOI] [PubMed] [Google Scholar]
- 2. Ghanbari H, Dalloul G, Hasan R, Daccarett M, Saba S, David S, Machado C. Effectiveness of implantable cardioverter‐defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2009;169:1500–1506. [DOI] [PubMed] [Google Scholar]
- 3. MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P, Birnie D, Simpson CS, Khaykin Y, Pinter A, Nanthakumar K, Calzavara AJ, Austin PC, Tu JV, Lee DS. Sex differences in implantable cardioverter‐defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. [DOI] [PubMed] [Google Scholar]
- 4. Costanzo MR. Cardiac resynchronization therapy in women. Card Electrophysiol Clin. 2015;7:721–734. [DOI] [PubMed] [Google Scholar]
- 5. Dhruva SS, Redberg RF. Evaluating sex differences in medical device clinical trials: time for action. JAMA. 2012;307:1145–1146. [DOI] [PubMed] [Google Scholar]
- 6. FDA . Use of real‐world evidence to support regulatory decision‐making for medical devices. 2016. Available at: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm513027.pdf. Accessed May 4, 2017.
- 7. Piccini JP, Mittal S, Snell J, Dalal N, Varma N. Impact of remote monitoring on clinical events and healthcare utilization: a nationwide assessment. Heart Rhythm. 2016;13:2279–2286. [DOI] [PubMed] [Google Scholar]
- 8. Huntington JT, Butterfield M, Fisher J, Torrent D, Bloomston M. The Social Security Death Index (SSDI) most accurately reflects true survival for older oncology patients. Am J Cancer Res. 2013;3:518–522. [PMC free article] [PubMed] [Google Scholar]
- 9. Masoudi FA, Ponirakis A, Yeh RW, Maddox TM, Beachy J, Casale PN, Curtis JP, De Lemos J, Fonarow G, Heidenreich P, Koutras C, Kremers M, Messenger J, Moussa I, Oetgen WJ, Roe MT, Rosenfield K, Shields TP Jr, Spertus JA, Wei J, White C, Young CH, Rumsfeld JS. Cardiovascular care facts: a report from the National Cardiovascular Data Registry: 2011. J Am Coll Cardiol. 2013;62:1931–1947. [DOI] [PubMed] [Google Scholar]
- 10. Lindvall C, Chatterjee NA, Chang Y, Chernack B, Jackson VA, Singh JP, Metlay JP. National trends in the use of cardiac resynchronization therapy with or without implantable cardioverter‐defibrillator. Circulation. 2016;133:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zusterzeel R, Curtis JP, Canos DA, Sanders WE, Selzman KA, Pina IL, Spatz ES, Bao H, Ponirakis A, Varosy PD, Masoudi FA, Strauss DG. Sex‐specific mortality risk by QRS morphology and duration in patients receiving CRT: results from the NCDR. J Am Coll Cardiol. 2014;64:887–894. [DOI] [PubMed] [Google Scholar]
- 12. Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. [DOI] [PubMed] [Google Scholar]
- 13. Curtis LH, Al‐Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter‐defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298:1517–1524. [DOI] [PubMed] [Google Scholar]
- 14. Hernandez AF, Fonarow GC, Liang L, Al‐Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter‐defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. [DOI] [PubMed] [Google Scholar]
- 15. Mehra MR, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, O'Connor CM, Reynolds D, Walsh MN, Fonarow GC. Evidence of clinical practice heterogeneity in the use of implantable cardioverter‐defibrillators in heart failure and post‐myocardial infarction left ventricular dysfunction: findings from IMPROVE HF. Heart Rhythm. 2009;6:1727–1734. [DOI] [PubMed] [Google Scholar]
- 16. Peterson PN, Daugherty SL, Wang Y, Vidaillet HJ, Heidenreich PA, Curtis JP, Masoudi FA. Gender differences in procedure‐related adverse events in patients receiving implantable cardioverter‐defibrillator therapy. Circulation. 2009;119:1078–1084. [DOI] [PubMed] [Google Scholar]
- 17. Arshad A, Moss AJ, Foster E, Padeletti L, Barsheshet A, Goldenberg I, Greenberg H, Hall WJ, McNitt S, Zareba W, Solomon S, Steinberg JS. Cardiac resynchronization therapy is more effective in women than in men: the MADIT‐CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) trial. J Am Coll Cardiol. 2011;57:813–820. [DOI] [PubMed] [Google Scholar]
- 18. Russo AM, Daugherty SL, Masoudi FA, Wang Y, Curtis J, Lampert R. Gender and outcomes after primary prevention implantable cardioverter‐defibrillator implantation: findings from the National Cardiovascular Data Registry (NCDR). Am Heart J. 2015;170:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rho RW, Patton KK, Poole JE, Cleland JG, Shadman R, Anand I, Maggioni AP, Carson PE, Swedberg K, Levy WC. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter‐defibrillator. Circulation. 2012;126:2402–2407. [DOI] [PubMed] [Google Scholar]
- 20. Santangeli P, Pelargonio G, Dello Russo A, Casella M, Bisceglia C, Bartoletti S, Santarelli P, Di Biase L, Natale A. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta‐analysis. Heart Rhythm. 2010;7:876–882. [DOI] [PubMed] [Google Scholar]
- 21. Zeitler EP, Hellkamp AS, Schulte PJ, Fonarow GC, Hernandez AF, Peterson ED, Sanders GD, Yancy CW, Al‐Khatib SM. Comparative effectiveness of implantable cardioverter defibrillators for primary prevention in women. Circ Heart Fail. 2016;9:e002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, Feldman AM, Galle E, Ecklund F. Predictors of sudden cardiac death and appropriate shock in the comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) trial. Circulation. 2006;114:2766–2772. [DOI] [PubMed] [Google Scholar]
- 23. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 24. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang AS. An individual patient meta‐analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng YJ, Zhang J, Li WJ, Lin XX, Zeng WT, Tang K, Tang AL, He JG, Xu Q, Mei MY, Zheng DD, Dong YG, Ma H, Wu SH. More favorable response to cardiac resynchronization therapy in women than in men. Circ Arrhythm Electrophysiol. 2014;7:807–815. [DOI] [PubMed] [Google Scholar]
- 26. Zabarovskaja S, Gadler F, Braunschweig F, Stahlberg M, Hornsten J, Linde C, Lund LH. Women have better long‐term prognosis than men after cardiac resynchronization therapy. Europace. 2012;14:1148–1155. [DOI] [PubMed] [Google Scholar]
- 27. Wilcox JE, Fonarow GC, Zhang Y, Albert NM, Curtis AB, Gheorghiade M, Heywood JT, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Clinical effectiveness of cardiac resynchronization and implantable cardioverter‐defibrillator therapy in men and women with heart failure: findings from IMPROVE HF. Circ Heart Fail. 2014;7:146–153. [DOI] [PubMed] [Google Scholar]
- 28. Varma N, Boehmer J, Costanzo M, Gold M, Singh J, Polosajian L, Bhargava K, Farazi T, Gill J, Auricchio A. Contrasting profiles of USA versus outside of USA CRT recipients: insights from the International Advance CRT Registry. Heart Rhythm. 2016;13:S58. [Google Scholar]
- 29. Varma N, Manne M, Nguyen D, He J, Niebauer M, Tchou P. Probability and magnitude of response to cardiac resynchronization therapy according to QRS duration and gender in nonischemic cardiomyopathy and LBBB. Heart Rhythm. 2014;11:1139–1147. [DOI] [PubMed] [Google Scholar]
- 30. Varma N, Lappe J, He J, Niebauer M, Manne M, Tchou P. Sex‐specific response to cardiac resynchronization therapy: effect of left ventricular size and QRS duration in LBBB. 2017. Available at: http://www.sciencedirect.com/science/article/pii/S2405500X17302591. Accessed May 6, 2017. [DOI] [PubMed]
- 31. Sheridan DJ, Julian DG. Achievements and limitations of evidence‐based medicine. J Am Coll Cardiol. 2016;68:204–213. [DOI] [PubMed] [Google Scholar]
- 32. Chen CE, Dhruva SS, Redberg RF. Inclusion of comparative effectiveness data in high‐risk cardiovascular device studies at the time of premarket approval. JAMA. 2012;308:1740–1742. [DOI] [PubMed] [Google Scholar]
- 33. Akar JG, Bao H, Jones P, Wang Y, Chaudhry SI, Varosy P, Masoudi FA, Stein K, Saxon LA, Curtis JP. Use of remote monitoring of newly implanted cardioverter‐defibrillators: insights from the patient related determinants of ICD remote monitoring (PREDICT RM) study. Circulation. 2013;128:2372–2383. [DOI] [PubMed] [Google Scholar]
- 34. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65:2601–2610. [DOI] [PubMed] [Google Scholar]
- 35. Eapen ZJ, McCoy LA, Fonarow GC, Yancy CW, Miranda ML, Peterson ED, Califf RM, Hernandez AF. Utility of socioeconomic status in predicting 30‐day outcomes after heart failure hospitalization. Circ Heart Fail. 2015;8:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agarwal S, Menon V, Jaber WA. Outcomes after acute ischemic stroke in the United States: does residential ZIP code matter? J Am Heart Assoc. 2015;4:e001629 DOI: 10.1161/JAHA.114.001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yancy CW, Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Mehra MR, O'Connor CM, Reynolds D, Walsh MN. Influence of patient age and sex on delivery of guideline‐recommended heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Am Heart J. 2009;157:754–762.e752. [DOI] [PubMed] [Google Scholar]


