Abstract
Background
Ischemic and hemorrhagic stroke are increasingly recognized as heterogeneous diseases with distinct subtypes and etiologies. Information on variation in distribution of vascular risk factors according to age in stroke subtypes is limited. We investigated the prevalence of vascular risk factors in stroke subtypes in relation to age.
Methods and Results
We studied a prospective multicenter university hospital–based cohort of 4033 patients. For patients with ischemic stroke caused by large artery atherosclerosis, small vessel disease, or cardioembolism and for patients with spontaneous intracerebral hemorrhage or aneurysmal subarachnoid hemorrhage, we calculated prevalences of vascular risk factors in 4 age groups: <55, 55 to 65, 65 to 75, and ≥75 years, and mean differences with 95% CIs in relation to the reference age group. Patients aged <55 years were significantly more often of non‐white origin (in particular in spontaneous intracerebral hemorrhage and aneurysmal subarachnoid hemorrhage patients) and most often smoked (most prominent for aneurysmal subarachnoid hemorrhage patients). Patients aged <55 years with ischemic stroke caused by large artery atherosclerosis or small vessel disease more often had hypertension, hyperlipidemia, and diabetes mellitus than patients with ischemic stroke of cardiac origin. Overall, the frequency of hypertension, hyperlipidemia, and diabetes mellitus increased with age among all stroke subtypes, whereas smoking decreased with age. Regardless of age, accumulation of potentially modifiable risk factors was most pronounced in patients with ischemic stroke caused by large artery atherosclerosis or small vessel disease.
Conclusions
The prevalence of common cardiovascular risk factors shows different age‐specific patterns among various stroke subtypes. Recognition of these patterns may guide tailored stroke prevention efforts aimed at specific risk groups.
Keywords: cerebrovascular disease/stroke, intracerebral hemorrhage, ischemic stroke, risk factor, subarachnoid hemorrhage
Subject Categories: Risk Factors, Ischemic Stroke, Intracranial Hemorrhage
Introduction
Stroke is the second cause of death worldwide,1 and the third most common cause of disability.2 Traditionally, the main stroke subtypes are ischemic stroke (IS), spontaneous intracerebral hemorrhage (sICH), and aneurysmal subarachnoid hemorrhage (aSAH). Eighty to 90% of the risk of both IS and intracerebral hemorrhage (ICH) is attributable to common vascular risk factors, including hypertension, smoking, obesity, diet, physical inactivity, diabetes mellitus, alcohol intake, and unfavorable lipid profile.3 IS and sICH are increasingly recognized as heterogeneous diseases with distinct subtypes, etiologies, and epidemiological patterns. Consequently, vascular risk profiles may differ according to stroke subtype and age. However, information on the variation in distribution of vascular risk factors according to age in stroke subtypes is limited,3, 4 although there are studies that report specifically on risk factors for stroke in the young and in the elderly.5, 6, 7, 8, 9
We investigated age‐specific prevalences of vascular risk factors in patients with IS and its subtypes, sICH, and aSAH in a prospective multicenter hospital‐based cohort in The Netherlands.
Methods
Study Population and Definition of Stroke
We included patients with cerebral ischemia, including both IS and transient ischemic attack (TIA), sICH, or aSAH who were enrolled in an ongoing prospective registry of 8 university hospitals between September 2009 and November 2014 in The Netherlands.10 In this study, the so‐called Parelsnoer Initiative, clinical data, imaging, and biomaterials of patients with stroke are prospectively and uniformly collected.10 We approached all eligible patients, or a next of kin when patients were unconsciousness or mentally incompetent, for informed consent within the first 3 months after the event. For simplicity, we used IS to refer to patients with IS or TIA. IS, sICH, and aSAH were defined according to the World Health Organization criteria for stroke and TIA,11 and confirmation on computed tomography or magnetic resonance imaging was required for sICH and aSAH.
We further classified IS into specific subtypes according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification:12 large artery atherosclerosis (LAA), small vessel disease (SVD), cardioembolic stroke, other determined cause, and undetermined cause.12 For the current analyses we only included the IS subtypes LAA, SVD, and cardioembolic stroke because both IS of other determined cause and of undetermined origin represent a variety of diseases. ICH was defined as spontaneous when presumably caused by rupturing of small vessels in the presence of hypertension or cerebral amyloid angiopathy. We excluded ICH patients with known macrovascular causes (eg, arteriovenous malformation, dural arteriovenous fistula, cavernoma, cerebral venous sinus thrombosis), tumor, or other rare causes. We defined aSAH by the presence of subarachnoid blood on noncontrast computed tomography compatible with a ruptured aneurysm and an aneurysm on computed tomography, magnetic resonance imaging, or digital subtraction angiography.
The ethics committees of all participating centers approved the study and all patients provided written informed consent.
Assessment of Risk Factors
We dichotomized ethnicity between white versus non‐white origin. We defined obesity as a BMI ≥30 kg/m2, calculated by dividing weight in kilograms by the square of height in meters. We defined hypertension as ever or current diagnosis or treatment with antihypertensive drugs, whereas diabetes mellitus was defined as ever or current diagnosis or treatment with antidiabetic drugs. Total cholesterol >3.5 mmol/L, low‐density lipoprotein cholesterol >2.5 mmol/L, or treatment with lipid‐lowering agents was considered as hypercholesterolemia. Information about these modifiable risk factors was obtained by consulting the patients' medical records or general practitioners. We defined smoking as current or recently stopped, in which “recently” was defined as less than 6 months. Lastly, family history of early‐onset cardiovascular disease was considered positive in the case of a first‐degree relative with a past vascular event, which included IS, ICH, SAH, myocardial infarction, and peripheral artery disease, before the age of 60 years for patients with either IS or sICH. For patients with aSAH, we considered the family history as positive in the case of at least one first‐degree relative with aSAH.
Statistical Analysis
We defined 4 age groups of comparable size: <55 years (n=1004, 24.9%), 55 to 65 years (n=943, 23.4%), 65 to 75 years (n=1053, 26.1%), and ≥75 years (n=1033, 25.6%). For each stroke subtype, we used the age group that contained the median age of that specific stroke type as a reference. For IS and sICH, the reference was the age group of 65 to 75 years, and for aSAH this was the age group of 55 to 65 years. For each stroke subtype, we calculated frequencies of risk factors in each age group and subsequently mean differences (MDs) in prevalence with corresponding 95% CIs for each age group in relation to the reference age group. Finally, we determined the number of potentially modifiable risk factors (obesity, hypertension, hyperlipidemia, diabetes mellitus, and smoking) in each age group for each stroke subtype. For this final analysis, we imputed missing data of height (21.8%), weight (19.6%), hypertension (1.2%), hyperlipidemia (2.6%), diabetes mellitus (1.5%), and smoking status (5.2%) by multiple imputation using linear regression.
Results
Baseline Characteristics and Distribution of Stroke
We included 4033 patients: 3311 patients with IS, 294 patients with sICH, and 428 patients with aSAH. In 58.5% of the patients with cerebral ischemia, symptoms persisted for more than 24 hours. Distribution of stroke subtypes, and their age distribution, is shown in Table 1. The median age of patients was 65.6 years (interquartile range, 55.1–73.3) and 2252 (55.8%) were men. Baseline characteristics of the cohort are presented in Table 2. History of cardiovascular disease was present in 1288 (31.9%) patients, and in 943 (23.4%) patients this history included stroke.
Table 1.
Stroke Distribution in the Dutch Parelsnoer Institute‐Cerebrovascular Accident Study
| No. (%) | Age, Median (IQR) | |
|---|---|---|
| Ischemic strokea | 3311 (82.1) | 66.7 (56.4–76.2) |
| Large artery atherosclerosisb | 815 (24.6) | 68.2 (61.6–76.7) |
| Small vessel diseaseb | 632 (19.1) | 66.1 (58.0–75.8) |
| Cardioembolic strokeb | 467 (14.1) | 72.2 (62.2–80.4) |
| Other determined causeb | 214 (6.5) | 51.1 (42.8–64.4) |
| Undetermined strokeb | 1130 (34.1) | 65.6 (54.0–75.3) |
| Spontaneous intracerebral hemorrhage | 294 (7.3) | 65.7 (55.0–73.8) |
| Aneurysmal subarachnoid hemorrhage | 428 (10.6) | 56.7 (48.4–65.5) |
IQR indicates interquartile range.
In 53 (1.6%) patients with ischemic stroke, a Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification was lacking.
The percentages reported are as part of total ischemic stroke.
Table 2.
Baseline Characteristics of Patients Included in the Dutch Parelsnoer Institute‐Cerebrovascular Accident Study
| N=4033 | |
|---|---|
| Men | 2252 (55.8) |
| Age, median (IQR) | 65.6 (55.1–75.3) |
| Non‐white | 187/3507 (5.3) |
| Cardiovascular risk factors: | |
| Hypertension | 2102/3985 (52.7) |
| Hyperlipidemia | 1277/3928 (32.5) |
| Diabetes mellitus | 573/3973 (14.4) |
| Current smoker | 1201/3822 (31.4) |
| Family history of early‐onset vascular eventsa | 951/2621 (36.3) |
| Familial history of subarachnoid hemorrhageb | 27/301 (8.2) |
| Obesity | 547/3108 (17.6) |
| Cardiovascular history | |
| Ischemic stroke (including TIA) | 939/3768 (24.9) |
| Intracerebral hemorrhage | 63/3754 (1.7) |
| Subarachnoid hemorrhage | 50/3759 (1.3) |
| Myocardial infarction | 409/3984 (10.3) |
| Atrial fibrillationc | 398/3232 (12.3) |
| Peripheral artery disease | 307/3954 (7.8) |
| Any cardiovascular history | 1288/3667 (35.1) |
Values are expressed as numbers (percentages) unless otherwise indicated. IQR indicates interquartile range; TIA, transient ischemic attack.
Any vascular bed included; not collected in patients with aneurysmal subarachnoid hemorrhage.
In a first‐degree relative; only collected in patients with aneurysmal subarachnoid hemorrhage.
Medical history of atrial fibrillation was only routinely collected in patients with cerebral ischemia.
Ischemic Stroke Overall
We found male predominance among all age groups, most prominently in patients aged 55 to 75 years (Figure 1A; Table S1). Patients aged <55 years (7.5%) or 55 to 65 years (6.8%) were significantly more often non‐white than those in the reference age group of 65 to 75 years (3.8%; Figure 2A; Table S2). Compared with the reference group, patients aged ≥75 years were significantly less often obese (MD, −7.0%; 95% CI, −11.0 to −3.1), whereas prevalences of obesity were similar among the other age groups (≈20%) (Figure 2A). Prevalence of hypertension increased with age. Prevalences of hyperlipidemia and diabetes mellitus increased up to the age of 75 years, and declined thereafter. Smoking was the most prevalent modifiable risk factor among the youngest (43.9%) group, and decreased with age, especially after the age of 65 years (Figures 1A and 2A). Patients aged ≥75 years significantly less often reported a family history of early‐onset cardiovascular disease (Figure 2A) than younger patients.13 The total number of potentially modifiable risk factors increased up to the age of 75 years, and declined thereafter (Figure 3A; Table S3).
Figure 1.
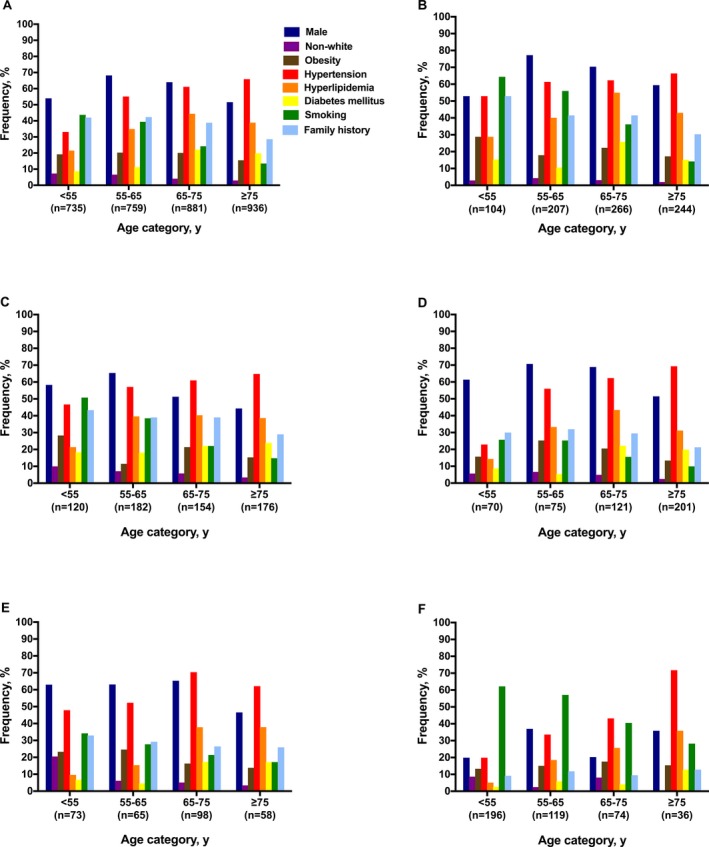
Prevalences of vascular risk factors according to age group. Ischemic stroke overall (A); ischemic stroke caused by large artery atherosclerosis (B), small vessel disease (C), or cardioembolism (D); spontaneous intracerebral hemorrhage (E); and aneurysmal subarachnoid hemorrhage (F).
Figure 2.
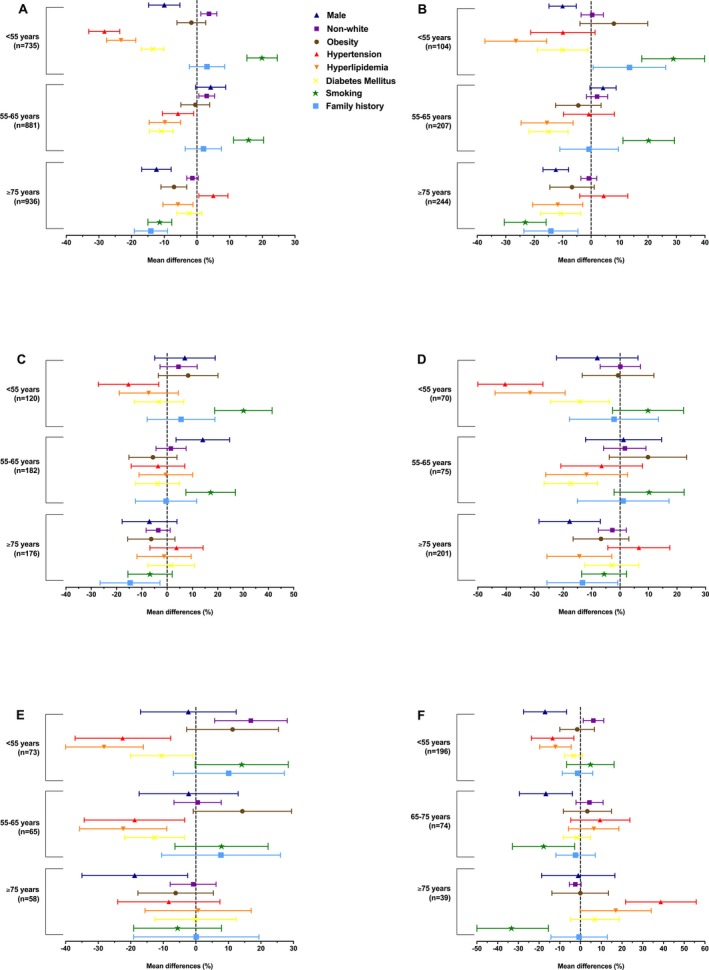
Age‐specific trends in vascular risk factors. The forest plots show the mean differences with accompanying 95% CIs in the prevalences of the vascular risk factors for each age group in relation to that of the reference age group (65–75 years for ischemic stroke and intracerebral hemorrhage and 55–65 years for aneurysmal subarachnoid hemorrhage). Ischemic stroke overall (A); ischemic stroke caused by large artery atherosclerosis (B), small vessel disease (C), or cardioembolism (D); spontaneous intracerebral hemorrhage (E); and aneurysmal subarachnoid hemorrhage (F).
Figure 3.
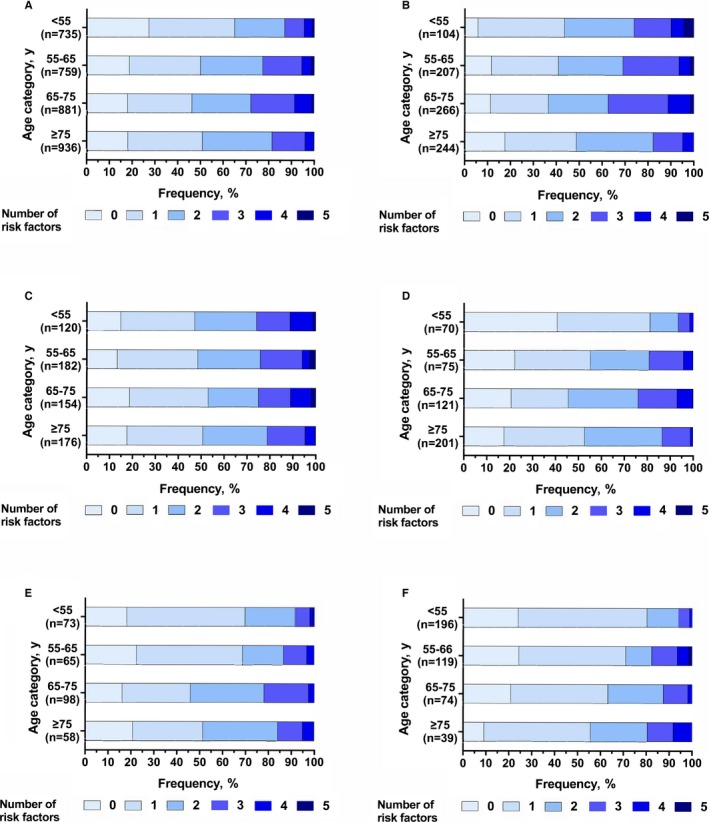
Number of potentially modifiable risk factors (obesity, hypertension, hyperlipidemia, diabetes mellitus, and smoking) present in each age group in ischemic stroke overall (A); ischemic stroke caused by large artery atherosclerosis (B), small vessel disease (C), or cardioembolism (D); spontaneous intracerebral hemorrhage (E); and aneurysmal subarachnoid hemorrhage (F).
Ischemic Stroke Caused by Large Artery Atherosclerosis
We found male predominance among all age groups (Figure 1B; Table S4). Obesity was most prevalent in the youngest group (30.3%). Hypertension was present in >50% in all age groups. The prevalence of hyperlipidemia increased up to the age of 75 years, whereas it declined thereafter (Figure 2B; Table S5). Diabetes mellitus was found most often in patients aged 65 to 75 years (25.7%), but also in 15.7% of those aged <55 years (Figure 1B). Compared with the reference age group, patients <65 years smoked significantly more often, and those ≥75 years significantly less (Figure 2B). A family history of early‐onset cardiovascular disease was significantly more often reported by those aged <55 years (55.6%) in comparison with the reference group (42.1%), and significantly less by those aged ≥75 years (27.7%) (Figure 2B). Accumulation of at least 2 potentially modifiable risk factors was seen in more than half the patients (Figure 3B; Table S3).
Ischemic Stroke Caused by Small Vessel Disease
In patients with IS caused by SVD, we found a higher prevalence of men than women only in those aged <65 years (Figure 1C; Table S6). The proportion of patients of non‐white origin declined with age. Obesity was most frequent among the youngest age group (28.3%). Hypertension (45.8%), hyperlipidemia (32.5%), and diabetes mellitus (18.6%) were all frequent in patients with IS caused by SVD aged <55 years and the prevalence of these risk factors generally increased with age (Figure 1C). Smoking was most prevalent among those aged <55 years (51.3%) and declined with age (Figure 2C; Table S7). Approximately half the patients had at least 2 potentially modifiable risk factors (Figure 3C). In contrast with other IS subtypes, in patients with IS caused by SVD we observed little variation in the distribution of the total number of risk factors present across the age groups (Figure 3B through 3F; Table S3).
Ischemic Stroke Caused by Cardioembolism
In patients with IS caused by cardioembolism, we observed male predominance except in patients aged ≥75 years (Figure 1D; Table S8). The prevalence of non‐white origin showed little variation with age (Figure 2D; Table S9), whereas obesity peaked markedly (26.9%) between 55 to 65 years (Figure 1D). In the youngest age group, hypertension (22.9%), hyperlipidemia (13.0%), and diabetes mellitus (8.7%) were all less prevalent in comparison with either LAA or SVD. These differences became less marked with increasing age (Figure 1B through 1D). Smoking and a family history of early‐onset cardiovascular disease were less prevalent in all age groups compared with patients with IS caused by either LAA or SVD (Figure 1B through 1D). Patients with IS of cardiac origin aged <65 years most often had <2 modifiable risk factors, in particular those aged <55 years; these differences were no longer prominent in those aged ≥65 years (Figure 3D; Table S3).
Spontaneous Intracerebral Hemorrhage
A male predominance was noticed up to 75 years of age, but not thereafter (Figure 1E; Table S10). Patients aged <55 years were significantly more often of non‐white origin (21.5%) compared with the reference group (4.6%; MD, 16.9% [95% CI, −28.1 to −5.8]) (Figures 1E and 2E; Table S11). Prevalence of obesity was considerably higher among those aged <55 years (25.0%; MD, 11.3% [95% CI, −2.8 to 25.4]) and aged 55 to 65 years (28.0%; MD, 14.3% [95% CI, −0.8 to 29.4]) than in the reference group (13.7%) (Figure 2E). Hypertension was frequent among all age groups, with prevalences rising up to 75 years of age; thereafter, we noticed a declining prevalence. Prevalence of hyperlipidemia and diabetes mellitus increased with age. Again, smoking was most prevalent (34.8%) in the youngest age group and declined with increasing age. About a third of those aged <65 years reported a family history of early‐onset cardiovascular disease (Figure 1E). The majority of patients aged <65 years had only one modifiable risk factor, whereas approximately half of the patients aged ≥65 years had at least 2 potentially modifiable risk factors (Figure 3E; Table S3).
Aneurysmal Subarachnoid Hemorrhage
Patients with aSAH showed a strong female preponderance (73.8%; Figure 1F; Table S12). aSAH was most pronounced in patients aged <55 years (81.1%) and patients aged 65 to 75 years (79.7%). Patients aged <55 years were significantly more often of non‐white origin (8.8%) as compared with the reference group (55–65 years, 2.5%; MD, 6.2% [95% CI, 1.3–11.2]). Smoking was prevalent in more than half of those aged <65 years (Figure 1F) and decreased to about 40% in those aged 65 to 75 years. In contrast, hypertension strongly increased with age and was observed significantly less often (19.6%) among the youngest as compared with the reference group (33.1%; MD, −13.5% [95% CI, −23.7 to −3.2]) (Figure 2F; Table S13). In all groups, prevalences of hyperlipidemia and diabetes mellitus were low and showed a gradual increase with age. Familial occurrence of aSAH was found stable among the age groups (≈8%). Regardless of age, most patients had only one known potentially modifiable risk factor, but the proportion of patients with more than one risk factor increased with age (Figure 3F; Table S3).
Discussion
In this prospective multicenter university hospital–based cohort in The Netherlands we found that the prevalence of cardiovascular risk factors shows age‐specific patterns among various stroke subtypes. Except for aSAH, we found a male predominance in all stroke subtypes, which attenuated with advancing age. IS, sICH, and aSAH patients aged <55 years were significantly more often of non‐white origin. Obesity was present in more than one fifth of patients aged <55 years with sICH and in those with IS caused by either LAA or SVD. Age‐specific prevalences of potentially modifiable risk factors showed a comparable pattern for LAA and SVD. These patients more often had hypertension, hyperlipidemia, and diabetes mellitus than patients with IS of cardiac origin if they were aged <55 years, but these differences became less marked with increasing age. Accumulation of potentially modifiable risk factors was generally most pronounced in patients with LAA or SVD as compared with patients with cardioembolic stroke. In most IS subtypes, we observed declining prevalences of hyperlipidemia and diabetes mellitus in patients aged ≥75 years. In all stroke subtypes, smoking was most prevalent among the youngest group; the proportion of smokers being highest in patients with aSAH.
Ischemic Stroke Overall
The declining proportion of patients with hyperlipidemia, diabetes mellitus, and smoking in the elderly IS is in line with previous population‐ and hospital‐based cohorts4, 7, 8, 9 and may reflect mortality displacement.4 Excess (vascular‐related) mortality in patients with vascular risk factors ultimately results in low prevalence rates among survivors. In contrast with our findings, a Danish nationwide hospital‐based cohort including 40 102 first‐ever IS patients found a declining prevalence of hypertension in the elderly (>70–80 years).4 A difference with our study is that we also included patients with previous strokes (23.4%), in whom hypertension may already have been diagnosed. We consistently found smoking to be most prevalent in patients aged <55 years (43.9%). In the nationwide study in Denmark, smoking prevalence peaked (55%) at 50 years of age and declined rapidly thereafter, conforming to our observation.4 Previous studies focusing on young stroke patients found similar figures on smoking prevalence. One study (sifap1 [Stroke in Young Fabry Patients]) consisting of 4467 IS patients aged 18 to 55 years reported a smoking prevalence of 55.5%,6 and another study from Helsinki including 1008 consecutive first‐ever IS patients aged 15 to 49 years reported a rate of 44%.5
Ischemic Stroke Caused by Large Artery Atherosclerosis
Almost a third of patients with IS caused by LAA were obese; this is twice as frequent as the proportion of obesity reported for Dutch persons of similar age.14 For IS in general, a previous study found a similar age‐specific pattern.4 Obesity predisposes to the so‐called metabolic syndrome,15, 16 consisting of hypertension, hyperlipidemia, and diabetes mellitus, which may be reflected by the accumulation of risk factors observed in patients with IS caused by LAA. Such risk factor accumulation has been previously observed, but not specifically for IS subtypes. The Helsinki cohort also showed accelerated accumulation of traditional risk factors from the mid‐40s, most prominent in men,5, 17 whereas only 5% of the young stroke patients in sifap1 were devoid of any modifiable risk factor.6
Ischemic Stroke Caused by Small Vessel Disease
Female predominance in patients with IS caused by SVD aged ≥75 years likely reflects their longer life expectancy. Only in this IS subtype, diabetes mellitus showed no decline in prevalence in the elderly. In line with our study, most previous hospital‐ and population‐based cohorts found that hypertension, diabetes mellitus, and smoking appear equally common in SVD and LAA and that these subtypes do not harbor different risk profiles.18, 19, 20, 21 However, by specifying the prevalences of risk factors for age, we observed that accumulation of modifiable risk factors in the young is more pronounced in those with SVD than in other IS subtypes.
Ischemic Stroke Caused by Cardioembolism
Patients with IS of cardiac origin aged <55 years carried less potentially modifiable risk factors than those with IS caused by LAA or SVD. This is probably explained by cardiac disorders such as patent foramen ovale, atrial septal aneurysms, and (inherited) cardiomyopathies that are not associated with vascular risk factors.5, 22 The observed steep rise in prevalence of risk factors with age in this group is compatible with atrial fibrillation as the most common source of cardioembolism in the elderly and its prevalence increasing with age.23 The peaking prevalence of obesity in those aged 55 to 65 years may herald the trend of an increasing attributable risk for atrial fibrillation due to obesity.23
Spontaneous Intracerebral Hemorrhage
The finding that the proportion of patients of non‐white origin is high in the young is consistent with previous reports showing that blacks and Hispanics have a higher risk of (deep) sICH than white.24, 25 Our finding of a trend towards higher prevalence of obesity in those aged <65 years than in the elderly suggests a role for unclarified (age‐related) metabolic factors in sICH etiology. A previous study among 384 consecutive sICH patients aged ≥55 years found that high BMI values >30 kg/m2 but also low values of <18.5 kg/m2 were associated with deep but not lobar ICH risk, most prominently in men.26 Despite the frequent association between obesity and hyperlipidemia, higher levels of total cholesterol and particularly triglycerides have repeatedly been suggested to protect against ICH.26, 27 We noticed hyperlipidemia in only 12.5% of patients aged <65 years. Consistent with previous observations,24, 28 diabetes mellitus and smoking, but not hypertension, were less prevalent among all age groups with sICH than in IS.
Aneurysmal Subarachnoid Hemorrhage
Our observation of female preponderance and a high prevalence of smoking is in line with previous observations.29, 30, 31 Hypertension, another well‐established risk factor for aSAH,30, 32 was found to be least prevalent (19.6%) in the youngest age group. Many young aSAH patients may be unaware of preexisting hypertension at the time of hemorrhage since aSAH occurs at a relatively young age, often as a first vascular event. Familial occurrence of aSAH was found to be stable among the different age groups, while, in contrast, older IS and sICH patients less often reported a family history of cardiovascular disease. A positive family history may reflect the involvement of genetic risk factors in aSAH pathophysiology and its involvement may be regardless of age. Alternatively, the proportion of a positive family history in the young may be low in these patients because aSAH has yet to occur in a next of kin, as aSAH incidence increases with age.33
Strengths and Limitations
Strengths of our study are its large size, prospective design, and application of standardized phenotyping of stroke subtypes and definitions of vascular risk factors. Our study also has limitations. First, it represents a university hospital–based cohort and is not population based. At the same time, this has the advantage of our study being enriched for young stroke patients. The observed higher proportion of non‐white among young stroke patients may be due to socioeconomic inequalities among ethnic minority groups34 or differences in genetic risk factors or may reflect the increasing number of next‐generation immigrants among the Dutch population,35 but this requires further study. Second, selection bias may play a role in that patients with a poor prognosis may have died before informed consent could be obtained. The sample size of patients with sICH was limited and we were therefore unable to distinguish lobar from nonlobar sICH, which have different risk profiles.36, 37 Third, assessment of IS caused by SVD may have been prone to misclassification since, according to TOAST,12 support from imaging is not required. Because hypertension and diabetes mellitus are considered supportive for the diagnosis of SVD in the TOAST classification, this may have inflated prevalences of these risk factors.19 Fourth, cardiovascular disease in first‐degree relatives aged <60 years was self‐reported by the patient or a next of kin and not verified by consulting medical records.
Conclusions
We show in a large, well‐phenotyped cohort that stroke subtypes have different risk profiles that vary according to age. Recognition of these patterns may guide more tailored primary and secondary stroke prevention efforts, including targeted prevention campaigns for specific age groups. For example, our study emphasizes the importance of premature atherosclerosis in the etiology of stroke in the young. Since young stroke patients have an increased risk of vascular‐related mortality over the course of 20 years,38, 39 and the incidence of stroke in young patients is rising,40 there is a large need for specific strategies to prevent cardiovascular disease in these patients.
Disclosures
None.
Supporting information
Table S1. Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke
Table S2. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke
Table S3. Number of Potentially Modifiable Risk Factors* in Stroke Patients According to Subtype
Table S4. Prevalence of Vascular Risk Factors in Patients Ischemic Stroke Caused by Large Artery Atherosclerosis
Table S5. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke Caused by Large Artery Atherosclerosis
Table S6. Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke Caused by Small Vessel Disease
Table S7. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke Caused by Small Vessel Disease
Table S8. Prevalence of Vascular Risk Factors in Patients With Cardioembolic Stroke
Table S9. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Cardioembolic Stroke
Table S10. Prevalence of Vascular Risk Factors in Patients With Spontaneous Intracerebral Hemorrhage
Table S11. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Spontaneous Intracerebral Hemorrhage
Table S12. Prevalence of Vascular Risk Factors in Patients With Aneurysmal Subarachnoid Hemorrhage
Table S13. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Aneurysmal Subarachnoid Hemorrhage
Acknowledgments
The work described in this study was carried out in the context of the Parelsnoer Institute (PSI). Funding of the PSI was cofinanced by the Dutch Government, the Dutch Federation of University Medical Centers (UMCs), and the 8 UMCs (from 2007–2011). Continuation of the PSI is financed by the UMCs. Ruigrok was supported by a clinical fellowship grant of The Netherlands Organization for Scientific Research (project No. 40‐00703‐98‐13533). Klijn is supported by a clinical established investigator grant from the Dutch Heart Foundation (2012T077), and an ASPASIA grant from The Netherlands Organisation for Health Research and Development (ZonMw; grant No. 015008048).
(J Am Heart Assoc. 2017;6:e005090 DOI: 10.1161/JAHA.116.005090.)28483775
References
- 1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker‐Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez‐Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez‐Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui‐Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker‐Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan‐Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere‐Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz‐Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez‐Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina‐Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi‐Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez‐Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez‐Riera L, Sanman E, Schwebel DC, Scott JG, Segui‐Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 3. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S; INTERSTROKE investigators . Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 4. Andersen KK, Andersen ZJ, Olsen TS. Age‐ and gender‐specific prevalence of cardiovascular risk factors in 40 102 patients with first‐ever ischemic stroke: a Nationwide Danish Study. Stroke. 2010;41:2768–2774. [DOI] [PubMed] [Google Scholar]
- 5. Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first‐ever ischemic stroke the Helsinki Young Stroke Registry. Stroke. 2009;40:1195–1203. [DOI] [PubMed] [Google Scholar]
- 6. von Sarnowski B, Putaala J, Grittner U, Gaertner B, Schminke U, Curtze S, Huber R, Tanislav C, Lichy C, Demarin V, Basic‐Kes V, Ringelstein EB, Neumann‐Haefelin T, Enzinger C, Fazekas F, Rothwell PM, Dichgans M, Jungehulsing GJ, Heuschmann PU, Kaps M, Norrving B, Rolfs A, Kessler C, Tatlisumak T; sifap1 Investigators . Lifestyle risk factors for ischemic stroke and transient ischemic attack in young adults in the Stroke in Young Fabry Patients study. Stroke. 2013;44:119–125. [DOI] [PubMed] [Google Scholar]
- 7. Rojas JI, Zurrú MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very old–risk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. Eur J Neurol. 2007;14:895–899. [DOI] [PubMed] [Google Scholar]
- 8. Marini C, Baldassarre M, Russo T, De Santis F, Sacco S, Ciancarelli I, Carolei A. Burden of first‐ever ischemic stroke in the oldest old: evidence from a population‐based study. Neurology. 2004;62:77–81. [DOI] [PubMed] [Google Scholar]
- 9. Béjot Y, Rouaud O, Jacquin A, Osseby GV, Durier J, Manckoundia P, Pfitzenmeyer P, Moreau T, Giroud M. Stroke in the very old: incidence, risk factors, clinical features, outcomes and access to resources—a 22‐year population‐based study. Cerebrovasc Dis. 2010;29:111–121. [DOI] [PubMed] [Google Scholar]
- 10. Nederkoorn PJ, van Dijk EJ, Koudstaal PJ, Luijckx GJ, van Oostenbrugge RJ, Visser MC, Wermer MJ, Ruigrok YM, Algra A, Kappelle LJ; Dutch String‐of‐Pearls Stroke Study‐Group . The Dutch String‐of‐Pearls Stroke Study: protocol of a large prospective multicenter genetic cohort study. Int J Stroke. 2015;10:120–122. [DOI] [PubMed] [Google Scholar]
- 11. WHO Monica Project Investigators . The World Health Organization MONICA Project (Monitoring trends and determinants in cardiovascular disease). J Clin Epidemiol. 1988;41:105–114. [DOI] [PubMed] [Google Scholar]
- 12. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 13. Jerrard‐Dunne P, Markus HS, Steckel DA, Buehler A, Von Kegler S, Sitzer M. Early carotid atherosclerosis and family history of vascular disease: specific effects on arterial sites have implications for genetic studies. Arterioscler Thromb Vasc Biol. 2003;23:302–306. [DOI] [PubMed] [Google Scholar]
- 14. Statistics Netherlands . Available at: www.cbs.nl/. http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=81175NED&D1=14-43&D2=a&D3=a&D4=0&D5=l&VW=T. Accessed June 27, 2016.
- 15. DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. [DOI] [PubMed] [Google Scholar]
- 17. Putaala J, Haapaniemi E, Kaste M, Tatlisumak T. How does number of risk factors affect prognosis in young patients with ischemic stroke? Stroke. 2012;43:356–361. [DOI] [PubMed] [Google Scholar]
- 18. Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke. 2005;36:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, Anderson CS, Hankey GJ, Jamrozik K, Appelros P, Sudlow CL. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–629. [DOI] [PubMed] [Google Scholar]
- 20. Schulz UGR, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population‐based studies. Stroke. 2003;34:2050–2059. [DOI] [PubMed] [Google Scholar]
- 21. Bejot Y, Caillier M, Ben Salem D, Couvreur G, Rouaud O, Osseby GV, Durier J, Marie C, Moreau T, Giroud M. Ischaemic stroke subtypes and associated risk factors: a French population based study. J Neurol Neurosurg Psychiatry. 2008;79:1344–1348. [DOI] [PubMed] [Google Scholar]
- 22. Yesilot Barlas N, Putaala J, Waje‐Andreassen U, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, Kloss M, Ferrari J, Lemmens R, Coban O, Haapaniemi E, Maaijwee N, Rutten‐Jacobs L, Bersano A, Cereda C, Baron P, Borellini L, Valcarenghi C, Thomassen L, Grau AJ, Palm F, Urbanek C, Tuncay R, Durukan Tolvanen A, van Dijk EJ, de Leeuw FE, Thijs V, Greisenegger S, Vemmos K, Lichy C, Bereczki D, Csiba L, Michel P, Leys D, Spengos K, Naess H, Tatlisumak T, Bahar SZ. Etiology of first‐ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol. 2013;20:1431–1439. [DOI] [PubMed] [Google Scholar]
- 23. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;6736:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howard G, Cushman M, Howard VJ, Kissela BM, Kleindorfer DO, Moy CS, Switzer J, Woo D. Risk factors for intracerebral hemorrhage: the reasons for geographic and racial differences in stroke (REGARDS) study. Stroke. 2013;44:1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Labovitz DL, Halim A, Boden‐Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–522. [DOI] [PubMed] [Google Scholar]
- 26. Biffi A, Cortellini L, Nearnberg CM, Ayres AM, Schwab K, Gilson AJ, Rost NS, Goldstein JN, Viswanathan A, Greenberg SM, Rosand J. Body mass index and etiology of intracerebral hemorrhage. Stroke. 2011;42:2526–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, Breteler MM, Ikram MA. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2011;31:2982–2989. [DOI] [PubMed] [Google Scholar]
- 28. Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 29. Kelly PJ, Crispino G, Sheehan O, Kelly L, Marnane M, Merwick A, Hannon N, Ní Chróinín D, Callaly E, Harris D, Horgan G, Williams EB, Duggan J, Kyne L, McCormack P, Dolan E, Williams D, Moroney J, Kelleher C, Daly L. Incidence, event rates, and early outcome of stroke in Dublin, Ireland: the North Dublin population stroke study. Stroke. 2012;43:2042–2047. [DOI] [PubMed] [Google Scholar]
- 30. Korja M, Lehto H, Juvela S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke. 2014;45:1958–1963. [DOI] [PubMed] [Google Scholar]
- 31. Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, Anderson CS. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36:2773–2780. [DOI] [PubMed] [Google Scholar]
- 32. Ruigrok YM, Buskens E, Rinkel GJ. Attributable risk of common and rare determinants of subarachnoid hemorrhage. Stroke. 2001;32:1173–1175. [DOI] [PubMed] [Google Scholar]
- 33. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agyemang C, van Oeffelen AA, Norredam M, Kappelle LJ, Klijn CJ, Bots ML, Stronks K, Vaartjes I. Socioeconomic inequalities in stroke incidence among migrant groups: analysis of nationwide data. Stroke. 2014;45:2397–2403. [DOI] [PubMed] [Google Scholar]
- 35. Statistics Netherlands . Available at: www.cbs.nl/. http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=37325&D1=0-2&D2=a&D3=0,124-130&D4=0&D5=0-4&D6=0-1,1920&HDR=G5,T,G3,G2,G4&STB=G1&VW=T. Accessed July 27, 2016.
- 36. Béjot Y, Cordonnier C, Durier J, Aboa‐Eboulé C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population‐based study. Brain. 2013;136:658–664. [DOI] [PubMed] [Google Scholar]
- 37. Kremer PH, Jolink WM, Kappelle LJ, Algra A, Klijn CJ; SMART and ESPRIT Study Groups . Risk factors for lobar and non‐lobar intracerebral hemorrhage in patients with vascular disease. PLoS One. 2015;10:e0142338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rutten‐Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, de Leeuw FE. Long‐term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309:1136–1144. [DOI] [PubMed] [Google Scholar]
- 39. Rutten‐Jacobs LC, Maaijwee NA, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, van der Vlugt MJ, van Dijk EJ, de Leeuw FE. Long‐term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol. 2013;74:592–601. [DOI] [PubMed] [Google Scholar]
- 40. Béjot Y, Daubail B, Jacquin A, Durier J, Osseby GV, Rouaud O, Giroud M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. J Neurol Neurosurg Psychiatry. 2014;85:509–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke
Table S2. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke
Table S3. Number of Potentially Modifiable Risk Factors* in Stroke Patients According to Subtype
Table S4. Prevalence of Vascular Risk Factors in Patients Ischemic Stroke Caused by Large Artery Atherosclerosis
Table S5. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke Caused by Large Artery Atherosclerosis
Table S6. Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke Caused by Small Vessel Disease
Table S7. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Ischemic Stroke Caused by Small Vessel Disease
Table S8. Prevalence of Vascular Risk Factors in Patients With Cardioembolic Stroke
Table S9. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Cardioembolic Stroke
Table S10. Prevalence of Vascular Risk Factors in Patients With Spontaneous Intracerebral Hemorrhage
Table S11. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Spontaneous Intracerebral Hemorrhage
Table S12. Prevalence of Vascular Risk Factors in Patients With Aneurysmal Subarachnoid Hemorrhage
Table S13. Mean Differences in the Prevalence of Vascular Risk Factors in Patients With Aneurysmal Subarachnoid Hemorrhage


