Abstract
Background
Obesity and its association with reduced life expectancy are well established, with cardiovascular disease as one of the major causes of fatality. Metabolic surgery is a powerful intervention for severe obesity, resulting in improvement in comorbid diseases and in cardiovascular risk factors. This study investigates the relationship between metabolic surgery and long‐term cardiovascular events.
Methods and Results
A cohort of Roux‐en‐Y gastric bypass surgery (RYGB) patients was tightly matched by age, body mass index, sex, Framingham Risk Score, smoking history, use of antihypertension medication, diabetes mellitus status, and calendar year with a concurrent cohort of nonoperated control patients. The primary study end points of major cardiovascular events (myocardial infarction, stroke, and congestive heart failure) were evaluated using Cox regression. Secondary end points of longitudinal cardiovascular risk factors were evaluated using repeated‐measures regression. The RYGB and matched controls (N=1724 in each cohort) were followed for up to 12 years after surgery (overall median of 6.3 years). Kaplan–Meier analysis revealed a statistically significant reduction in incident major composite cardiovascular events (P=0.017) and congestive heart failure (0.0077) for the RYGB cohort. Adjusted Cox regression models confirmed the reductions in severe composite cardiovascular events in the RYGB cohort (hazard ratio=0.58, 95% CI=0.42–0.82). Improvements of cardiovascular risk factors (eg, 10‐year cardiovascular risk score, total cholesterol, high‐density lipoprotein, systolic blood pressure, and diabetes mellitus) were observed within the RYGB cohort after surgery.
Conclusions
Gastric bypass is associated with a reduced risk of major cardiovascular events and the development of congestive heart failure.
Keywords: blood vessel, cardiovascular events, coronary artery disease, endothelium, heart failure, metabolic syndrome, stroke
Subject Categories: Heart Failure, Cardiomyopathy, Remodeling, Metabolic Syndrome, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
For patients with severe obesity, treatment with Roux‐en‐Y gastric bypass, when compared with nonsurgical management, is associated with reduced cardiovascular risk, and lower long‐term risk of congestive heart failure.
What Are the Clinical Implications?
In comparison with nonsurgical management of patients with severe obesity, Roux‐en‐Y gastric bypass may improve long‐term cardiovascular outcomes.
Introduction
There has been an alarming slowing in the decline trajectory for cardiovascular disease (CVD) mortality rates in the last 5 years, attributed in large part to the substantial increases in the prevalence of obesity and diabetes mellitus.1 The pathophysiology of obesity‐related CVD is felt to be rooted in altered adipose tissue function, which develops with caloric excess in genetically and environmentally predisposed individuals. An altered adipose tissue secretory profile results in a low‐grade inflammatory state, poorly regulated lipolysis, and ectopic lipid deposition, which predispose to metabolic dysfunction and additional disease burden.2, 3, 4 Metabolic dysfunction leads to hypertension, type II diabetes mellitus, and dyslipidemias, which are known cardiovascular and coronary disease risk factors. In addition, the states of chronic inflammation and metabolic dysfunction result in altered endothelial homeostasis, which establishes a pro‐atherogenic phenotype in the endothelium termed endothelial dysfunction.5
Weight loss achieved by any method will improve cardiovascular risk and reverse the abovementioned pathophysiologic sequence.6 The shortcomings of diet and other lifestyle modifications have led to the emergence of metabolic surgery, which is recognized as the most effective option for achieving significant and lasting weight control. Investigations into the mechanisms of cardiovascular protection afforded by metabolic surgery have revealed a reduction in markers of inflammation and thrombosis,7, 8, 9 restoration of more favorable adipokine secretory profile,7, 10 improved endothelial function,10, 11, 12, 13 reduction in cardiovascular risk factors,14, 15 restoration of normal metabolism,16 and improvements in cardiac structure and function.17
While there is substantial evidence demonstrating that surgical weight loss reduces a number of CVD risk factors, the clinical evidence that metabolic surgery results in improved cardiovascular outcomes is more limited. While prior studies have demonstrated the protective effects of metabolic surgery on cardiovascular events, these studies have been limited by short follow‐up, the evaluation of surgical procedures that have since been replaced by more effective options, and the absence of an appropriate comparison group.18, 19, 20, 21, 22, 23 The purpose of this study is to investigate the effects of Roux‐en‐Y gastric bypass (RYGB) surgery on long‐term CVD in a large clinical population with long‐term follow‐up, using strict matching criteria.
Methods
This is a retrospective longitudinal cohort study utilizing a prospective surgical registry with linkage to clinical information provided by the electronic health record of Geisinger Health System, a large integrated health system. Beginning in 2004, all bariatric surgery candidates at Geisinger Medical Center are offered participation in an Institutional Review Board–approved prospective research program whereby preoperative clinical information, perioperative outcome data, and longitudinal clinical as well as laboratory data are collected and stored. Patients with surgery completed before 2004 were invited retrospectively to participate in this clinical registry. The exact details of the electronic extraction of clinical data for research purposes and the overall research consent rate of >90% have been previously described.24 This study was approved by the Health System's Institutional Review Board.
Case Selection
Study patients in the surgical registry who met all of the following criteria (N=2503) were selected: provided signed informed consent for participation in the surgical registry, completed RYGB surgery between January 1, 2002 and December 31, 2012, age 20 to 80 years at time of surgery, presurgery body mass index (BMI) >35 kg/m2, and no evidence of pre‐existing CVD (defined as clinical diagnosis of ICD9 410–449, which includes ischemic heart disease, cardiomyopathy, cardiac dysrhythmias or heart failure, cerebrovascular disease, or peripheral vascular disease). These 2503 patients were further limited to 2420 (97%) who had available data to calculate the predicted 10‐year risk of CVD using the Framingham Risk Score,25 which requires sex, age, cholesterol level, high‐density lipoprotein (HDL) level, smoking history, systolic blood pressure, and use of hypertension medication.
Control Selection
The Geisinger Health System primary care cohort was used to identify a set of nonsurgical controls. Potential controls (n=26 946) were active in the Geisinger primary care system (3+ office visits over >2‐year period) without CVD, age 18 to 80, meeting BMI and comorbidity criteria for eligibility for bariatric surgery (BMI >40 or >35 kg/m2 with comorbidity of diabetes mellitus, hypertension, hyperlipidemia, or sleep apnea), no prior history of bariatric surgery, no diagnosis of serious mental health disorder or illegal drug use, and sufficient data available to calculate predicted 10‐year risk of CVD using the Framingham Risk Score.25
The 2420 RYGB patients were individually matched to the eligible nonsurgical controls by age (±1 year), BMI (within 3 kg/m2), sex, Framingham Risk Score (10‐year risk of CVD within ±1%), smoking history (ever/never), use of antihypertension medication (yes/no), diabetes mellitus status (yes/no), and active status in a primary care clinic without pre‐existing CVD at the time of the corresponding matched patient's surgery. If >1 control was available, the matched control was randomly selected.
Definition of Severe CVD
The primary outcome for this study was time until a severe CVD event defined as development of myocardial infarction (MI, ICD9 410), congestive heart failure (CHF, ICD9 428), or stroke (ICD9 430–434). For the RYGB cases, time of follow‐up started at time of surgery and concluded with their most recent visit occurring between January 1, 2002 and December 31, 2015. For the control cohort, the follow‐up period started at the time of the corresponding matched‐case surgery and concluded with their most recently recorded visit occurring between January 1, 2002 and December 31, 2015.
Statistical Methods
Characteristics of the case and control cohorts were compared using 2‐sample tests or Wilcoxon rank sum test for continuous data and χ2 tests for categorical data. Comparisons were made between items that were included in the matching criteria (eg, age, BMI, 10‐year CVD risk), other items in the Framingham Risk Score but not used in the matching (eg, systolic blood pressure, cholesterol level, HDL level), and other clinically relevant patient characteristics (eg, race/ethnicity, diastolic blood pressure, use of statins). Kaplan–Meier analysis was used to evaluate time until composite severe CVD, time until stroke, time until MI, and time until CHF. The sum of the subtypes of cardiovascular events was greater than the composite total because some individuals experienced more than 1 subtype of cardiovascular event. The Kaplan–Meier curves were compared between cases and controls using log‐rank tests. Cox regression was used to calculate unadjusted and adjusted hazard ratios (HR) with 95% CI for time until severe CVD, stroke, MI, and CHF. This analysis was conducted to determine whether residual confounding remained when adjusted for variables not included within the matching criteria. The proportional hazards assumption was tested and confirmed before Cox modeling.
As a secondary analysis, changes from baseline to 5 years after surgery in 10‐year CVD risk (using Framingham Risk Score25), cholesterol level, HDL level, systolic blood pressure, and BMI were compared between RYGB cases and controls using repeated‐measures linear regression. This analysis was limited to the subset of matched cases/controls with sufficient data to calculate at least 1 postsurgery Framingham risk score in the respective case and control. Similarly, the rate of diabetes mellitus remission after surgery, defined as hemoglobin A1c <6.5 without hypoglycemic therapy for 1 year, was compared between RYGB diabetic cases and diabetic controls using Kaplan–Meier analysis and log‐rank test. SAS version 9.4 was used for statistical analysis.
Results
Of the 2420 eligible RYGB cases, a matched control was identified for 1724 (71%) (Figure 1). In comparison with the 696 RYGP cases that did not have a match, the 1724 were older, had a lower percentage of males, had a lower BMI, had less diabetes mellitus, had a lower 10‐year CVD risk score, and had a lower percentage of smokers (Table S1). The characteristics of the RYGB cases and controls were similar for items within the matching criteria and for Framingham Risk Score components (Table 1). Race and use of statins were similar between the groups but diastolic blood pressure tended to be higher in the control group. The median follow‐up time for cases was 5.8 years (range 0.1–12.0) and 6.7 years for controls (range 0.5–12.6).
Figure 1.
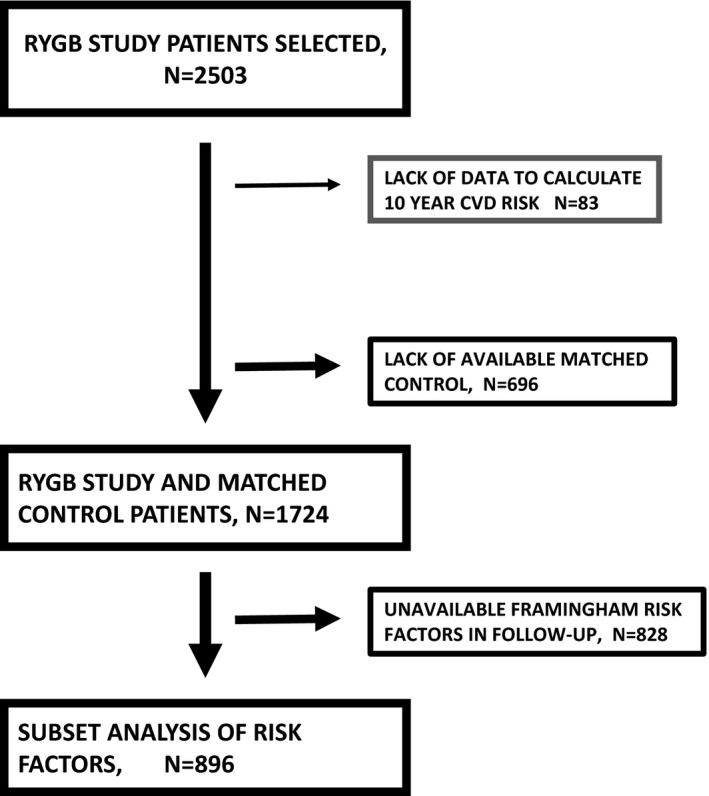
Study flow diagram. CVD indicates cardiovascular disease; RYGB, Roux‐en‐Y gastric bypass surgery.
Table 1.
Characteristics of the Study Cohorts (N=1724 Per Group)
| RYGB (n=1724) | Control (n=1724) | P Value | |
|---|---|---|---|
| Used in matching criteria | |||
| Age, y—mean (SD) | 45.0 (10.6) | 45.1 (10.6) | 0.986a |
| Sex | |||
| Female, % (n) | 87% (n=1493) | 87% (n=1493) | NA |
| Male, % (n) | 13% (n=231) | 13% (n=231) | |
| BMI, kg/m2—mean (SD) | 46.5 (6.0) | 46.5 (6.1) | 0.930a |
| Diabetes mellitus, % (n) | 28% (n=486) | 28% (n=486) | NA |
| 10‐y CVD risk (FRS), %—mean (SD) | 9.40 (8.11) | 9.35 (8.05) | 0.851a |
| Smoking history | |||
| Ever, % (n) | 42% (n=724) | 42% (n=724) | NA |
| Never, % (n) | 58% (n=1000) | 58% (n=1000) | |
| Anti‐HTN med. use, % (n) | 64% (n=1097) | 64% (n=1097) | NA |
| Other items within FRS | |||
| Systolic BP, mm Hg—median (IQR) | 128 (120, 140) | 128 (121, 134) | 0.100b |
| Cholesterol, mg/dL—median (IQR) | 183 (161, 210) | 186 (165, 209) | 0.062b |
| HDL, mg/dL—median (IQR) | 46 (39, 54) | 46 (40, 53) | 0.392b |
| Other patient characteristics | |||
| Race | |||
| White, % (n) | 96% (n=1656) | 97% (n=1669) | 0.581c |
| Black, % (n) | 2% (n=35) | 2% (n=26) | |
| Hispanic, % (n) | 2% (n=28) | 1% (n=23) | |
| Other, % (n) | <1% (n=5) | <1% (n=6) | |
| Diastolic BP, mm Hg—median (IQR) | 78 (70, 82) | 78 (74, 81) | <0.0001b |
| Statin use, % (n) | 31% (n=534) | 32% (n=547) | 0.633c |
Anti‐HTN medications included ACE inhibitors, β‐blockers, diuretics, thiazides, calcium channel blockers, and angiotensin II receptor antagonists. Median follow‐up time=5.7 years, range=[0.1, 12.0]. ACE indicates angiotensin‐converting enzyme; BMI, body mass index; CVD, cardiovascular disease; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; HTN, hypertension; IQR, interquartile range;. NA, not applicable; RYGB, Roux‐en‐Y gastric bypass.
Two‐sample t test.
Wilcoxon rank sum test.
χ2 test.
The total number of composite severe CVD events was 63 in the RYGB group and 110 in the control group. Kaplan–Meier analysis indicated that the percent of RYGB patients with severe CVD was 0.4% at 1 year, 1.5% at 3 years, 2.3% at 5 years, and 4.8% at 8 years after surgery (Figure 2). These rates were similar to those found in the control group at 1 year (0.3%) but were lower than the control group at 3+ years after surgery (2.4% at 3 years, 4.4% at 5 years, and 8.4% at 8 years after surgery, log‐rank P=0.017). When considering each subtype of severe composite CVD individually, the number of patients with stroke, MI, and CHF was 31, 12, and 24 in the RYGB group and 49, 17, and 55 in the control group. The Kaplan–Meier estimated rates in the RYGB group were lower than the control group (Figure 3) but was only significant for CHF (P=0.0077).
Figure 2.
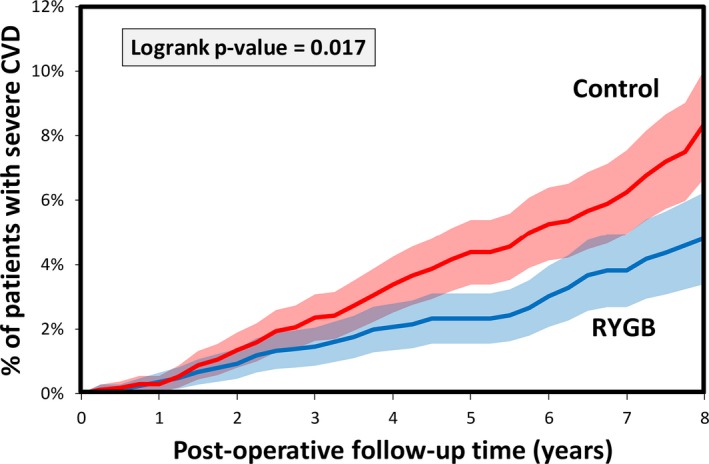
Kaplan–Meier curve estimated severe CVD rates in RYGB patients (n=1724) and controls (n=1724). CVD indicates cardiovascular disease; RYGB, Roux‐en‐Y gastric bypass surgery.
Figure 3.
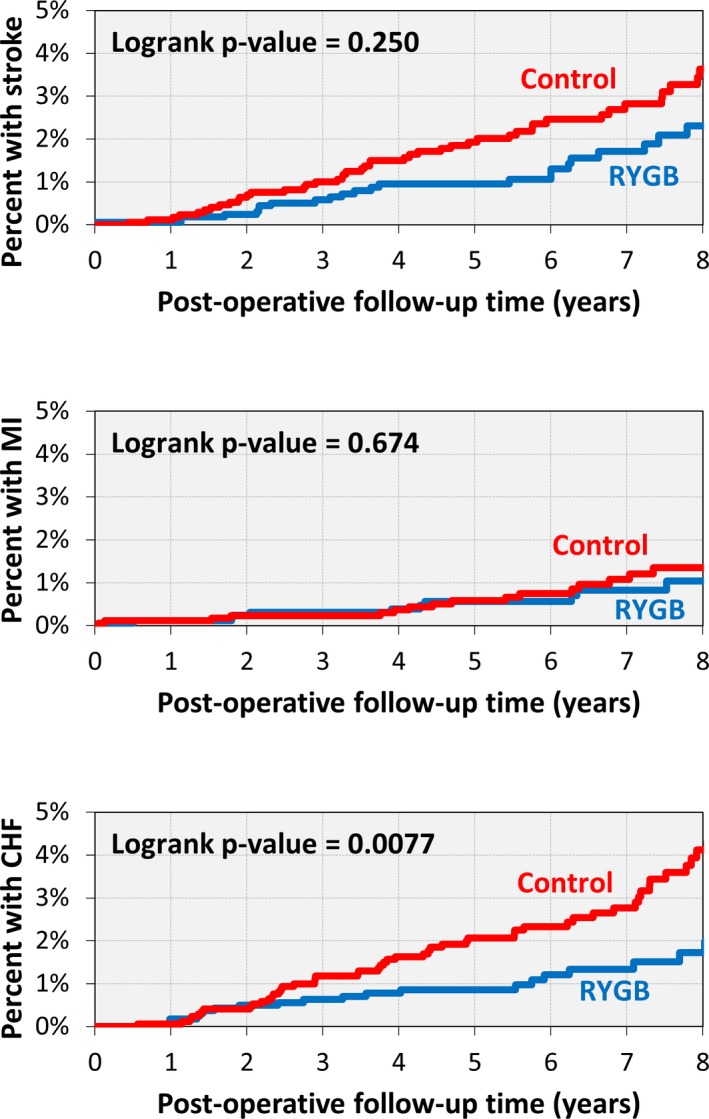
Kaplan–Meier curve for stroke, MI, and CHF in RYGB patients (n=1724) and controls (n=1724). CVD indicates cardiovascular disease; MI, myocardial infarction; RYGB, Roux‐en‐Y gastric bypass surgery.
In an unadjusted Cox regression model, the severe composite CVD risk differences between RYGB and control translated into a HR of 0.69 (95% CI =0.50–0.94, Table 2). Adjusted Cox regression modeling indicated that the effect of RYGB on severe CVD remained significant and strengthened after adjusting for additional patient characteristics (Table 2, HR=0.58, 95% CI=0.42–0.82). A similar result was found for CHF. In adjusted analysis, the effect of RYGB on stroke and MI remained not statistically significant.
Table 2.
Cox Regression Models for Severe CVD, Stroke, MI, and CHF Comparing RYGB to Controls
| Severe Composite CVD (N=173) | Stroke (N=80) | MI (N=29) | CHF (N=79) | |||||
|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | P Value | HR [95% CI] | P Value | HR [95% CI] | P Value | HR [95% CI] | P Value | |
| Unadjusted | 0.69 [0.50–0.94] | 0.018 | 0.77 [0.49–1.21] | 0.251 | 0.85 [0.41–1.79] | 0.675 | 0.53 [0.33–0.85] | 0.0089 |
| Adjusteda | 0.58 [0.42–0.82] | 0.0018 | 0.73 [0.45–1.17] | 0.188 | 0.89 [0.41–1.92] | 0.764 | 0.38 [0.22–0.64] | 0.0003 |
BMI indicates body mass index; BP, blood pressure; CHF, congestive heart failure; CHOL, cholesterol; CVD, cardiovascular disease; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; HTN, hypertension; HR, hazard ratios; MI, myocardial infarction; RYGB, Roux‐en‐Y gastric bypass.
The hazard ratios (HR) are for risk of CVD within the RYGB group (HR <1 indicates lower risk in the RYGB group).
Adjusted for FRS, sex, diabetes mellitus, age, BMI, smoking, HTN treatment, systolic BP, CHOL, HDL, race, diastolic BP, and statin use.
For the secondary analysis, a subset of 894 matched cases and controls had at least 1 Framingham Risk Score available during the follow‐up period. Statistical comparisons of demographic, ethnic, and clinical characteristics of the RYGB and control cohorts in the secondary analysis are available in Table S2. For these 894 matches, improvements in 10‐year CVD risk, total cholesterol level, HDL level, and systolic blood pressure were higher in the RYGB group (P<0.0001 for each, Figure 4A through 4D). For the subset of diabetic patients (n=486 per group, Table S3), the rate of diabetes mellitus remission (1 year with A1c <6.5) was greater in the RYGB group than in the control group (66.1% versus 4.8% at 3 years after surgery, log‐rank P<0.0001, Figure 4E). For the RYGB cases, BMI decreased from 46.5 kg/m2 at baseline to 32.5 kg/m2 at 5 years after surgery. This weight loss was significantly greater than in the control group (P<0.0001), which remained weight stable (46.5 kg/m2 at baseline versus 46.1 kg/m2 at 5 years of follow‐up, (Figure 4F).
Figure 4.

Change in 10‐year CVD risk (A), cholesterol (B), HDL (C), systolic BP (D), diabetes mellitus status (E), and BMI (F) from baseline to 5 years after surgery compared between RYGB patients matched with controls. P<0.0001 in each outcome for overall difference between RYGB and controls across time postsurgery (calculated using a repeated‐measure regression model). BMI indicates body mass index; BP, blood pressure; CVD, cardiovascular disease; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; RYGB, Roux‐en‐Y gastric bypass.
Discussion
Our study found that individuals undergoing RYGB surgery are at nearly half the risk of a severe cardiovascular event 8 years after surgery compared with similar patients who did not have surgery. While prior studies18, 19, 20, 21, 22, 23 have reported beneficial effects of surgery on MI and stroke, this is the first study to demonstrate the long‐term protective effect of RYGB surgery on CHF. Several important CVD risk factors and calculated Framingham Risk Score were also lower among metabolic surgery patients up to 8 years after surgery. These findings provide evidence for a durable impact of metabolic surgery on cardiovascular outcomes.
Our findings are consistent with previous studies reporting the benefits of metabolic surgery on cardiovascular health. Johnson and colleagues reported a short‐term reduction of 60% to 70% in cardiovascular events, with a median follow‐up of 19 to 21 months.21 Scott and colleagues reported a long‐term benefit of surgery on hospitalization and death from MI and stroke among a subgroup of metabolic surgery patients with type II diabetes mellitus.19 The Swedish Obesity Study reported fewer cardiovascular fatalities (HR=0.47) and fewer cardiovascular events (MI or stroke, HR=0.67) after bariatric surgery; however, 68.1% of surgeries were vertical banded gastroplasty, a surgical procedure that has largely been replaced by newer and more effective bariatric surgery procedures.20 Our study expands on the findings of these prior studies, demonstrating a long‐term cardiovascular benefit of RYGB surgery in a surgical population that included patients with and without type II diabetes mellitus.
Our findings are also consistent with studies of metabolic surgery and cardiovascular mortality. Adams reported a 56% reduction in deaths caused by coronary artery disease, with a mean follow‐up of 7.1 years.18 While reduced mortality is an important outcome, individuals living with CVD and recovering from severe cardiovascular events frequently suffer from poor quality of life, lost productivity, and high medical costs.26 We report a 45% reduction in the risk of major cardiovascular events (composite of MI, stroke, and CHF). Our study thus provides new understanding of the impact of metabolic surgery on these highly burdensome CVD outcomes. We are currently in the process of compiling our longitudinal comparative mortality results broken down by individual causes of mortality and hypothesize that the differential cardiovascular mortality rates will be consistent with our measured cardiovascular events.
A strong association between obesity and CHF,27 the adverse effects of obesity on myocardial structure and function,28 and the adverse effects of obesity on blood vessel homeostasis7, 8, 9, 10, 11, 12, 13, 14, 29 have been reported previously. These effects are consistent with our finding of a significant reduction in CHF after metabolic surgery. In this study, CHF risk reduction appeared to be the primary driving force for the overall composite cardiovascular event risk reduction. MI and stroke risk were lower after metabolic surgery, but these findings were not statistically significant. This nonsignificant finding may be partially explained by previous research suggesting that the association between BMI and MI/stroke may not be as strong as its association with CHF.30 In addition, the inclusion of only those individuals with no prior history of prior CVD likely limited the number of stroke and MI events, thus rendering the study underpowered to identify a statistically significant risk reduction for stroke and MI.
The longitudinal analysis of individual cardiovascular risk factors after bariatric surgery confirms the beneficial effects of metabolic surgery on known CVD risk factors. Other potential mechanisms for the cardiovascular protection conferred by metabolic surgery involve restoration of a normal adipokine secretory profile8, 9, 10 and a reduction in systemic inflammation,8, 29 both of which exert direct beneficial effects on the vascular endothelium.11, 12, 13 Recent evidence suggests that some of the beneficial effects of metabolic surgery occur promptly after surgery, are independent of weight loss, and may involve the enhanced protective function of HDL in addition to increased HDL levels.31 It is likely that additional study will reveal other mechanisms of cardiovascular protection related to bariatric surgery that are unrelated to weight loss and are a function of altered foregut anatomy.
Strengths of this study include the use of a tightly matched control group with a long‐term follow‐up comparable to the RYGB case group (Kaplan–Meier estimated follow‐up at 3, 5, and 8 years was 80%, 72%, and 67%, respectively). Additional strengths include the use of outcomes for specific subtypes of severe CVD and the implementation of multiple Cox regression to support the conclusions of the unadjusted analysis. Finally, the analysis evaluating longitudinal changes in CVD risk factors from pre‐ to post‐follow‐up was consistent with the large effect size found with severe composite CVD events and provides some insight into potential mechanisms for this observation.
This study has a few limitations. As with the other longitudinal studies of bariatric surgery, this was a retrospective observational study. While we were unable to adjust for unmeasured factors that potentially differ between the individuals who did and did not undergo surgery (eg, readiness for lifestyle change), we used a control group that was tightly matched to surgical cases on the most likely confounders (eg, smoking status, baseline CVD risk), reducing the impact of confounding on our findings. An additional limitation in this study is that using diagnosis codes for the identification of clinical events may increase the risk of misclassification.
Our secondary analysis of CVD risk factors was limited to a subset of eligible patients because of missing postsurgery lipid values. We determined that this subset was older, had more diabetes mellitus, had a higher 10‐year CVD risk, and more hypertension medication use as compared with those without available postsurgery lipid values. In sensitivity analysis, the association between RYGB and risk of future CVD was consistent within this subgroup as compared to the overall cohort. It is unclear whether these findings are generalizable across the entire population, but there is no evidence that the mechanisms driving our observed association between surgery and CVD risk would differ between patients included and excluded from this analysis.
The rate of visits per year was slightly higher (6.3 office visits per year) in the control group than in the RYGB case group (4.9 office visits per year). Since visit frequency was associated with greater chance of CVD, this could have led to a detection bias (ie, more visits in control group results in greater chance of finding CVD in control group). However, a sensitivity analysis revealed that adjusting for visit frequency within the multiple Cox model did not change the overall conclusion (the adjusted HR increased from 0.58 to 0.66 but remained significant, P=0.015).
Finally, although a match was found for 71% of the bariatric surgery patients, those without a matched control had different characteristics as compared with those included in this study (Table S1). However, the rate of postoperative CVD was not significantly different between the groups. It is unclear whether the differences in patient characteristics impacted the results.
In conclusion, these results support the growing body of evidence that metabolic surgery can improve health, including a substantial reduction in CVD risk factors and major cardiovascular outcomes.
Sources of Funding
This research was supported by research funds from the Geisinger Health System and the National Institutes of Health (grant no. DK072488).
Disclosures
None.
Supporting information
Table S1. Comparison of RYGB Patients Who Had a Matched Control Versus RYGB Patients Without a Matched Control
Table S2. Comparison of RYGB Patients With a Follow‐Up FRS Versus Control Patients With a Follow‐Up FRS
Table S3. Comparison of RYGB Patients With Diabetes Mellitus Versus Control Patients With Diabetes Mellitus
(J Am Heart Assoc. 2017;6:e005126 DOI: 10.1161/JAHA.116.005126.)28536154
References
- 1. Sidney S, Quesenberry C, Jaffe M, Sorel M, Nguyen‐Huynh M, Kushi L, Go A, Rana J. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–599. [DOI] [PubMed] [Google Scholar]
- 2. Van Gaal L, Mertens I, De Block C. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. [DOI] [PubMed] [Google Scholar]
- 3. Bays H. Adiposopathy: is sick fat a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. [DOI] [PubMed] [Google Scholar]
- 4. Rosen E, Spiegleman B. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prieto D, Contreras C, Sanchez A. Endothelial dysfunction, obesity, and insulin resistance. Curr Vasc Pharmacol. 2014;12:412–426. [DOI] [PubMed] [Google Scholar]
- 6. Magkos F, Fraterrigo G, Yoshino J, Lueckijng C, Kirbach K, Kelly S, de las Fuentas L, He S, Okunade A, Patterson B, Klein S. Effects of moderate and subsequent progressive weight loss on metabolic function and adiopose tissue biology in humans with obesity. Cell Metab. 2016;23:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Appachi S, Kashyap S. ‘Adiposopathy’ and cardiovascular disease: the benefits of bariatric surgery. Curr Opin Cardiol. 2013;28:540–546. [DOI] [PubMed] [Google Scholar]
- 8. Domienik‐Karlowicz J, Rymarczyk Z, Dzikowska‐Diduch O, Wojciech L, Chmura A, Demkow U, Pruszczyk P. Emerging markers of atherosclerosis before and after bariatric surgery. Obes Surg. 2015;25:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Netto B, Bettini S, Clemente A, Ferreira J, Boritza K, Souza S, Von der Heyde M, Earthman C, Damaso A. Roux‐en‐Y Gastric bypass decreases pro‐inflammatory and thrombotic biomarkers in individuals with extreme obesity. Obes Surg. 2015;25:1010–1018. [DOI] [PubMed] [Google Scholar]
- 10. Boido A, Ceriani V, Cetta F, Lombardi F, Pontiroli A. Bariatric surgery and prevention of cardiovascular events and mortality in morbid obesity: mechanisms of action and choice of surgery. Nutr Metab Cardiovasc Dis. 2015;25:437–443. [DOI] [PubMed] [Google Scholar]
- 11. Nerla R, Tarzia P, Sestito A, Di Monaco A, Infusino F, Matera D, Greco R, Tacchino R, Lanza G, Crea F. Effect of bariatric surgery on peripheral flow‐mediated dilatation and coronary microvacular function. Nutr Metab Cardiovasc Dis. 2012;22:626–634. [DOI] [PubMed] [Google Scholar]
- 12. Vasquez L, Pazos F, Berrazueta R, Fernandez‐Escalante C, Garcia‐Unzueta M, Freijanes J, Amado J. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab. 2005;90:316–322. [DOI] [PubMed] [Google Scholar]
- 13. Bigornia S, Mott M, Hess D, Apovian C, McDonnell M, Duess M, Kluge M, Fiscale A, Vita J, Gokce N. Long‐term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity (Silver Spring). 2010;18:754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vogel J, Franklin B, Zalesin K, Trivax J, Krause K, Chengelis D, Mccullough P. Reduction in predicted coronary heart disease risk after substantial weight reduction after bariatric surgery. Am J Cardiol. 2007;99:222–226. [DOI] [PubMed] [Google Scholar]
- 15. Arteburn D, Schauer D, Wise R, Gersin K, Fischer D, Selwyn C, Erisman A, Tsevat J. Change in predicted 10‐year cardiovascular risk following laparoscopic Roux‐en‐Y gastric bypass surgery. Obes Surg. 2009;19:184–189. [DOI] [PubMed] [Google Scholar]
- 16. Chen Y, Corsino L, Shantavasinkul P, Grant J, Portenier D, Ding L, Torquati A. Gastric bypass surgery leads to long‐term remission or improvement of type 2 diabetes and significant decrease of microvascular and macrovascular complications. Ann Surg. 2016;263:1138–1142. [DOI] [PubMed] [Google Scholar]
- 17. Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. The effects of bariatric surgery on cardiac structure and function: a systematic review of cardiac imaging outcomes. Obes Surg. 2016;26:1030–1040. [DOI] [PubMed] [Google Scholar]
- 18. Adams T, Gress R, Smith S, Halverson C, Simper S, Rosamond W, LaMonte M, Stroup A, Hunt S. Long‐term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. [DOI] [PubMed] [Google Scholar]
- 19. Scott J, Johnson B, Blackhurst D, Bour E. Does bariatric surgery reduce the risk of major cardiovascular events? A retrospective cohort study of morbidly obese surgical patients. Surg Obes Relat Dis. 2013;9:32–41. [DOI] [PubMed] [Google Scholar]
- 20. Sjostrom L, Peltonen M, Jacobson P, Sjostrom C, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos A, Lonroth H, Narbo K, Naslund I, Olbers T, Svensson P, Carlsson L. Bariatric surgery and long‐term cardiovascular events. JAMA. 2012;307:56–65. [DOI] [PubMed] [Google Scholar]
- 21. Johnson B, Blackhurst D, Latham B, Cull D, Bour E, Oliver T, Williams B, Taylor S, Scott J. Bariatric surgery is associated with a reduction in major macrovascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216:545–558. [DOI] [PubMed] [Google Scholar]
- 22. Kwok C, Pradhan A, Khan M, Anderson S, Keavney B, Myint P, Mamas M, Loke Y. Bariatric surgery and its impact on cardiovascular disease and mortality: a systemic review and meta‐analysis. Int J Cardiol. 2014;173:20–28. [DOI] [PubMed] [Google Scholar]
- 23. Vest A, Heneghan H, Agarwal S, Schauer P, Young J. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–1777. [DOI] [PubMed] [Google Scholar]
- 24. Wood G, Chu X, Manney C, Strodel W, Petrick A, Gabrielsen J, Seiler J, Carey D, Argyropoulos G, Benotti P, Still C, Gerhard G. An electronic health record‐enabled obesity database. BMC Med Inform Decis Mak. 2012;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Agostino R, Ramachandran V, Pencina M, Wolf P, Cobain M, Massaro J, Kannel W. General cardiovascular risk profile for use in primary care. The Framingham Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 26. Heidenreich P, Albert N, Allen L, Bluemke D, Butler J, Fonarow G, Ikonomidis J, Khavjou O, Konstam M, Maddox T, Nichol G, Pham M, Pina I, Trogdon J. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, Folsom AR, Coresh J. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. 2016;5:e003921 DOI: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alpert M, Terry B, Mulekar M, Cohen M, Massey C, Fan M, Panayiotou H, Mukerji V. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80:736–740. [DOI] [PubMed] [Google Scholar]
- 29. Hafida S, Mirshahi T, Nikolajczyk B. The impact of bariatric surgery on inflammation: quenching the fire of obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Donnell M, Xavier D, Lisheng L, Zhang H, Chin S, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen M, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey G, Dans A, Yusoff K, Truelsen T, Diener H, Sacco R, Ryglewicz D, Czlonkowska A, Weimar C, Wang CX, Yusuf S. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 31. Osto E, Doytcheva P, Corteville C, Bueter M, Dorig C, Stivala S, Buhmann H, Colin S, Rohrer L, Hasballa R, Tailleux A, Wolfrum C, Tona F, Manz J, Vetter D, Spliethoff K, Vanhoutte P, Landmesser U, Pattou F, Staels B, Matter C, Lutz T, Luscher T. Rapid and body weight‐independent improvement of endothelial and high‐density lipoprotein function after Roux‐en‐Y gastric bypass. Role of glucagon‐like peptide‐1. Circulation. 2015;131:871–881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of RYGB Patients Who Had a Matched Control Versus RYGB Patients Without a Matched Control
Table S2. Comparison of RYGB Patients With a Follow‐Up FRS Versus Control Patients With a Follow‐Up FRS
Table S3. Comparison of RYGB Patients With Diabetes Mellitus Versus Control Patients With Diabetes Mellitus


