Abstract
Background
Incidence and prevalence of atrial fibrillation (AF) are expected to increase dramatically; however, we currently lack comprehensive data on temporal trends in unselected clinical populations.
Methods and Results
Analysis of the UK Clinical Practice Research Datalink (CPRD) from 1998 to 2010 of patients with incident AF, excluding major valvular disease, linked to hospital admission data and national statistics. Fifty‐seven thousand eight hundred eighteen adults were identified with mean age 74.2 (SD, 11.7) years and 48.3% women. Overall age‐adjusted incidence of AF per 1000 person years was 1.11 (95% CI, 1.09–1.13) in 1998–2001, 1.33 (1.31–1.34) in 2002–2006, and 1.33 (1.31–1.35) in 2007–2010. Ongoing increases in incidence were noted for patients aged ≥75 years, with similar temporal patterns in women and men. Associated comorbidities varied over time, with a constant prevalence of previous stroke, increases in hypertension and diabetes mellitus, and decreases in ischemic heart disease. Among patients aged 55 to 74 years, there was a significant reduction in mortality over time (P<0.001), but mortality rates in patients aged ≥75 years remained static at 14% to 15% per year (P=0.84). Projections of AF prevalence demonstrated a constant yearly rise, increasing from 700 000 patients in 2010 to between 1.3 and 1.8 million patients with AF in the United Kingdom by 2060.
Conclusions
In a large general practice population, incident AF increased and then plateaued overall, with a continued increase in patients aged ≥75 years. The large projected increase in AF prevalence associated with temporal changes in AF‐related comorbidities suggests the need for comprehensive implementation of AF prevention and management strategies.
Keywords: atrial fibrillation, epidemiology, incidence, mortality, prevalence, primary care
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Mortality/Survival, Quality and Outcomes
Introduction
The lifetime risk of developing atrial fibrillation (AF) among those aged ≥40 years is ≈1 in 4.1, 2 The incidence of AF increases dramatically with age and is higher in men than women.1, 2 Although incidence and prevalence rates vary from country to country, virtually every reported study has demonstrated an increased incidence and prevalence, which is projected to rise substantially in the ensuing decades.1, 2, 3, 4, 5, 6, 7 The majority of these observations have included AF patients as part of cohort studies or AF registries. There are scant data reflecting unselected clinical populations, such as those seen in general practice.
Recent estimates suggest that 12.1 to 15.9 million patients will have AF in the United States by 20506 and 17.9 million people in Europe by 2060.3, 5 Given the increased risk of stroke, morbidity, and death related to AF, these increases in prevalence will have a considerable public health burden. A recent analysis of the Framingham cohort demonstrated a 3‐ to 4‐fold rise in age‐adjusted incidence between 1958 and 1967 and 1998 and 2007, with temporal changes in AF‐associated risk factors, such as increases in the prevalence of diabetes mellitus and obesity, despite decreases in heart failure, heavy alcohol intake, and smoking.8 Increased AF awareness and initiatives to improve detection of AF9, 10, 11 have contributed to the greater incidence and reported prevalence of AF, in addition to an aging population and improved survival from other cardiovascular diseases. The greater impetus to detect and treat AF has been advocated in clinical guidelines.12, 13, 14, 15 In the UK, AF has been added to the Quality Outcomes Framework, where appropriate identification and management of AF in primary care is rewarded and incentivized.
The purpose of the present analysis was to investigate temporal trends in AF incidence, comorbidities, and mortality in a primary care population representative of contemporary clinical practice. Our aim was to gain insight into the future AF population profile and make projections of the likely change in AF prevalence to 2060, in the UK.
Methods
Data Source
Data for this study were obtained from the UK Clinical Practice Research Datalink (CPRD), a primary care database (GOLD) linked to the Hospital Episode Statistics (HES) and the Office for National Statistics (ONS). The CPRD GOLD contains the computerized medical records from representative primary care practices in the UK, currently providing care to ≈8% of the UK population. All patients in the UK are registered with a general practitioner (GP). Information comprising patient demography, medical history, medications, hospitalizations, specialist referrals, and clinical events are captured electronically.16 The CPRD validates the data quality from each practice primarily from death records and gaps in data transfer, and analyses are only performed on data for periods where the practices are considered to deliver data “up‐to‐standard.” Systematic reviews of the medical diagnoses in the database, including AF, have demonstrated high overall validity,17, 18 with a median positive predictive value of 89%.17 All admissions to National Health Service (NHS) hospitals in England are captured by the HES, which records the date of admission and discharge and the main diagnoses, with data available from 1997 onward. UK population data and projections were extracted from reference tables available from the ONS (www.ons.gov.uk). Ethics approval for the study was not required because these were secondary analyses of anonymized data.
Study Population
The study population comprised adults aged ≥18 years with a first (incident) diagnosis of AF either based on read codes identified by the CPRD or on hospital discharge code (International Classification of Diseases, Tenth Revision [ICD‐10]: I48) or both. Patients with any type of valvular heart disease or past valve interventions were excluded. The inclusion period was from January 1, 1998 to December 31, 2010 to ensure availability of corresponding HES data.
The presence of comorbid conditions were recorded, including hypertension, diabetes mellitus, ischemic heart disease, cerebrovascular disease, heart failure (clinical diagnosis) vascular disease, and renal disease, as defined by their corresponding Read code or ICD‐10 code, either preceding or during the same visit as the incident AF diagnosis. Individual baseline CHA2DS2‐VASc scores were calculated to assess stroke risk.19 The CHA2DS2‐VASc score assigns 2 points each for age ≥75 years and previous stroke or transient ischemic attack (TIA) and 1 point each for the presence of congestive heart failure, hypertension, diabetes mellitus, vascular disease, age 65 to 74 years, and female sex (scores range from 0 to 9). Only year of birth is provided by the CPRD; therefore, date of birth was assumed as June 15 for age calculation. Prescribed medications at AF diagnosis, including oral anticoagulation (OAC) and cardiovascular medications, were also recorded. A prescription within 3 months preceding AF diagnosis was assumed as an indication for active treatment at the date of AF diagnosis.
Statistical Analysis
Baseline demographic and clinical characteristics of the incident AF population are presented as number and percentage, mean (SD), or median (interquartile range), as appropriate. We prespecified 3 time periods (1998–2001, 2002–2006, and 2007–2010). Age‐adjusted incidence rates were calculated as weighted averages on crude rates for each age, with weights given as proportions of persons with the corresponding age in the UK population. CIs were calculated according to Fay and Feuer.20 Age‐adjusted incidence rates are reported at the population level and for 5 age groups (<55, 55–64, 65–74, 75–84, and ≥85 years). Data on those aged 18 to 54 years were combined because of small numbers. Effect of age on incidence is represented by a natural cubic spline and estimated by Poisson regression on the number of AF events on age and the year of diagnosis, with the natural logarithm of person time at risk as offset. The person time at risk per year and age group in each center was provided by the CPRD and was subsequently scaled to the UK proportion in each year. Trend over years was tested by estimating the linear effect of year, complemented by a likelihood ratio test for reducing from a factorial to linear effect of year.
The projection of the prevalence of AF, p ij, at age level i in year j was calculated iteratively based on age level incidence r i and mortality M i as
with the overall relative mortality of AF patients, RRAF, assumed to be constant across all ages. This is a slightly modified version of the formula presented by Miyasaka et al.6 An annual constant increase in incidence may be given by q>0, also implying equal relative increase across age groups. Incidence was estimated as described above, whereas mortality rates were extracted from the UK population statistics provided by the ONS in 2010. An excess mortality for AF compared to non‐AF patients of 50% was assumed, that is, RRAF=1.5. This value was chosen as a conservative representation of previously published data. We also performed a range of sensitivity analyses (including mortality excesses of 20% and 100% and a more‐detailed age‐dependent mortality model based on the comparison of observed and expected deaths according to ONS statistics). Prevalence in 2010 was estimated directly from the CPRD cohort and used as a basis for the projection of prevalence from 2011 to 2060 on the assumption of a constant incidence (q=0), as well as with an assumed 1% annual increase in incidence (q=0.01). Using ONS projections of the UK population size, N UK,ij, at age group i in year j, the expected number of AF patients at year j was obtained as
and the overall prevalence p j=N AF,j/∑iNUK,ij.
AF‐related mortality was described in terms of number of deaths divided by the total person time at risk 1 year after the date of diagnosis. Person time was censored at the time of transfer out of clinic, the last data transfer from the clinic, or end of study (December 31, 2010). The trend for crude mortality across the time period studied in patients with 3 years of follow‐up was calculated using an incidence rate ratio (IRR) based on Poisson regression.
All statistical analyses were performed using Stata software (version 13.1; StataCorp LP, College Station, TX).
Results
Population and Comorbidities
A total of 57 818 patients were identified with incident AF between January 1, 1998 and December 31, 2010. This included 27 943 women (48.3%) with a mean age of 77.2 (SD, 10.7) and 29 875 men (51.7%) with a mean age of 71.5 (12.0) years. Table 1 presents the baseline characteristics of the cohort overall and by time period (1998–2001, 2002–2006, and 2007–2010). There was clear evidence of an ageing AF population, with the proportion of over 85‐year‐olds increasing from 15.5% in 1998–2001 to 19.0% in 2007–2010.
Table 1.
Baseline Demographic and Clinical Characteristics of Incident AF
| Baseline Characteristic | Overall, n (%) | 1998–2001, n (%) | 2002–2006, n (%) | 2007–2010, n (%) |
|---|---|---|---|---|
| No. of patients | 57 818 | 12 035 | 24 824 | 20 959 |
| Age, y, mean (SD) | 74.2 (11.7) | 74.0 (11.6) | 74.1 (11.8) | 75.0 (11.8) |
| Age group, y | ||||
| 18 to 40 | 650 (1.1) | 155 (1.3) | 287 (1.2) | 208 (1.0) |
| 41 to 54 | 3156 (5.5) | 656 (5.5) | 1376 (5.5) | 1124 (5.4) |
| 55 to 64 | 7697 (13.3) | 1507 (12.5) | 3311 (13.3) | 2879 (13.7) |
| 65 to 74 | 15 483 (26.8) | 3414 (28.4) | 6641 (26.8) | 5428 (25.9) |
| 75 to 84 | 20 990 (36.3) | 4442 (36.9) | 9214 (37.1) | 7334 (35.0) |
| ≥85 | 9842 (17.0) | 1861 (15.5) | 3995 (16.1) | 3986 (19.0) |
| Women | 27 943 (48.3) | 5882 (48.9) | 12 146 (48.9) | 9915 (47.3) |
| Previous stroke or TIA | 8440 (14.6) | 1805 (15.0) | 3559 (14.3) | 3076 (14.7) |
| Ischemic stroke | 6464 (11.2) | 1499 (12.5) | 2619 (10.6) | 2346 (11.2) |
| Hypertension | 31 962 (55.3) | 5540 (46.0) | 13 645 (55.0) | 12 777 (61.0 |
| Diabetes mellitus | 6595 (11.4) | 1014 (8.4) | 2743 (11.0) | 2838 (13.5) |
| Heart failurea | 7372 (12.8) | 2099 (17.4) | 3261 (13.1) | 2012 (9.6) |
| Ischemic heart disease | 23 242 (40.2) | 5309 (44.1) | 10 120 (40.8) | 7813 (37.3) |
| Vascular disease | 8162 (14.1) | 1760 (14.6) | 3464 (14.0) | 2938 (14.0) |
| Peripheral arterial disease | 1880 (3.3) | 420 (3.5) | 768 (3.1) | 692 (3.3) |
| Mean (SD) CHA2DS2‐VASc score | 3.2 (1.9) | 3.1 (1.9) | 3.2 (1.9) | 3.0 (1.9) |
| ACE inhibitorb | 14 141 (24.5) | 2114 (17.6) | 5880 (23.7) | 6147 (29.3) |
| Beta‐blockersb | 13 592 (23.5) | 2159 (17.9) | 6042 (24.3) | 5391 (25.7) |
| Digoxinb | 3839 (6.6) | 1468 (12.2) | 1762 (7.1) | 609 (2.9) |
ACE indicates angiotensin‐converting enzyme; TIA, transient ischemic attack.
A change in the Quality Outcomes Framework (QOF) definition of heart failure between 2002–2006 and 2007–2010 means that heart failure diagnosis is not directly comparable across the 3 time points.
A prescription within 3 months before atrial fibrillation (AF) diagnosis was assumed as an indication for active treatment on the date of AF diagnosis.
Prevalence of associated comorbidities at the time of AF diagnosis varied over time (Table 1). The proportions of AF patients with a previous stroke/TIA and vascular disease remained fairly constant (11–15%), whereas clear increases were observed in the prevalence of hypertension and diabetes mellitus (46–61% and 9–14%, respectively). Ischemic heart disease at the time of AF diagnosis decreased (44–37%). Although we noted a decrease in the number of incident AF patients with a clinical diagnosis of heart failure (17–10%), the adoption of more‐stringent diagnostic criteria during the study period was a confounding factor. Overall, the CHA2DS2‐VASc score remained similar across all time periods (mean, 3.2; SD, 1.9). Type of AF was not consistently reported.
Medications at the time of incident AF diagnosis are displayed in Table 1. Anticoagulation pre‐existing the diagnosis of AF was uncommon (n=2709; 4.7%). There was a steady increase in use of angiotensin‐converting enzyme (ACE) inhibitors and beta‐blockers from 1998 to 2010 and a steep decline in the use of digoxin.
Incidence of AF
The incidence of AF increased with age from 0.13 per 1000 person‐years in those aged <55 years to 7.65 per 1000 person‐years in those aged ≥85 years (Table 2). Incident AF was greater in men compared to women (1.33 vs 1.18 per 1000 person‐years, respectively).
Table 2.
Age‐Adjusted Incidence Rates (With 95% Confidence Intervals) of AF in the UK Overall and by Sex, Stratified by Calendar‐Year of Diagnosis
| Age‐Adjusted Incidence Per 1000 (95% CI) | Overall | 1998–2001 | 2002–2006 | 2007–2010 |
|---|---|---|---|---|
| All patients; overall, y | 1.26 (1.25–1.27) | 1.11 (1.09–1.13) | 1.33 (1.31–1.34) | 1.33 (1.31–1.35) |
| <55 | 0.13 (0.13–0.13) | 0.12 (0.11–0.13) | 0.14 (0.13–0.15) | 0.13 (0.13–0.14) |
| 55 to 64 | 1.16 (1.13–1.19) | 1.06 (1.00–1.12) | 1.20 (1.16–1.24) | 1.22 (1.17–1.26) |
| 65 to 74 | 3.24 (3.19–3.30) | 3.02 (2.92–3.13) | 3.42 (3.34–3.50) | 3.26 (3.18–3.35) |
| 75 to 84 | 6.42 (6.33–6.52) | 5.72 (5.55–5.89) | 6.84 (6.71–6.99) | 6.66 (6.51–6.82) |
| ≥85 | 7.65 (7.48–7.81) | 6.27 (5.98–6.58) | 8.05 (7.80–8.31) | 8.73 (8.46–9.01) |
| Men; all ages, y | 1.33 (1.32–1.35) | 1.17 (1.14–1.20) | 1.39 (1.37–1.42) | 1.43 (1.41–1.46) |
| <55 | 0.19 (0.18–0.19) | 0.17 (0.15–0.18) | 0.20 (0.19–0.21) | 0.19 (0.18–0.20) |
| 55 to 64 | 1.55 (1.51–1.60) | 1.43 (1.34–1.53) | 1.60 (1.53–1.67) | 1.62 (1.55–1.70) |
| 65 to 74 | 3.97 (3.89–4.06) | 3.78 (3.62–3.96) | 4.06 (3.94–4.20) | 4.05 (3.91–4.20) |
| 75 to 84 | 7.12 (6.98–7.28) | 6.34 (6.06–6.64) | 7.54 (7.32–7.78) | 7.45 (7.20–7.70) |
| ≥85 | 8.24 (7.93–8.56) | 6.65 (6.08–7.26) | 8.69 (8.21–9.19) | 9.57 (9.06–10.09) |
| Women; all ages, y | 1.18 (1.16–1.19) | 1.05 (1.02–1.08) | 1.26 (1.24–1.28) | 1.22 (1.20–1.25) |
| <55 | 0.07 (0.07–0.07) | 0.06 (0.06–0.07) | 0.07 (0.07–0.08) | 0.07 (0.06–0.08) |
| 55 to 64 | 0.76 (0.73–0.80) | 0.68 (0.62–0.74) | 0.80 (0.75–0.85) | 0.81 (0.76–0.87) |
| 65 to 74 | 2.58 (2.51–2.64) | 2.35 (2.22–2.48) | 2.82 (2.72–2.93) | 2.53 (2.42–2.64) |
| 75 to 84 | 5.93 (5.81–6.04) | 5.30 (5.09–5.52) | 6.35 (6.18–6.53) | 6.08 (5.89–6.27) |
| ≥85 | 7.37 (7.18–7.57) | 6.11 (5.77–6.47) | 7.77 (7.48–8.07) | 8.33 (8.01–8.66) |
AF indicates atrial fibrillation.
Comparing the 3 time periods, the overall age‐adjusted incidence rate of AF per 1000 person‐years was 1.11 in 1998–2001 (95% CI, 1.09–1.13), 1.33 in 2002–2006 (1.31–1.34), and 1.33 in 2007–2010 (1.31–1.35; Table 2). Although incidence rates were fairly static for younger patients, they continued to increase in older patients, both in women and men (Figure 1).
Figure 1.
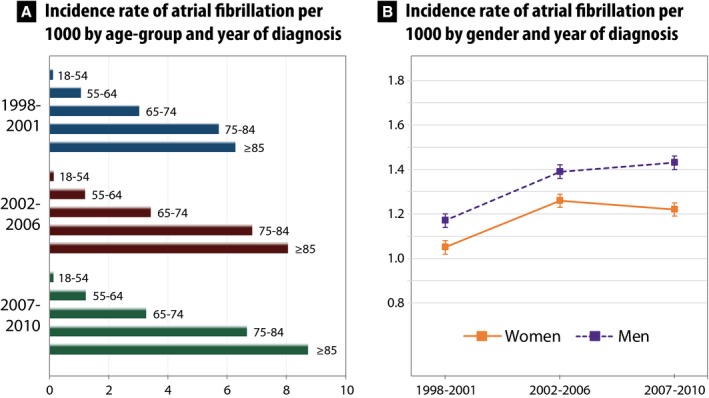
Age‐adjusted annual incidence rate of atrial fibrillation per 1000 in the UK (1998–2010) showing (A) increases in incidence over time with increasing age, particularly in older patients, and (B) sex‐stratified incidence of atrial fibrillation with higher rates in men.
Mortality in Patients With Incident AF
Averaged over all time periods, the crude 1‐year mortality rate was 8.8% in women with incident AF (95% CI, 8.4–9.1%) and 10.6% in men (95% CI, 10.2–11.0%). As expected, older patients had substantially higher mortality: 1.0% (18–39 years), 2.2% (40–54 years), 3.2% (55–64 years), 6.0% (65–74 years), 10.4% (75–84 years), and 23.7% (≥85 years) per year.
Two age groups were modeled to assess mortality over time (Figure 2). In patients aged 55 to 74 there was a reduction in mortality (IRR per calendar year, 0.97; 95% CI, 0.95–0.99; P<0.001). In contrast, patients aged ≥75 years had similar mortality between 1998 and 2010 (IRR, 1.00; 95% CI, 0.99–1.01; P=0.84).
Figure 2.
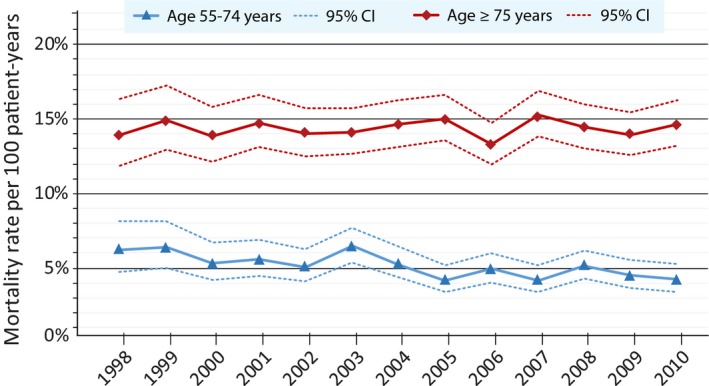
One‐year crude mortality rates and 95% CIs according to the year of incident atrial fibrillation. In patients aged 55 to 74 years, there was a significant reduction over time (incidence rate ratio per calendar year, 0.97; 95% CI, 0.95–0.99; P<0.001), whereas no difference in mortality was observed in patients aged ≥75 years (incidence rate ratio, 1.00; 95% CI, 0.99–1.01; P=0.84).
Sensitivity analyses for mortality all identified similar and consistent results (data not shown).
Projected Prevalence of AF
Based on the study population, the number of AF patients in the UK in 2010 is estimated at 718 334 adults (corresponding to an overall prevalence of 14.5 per 1000 and 83.6 per 1000 among the population aged 75 and above). Using the prevalence model described and assuming a constant incidence rate from 2010 onward, the projected number of adults with AF in the UK will be 1 085 078 by 2040 and 1 258 705 by 2060. With a constant 1% increase in the incidence rate, the projected number of AF patient is 1 322 694 by 2040 and 1 846 960 by 2060. Prevalence rates are projected to rise faster in men than women (Table 3). Overall, the burden of AF as a proportion of the population is projected to dramatically increase year on year (Figure 3).
Table 3.
Projected Prevalence of AF Per 1000 Persons
| Model | Projected Prevalence (Per 1000) | |||||
|---|---|---|---|---|---|---|
| Age‐Dependent Mortality Constant Incidence | Constant Excess Mortality Increased Incidence | |||||
| Year | Overall | Females | Males | Overall | Females | Males |
| 2010 | 14.5 | 12.9 | 16.3 | 14.5 | 12.9 | 16.3 |
| 2020 | 15.5 | 13.8 | 17.2 | 15.5 | 13.5 | 17.7 |
| 2040 | 20.5 | 18.7 | 22.3 | 23.1 | 20.4 | 25.8 |
| 2060 | 25.4 | 23.9 | 26.9 | 32.0 | 29.0 | 34.9 |
AF indicates atrial fibrillation.
Figure 3.
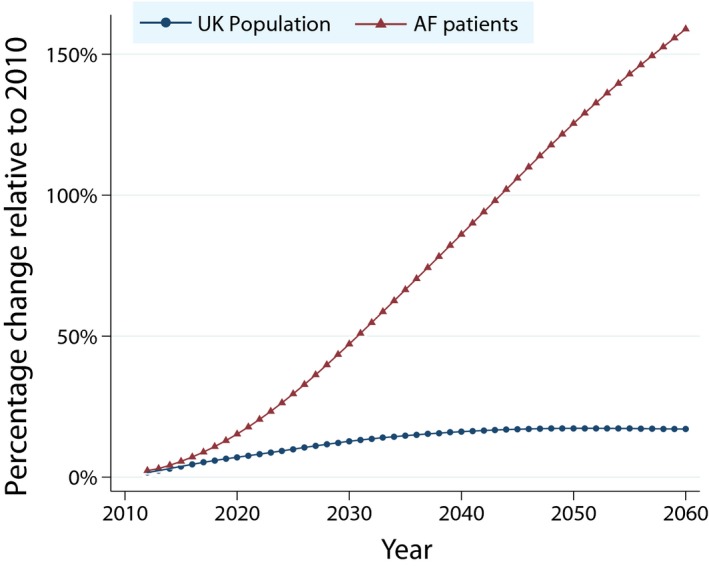
Estimated change from 2010 in the UK population and numbers of patients with AF in comparison to the predicted United Kingdom population, assuming increased incidence of AF. AF indicates atrial fibrillation.
Discussion
Using a general practice database linked to hospital records and national epidemiological data, our principal findings show an increase in the overall age‐adjusted incidence of AF between 1998 and 2001 and 2002 and 2006 (from 1.11 to 1.33 per 1000, respectively), followed by a plateau between 2007 and 2010; however, the incidence continued to rise in those patients over the age of 75 years, both in women and men. Second, mortality in these older patients has not decreased in line with younger patients with incident AF, despite apparent improvements in management, both pharmacological and interventional. Third, the projected prevalence of AF based on our data will dramatically increase and pose a considerable public health burden, and even assuming no increase in the incident AF rate, this will still equate to almost 1.3 million patients with AF by 2060 in the UK alone. Accounting for the increasing incidence of AF, this figure could rise to over 1.8 million patients.
A number of studies have explored the incidence of AF, including the Netherlands,1 Germany,21 Iceland,7 and the United States.4, 6, 8, 22, 23, 24 A global systematic review reported a significant rise in the incidence of AF between 1990 and 2010. The incidence of AF in 2010 was 59.5 per 100 000 population (95% uncertainty interval [UI], 49.9–74.9) in women and 77.5 (95% UI 65.2–95.4) in men, an increase of 35% for women and 28% for men from 1990.3 All of these cohorts differ markedly in their patient population, including hospitalized patients,7 administrative databases,6, 24 health insurance cohorts,4, 21, 23 and population‐based prospective cohorts.1, 8 Five previous studies6, 7, 8, 22, 25 have examined the incidence of AF at multiple time points, whereas others have taken a “snapshot” of the overall incidence of AF and by sex and age group at 1 particular period of time.1, 4, 23, 26, 27 All studies, with the exception of 1,24 demonstrate an increase in the overall incidence of AF over time, with increasing age, and a greater incidence in men than women.1, 6, 7, 8, 21, 22, 23, 25, 26, 27
CPRD data linkage with hospital records and national epidemiological data provides us with a unique opportunity to assess incidence rates and comorbidity patterns in a sample of patients' representative of general practice in a developed country (the UK). Several studies have validated CPRD data and confirmed the data quality and completeness of the clinical records.17, 18, 25, 28 Comparison with both the Framingham8 and Olmsted County6 cohorts in the United States suggest that incident AF is much lower in our UK primary care cohort. The 2 US Medicare beneficiary databases of people aged ≥65 years report higher incident rates of AF per 1000 person‐years22, 23 than are evident in a similar German population21 and other European cohorts.1, 7 Some variation in incident AF rates may be attributed to the heterogeneity and source of the cohorts, diverse follow‐up periods, and different definitions of incident AF.
Three previous analyses have been conducted using general practice data. Analysis of CPRD data captured between 1993 and 2005 show a temporal increase in incident AF, with a similar pattern over time by age group and sex to our findings.27 In a Scottish primary care cohort examining the incidence of AF in men and women in 2001–2002, analogous rates of incident AF were demonstrated compared to our cohort.26 In a more‐recent UK analysis, identification of AF and initiation of anticoagulant therapy was observed to improve over a 12‐year period.25 Although this is important for the prevention of stroke and thromboembolism, our finding of no substantial change in mortality from 1998 to 2010 in patients aged 75 years and older is an important reminder that death in AF patients is typically a consequence of sudden death or progressive heart failure.29, 30 Thus, additional attention is warranted to impact on the static, high death rates observed in older AF patients. Indeed, a recent analysis of Olmstead County residents revealed no change in survival between 2000 and 2010 among those AF patients who survived the first 90 days after AF diagnosis (hazard ratio [HR], 1.05; 95% CI, 0.85–1.31).24
We also observed changes in the comorbidity patterns of patients with incident AF across the 12 years of our study. Whereas some are linked to an ageing population, others require further consideration of their impact on clinical management. For example, the increase in diabetes mellitus and hypertension we identified were out of proportion with the increase in age, probably reflecting better diagnosis or changes in prevalence. Obesity, diabetes mellitus, and the risk of AF are closely related, and diabetes mellitus is both an independent risk factor for incident AF and associated with stroke in AF patients.31, 32 We also saw unexpected decreases in the rate of heart failure in patients diagnosed with incident AF, similar to that observed in the Framingham cohort.8 This may represent earlier diagnosis of AF, before the onset of heart failure, or more‐stringent diagnostic definitions. Indeed, AF and heart failure are closely linked in pathophysiology and the combination is associated with high levels of morbidity and mortality,33 regardless of ejection fraction.34 Earlier diagnosis of AF and the prevention of heart failure are important treatment goals that may lead to an improvement in prognosis. The temporal decrease we observed in ischemic heart disease is compatible with UK national statistics for the general population.35 The rates of OAC prescription reported here are very low because the data reflect the proportion of patients receiving this medication at the time of AF diagnosis and in the 3 months beforehand, not after AF diagnosis.
With regard to the future prevalence of AF, 4 studies have reported a dramatic increase in projected AF.4, 6, 7, 36 Data from the most recent retrospective health insurance claim database estimated that 1.8 million US citizens would be newly diagnosed with AF by 2030 without any increase in incident AF.4 However, a logarithmic progression in AF incidence would result in 2.6 million new cases by 2030, translating into a prevalence of 12.3 million,4 similar to projections made from the Olmsted County cohort.6 In an Icelandic study, the projected prevalence of AF in 2030 will be 2.9% (95% CI, 2.8–2.9; assuming constant incidence and mortality rates), rising to 3.8% in 2050 or 4.3% if incidence and mortality rates increase.7 Our data confirm that the UK will also see substantial increases in AF prevalence relative to population growth and highlights the enormous potential impact this will have on health care provision and expenditure. Attributed to increased AF awareness and more‐intensive monitoring (eg, implantable devices and smartphone applications), the detection of AF is also improving rapidly.10, 37, 38 The costs of care associated with AF are high and continue to increase, adding to the global health care burden of AF.39, 40
Strengths and Limitations
The strength of our analysis was the use of one of the largest unselected population cohorts of patients with incident AF, using a well‐validated general practice database with linkage to hospital records. The sample size allowed us to investigate differences in incidence, comorbidities, and mortality across a 12‐year period in a contemporary population and project prevalence of AF with a high degree of confidence. As with any data linkage study, we are reliant on accurate coding of diagnoses and have limited capability to interrogate any discrepancy in diagnosis, type of AF, comorbidities, or prescribing data. Nevertheless, CPRD has demonstrated excellent levels of external validity.17 Although we had no electrocardiogram data to corroborate the AF diagnosis, a previous validation study of diagnostic coding for AF in CPRD (undertaken by manually reviewing computerized patient records and externally confirmed by a questionnaire to the GP) has shown that the recording of AF in primary care is consistent and valid, with a positive predictive value of 98%.41 In addition, we were able to confirm diagnoses of AF made in the hospital by linkage to the HES. Although the general practices that supply data to CPRD are self‐selected, they appear to be representative of the general UK population. Data on ethnicity were missing for 19.7%, and therefore the impact of ethnicity on incidence and prevalence could not be examined. Given that ethnicity has been shown to influence AF incidence in other studies, this may impact future incidence and prevalence estimates. Finally, awareness of AF has the potential to impact on incidence in a nonlinear way that would not be accounted for in our incidence and prevalence projections. Conversely, patients with asymptomatic AF may be less likely to come to the attention of their general practitioner, and hence our estimates on incidence and prevalence could be underestimates of the true burden of AF in the community.
Conclusions
In a large general practice population, incident AF increased and then plateaued overall, with a continued increase in patients aged ≥75 years and was more common in men than women. Projections suggest that between 1.3 and 1.8 million people in the UK will have AF by 2060, constituting a considerable public health burden. The large projected increase in AF prevalence associated with temporal changes in AF‐related comorbidities (particularly, hypertension and diabetes mellitus) suggests the need for comprehensive implementation of AF prevention and management strategies.
Author Contributions
Lane and Lip contributed to the conception and design of the study, acquisition of the data, and obtaining funding. Lane, Skjøth, and Kotecha contributed to the statistical analysis. All authors contributed to the analysis and interpretation of the data, drafting and critical revision of the manuscript for important intellectual content, and supervision and material support. Data Access, Responsibility and Analysis: Lane and Skjøth had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
The license to access the CPRD database was funded by Bristol‐Myers Squibb. The funder did not participate in the design or conduct of the study, nor did they have access to the data or participate in the data management, analysis, or interpretation, or in the preparation or review of the manuscript, or the decision to submit the manuscript for publication.
Disclosures
Lane has received investigator‐initiated educational grants from Bristol‐Myers Squibb and Boehringer Ingelheim and has been a speaker and consultant for Boehringer Ingelheim, Bayer, and Bristol‐Myers Squibb/Pfizer. Skjøth has served as a consultant for Bayer. Kotecha has received research grants from Menarini, lecture fees from AtriCure, professional development support from Daiichi Sankyo, and is the lead for the Beta‐blockers in Heart Failure Collaborative Group (BB‐meta‐HF) and the RAte‐control Therapy Evaluation in Atrial Fibrillation trial group (RATE‐AF). Larsen has served as an investigator for Janssen Scientific Affairs, LLC and Boehringer Ingelheim and has been on the speaker bureaus for Bayer, BMS/Pfizer, Roche Diagnostics, Takeda, and Boehringer Ingelheim. Lip reports consulting for Bayer/Janssen, Astellas, Merck, Sanofi, BMS/Pfizer, Biotronik, Medtronic, Portola, Boehringer Ingelheim, Microlife, and Daiichi‐Sankyo. He is a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi‐Sankyo.
(J Am Heart Assoc. 2017;6:e005155 DOI: 10.1161/JAHA.116.005155.)28455344
References
- 1. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 3. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 5. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 7. Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–1117. [DOI] [PubMed] [Google Scholar]
- 8. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitzmaurice DA, Hobbs FD, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GY, Allan TF. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110:213–222. [DOI] [PubMed] [Google Scholar]
- 11. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallwnhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost‐effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH‐AF study. Thromb Haemost. 2014;111:1167–1176. [DOI] [PubMed] [Google Scholar]
- 12. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alferi O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohnloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 13. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS; The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the sepcial contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J. 2016;37:2893–2962.27567408 [Google Scholar]
- 14. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence . Atrial fibrillation: the management of atrial fibrillation. NICE clinical guideline 180. 2014. Available at: http://www.nice.org.uk/guidance/cg180/. Accessed September 15, 2016.
- 16. Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the general practice research database as an example of a UK primary care data resource. Ther Adv Drug Saf. 2012;3:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60:e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 20. Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16:791–801. [DOI] [PubMed] [Google Scholar]
- 21. Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Briethardt G. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–493. [DOI] [PubMed] [Google Scholar]
- 22. Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 24. Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM, Killian JM, Weston SA, Roger VL. Decade‐long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128:260–267.e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scowcroft AC, Cowie MR. Atrial fibrillation: improvement in identification and stroke preventive therapy—data from the UK Clinical Practice Research Datalink, 2000–2012. Int J Cardiol. 2014;171:169–173. [DOI] [PubMed] [Google Scholar]
- 26. Murphy NF, Simpson CR, Jhund PS, Stewart S, Kirkpatrick M, Chalmers J, MacIntyre K, McMurray JJ. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart. 2007;93:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the congestive heart failure, hypertension, age >75, diabetes mellitus, and prior stroke or transient ischemic attack (CHAD2) risk stratification scheme. Am Heart J. 2008;156:57–64. [DOI] [PubMed] [Google Scholar]
- 28. Jick SS, Kaye JA, Vasilakis‐Scaramozza C, Garcia Rodriguez LA, Ruigomez A, Meier CR, Schlienger RG, Black C, Jick H. Validity of the General Practice Research Database. Pharmacotherapy. 2003;23:686–689. [DOI] [PubMed] [Google Scholar]
- 29. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder TG, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD; Beta‐Bolockers in Heart Failure Collaborative Group . Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet. 2014;384:2235–2243. [DOI] [PubMed] [Google Scholar]
- 30. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Walletin L, Yusuf S. Causes of death and influencing factors in patients with atrial fibrillation: a competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013;128:2192–2201. [DOI] [PubMed] [Google Scholar]
- 31. Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, Bigger T, Cushman W, Goff D, Soliman EZ, Thomas A, Papademetriou V. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol. 2014;114:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36:3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GY. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta‐analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660–666. [DOI] [PubMed] [Google Scholar]
- 35. British Heart Foundation (BHF) . Trends in Coronary Heart Disease, 1961–2011. London, UK: BHF; 2012. [Google Scholar]
- 36. Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. [DOI] [PubMed] [Google Scholar]
- 37. Chen‐Scarabelli C, Scarabelli TM, Ellenbogen KA, Halperin JL. Device‐detected atrial fibrillation: what to do with asymptomatic patients? J Am Coll Cardiol. 2015;65:281–294. [DOI] [PubMed] [Google Scholar]
- 38. Kaasenbrood F, Hollander M, Rutten FH, Gerhards LJ, Hoes AW, Tieleman RG. Yield of screening for atrial fibrillation in primary care with a hand‐held, single‐lead electrocardiogram device during influenza vaccination. Europace. 2016;18:1514–1520. DOI: 10.1093/europace/euv426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wodchis WP, Bhatia RS, Leblanc K, Meshkat N, Morra D. A review of the cost of atrial fibrillation. Value Health. 2012;15:240–248. [DOI] [PubMed] [Google Scholar]
- 41. Ruigomez A, Johansson S, Wallander MA, Rodriguez LA. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc Disord. 2005;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]


