Abstract
Background
To address the need for personalized prevention, we conducted a subject‐level meta‐analysis within the framework of the Heart “OMics” in AGEing (HOMAGE) study to develop a risk prediction model for heart failure (HF) based on routinely available clinical measurements.
Methods and Results
Three studies with elderly persons (Health Aging and Body Composition [Health ABC], Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiaco [PREDICTOR], and Prospective Study of Pravastatin in the Elderly at Risk [PROSPER]) were included to develop the HF risk function, while a fourth study (Anglo‐Scandinavian Cardiac Outcomes Trial [ASCOT]) was used as a validation cohort. Time‐to‐event analysis was conducted using the Cox proportional hazard model. Incident HF was defined as HF hospitalization. The Cox regression model was evaluated for its discriminatory performance (area under the receiver operating characteristic curve) and calibration (Grønnesby‐Borgan χ2 statistic). During a follow‐up of 3.5 years, 470 of 10 236 elderly persons (mean age, 74.5 years; 51.3% women) developed HF. Higher age, BMI, systolic blood pressure, heart rate, serum creatinine, smoking, diabetes mellitus, history of coronary artery disease, and use of antihypertensive medication were associated with increased HF risk. The area under the receiver operating characteristic curve of the model was 0.71, with a good calibration (χ2 7.9, P=0.54). A web‐based calculator was developed to allow easy calculations of the HF risk.
Conclusions
Simple measurements allow reliable estimation of the short‐term HF risk in populations and patients. The risk model may aid in risk stratification and future HF prevention strategies.
Keywords: heart failure, meta‐analysis, risk factor, risk prediction
Subject Categories: Heart Failure, Risk Factors
Introduction
Heart failure (HF) is a complex syndrome often characterized by an abnormal cardiac structure and function leading to diminished quality of life, recurrent and costly hospital admissions, and premature death.1 HF remains a major public health concern because of the ageing of the populations and the increasing prevalence of HF‐associated comorbidities such as diabetes mellitus and obesity.2 The estimated prevalence of HF amounts to 1% to 2% of the adult population in developed countries, with the prevalence rising to >10% in those 70 years and older. The incidence is ≈5 to 10 per 1000 person‐years.3 Remarkable therapeutic advances in the past decades entailed a ≈3‐fold decrease in fatal HF, thereby increasing its prevalence and incidence. However, these advances are limited to HF with reduced ejection fraction, while its counterpart, HF with preserved ejection fraction, representing 50% of all HF cases, still lacks specific therapeutic strategies other than treating risk factors.4
Given the overall magnitude and lack of specific treatment for >50% of cases, identifying individuals at high risk for HF remains a priority in clinical research. Early identification will potentially allow testing preventive strategies in at‐risk populations thereby delaying or preventing HF onset and subsequently the disease burden. In this regard, a well‐calibrated, easily applicable, and generalizable risk model will help clinicians and trialists identify “high‐risk” patients. The former should concentrate on risk factor control with potential to delay HF onset, while the latter must focus on developing and testing novel therapeutic approaches.5 In this study, we aimed to identify routinely measured risk factors that reproducibly predict incident HF in an elderly population. We developed an easy‐to‐use calculator, allowing estimation of an individuals’ risk for incident HF in daily, routine clinical practice. The Heart “OMics” in AGEing (HOMAGE) study6 was used to conduct this subject‐level meta‐analysis and to develop an adequate scoring model.
Methods
Heart “OMics” in AGEing Database
The HOMAGE study database is constructed and maintained at the Studies Coordinating Centre in Leuven, Belgium. By sending anonymized data, the contributing partners confirmed that their study complies with good clinical practice (Helsinki Declaration), that all participants provided written informed consent, and that at the time of its conduct the study conformed to national regulations on clinical research in humans and on the protection of privacy. A detailed description of the database is available elsewhere.6 The database used for this analysis was locked on January 16, 2016.
Cohorts Analyzed
The entire HOMAGE database consists of 21 studies (n=46 134). Studies were eligible for inclusion in this analysis if information was available on fatal and/or nonfatal HF, with at least 20 events. We excluded 4 HF cohorts (n=4312), as well as 9 studies (n=7565) without information on HF outcome, and 4 studies with <20 HF events (n=4120). Among the 4 included studies (n=30 137), persons with a history of HF at baseline (n=223), those with missing information on incident HF (n=144), or those lost to follow‐up (n=279) were excluded. Figure 1 gives a detailed overview of the selection of the studies. Table 1 describes the 4 cohorts included in this analysis.
Figure 1.
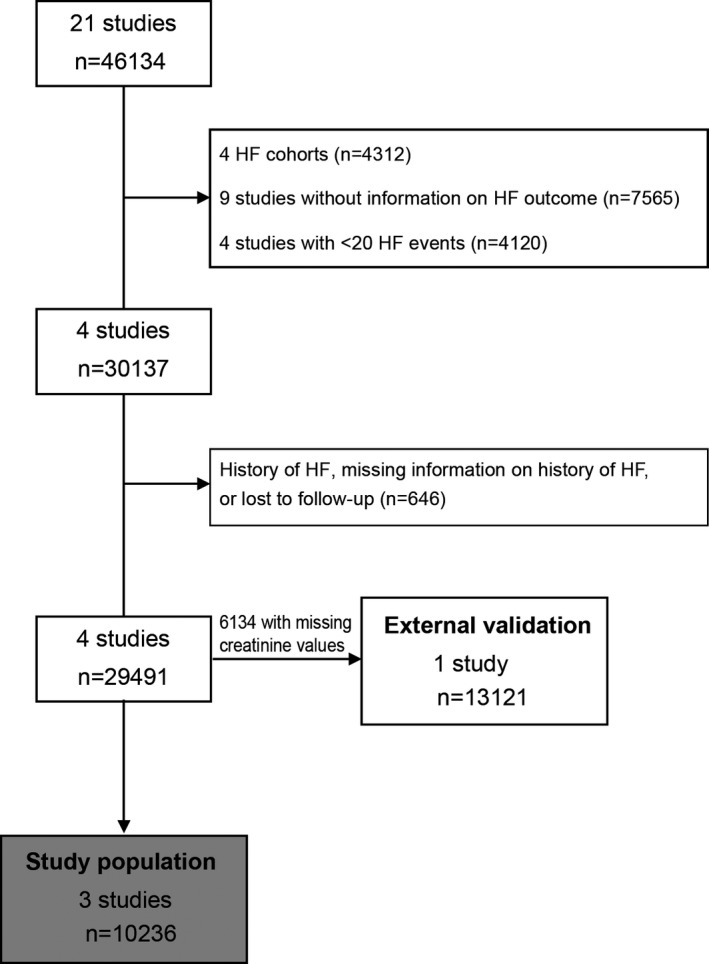
Flow chart of studies and participants included in the analysis. HF indicates heart failure.
Table 1.
Description of Included Studies
| Study | Type of Patients | Age Range, y | Total No. (HF Events) | Median Follow‐Up (5th–95th Percentile) | Definition of HF | |
|---|---|---|---|---|---|---|
| Study population | Health ABC | Population‐based (elderly persons) | 70.0–80.0 | 3075 (574) | 12.1 (2.0–14.1) | HF hospitalization: confirmation by reviewing hospital records |
| PREDICTOR | Population‐based (elderly persons) | 65.0–86.4 | 2001 (44) | 3.9 (2.5–5.0) | HF hospitalization: main cause of hospital admission=ICD‐9‐CM code 428 | |
| PROSPER | Patients at high risk of developing vascular disease | 70.0–83.4 | 5804 (234) | 3.3 (1.4–3.8) | HF hospitalization: confirmation by reviewing hospital records | |
| External validation | ASCOT | Hypertensive patients | 40.0–80.0 | 19 257 (292) | 5.6 (3.5–6.6) | HF hospitalization and physician‐based diagnosis/HF death |
ASCOT indicates Anglo‐Scandinavian Cardiac Outcomes Trial; Health ABC, Health Aging and Body Composition; HF, heart failure; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; PREDICTOR, Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiac; PROSPER, Prospective Study of Pravastatin in the Elderly at Risk.
Description of cohorts
The Health Aging and Body Composition (Health ABC) study is a cohort of 1584 women and 1491 men (aged ≥70 years), randomly recruited among Medicare beneficiaries residing in Pittsburg, Pennsylvania, and Memphis, Tennessee (April 1997–June 1998).7
The Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiaco (PREDICTOR) trial is a population study of a random sample of 5940 people from the Regional Population Registry of 4 Italian cities in the Lazio region (June 2007–January 2010). A total of 2001 people (33.7% of those invited), older than 65 years, provided written informed consent and were referred to 8 cardiology centers for clinical examination, blood tests, electrocardiography, and echocardiography.8, 9
PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) is a randomized, double‐blind, placebo‐controlled trial designed to test the hypothesis that treatment with pravastatin compared with placebo would reduce the risk of cardiovascular and cerebrovascular events in a cohort of 2000 women and 2804 men older than 70 years, with a history of vascular disease or at high risk for developing vascular disease. From 1997 until 1999, patients were enrolled via 3 coordinating centers in Scotland, Ireland, and The Netherlands.10, 11
Validation cohort
ASCOT (Anglo‐Scandinavian Cardiac Outcomes Trial) is a prospective, randomized, open, blinded end point trial, which recruited 19 257 high‐risk hypertensive patients aged 40 to 80 years, in Scandinavian countries, the United Kingdom, and Ireland (1998–2000).12, 13
Definition of Events
Table 1 gives an overview of the definition of events within each study. In all 3 studies, incident HF was defined as HF hospitalization. HF hospitalizations in the Health ABC study were confirmed by reviewing medical records of the participants.14 In the PREDICTOR study, HF hospitalizations were defined by International Classification of Diseases, Ninth Revision, Clinical Modification code 428 as the main cause of hospital admission. For the PROSPER trial, incident HF was defined as HF hospitalizations. Hospitalization records were examined and defined based on a combination of symptoms and signs, including chest radiography with fluid congestion or echocardiogram with severely diminished left ventricular function.15
Validation cohort
ASCOT defined HF events based on clinical signs and symptoms, chest x‐rays and echocardiography, or diagnosis of HF by the attending physician.
Statistical Analysis
For database management and statistical analyses, SAS software version 9.3 (SAS Institute, Cary, NC, USA) was used. Continuous variables were expressed as mean±SD and categorical variables as proportions. Missing values for BMI, systolic blood pressure, diastolic blood pressure, heart rate, glucose, cholesterol, and creatinine were imputated from the regression slope on age after stratification for cohort and sex. For comparison of means and proportions, the large sample Z‐test and chi‐square test were used, respectively. Statistical significance was a 2‐sided P value of <0.05.
Time‐to‐event analysis was conducted using the Cox proportional hazard model. Follow‐up time in the Health ABC study was limited to the maximum follow‐up time of the other 2 included cohorts, ie, 5.5 years. Log‐linearity was checked by testing the functional forms of the covariable by the Kolmogorov‐type supremum test. Covariables were entered into the multivariable model according to a stepwise selection procedure with P values for covariables to enter and stay in the model set to P=0.15 and P=0.05, respectively. Sex and age were forced into all models and other covariables considered to be of potential relevance and offered to the model were BMI, systolic and diastolic blood pressure, heart rate, blood glucose, serum total, high‐density lipoprotein and low‐density lipoprotein cholesterol, serum creatinine, smoking and drinking, use of antihypertensive medication, and history of diabetes mellitus, cardiovascular, coronary artery disease, peripheral artery, and cerebrovascular disease. In a sensitivity analysis, specific drug classes of antihypertensive treatment were added to the Cox models. Antihypertensive drug classes included β‐blockers, inhibitors of the renin‐angiotensin‐aldosterone system (RAAS), vasodilators, and diuretics. RAAS inhibitors included angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers, while vasodilators included calcium channel blockers and α‐blockers. All models were additionally adjusted for study.
Discrimination of the Cox regression models was assessed by calculating the area under the receiver operating characteristic curve (AUC), through a method taking into account time dependence and censoring, as previously described by Chambles and Diao.16 Assessment of the calibration, which demonstrates the goodness of fit of the models, was performed with the Grønnesby‐Borgan χ2 statistic. This test measures the level of agreement between observed versus predicted number of events, which were visually plotted across deciles of the predicted risk. SAS macros developed in the ARIC (Atherosclerosis Risk in Communities) study17 were used for these calculations. To internally validate our models, 100 bootstrap samples were generated, sampling subjects with replacement. We adjusted our AUC obtained from the internal validation for optimism. The external validation of our model was done in the ASCOT cohort.
Risk calculator
The formula below was used to calculate the risk :
where S0(t) is average survival at follow‐up time t (t=5 years), q is the number of continuous risk factors, p is the total number of risk factors (continuous and categorical), βi is the regression coefficient of the i‐th risk factor as estimated by the Cox regression model, wi,ref is the reference value of the base category of the i‐th risk factor, is the mean of the i‐th risk factor. We implemented this formula in Excel to develop an easy‐to‐use risk calculator (Figure S1).
Results
Study Population
A study population of 10 236 elderly people (51.3% women) with a mean age±SD of 74.5±3.7 years were included. Mean BMI±SD was 26.9±4.4 kg/m2 (Table 2). Except for the distribution of smoking and drinking status and diastolic blood pressure, all variables listed in Table 2 differed significantly between persons with incident HF and those without.
Table 2.
Baseline Characteristics by Incident HF
| All | HF | No HF | |
|---|---|---|---|
| No. of patients | 10 236 | 470 | 9766 |
| Mean of characteristics±SD | |||
| Age, y | 74.5±3.7 | 75.3±3.7 | 74.5±3.6§ |
| BMI, kg/m2 | 26.9±4.4 | 27.7±4.7 | 26.8±4.3§ |
| Systolic blood pressure, mm Hg | 146.9±22.7 | 149.5±24.9 | 146.8±22.5‡ |
| Diastolic blood pressure, mm Hg | 79.9±12.3 | 79.1±14.1 | 80.0±12.2 |
| Heart rate, beats per min | 66.7±11.5 | 68.9±12.5 | 66.6±11.5§ |
| Blood glucose, mmol/L | 5.60±1.60 | 5.92±2.13 | 5.58±1.57§ |
| Cholesterol, mmol/L | 5.50±0.97 | 5.37±0.98 | 5.51±0.97† |
| LDL cholesterol, mmol/L | 3.54±0.89 | 3.44±0.90 | 3.54±0.89* |
| HDL cholesterol, mmol/L | 1.33±0.40 | 1.29±0.41 | 1.33±0.38* |
| Serum creatinine, μmol/L | 96.2±27.7 | 106.6±42.0 | 95.8±26.7§ |
| No. with characteristics, % | |||
| Women | 5248 (51.3) | 212 (45.1) | 5036 (51.6)† |
| Smokers | 2052 (20.1) | 100 (21.3) | 1952 (20.0) |
| Consume alcohol | 5592 (54.6) | 237 (50.4) | 5355 (54.8) |
| Hypertension | 6577 (64.3) | 334 (71.1) | 6243 (63.9)† |
| Diabetes mellitus | 1299 (12.7) | 90 (19.2) | 1209 (12.4)§ |
| History of cardiovascular disease | 3701 (36.2) | 254 (54.0) | 3447 (35.3)§ |
| History of coronary artery disease | 2606 (25.5) | 209 (44.5) | 2397 (24.5)§ |
| History of peripheral artery disease | 257 (2.5) | 25 (5.3) | 232 (2.4)§ |
| History of cerebrovascular incident | 856 (8.4) | 55 (11.7) | 801 (8.2)† |
| Use of antihypertensive medication | 6596 (64.4) | 355 (75.5) | 6241 (63.9)§ |
| Use of ACEIs | 1697 (16.6) | 121 (25.7) | 1576 (16.1)§ |
| Use of calcium channel blockers | 2345 (22.9) | 159 (33.8) | 2186 (22.4) |
| Use of diuretics | 3372 (32.9) | 192 (40.9) | 3180 (32.6)‡ |
| Use of β‐blockers | 2087 (20.4) | 94 (20) | 1993 (20.4) |
| Use of angiotensin receptor blockers | 502 (4.9) | 26 (5.5) | 476 (4.9) |
The study population includes data from the Health ABC (Health Aging and Body Composition), PREDICTOR (Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiac), and PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) studies. Data are reported as mean±SD or number (percentage). ACEIs indicates angiotensin‐converting enzyme inhibitors; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Significance for the comparison between patients developing heart failure (HF) and those not developing HF: *P≤0.05; † P≤0.01; ‡ P≤0.001; § P≤0.0001.
After a median follow‐up time of 3.5 years (5th to 95th percentile: 2.3–5.5 years), 470 incident HF cases were present. Overall sex‐ and age‐adjusted incidence of HF was 12.1 (95% CI, 11.0–13.2) per 1000 person‐years. In persons younger than 75 years, the sex‐adjusted incidence rate was 9.8 (95% CI, 8.6–11.1) per 1000 person‐years, while the sex‐adjusted incidence rate for HF in persons older than 75 years was 15.3 (95% CI, 13.5–17.3) per 1000 person‐years. The sex‐adjusted cumulative incidence per age group is shown in Figure 2.
Figure 2.
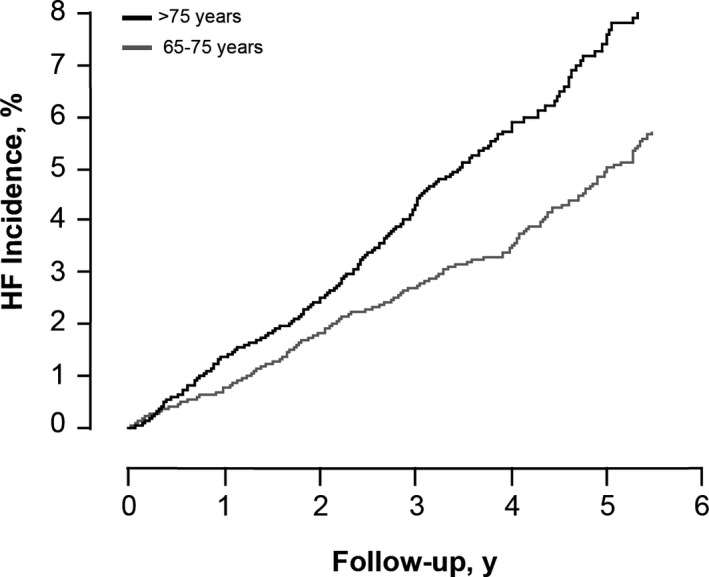
Sex‐adjusted cumulative heart failure (HF) incidence per age group.
The Cox regression analysis, corrected for study, showed that higher age, BMI, smoking, diabetes mellitus, history of coronary artery disease, use of antihypertensive medication, higher systolic blood pressure, heart rate, and serum creatinine were significantly associated with an increased risk of HF onset. Hazard ratios for a 1‐unit increase in risk factor are given in Table 3.
Table 3.
Hazard Ratios Relating HF Outcome to Risk Factors
| Study Population (Health ABC, PREDICTOR, PROSPER) | |||
|---|---|---|---|
| Risk Factor | Hazard Ratio | 95% CI | P Value |
| Female | 0.86 | 0.71–1.04 | 0.12 |
| Age, per y | 1.08 | 1.05–1.11 | <0.0001 |
| BMI, per kg/m2 | 1.03 | 1.01–1.06 | 0.002 |
| Smoking (0,1) | 1.84 | 1.46–2.32 | <0.0001 |
| Diabetes mellitus (0,1) | 1.41 | 1.12–1.79 | 0.004 |
| History of coronary artery disease (0,1) | 2.49 | 2.06–3.01 | <0.0001 |
| Use of antihypertensive medication (0,1) | 1.65 | 1.33–2.06 | <0.0001 |
| Systolic blood pressure, per mm Hg | 1.009 | 1.005–1.013 | <0.0001 |
| Heart rate, per beats per min | 1.02 | 1.01–1.03 | <0.0001 |
| Serum creatinine, per μmol/L | 1.005 | 1.004–1.006 | <0.0001 |
Hazard ratios are expressed per 1‐unit increase in continuous risk factors. All analyses are corrected for study. Health ABC indicates Health Aging and Body Composition; HF, heart failure; PREDICTOR, Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiac; PROSPER, Prospective Study of Pravastatin in the Elderly at Risk.
Model Assessment
The AUC for discriminating HF risk based on our Cox model was 0.706. After bootstrapping, we corrected the AUC for overoptimism and obtained an AUC of 0.702. Observed versus expected numbers of HF according to deciles of the 5‐year risk show good agreement (Grønnesby‐Borgan χ2 7.9; P=0.54). Figure 3 shows the calibration of the Cox model by plotting deciles of HF risk.
Figure 3.
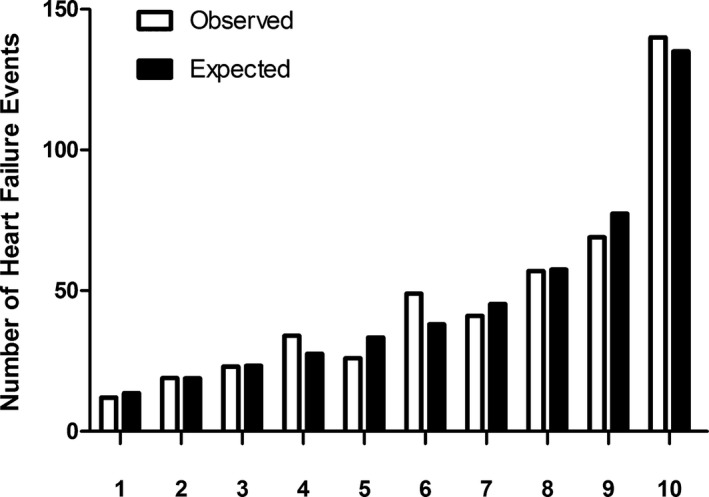
Calibration is shown. Observed (open bar) vs expected (solid bar) number of heart failure events across deciles of 5‐year heart failure risk.
We assessed calibration among the individual studies. Grønnesby‐Borgan χ2 statistics for PREDICTOR, Health ABC, and PROSPER were 2.9 (P=0.97), 5.1 (P=0.83), and 16.2 (P=0.06), respectively. Figure 4 shows the observed versus expected number of events for each individual study.
Figure 4.

Calibration per individual study. Observed (open bar) vs expected (solid bar) number of heart failure events across deciles of 5‐year risk for the Health ABC (Health Aging and Body Composition), PREDICTOR (Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiac), and PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) studies, which were used to develop the heart failure risk function. ASCOT (Anglo‐Scandinavian Cardiac Outcomes Trial) was used as an external validation cohort.
The risk function was externally validated using data from ASCOT. In 13 121 participants (mean age 63.3 years, 23.0% women), 191 HF events (HF hospitalization or death) occurred over a median follow‐up of 5.6 years (5th–95th percentile: 3.4–6.6). The AUC was 0.692 when we applied our risk function to this cohort.
Sensitivity Analyses
We examined the effect of antihypertensive treatment on incident HF in more detail by using specific classes of antihypertensive treatment: β‐blockers, RAAS inhibitors (including angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers), vasodilators (calcium channel blockers and α‐blockers), and diuretics. Results (Table 4) show that RAAS inhibitors, diuretics, and vasodilators were significantly associated with risk of incident HF, while use of β‐blockers were not.
Table 4.
Hazard Ratios Relating HF Outcome to Risk Factors, Including Classes of Antihypertensive Treatment
| Study Population (Health ABC, PREDICTOR, PROSPER) | |||
|---|---|---|---|
| Risk Factor | Hazard Ratio | 95% CI | P Value |
| Female | 0.87 | 0.71–1.05 | 0.15 |
| Age, per y | 1.08 | 1.05–1.11 | <0.0001 |
| BMI, kg/m2 | 1.03 | 1.01–1.05 | 0.007 |
| Smoking (0,1) | 1.84 | 1.46–2.32 | <0.0001 |
| Diabetes mellitus (0,1) | 1.36 | 1.07–1.72 | 0.012 |
| History of coronary artery disease (0,1) | 2.52 | 2.08–3.06 | <0.0001 |
| Use of β‐blockers (0,1) | 1.03 | 0.81–1.30 | 0.84 |
| Use of RAAS inhibitors (0,1) | 1.55 | 1.27–1.90 | <0.0001 |
| Use of vasodilators (0,1) | 1.35 | 1.13–1.67 | 0.003 |
| Use of diuretics (0,1) | 1.37 | 1.13–1.67 | 0.002 |
| Systolic blood pressure, per mm Hg | 1.009 | 1.005–1.013 | <0.0001 |
| Heart rate, per beats per min | 1.02 | 1.01–1.03 | <0.0001 |
| Serum creatinine, per μmol/L | 1.005 | 1.004–1.006 | <0.0001 |
Hazard ratios are expressed per 1‐unit increase in continuous risk factors. All analyses are corrected for study; the analysis in the patient cohort was further corrected for randomization group. Health ABC indicates Health Aging and Body Composition; HF, heart failure; PREDICTOR, Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso cardiac; PROSPER, Prospective Study of Pravastatin in the Elderly at Risk; RAAS, renin‐angiotensin‐aldosterone system.
Heart Failure Risk Calculator
Figure S1 is a calculator to compute a person's HF risk. Using the calculator, we obtain an HF risk of 2.58% for a 71‐year‐old woman, with a BMI of 26.4 kg/m2, nonsmoker, with diabetes mellitus, no history of coronary artery disease, who needs to use antihypertensive medication, with a systolic blood pressure of 150 mm Hg, heart rate of 62 beats per minute, and serum creatinine of 151.2 μmol/L. The calculation is done as described below:
Value of the risk factor of the individual
Mean value of the risk factors
5‐year estimated survival=S0(t)= 0.9738
Discussion
In this study, we developed a multivariable risk prediction tool to be used in daily clinical practice. The developed risk calculator estimates the risk of new‐onset HF in older people. Our stepwise approach identified several routinely available risk factors such as smoking, blood pressure, heart rate, diabetes mellitus, and creatinine levels, as well as nonmodifiable factors such as sex and age.
Risk Factors
The risk factors identified were in line with expectations and have been previously shown to be associated with incident HF.18, 19, 20 Older individuals had a higher risk for incident HF, explained by the age‐related cardiac structural and functional changes. The Framingham Study showed that per decade, the risk of HF increased by ≈24% in women and by 37% in men.21 Several studies examined the effect of smoking in older adults and found a higher risk for HF in both current and former heavy cigarette smokers.22, 23 Elevated systolic blood pressure is associated with increased risk of cardiovascular events and death in the general population.24 In older people and high‐risk patients, higher systolic blood pressure was a consistent risk factor. Resting heart rate is another variable with potential predictive capacity in HF and cardiovascular disease development. A recent study showed that resting heart rate was independently associated with incident HF in men during follow‐up and this association was found to be significant in men with both single and repeated heart rate measurement over time.25 These findings are replicated in our analyses as well. We also showed that diabetes mellitus were associated with increased HF risk. Hyperglycemia and insulin resistance are known to have unfavorable effects on the heart by increasing left ventricular mass index and wall thickness and promoting myocardial fibrosis and left ventricular dysfunction, which may be caused by increased oxidative stress and cardiac autonomic neuropathy.26, 27 In fact, incident HF was among the most frequent events among other cardiovascular events in recently published diabetes cardiovascular outcome trials on new oral hypoglycemic drugs in patients with type 2 diabetes mellitus.28, 29, 30 In addition, in a subgroup of the Health ABC study, in persons without known diabetes mellitus, glucose was a strong predictor of incident HF.31 Subsequently, serum creatinine was identified as a risk factor for incident HF. Creatinine is widely used to measure kidney function, and kidney damage has been associated with increased HF risk.32 Our results further reveal that persons who are taking antihypertensive treatment are at higher risk for incident HF. Previous studies showed that treatment with antihypertensive medication was associated with an increased residual cardiovascular risk.33, 34, 35, 36 It seems that the cardiovascular risk associated with hypertension cannot be totally diminished by intake of medication. Probably, patients taking hypertensive treatment at entry already had a higher cardiovascular risk profile, independent of blood pressure control. Looking into specific classes of antihypertensive treatment, we identified vasodilators, diuretics, and RAAS inhibitors as being associated with higher HF risk. This counterintuitive observation might be explained by reverse epidemiology: persons who need to take these drugs are most likely the ones who are already at higher risk for HF. Indeed, persons with symptoms of angina pectoris or previous coronary heart disease, who already have an increased HF risk, are often under treatment with vasodilators.37 Similarly, persons with severe hypertension, treated with diuretics, are at higher risk. Moreover, physicians might prescribe these medications to patients with suspected HF, or with mild symptoms, before the diagnosis of overt HF is made.
Prediction Models
Rather than assessing single risk factors, risk prediction models allow the identification of a broader segment of the population. Previously, risk models have been developed to predict HF risk, including mostly US and European cohorts.19 Most HF prediction models were described as mathematical equations, with only 2 US studies providing a point‐based risk score.14, 21 We provide an easy‐to‐use risk calculator in Excel to allow simple risk calculation for clinicians and facilitate its use in daily practice. Several HF prediction models assessed the discriminatory ability of B‐type natriuretic peptide or N‐terminal pro–B‐type natriuretic peptide on top of traditional risk factors.38, 39, 40, 41, 42 Overall, a modest improvement in discrimination was observed.40, 41, 42, 43 This does not mean that biomarkers could not be useful in further refining the identification of at‐risk patients. Adding biomarker information should be the next step to further categorize persons initially identified at high risk, based on our available clinical risk parameters. The HOMAGE consortium remains dedicated to discovering biomarkers that could lead to new insights and improve risk stratification, mechanistic profiling, and early detection of HF. Data of the Health ABC study were previously used to develop an HF risk score.14 We pooled the Health ABC data with data from the European PREDICTOR and PROSPER studies, which also include elderly people. Inclusion of both US and European persons adds to the generalizability of the developed risk score in elderly persons.
Limitations
Limitations of our study include the inability to systematically distinguish between participants with incident HF according to the type of left ventricular dysfunction (systolic versus diastolic). Recently, a large study including 4 population‐based cohorts identified differences in risk factors predicting HF with preserved ejection fraction and HF with reduced ejection fraction.44 Although age, BMI, systolic blood pressure, previous myocardial infarction, and antihypertensive treatment were identified as risk factors for both HF subtypes, male sex, smoking, left ventricular hypertrophy, left bundle branch block, and diabetes mellitus were found to only predict HF with reduced ejection fraction.20
Next, the diagnosis of HF is less clear and specific then, for example, myocardial infarction. The HF end point in our cohort was based on nonfatal HF hospitalization, which implicates that HF diagnosed at an outpatient clinic or by general practitioners was not included. Moreover, differences in HF hospitalization definition between the cohorts occur due to factors such as hospital admissions policy, treating physicians, adherence to guidelines for therapy, and support by dedicated HF nurses.
Finally, our risk score was developed in elderly patients at risk for vascular disease. Particularly in this population, aggravating factors related to rehospitalizations such as intercurrent infections, atrial fibrillation, anemia, or hyperthyroidism could play a role. We did not take into account these factors, since we focused on first hospitalization for HF and aimed to provide a risk score based on readily available clinical risk factors, to facilitate its use in daily practice.
Strengths
The strengths of our study are that we included persons from well‐characterized populations, with an adequate follow‐up period. Risk factors included are routinely available and our estimates are derived from a large sample. The risk function calculator is easy to use by clinicians or nurses and could be useful as a tool in risk factor modification screening within a public health or clinical setting. We assessed the validity of our HF risk model in the ASCOT cohort, showing good discrimination and calibration.
According to a recent systematic review, there are no recommendations in current guidelines for using risk prediction models for HF or studies assessing the effect of such models in routine clinical practice.19 Research into a practical application of our risk prediction scores remains necessary to give a definitive answer to whether improvement of outcome can be expected by using the risk tool. Indeed, for example, collaborative care in patients at risk for developing HF based on B‐type natriuretic peptide screening reduced the combined rates of left ventricular systolic dysfunction, diastolic dysfunction, and HF.45
Conclusions
An increase in HF over the next decades will translate to more than a 200% increase in direct medical costs of care.46 To mitigate mortality and healthcare costs, effective prevention of HF that can offer early correction of predisposing conditions and risk factors in susceptible persons is required. Our HF risk model allows reliable identification of high‐risk populations, contributing to a more targeted and cost‐effective implementation of preventive measures for HF.
Appendix
We would like to thank all contributing investigators.
HOMAGE‐Investigators
Inserm—Alexandre Mebazza, Florence Pinet, Anne Pizard, Philippe Rouet, Faiez Zannad; IT—Catherine Clusel; EDDH—Stephanie Grosjean; ACS Biomarker—Heico Breek, Joost Leenders; FIMA—Javier Diez; UCD—Kenneth McDonald; HULL—Andrew Clark; UM—Stephane Heymans; IRFMN—Roberto Latini, Serge Masson; MHH—Thomas Thum; KUL—Nicholas Cauwenberghs, Ljupcho Efremov, Lutgarde Thijs, Lotte Jacobs, Tatiana Kuznetsova, Augustine Odili, Jan A. Staessen, Fang‐Fei Wei, Wen‐Yi Yang, Zhen‐Yu Zhang; LSHTM—Tim Collier; UG—Christian Delles, Naveed Sattar; MOS—Harald Mischak; Stony Brook—Javed Butler; KU—Mamas Mamas; TATAA—Jens Bjorkman; Charité—Burkert Pieske; for Health ABC—Stephen Kritchevsky, Anne Newman, Lampros Papadimitrious; for PREDICTOR—Alessandro Boccanelli, Marina Davoli, Gian Francesco Mureddu; for PROSPER—Brendan Buckley, Ian Ford, Wouter Jukema, David J. Stott; and for ASCOT—Bjorn Dahlof, Neil Poulter, Peter Sever.
Sources of Funding
This work is supported by the European Union: HEALTH‐F7‐305507 HOMAGE (EU FP7 305507 http://www.homage-hf.eu). The European Research Council Advanced Researcher Grant‐2011‐294713‐EPLORE and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13 and G.088013), currently support the Studies Coordinating Centre in Leuven.
Disclosures
None.
Supporting information
Figure S1. Heart failure risk calculator.
(J Am Heart Assoc. 2017;6:e005231 DOI: 10.1161/JAHA.116.005231.)28465299
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Krum H, Abraham WT. Heart failure. Lancet. 2009;373:941–955. [DOI] [PubMed] [Google Scholar]
- 3. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poppe KK, Doughty RN. Outcomes in patients with heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:503–510. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, Lopez‐Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez‐Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E. Cholesterol lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 6. Jacobs L, Thijs L, Jin Y, Zannad F, Mebazaa A, Rouet P, Pinet F, Bauters C, Pieske B, Tomaschitz A, Mamas M, Diez J, McDonald K, Cleland JG, Rocca HP, Heymans S, Latini R, Masson S, Sever P, Delles C, Pocock S, Collier T, Kuznetsova T, Staessen JA. Heart ‘omics’ in AGEing (HOMAGE): design, research objectives and characteristics of the common database. J Biomed Res. 2014;28:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins‐Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the Health, Aging, and Body Composition Study. Arch Intern Med. 2009;169:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, Cesaroni G, Masson S, Cacciatore G, Colivicchi F, Uguccioni M, Perucci CA, Boccanelli A. Prevalence of preclinical and clinical heart failure in the elderly. A population‐based study in Central Italy. Eur J Heart Fail. 2012;14:718–729. [DOI] [PubMed] [Google Scholar]
- 9. Mureddu GF, Tarantini L, Agabiti N, Faggiano P, Masson S, Latini R, Cesaroni G, Miceli M, Forastiere F, Scardovi AB, Uguccioni M, Boccanelli A. Evaluation of different strategies for identifying asymptomatic left ventricular dysfunction and pre‐clinical (stage B) heart failure in the elderly. Results from ‘PREDICTOR’, a population based‐study in central Italy. Eur J Heart Fail. 2013;15:1102–1112. [DOI] [PubMed] [Google Scholar]
- 10. Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, Ford I, Jukema JW, Hyland M, Gaw A, Lagaay AM, Perry IJ, Macfarlane PW, Meinders AE, Sweeney BJ, Packard CJ, Westendorp RG, Twomey C, Stott DJ. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 1999;84:1192–1197. [DOI] [PubMed] [Google Scholar]
- 11. Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 12. Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 13. Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Rationale, design, methods and baseline demography of participants of the Anglo‐Scandinavian Cardiac Outcomes Trial. ASCOT investigators. J Hypertens. 2001;19:1139–1147. [DOI] [PubMed] [Google Scholar]
- 14. Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nanchen D, Stott DJ, Gussekloo J, Mooijaart SP, Westendorp RG, Jukema JW, Macfarlane PW, Cornuz J, Rodondi N, Buckley BM, Ford I, Sattar N, de Craen AJ. Resting heart rate and incident heart failure and cardiovascular mortality in older adults: role of inflammation and endothelial dysfunction: the PROSPER study. Eur J Heart Fail. 2013;15:581–588. [DOI] [PubMed] [Google Scholar]
- 16. Chambless LE, Diao G. Estimation of time‐dependent area under the ROC curve for long‐term risk prediction. Stat Med. 2006;25:3474–3486. [DOI] [PubMed] [Google Scholar]
- 17. Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med. 2011;30:22–38. [DOI] [PubMed] [Google Scholar]
- 18. Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, Burke G, Balfour P, Herrington D, Shi P, Post W, Olson J, Watson KE, Folsom AR, Lima JA. Heart failure risk prediction in the Multi‐Ethnic Study of Atherosclerosis. Heart. 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Echouffo‐Tcheugui JB, Greene SJ, Papadimitriou L, Zannad F, Yancy CW, Gheorghiade M, Butler J. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail. 2015;8:438–447. [DOI] [PubMed] [Google Scholar]
- 20. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new‐onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. [DOI] [PubMed] [Google Scholar]
- 22. Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins‐Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB, Butler J. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed AA, Patel K, Nyaku MA, Kheirbek RE, Bittner V, Fonarow GC, Filippatos GS, Morgan CJ, Aban IB, Mujib M, Desai RV, Allman RM, White M, Deedwania P, Howard G, Bonow RO, Fletcher RD, Aronow WS, Ahmed A. Risk of heart failure and death after prolonged smoking cessation: role of amount and duration of prior smoking. Circ Heart Fail. 2015;8:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 25. Nanchen D, Leening MJ, Locatelli I, Cornuz J, Kors JA, Heeringa J, Deckers JW, Hofman A, Franco OH, Stricker BH, Witteman JC, Dehghan A. Resting heart rate and the risk of heart failure in healthy adults: the Rotterdam Study. Circ Heart Fail. 2013;6:403–410. [DOI] [PubMed] [Google Scholar]
- 26. Khan H, Kunutsor SK, Kauhanen J, Kurl S, Gorodeski EZ, Adler AI, Butler J, Laukkanen JA. Fasting plasma glucose and incident heart failure risk: a population‐based cohort study and new meta‐analysis. J Card Fail. 2014;20:584–592. [DOI] [PubMed] [Google Scholar]
- 27. Kasznicki J, Drzewoski J. Heart failure in the diabetic population—pathophysiology, diagnosis and management. Arch Med Sci. 2014;10:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double‐blind trial. Lancet. 2015;385:2067–2076. [DOI] [PubMed] [Google Scholar]
- 29. McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–851. [DOI] [PubMed] [Google Scholar]
- 30. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalogeropoulos A, Georgiopoulou V, Harris TB, Kritchevsky SB, Bauer DC, Smith AL, Strotmeyer E, Newman AB, Wilson PW, Psaty BM, Butler J. Glycemic status and incident heart failure in elderly without history of diabetes mellitus: the Health, Aging, and Body Composition Study. J Card Fail. 2009;15:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:1307–1315. [DOI] [PubMed] [Google Scholar]
- 33. Andersson OK, Almgren T, Persson B, Samuelsson O, Hedner T, Wilhelmsen L. Survival in treated hypertension: follow up study after two decades. BMJ. 1998;317:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blacher J, Evans A, Arveiler D, Amouyel P, Ferrieres J, Bingham A, Yarnell J, Haas B, Montaye M, Ruidavets JB, Ducimetiere P. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens. 2010;24:19–26. [DOI] [PubMed] [Google Scholar]
- 35. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 36. Glynn RJ, L'Italien GJ, Sesso HD, Jackson EA, Buring JE. Development of predictive models for long‐term cardiovascular risk associated with systolic and diastolic blood pressure. Hypertension. 2002;39:105–110. [DOI] [PubMed] [Google Scholar]
- 37. Lanza GA, Crea F. Overview of management of myocardial ischemia: a mechanistic‐based approach. Cardiovasc Drugs Ther. 2016;30:341–349. [DOI] [PubMed] [Google Scholar]
- 38. Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalogeropoulos AP, Georgiopoulou VV, deFilippi CR, Gottdiener JS, Butler J. Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults: the Cardiovascular Health Study. JACC Cardiovasc Imaging. 2012;5:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith JG, Newton‐Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, Platonov PG, Hedblad B, Engstrom G, Wang TJ, Melander O. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, Selhub J, Jacques PF, Meigs JB, Tofler GH, Vasan RS. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122:1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. deFilippi CR, Christenson RH, Kop WJ, Gottdiener JS, Zhan M, Seliger SL. Left ventricular ejection fraction assessment in older adults: an adjunct to natriuretic peptide testing to identify risk of new‐onset heart failure and cardiovascular death? J Am Coll Cardiol. 2011;58:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail. 2016;9:e003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide‐based screening and collaborative care for heart failure: the STOP‐HF randomized trial. JAMA. 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 46. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd‐Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Heart failure risk calculator.


