Abstract
Background
Cardiac disease after mediastinal radiotherapy for thoracic malignancy (chest radiotherapy [XRT]) often manifests as progressive aortic stenosis. In patients with XRT‐induced severe aortic stenosis undergoing surgical aortic valve replacement (SAVR), we sought to: (1) study long‐term survival and compare these patients with a matched cohort undergoing SAVR during the same time frame; and (2) identify potential predictors of long‐term mortality.
Methods and Results
We studied patients with symptomatic severe aortic stenosis undergoing SAVR at our institution, of which there were 172 mediastinal XRT patients (63±13 years, 62% women) matched in a 1:1 fashion (based on age, sex, time of surgery, and aortic valve area) with 172 non‐XRT patients (comparison group). Baseline clinical and postoperative data were obtained. Society of Thoracic Surgeons score was calculated and mortality was recorded. In the XRT group, the median Society of Thoracic Surgeons score was 4% (interquartile range 2–13), while mean left ventricular ejection fraction, left ventricular stroke volume index, and mean aortic valve gradient were 54±11%, 38±14 mL/m2, and 39±11 mm Hg, respectively. In the entire cohort, 27% and 34% of patients underwent concomitant coronary artery bypass grafting and aortic surgery at the time of SAVR, respectively. Thirty‐day/in‐hospital deaths occurred in 4 (2%) patients in the XRT group and 0 patients in the comparison group. At 6±3 years of follow‐up, on matched group analysis, there were 95 (28%) deaths (83 [48%] in the XRT group versus 12 [7%] in the comparison group (log‐rank 89, P<0.001). On multivariable Cox survival analysis, in the whole cohort, higher Society of Thoracic Surgeons score (hazard ratio, 1.14; 95% CI, 1.03–1.26) and mediastinal XRT (hazard ratio, 8.12; 95% CI, 4.26–15.64) were associated with increased longer‐term mortality (both P<0.01).
Conclusions
In patients with severe aortic stenosis undergoing SAVR, patients with prior mediastinal XRT have significantly worse longer‐term survival versus a matched cohort.
Keywords: aortic stenosis, aortic valve replacement, chest radiotherapy, outcome, radiation risk, surgery
Subject Categories: Valvular Heart Disease
Introduction
Therapeutic advances in chest radiotherapy (XRT) over the past 50 years have dramatically improved survival in patients with thoracic malignancy. Increased longevity, however, is associated with the appearance of late effects of chest (especially mediastinal) XRT, otherwise known as radiation‐associated cardiac disease.1, 2, 3, 4, 5 This is a heterogeneous cardiovascular disorder, which covers a broad spectrum of cardiovascular disease processes and may involve any cardiac structure, including the pericardium, myocardium, valvular system, conduction system, and coronary arteries.1, 2, 3, 4, 5 The lungs, great vessels, and carotid arteries may also be involved.
Valvular heart disease occurs in as many as 81% of patients with previous mediastinal XRT, with the aortic and mitral valves most commonly affected.6 Radiation‐associated valvular disease has a varied spectrum with progressive thickening and calcification of the affected valves and resultant progressive stenosis and/or regurgitation. A substantial proportion of such patients develop severe symptomatic valvular (including aortic stenosis [AS]) disease requiring cardiovascular surgery. However, a recent study from our group found significantly increased long‐term mortality among all patients with prior radiation undergoing open heart surgery.7 This may be attributed to the presence of multiple cardiac lesions, primary myocardial/pericardial dysfunction, pulmonary and vascular comorbidities, and XRT‐induced fibrosis of lungs and surrounding tissues, all of which result in a hostile surgical environment. With the emergence of percutaneous technologies, there has been a heightened emphasis on elucidating outcomes in patients with radiation‐associated AS undergoing surgical aortic valve (AV) replacement (SAVR). In this study, we sought to: (1) study long‐term survival in patients with mediastinal XRT‐associated severe AS undergoing SAVR and compare these patients with a matched cohort undergoing SAVR during the same time frame; and (2) to identify potential predictors of long‐term mortality.
Methods
This was an observational cohort study (matched design) of patients with severe AS who underwent SAVR at our tertiary care referral center between January 1, 2000 and January 1, 2015. The study population consisted of two equally matched groups (an “XRT group” and a “comparison group”). The XRT group consisted of 172 symptomatic patients with a history of thoracic malignancy who underwent mediastinal irradiation and consequently developed AS severe enough to require SAVR. The diagnosis of radiation‐associated AS was made after a thorough clinical and echocardiographic evaluation by experienced cardiologists. The type of malignancy and the year of radiation were all documented, where available. The comparison group consisted of 172 symptomatic patients without a history of thoracic malignancy or prior chest/mediastinal irradiation who were 1:1 matched in terms of age (within 2 years), sex, type and timing of AV replacement (AVR; within 2 years), and AV area (within 0.1 cm2). The comparison group was identified from >10 000 patients who underwent SAVR during the same time frame. We excluded patients with significant multivalvular disease, documented constrictive pericarditis, and severe restrictive lung disease. However, in our contemporary experience of AS patients, concomitant coronary artery disease (CAD) is common and, as a result, we decided to include this subgroup.8 All procedures were elective and none were performed in an emergent/urgent setting.
Data were assembled through individual analysis of electronic medical records after obtaining appropriate institutional review board approval with waiver of individual informed consent. Clinical data and demographics were recorded prospectively in the electronic medical records at the time of initial clinical encounter and manually extracted for the current study. Baseline patient characteristics including demographics, symptoms, vital signs, medical history, oncologic history, and common laboratory and pulmonary function test values before SAVR were collected. Data were obtained as close to the date of SAVR as possible (within 1 month in the majority of cases). Medication use at the time of SAVR was ascertained by electronic medical record review. Based on the above data, the Society of Thoracic Surgeons (STS) score and Charlson comorbidity index were calculated. Even though the STS score has only been validated to predict 30‐day postoperative mortality, we used it in the survival analysis, as it is a composite of many factors that are known to be associated with adverse postoperative events in the longer‐term in AS patients.8 In addition, we calculated Charlson comorbidity index in all patients, as a surrogate for frailty and comorbidity.9 All patients were deemed free of any major comorbid condition such as recurrence of original tumor or subsequent development of a new tumor and cleared to undergo cardiac surgery after a thorough clinical evaluation by cardiology and cardiothoracic surgery.
Preoperative Echocardiography and Additional Imaging
All patients underwent a comprehensive echocardiogram with commercially available instruments (Philips Medical Systems, NA, Bothell, WA; General Electric Medical Systems, Milwaukee, WI; and Siemens Medical Solutions USA, Inc, Malvern, PA) as part of a standard clinical diagnostic evaluation. Measurements and recordings were obtained according to current recommendations.10, 11 Left ventricular ejection fraction was calculated using the Simpson's biplane method. We used a semiquantitative 5‐point scale (with grades of none, mild, moderate, moderately severe, and severe) to stratify valvular regurgitation on color 2‐dimensional Doppler echocardiography clips obtained in multiple standard views. Right ventricular systolic pressure was estimated in a standard fashion.12
For quantification of AS severity, LV outflow tract (LVOT) diameter was measured in a standard fashion on parasternal long‐axis views and the AV area was calculated using the continuity equation, according to guidelines.11 Severe AS was defined as AV area ≤1 cm2. LV stroke volume index (LV‐SVI) was measured using the following formula: LVOTVTI×LVOTarea/body surface area. A cutoff ≥35 mL/m2 was considered as preserved LV‐SVI.11, 13, 14, 15
Where available, data on the degree of ascending aortic calcification assessed on chest multidetector computed tomography were recorded. In patients with a history of cardiac surgery, preoperative planning using computed tomography was performed, as previously described.16
Surgery
The details of various surgical therapies for management of AS were recorded and categorized as follows8: (1) AVR, (2) AVR and coronary artery bypass grafting (CABG), and (3) AVR and other (concomitant aortic surgery). The type of valvular prosthesis (mechanical versus bioprosthesis) was recorded. The use of internal mammary artery was also recorded. Because chest and mediastinal XRT can potentially damage the internal mammary arteries, they were reviewed on preoperative coronary angiography and only the arteries that were deemed suitable were used. The final decision regarding the specific operative technique was made by the attending cardiothoracic surgeon. In patients with high‐risk features (eg, significant ascending aortic calcification, redo surgery), preventive surgical strategies were employed.17
Follow‐Up
The beginning of follow‐up was considered the date of AVR. Postoperative data, including the length of intensive care unit and hospital stay and postoperative complications such as pericardial effusion, arrhythmia, stroke, and myocardial infarction, were recorded. The primary end point was all‐cause mortality. Data on death and survival were obtained from the medical record, from the US Social Security Death Index, or the Ohio Death Index. We attempted to ascertain the cause of death from chart review or telephone follow‐up. Cardiovascular mortality was defined as any death due to cardiac arrest, myocardial infarction, arrhythmia, heart failure, or any other cardiovascular cause. In addition, if the cause of death could not be discerned, this was also categorized as cardiovascular in etiology unless the patient's proximal history strongly suggested a noncardiovascular cause.18
Statistical Analysis
Continuous variables were expressed as mean±SD or median. When appropriate, continuous variables were also divided into 2 groups based on median for further analyses. Categorical data are presented as percentage frequency. Differences in baseline and procedural characteristics between matched groups were assessed with Student paired t test or repeated‐measures ANOVA (for parametric variables) and the Wilcoxon signed‐rank test (for nonparametric variables). Differences in the distribution of categorical variables were compared using conditional logistic regression stratified by matched pair. Cox proportional hazards analysis was performed to determine the association of relevant predictors with mortality. Multivariable analysis ignoring matched pairs was performed to describe the impact of the matched variables and mediastinal XRT exposure on mortality. Subsequently, conditional Cox proportional hazards stratified by matched pair was performed to discern whether prior mediastinal XRT exposure remained an independent predictor of mortality.19 Multivariable models for mortality were built using variables thought to be most biologically important to the outcome, avoiding colinearity and overfitting (a 10 event to 1 variable ratio was used). Cox proportional hazard results are reported as hazard ratios (HRs) with 95% CIs. Cumulative survival probabilities as a function of time were obtained via the Kaplan–Meier method, and event curves were compared using the log‐rank test. Significance was determined at P<0.05. Statistical analysis was performed with R version 3.1.0 and SPSS version 11.5 (SPSS Inc, Chicago, IL).
Results
The clinical and echocardiographic characteristics of our matched study cohort are shown in Table 1. Due to the fact that the primary study population had a history of mediastinal XRT, they were relatively younger as compared with a standard population who develop severe AS requiring SAVR. The 2 groups were well matched in terms of the incidence of standard risk factors and obstructive CAD; however, as would be expected, the proportion of patients with significant left main and 3‐vessel CAD was higher in the XRT group. Similarly, results from pulmonary function tests were slightly worse in the XRT patients versus controls, as would be expected. While AV area and left ventricular ejection fraction were similar in both groups, peak and mean AV gradients were higher and the proportion of patients with an abnormal LV‐SVI was higher in the XRT group. On the other hand, the STS score and Charlson comorbidity index were similar in the 2 groups. A multidetector computed tomography scan was performed in all 172 patients in the XRT group versus 109 patients in the control group. Significant (at least partially circumferential) calcification of the ascending aorta was observed in 62 (36%) patients in the XRT group versus none in the control group (P<0.001).
Table 1.
Baseline Characteristics of the Matched Study Cohort
| Variable | XRT Group (n=172) | Comparison Group (n=172) | P Value |
|---|---|---|---|
| Clinical, demographic, and symptom variables | |||
| Age, y | 63±13 | 64±13 | N/A |
| Female sex | 106 (62) | 106 (62) | N/A |
| Hypertension | 105 (61) | 119 (69) | 0.073 |
| Diabetes mellitus | 35 (20) | 44 (26) | 0.154 |
| Hyperlipidemia | 104 (61) | 110 (64) | 0.291 |
| Obstructive CAD | 45 (26) | 47 (27) | 0.457 |
| ≥50% left main or 3‐vessel CAD | 40 (23) | 8 (5) | <0.001 |
| Atrial fibrillation | 9 (5) | 13 (8) | 0.263 |
| End‐stage renal disease | 2 (1) | 1 (0.6) | 0.502 |
| Smoking history | 76 (44) | 70 (40) | 0.496 |
| Prior stroke | 14 (8) | 15 (9) | 0.502 |
| Prior cardiac surgery | 34 (20) | 29 (17) | 0.492 |
| Dyspnea on exertion | 161 (94) | 153 (89) | 0.243 |
| Angina | 53 (31) | 56 (33) | 0.412 |
| Syncope | 11 (6) | 8 (5) | 0.319 |
| Median STS score with interquartile range, % | 4 [2–13] | 4 [3–11] | 0.323 |
| Charlson comorbidity index | 3.3±1.6 | 3.1±1.5 | 0.311 |
| Medications | |||
| Betablockers | 105 (61) | 98 (57) | 0.24 |
| Statins | 102 (60) | 81 (47) | 0.02 |
| Angiotensin‐converting enzyme inhibitors | 32 (19) | 43 (25) | 0.10 |
| Aspirin | 100 (59) | 96 (56) | 0.35 |
| Laboratory and pulmonary function data | |||
| Glomerular filtration rate, mL/min per 1.73 m2 | 74±24 | 77±23 | 0.168 |
| Hemoglobin, mg/dL | 13.2±02 | 13.1±2 | 0.209 |
| FEV1 (% of predicted) | 74±14 | 70±16 | 0.018 |
| FVC (% of predicted) | 78±13 | 74±16 | 0.026 |
| FEV1/FVC ratio | 94±9 | 94±8 | 0.502 |
| Echocardiographic data | |||
| Left ventricular ejection fraction, % | 54±11 | 56±9 | 0.049 |
| Aortic valve area, cm2 | 0.73±0.21 | 0.70±0.14 | N/A |
| Peak aortic valve gradient, mm Hg | 64±25 | 85±22 | <0.001 |
| Mean aortic valve gradient, mm Hg | 39±11 | 49±13 | <0.001 |
| LV‐SVI | 38±14 | 40±11 | 0.224 |
| Abnormal LV‐SVI (<35 mL/m2) | 89 (52) | 61 (35) | <0.001 |
| Aortic regurgitation (≥II+) | 16 (9) | 17 (10) | 0.842 |
| Right ventricular systolic pressure, mm Hg | 39±11 | 37±10 | 0.113 |
Values are expressed as number (percentage) or mean±SD.
Because the prior chest radiotherapy exposure (XRT) and comparison groups were matched based on age, sex, and aortic valve area, P values are not reported. CAD indicates coronary artery disease; FEV1, forced expiratory volume at 1 second; FVC, forced vital capacity; LV‐SVI, left ventricular stroke volume index; N/A, not applicable; STS, Society of Thoracic Surgeons.
The breakdown of surgeries in the entire cohort was as follows: isolated SAVR (n=134 [39%]), SAVR+CABG (n=92 [27%]), and SAVR+aorta (n=118 [34%]), with no differences between subgroups, by study design. Within the CABG group, internal mammary artery was utilized in 71 cases, with a significantly lower proportion of use in the XRT versus the control group (27 versus 44, P<0.01). The number of utilized coronary bypass grafts were significantly higher in the XRT versus control groups (1.8±1.2 versus 1.2±0.9, P<0.05). A significantly higher proportion of XRT patients needed hypothermic circulatory arrest versus the control patients (48 patients versus 9 patients, P<0.001).
The breakdown of prior malignancies and radiation doses in the XRT group was as follows: breast cancer (51 [30%], 50–60 Gy), lung cancer with mediastinal involvement (5 [3%], 60 Gy), Hodgkin's lymphoma (94 [55%], 40–45 Gy), non‐Hodgkin's lymphoma (10 [6%], 40–45 Gy), and others, including esophageal cancer (12 [7%], 40–45 Gy). The median time from XRT to surgery was 25 [18–32] years and 31 (18%) patients had received prior chemotherapy.
Outcomes
The immediate postoperative outcomes for both groups are demonstrated in Table 2. During surgery, mediastinal adhesions were observed in all XRT patients, while they were observed only in redo surgical cases in the control group (100% versus 17%, P<0.001). As expected, the cardiopulmonary bypass time (96±25 minutes versus 80±12 minutes) and total procedure time (incision to skin closure time, 233±40 minutes versus 175±35 minutes) were significantly higher in the XRT versus the control group (both P<0.001). While the majority of patients in the entire study cohort had a bioprosthetic AVR implanted, a significantly higher proportion of XRT patients had a mechanical valve implanted. As expected, patients in the XRT group had a longer intensive care unit and in‐hospital stay, a higher rate of readmission within the first 3 months postoperatively, and a higher rate of postoperative and permanent atrial fibrillation versus the comparison group. In addition, 10 patients had in‐hospital postoperative stroke (9 in the XRT and 1 in the comparison group, respectively; P=0.01), while 4 (2.3%) patients died during hospitalization for the procedure (all in the XRT group and none in the comparison group, P=0.01).
Table 2.
Perioperative and Postoperative Characteristics of the Matched Study Cohort
| Variable | XRT Group (n=172) | Comparison Group (n=172) | P Value |
|---|---|---|---|
| Type of aortic valve replacement | |||
| Bioprosthesis | 137 (80) | 157 (91) | <0.001 |
| Mechanical | 35 (20) | 15 (9) | |
| Perioperative blood transfusions, No. of units | 3.1±1.6 | 2±1.2 | <0.001 |
| Perioperative inotropic support | 74 (43) | 58 (34) | 0.03 |
| Length of hospital stay | 15±13 | 9±6 | <0.001 |
| Length of intensive care unit stay | 5±9 | 2±3 | <0.001 |
| ≥Moderate pericardial effusion | 51 (30) | 42 (24) | 0.274 |
| Cardiac tamponade | 1 (1) | 0 | 0.521 |
| In‐hospital myocardial infarction | 0 | 1 (1) | 0.502 |
| In‐hospital stroke | 9 (5) | 1 (1) | 0.005 |
| In‐hospital mortality | 4 (2) | 0 | 0.005 |
| In‐hospital atrial fibrillation | 86 (50) | 52 (30) | <0.001 |
| Persistent postoperative atrial fibrillation | 49 (29) | 24 (14) | <0.001 |
| Permanent pacemaker | 18 (11) | 8 (5) | 0.005 |
| Internal defibrillator | 1 (1) | 1 (1) | 0.504 |
| Left ventricular ejection fraction, % | 52±9 | 53±5 | 0.114 |
| Recurrent admission following initial discharge within 3 mo | 37 (22) | 14 (8) | <0.001 |
Values are expressed as number (percentage) or mean±SD.
XRT indicates prior chest radiotherapy exposure.
During a mean follow‐up of 5.7±3 years, there were 95 (28%) deaths in the total cohort, with a significantly higher proportion of events in the XRT group versus the comparison group (83 of 172 [48%] versus 12 of 172 [7%], P<0.001). Kaplan–Meier curves comparing the mortality between the XRT and comparison groups are shown in Figure 1 (Log‐rank statistic 89, P<0.001). Within the XRT group, only 9 patients died due to a documented noncardiac etiology (1 due to recurrent cancer, 1 due to septic shock, and 7 due to multiorgan failure). The remainder had a cardiorespiratory or unknown etiology (specifically, none of these patients had a documented noncardiac disorder, including malignancy, recognized before death that would be deemed as a cause of death). No patient in the comparison group had a noncardiac etiology to account for death.
Figure 1.
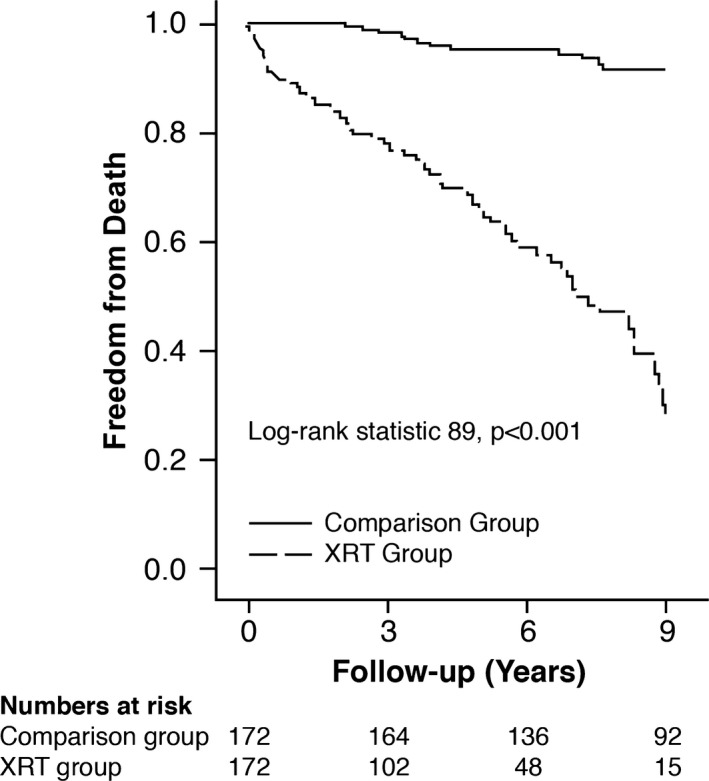
Kaplan–Meier survival curves of the entire study cohort separated into 2 subgroups: mediastinal radiotherapy (XRT group) vs the comparison group.
We performed univariable Cox proportional hazard analyses in the whole study cohort to identify potential variables associated with increased mortality. The results are shown in Table 3. Incorporating relevant univariable predictors, we subsequently performed multivariable Cox proportional hazard analyses in the whole study cohort, the results of which are shown in Table 4. Prior mediastinal XRT exposure (HR, 8.51; 95% CI, 4.46–16.23) and higher STS score (HR, 1.13; 95% CI, 1.03–1.26) were the only significant factors associated with higher longer‐term mortality (both P<0.001).
Table 3.
Univariable Cox Proportional Hazards Analysis for the Primary End Point of All‐Cause Mortality
| Variable | Hazard Ratio (95% CI) | Chi‐Square | P Value |
|---|---|---|---|
| Preoperative characteristics | |||
| Prior XRT exposure | 11.00 (5.89–20.53) | 59.2 | <0.001 |
| Age (for 10‐year increase) | 1.45 (1.23–1.70) | 20.1 | <0.001 |
| Female sex | 1.34 (0.86–2.09) | 1.8 | 0.178 |
| Hypertension | 1.09 (0.70–1.70) | 0.17 | 0.682 |
| Diabetes mellitus | 1.04 (0.64–1.68) | 0.03 | 0.857 |
| Hyperlipidemia | 1.05 (0.69–1.60) | 0.05 | 0.823 |
| Obstructive CAD | 1.58 (1.32–1.89) | 22.9 | <0.001 |
| ≥50% left main or ≥70% 3‐vessel CAD | 4.10 (2.23–7.51) | 21.7 | <0.001 |
| Atrial fibrillation | 1.64 (0.64–4.23) | 1.1 | 0.291 |
| Smoking history | 1.03 (0.61–1.72) | 0.02 | 0.923 |
| Prior stroke | 1.08 (0.55–2.13) | 0.05 | 0.818 |
| Type of prior cancer (lymphoma vs other) | 1.24 (0.98–1.98) | 2.4 | 0.092 |
| Prior cardiac surgery | 3.22 (2.07–5.01) | 27.9 | <0.001 |
| Glomerular filtration rate (for every unit reduction) | 1.04 (1.01–1.07) | 4.1 | 0.033 |
| FEV1 (for every unit reduction) | 1.02 (1.01–1.03) | 11.6 | 0.005 |
| FVC (for every unit reduction) | 1.02 (1.01–1.03) | 11.2 | 0.006 |
| FEV1/FVC ratio | 1.02 (1.01–1.03) | 4.5 | 0.009 |
| STS score (for every 1% increase) | 1.22 (1.11–1.35) | 15.2 | <0.001 |
| Charlson comorbidity index | 1.23 (1.09–1.39) | 13.9 | <0.001 |
| β‐Blockers | 0.75 (0.48–1.16) | 0.17 | 0.192 |
| Statins | 0.96 (0.63–1.46) | 0.03 | 0.864 |
| Angiotensin‐converting enzyme inhibitors | 0.84 (0.52–1.35) | 0.57 | 0.448 |
| Aspirin | 0.93 (0.61–1.42) | 0.12 | 0.732 |
| LVEF (for every 10% reduction) | 1.22 (1.02–1.46) | 4.7 | 0.029 |
| Abnormal vs normal LV‐SVI | 1.68 (1.11–2.54) | 6.3 | 0.013 |
| Aortic valve area (for every 0.1 cm2 worsening) | 1.87 (0.61–3.75) | 1.3 | 0.263 |
| Operative and postoperative characteristics | |||
| Extent of cardiac surgery | |||
| Isolated AVR | Reference | ||
| AVR+CABG | 3.67 (2.08–6.49) | 21 | <0.001 |
| AVR+aorta | 2.25 (0.78–2.00) | 0.91 | 0.233 |
| Use of internal mammary artery vs not | 1.09 (0.51–4.13) | 0.14 | 0.862 |
| Cardiopulmonary bypass time | 1.12 (0.62–1.59) | 0.20 | 0.582 |
| Perioperative inotropic support vs not | 1.74 (0.84–3.18) | 1.6 | 0.189 |
| No. of units of perioperative blood transfusions | 1.07 (0.53–1.83) | 0.15 | 0.722 |
| Bioprosthetic vs mechanical AVR | 1.31 (0.74–2.32) | 0.92 | 0.341 |
| Persistent postoperative atrial fibrillation | 2.03 (1.30–3.18) | 10.2 | <0.001 |
| Permanent pacemaker | 1.66 (0.83–3.51) | 2.07 | 0.148 |
| Postoperative LVEF (for every 10% reduction) | 1.10 (0.72–1.69) | 0.19 | 0.612 |
AVR indicates aortic valve replacement; CABG, coronary artery bypass grafting; CAD, coronary artery disease; FEV1, forced expiratory volume at 1 second; FVC, forced vital capacity; LVEF, left ventricular ejection fraction; LV‐SVI, left ventricular stroke volume index; STS, Society of Thoracic Surgeons; XRT, chest radiotherapy.
Table 4.
Multivariable Cox Proportional Hazards Analysis for the Primary End Point of All‐Cause Mortality
| Variable | Hazard Ratio (CI) | Chi‐Square | P Value |
|---|---|---|---|
| Prior XRT exposure | 8.12 (4.26–15.64) | 44.7 | <0.001 |
| STS score (for every 1% increase) | 1.14 (1.03–1.26) | 7.0 | 0.008 |
| Abnormal vs normal LV‐SVI | 1.33 (0.86–2.04) | 1.72 | 0.19 |
| FEV1 (for every unit decrease) | 1.01 (0.99–1.03) | 1.2 | 0.28 |
| Extent of cardiac surgery | |||
| Isolated AVR | Reference | ||
| AVR+CABG | 1.71 (0.94–3.11) | 3.2 | 0.07 |
| AVR+aorta | 1.13 (0.70–1.84) | 0.19 | 0.65 |
| Persistent postoperative atrial fibrillation | 1.22 (0.76–1.95) | 0.70 | 0.40 |
| Chi‐square for the overall model was 110 (P<0.001) | |||
The model does not account for matching. Please see text for results from the matched model. Predictors that constitute the Society of Thoracic Surgeons (STS) score were not individually entered into the analysis. When forced vital capacity (FVC) or forced expiratory volume at 1 second (FEV1)/FVC ratio were entered instead of FEV1, the results were similar. Due to collinearity between the Charlson comorbidity index and STS score, only the STS score was entered. Results were similar if the Charlson comorbidity index was entered instead of the STS score. AVR indicates aortic valve replacement; CABG, coronary artery bypass grafting; LV‐SVI, left ventricular stroke volume index; XRT, chest radiotherapy.
Differences in mortality were further compared among subgroups. As shown in Table 5, within all subgroups, the proportion of deaths were significantly higher in the XRT versus the comparison group. Specifically, 82% of the patients undergoing redo cardiac surgery in the XRT group died during long‐term follow‐up. In addition, the mortality in the XRT group was significantly higher than the comparison group, even when the patients were further stratified based on STS score higher or lower than a median of 4%. The Kaplan–Meier curves of the entire study cohort, separated into 4 subgroups (XRT versus comparison and STS score higher or lower than median) are shown in Figure 2 (log‐rank statistic 95, P<0.001).
Table 5.
Long‐Term All‐Cause Mortality Stratified by Prior XRT Exposure, Separated by Relevant Subgroups
| Variable | XRT Group (n=172) | Comparison Group (n=172) |
|---|---|---|
| Age, median 65 y | ||
| <65 y | 34/92 (37) | 0/83 |
| ≥65 y | 49/80 (61) | 12/89 (14) |
| Sex | ||
| Male | 28/66 (42) | 2/66 (3) |
| Female | 35/106 (33) | 10/106 (9) |
| Obstructive coronary artery disease | ||
| Yes | 57/127 (45) | 7/125 (6) |
| No | 26/45 (58) | 5/47 (11) |
| Left ventricular stroke volume index | ||
| <35 mL/m2 | 46/89 (52) | 6/61 (10) |
| ≥35 mL/m2 | 37/83 (45) | 6/111 (5) |
| Society of Thoracic Surgeons score, median 4% | ||
| <4% | 30/78 (39) | 0/91 |
| ≥4% | 53/94 (56) | 12/81 (15) |
| Surgery | ||
| AVR | 10/40 (25) | 7/94 (7) |
| AVR+CABG | 26/45 (58) | 5/47 (11) |
| AVR+aorta | 47/87 (54) | 0/91 |
| Redo cardiac surgery | ||
| No | 54/138 (39) | 9/143 (6) |
| Yes | 28/34 (82) | 3/29 (10) |
| AVR type | ||
| Bioprosthetic | 71/127 (52) | 12/157 (8) |
| Mechanical | 12/35 (34) | 0/15 |
| Permanent postoperative atrial fibrillation | ||
| No | 55/123 (45) | 10/148 (7) |
| Yes | 28/49 (57) | 2/24 (8) |
All differences between subgroups were significant (P<0.001), derived from univariable Cox proportional hazard analysis, shown in Table 3. Values are expressed as number (percentage) AVR indicates aortic valve replacement; CABG, coronary artery bypass grafting; XRT, chest radiotherapy.
Figure 2.
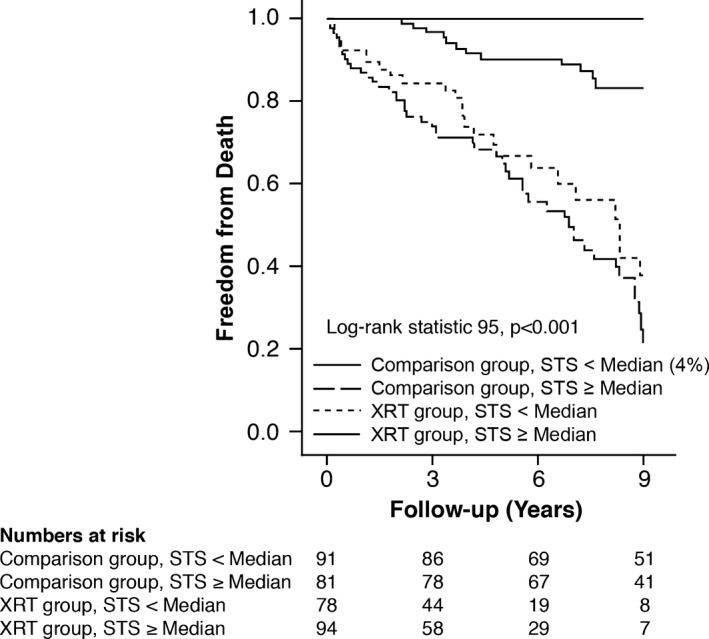
Kaplan–Meier survival curves of the entire study cohort separated into 4 subgroups, based on mediastinal radiotherapy (XRT) vs not and Society of Thoracic Surgeons (STS) score better or worse than median.
To account for matching status, a multivariable conditional Cox proportional hazards model was also built stratified by matched group. This model was adjusted for mediastinal XRT exposure, STS score, type of cardiac surgery, permanent postoperative atrial fibrillation, and forced expiratory volume at 1 second. Again, prior XRT exposure (HR, 4.21; 95% CI, 2.24–7.85) and higher STS score (HR, 1.07; 95% CI, 1.01–1.17) had a significant association with all‐cause mortality (both P<0.01).
Finally, in order to remove the potentially incremental deleterious impact of obstructive CAD on long‐term survival, we excluded patients who underwent concurrent CABG. In that subgroup (n=252, 64 deaths, follow‐up 6±3 years), multivariable Cox survival analysis also demonstrated that higher STS score (HR, 1.12; 95% CI, 1.02–1.33) and prior mediastinal XRT exposure (HR, 9.94; 95% CI, 4.84–22.3) were associated with increased long‐term mortality (P<0.01). A significantly higher proportion (P<0.001) of patients in the XRT group died as compared with the comparison group: 57 of 125 (46%) versus 7 of 125 (6%). The Kaplan–Meier survival curves for this subgroup of patients are shown in Figure 3 (log‐rank statistic 61, P<0.001).
Figure 3.
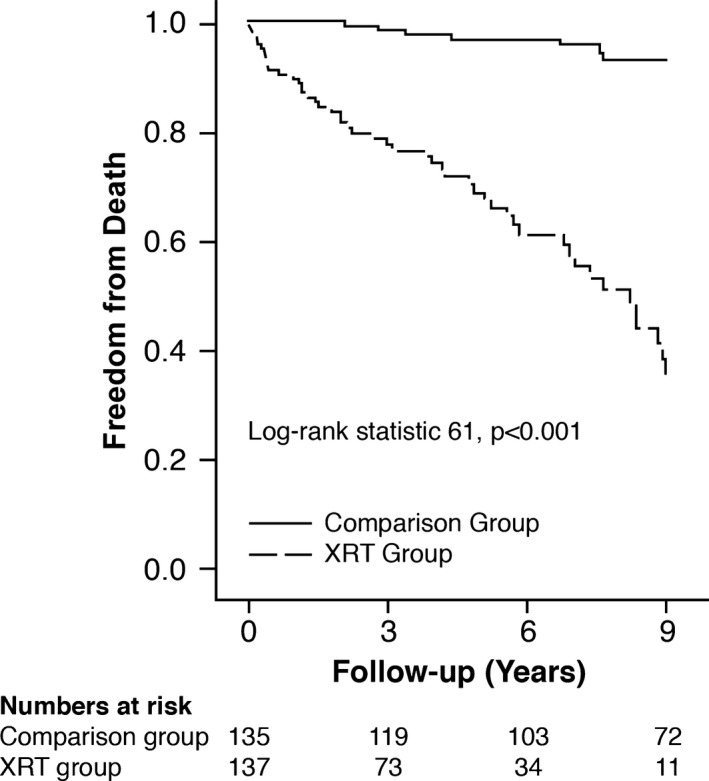
Kaplan–Meier survival curves of the subgroup without obstructive coronary artery disease separated into 2 subgroups: mediastinal radiotherapy (XRT group) vs comparison group.
Discussion
In the current study of patients with symptomatic severe AS undergoing SAVR, we demonstrate that patients with mediastinal radiation–associated severe AS (XRT group) have significantly worse longer‐term survival versus an age‐ and sex‐matched comparison group undergoing similar procedures around the same time frame. The majority of the deaths in the XRT group were due to cardiopulmonary disease or multiorgan failure, rather than recurrent malignancies. There were multiple factors associated with higher longer‐term mortality on univariable analysis, including abnormal LV‐SVI (suggesting a higher proportion of impaired contractile reserve) and abnormal findings on pulmonary function tests. However, on multivariable survival analysis, higher STS score and prior mediastinal XRT exposure were the only 2 factors independently associated with increased longer‐term mortality. In patients with radiation heart disease, standard risk stratification fares poorly for longer‐term outcomes and additional specific factors need to be considered. In addition, the finding of poor survival in the XRT group was similar when patients with obstructive CAD were excluded from the analysis (Figure 3).
Although long‐term survival was dramatically worse in the radiation group, there was no such difference in short‐term survival (although the current study may be underpowered to make conclusive assertions about short‐term survival). The perioperative stroke rate was significantly higher in the XRT versus the comparison group, likely due to a higher rate of significant ascending aortic calcification. As expected, immediately following SAVR, patients in the XRT group had significantly greater need for transfusion and inotropic support, along with longer intensive care unit and in‐hospital stay versus the comparison group. However, in spite of a median STS score of 4%, the in‐hospital mortality in the XRT group was only 2.3% (and 0% in the comparison group). However, XRT patients had a higher rate of recurrent admissions following perioperative discharge, due to recurrent heart failure and pleural effusions and a higher rate of permanent atrial fibrillation.
Findings from studies of long‐term outcomes (with or without cardiac surgery) in radiation heart disease patients are limited but do in fact demonstrate increased morbidity and mortality.1, 2, 3, 4, 5, 20, 21, 22 Previous surgical reports have demonstrated various predictors of short‐term (constrictive pericarditis, reduced preoperative ejection fraction, longer cardiopulmonary bypass times) and long‐term outcomes (radiation dose, duration of radiation, tangential versus mediastinal).4, 20, 22, 23 However, many of these studies had significantly smaller sample sizes and patients with complex surgeries (eg, CABG plus valve procedures) were excluded. We previously demonstrated that prior XRT exposure was an independent risk factor for increased long‐term mortality in patients undergoing cardiac surgery, including isolated CABG as well as combined CABG and valvular surgery.7 We have also reported that certain features may be potential predictors of adverse outcomes in patients with radiation‐associated cardiac disease, including abnormally thickened aortomitral curtain, reduced left ventricular global longitudinal strain, and worse degree of pulmonary fibrosis.7, 24, 25, 26
To the best of our knowledge, the current study is one of the largest to assess long‐term survival in patients with XRT‐associated severe AS undergoing SAVR and to compare them with a matched population. Although our data on cause of death after surgery in the radiation group are limited, it appears that cardiopulmonary disease was a common mechanism of death. This is likely because of the possibility that prior chest (especially mediastinal) XRT exposure introduces multiple technical problems at the time of surgery due to radiation‐induced fibrosis of surrounding tissues, adhesions, and the presence of multiple cardiac lesions.24 Radiation patients frequently develop pulmonary complications as a result of open heart surgery, not the least of which are recurrent pleural effusions and severe restrictive lung disease. It is our experience that respiratory complications may significantly compromise function and survival in patients with extensive prior radiation. In addition, the presence of myocardial disease either as a result of the underlying cardiac condition (potentially exacerbated by prior concomitant chemotherapy) or as a consequence of a restrictive‐type cardiomyopathy produced by the effects of radiation may play a role in impaired survival and is not necessarily improved by valvular surgery. Indeed, abnormal LV‐SVI was more frequently observed in the XRT group, despite similar left ventricular ejection fraction (likely suggesting more intrinsic myocardial disease).
The results of the current study raise the following question: Could patients who develop severe AS in the setting of a prior history of mediastinal XRT be best served by newer nonsurgical therapies such as transcatheter AVR? While promising trends in short‐ and intermediate‐term survival with transcatheter AVR have been noted in nonradiation cohorts at intermediate and high surgical risk,27, 28, 29 robust data on long‐term outcomes in patients with heart disease after radiation who have undergone transcatheter AVR are currently lacking. Indeed, an automatic assumption that a percutaneous approach in patients with heart disease after radiation would be better needs to be made with caution. In a recent report, we have demonstrated that patients who develop obstructive CAD due to prior XRT exposure and subsequently undergo percutaneous coronary intervention had high mortality compared with a matched control population.19 Another therapeutic consideration is heart transplantation or heart‐lung transplantation in selected patients with evidence of significant myocardial disease that appears disproportionate to the coronary or valvular issues or in whom there is significant restrictive lung disease. However, heart or heart‐lung transplantation in this group has significant associated concerns. Recurrent malignancy may be more common after graft‐versus‐host chemotherapy in these patients. In addition, the effect of radiation on the pulmonary vasculature and thoracic volume may render lung transplantation difficult or even impossible.
Strengths and Limitations
The present study had the following strengths and limitations. It was a large observational retrospective study conducted at a tertiary care referral center, which may have lent some degree of referral bias. The current control group represents a fraction of all SAVRs performed in our institution during the same time frame. While we attempted a thorough matching process, it is not possible to control for a history, occult presence, or severity of the specific malignancies, and, as such, it may be difficult to separate the effect of radiotherapy from the effect of malignancy. However, the majority of malignancies occurred decades ago and none of the patients had any evidence of a recurrent malignancy at the time of their SAVR. In addition, we were not able to match for all baseline comorbidities, which might have accounted for some differences in outcomes. However, the STS score was similar in both groups. The results of the current study should not be generalized to patients receiving localized radiation (eg, isolated peripheral lung/breast lesions) using modern radiation oncology protocols, who may have better outcomes following open surgical repair than the current population. Precise data on the total radiation dose, volume of the heart irradiated, and chemotherapy regimen, which influences outcomes in patients with radiation‐associated AS, was lacking, as most of the patients had these therapies years (and in some cases decades) before development and recognition of severe AS. We did not report frailty index30 in the current study as the various components of this index were not uniformly recorded at the time these patients were evaluated. However, all patients were deemed appropriate to undergo cardiac surgery after a thorough clinical evaluation by cardiology and cardiothoracic surgery. As a surrogate for frailty, we did report Charlson comorbidity index.9 A previous report demonstrated a strong association between these 2 indices in older hospitalized patients.31 Low‐dose dobutamine echocardiography to assess contractile reserve was not uniformly performed in the earlier part of this study, and hence the results are not reported. We chose all‐cause mortality because it is considered to be more objective than cardiac mortality.32 Data on cancer recurrence during follow‐up (at the site of radiation or a remote site) were not uniformly available.
Conclusions
In patients with symptomatic severe AS undergoing SAVR, we demonstrate that patients with radiation‐associated severe AS have significantly worse longer‐term survival rates versus an age‐ and sex‐matched comparison group undergoing similar procedures. Mediastinal XRT exposure was associated with worse longer‐term survival across all major subgroups separated based on median age, sex, normal versus abnormal LV‐SVI, median STS score, CAD, or redo cardiac surgery status. The current data require additional validation and raise the question of the possibility of considering alternative management strategies in this population.
Disclosures
No external funding was used for this study. Dr Desai is supported in part by the Haslam Family Endowed Chair in Cardiovascular Medicine. Dr Desai is a consultant for Myokardia, Inc. Dr Roselli is a consultant for Medtronic, Sorin, and St. Jude Medical. Dr Johnston is a consultant for Edwards, St. Jude Medical, KEF, and IVHR. The other authors have no financial conflicts of interest.
(J Am Heart Assoc. 2017;6:e005396 DOI:10.1161/JAHA.116.005396.)28476874
References
- 1. Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61:2319–2328. [DOI] [PubMed] [Google Scholar]
- 2. Mousavi N, Nohria A. Radiation‐induced cardiovascular disease. Curr Treat Options Cardiovasc Med. 2013;15:507–517. [DOI] [PubMed] [Google Scholar]
- 3. Bouillon K, Haddy N, Delaloge S, Garbay JR, Garsi JP, Brindel P, Mousannif A, Le MG, Labbe M, Arriagada R, Jougla E, Chavaudra J, Diallo I, Rubino C, de Vathaire F. Long‐term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57:445–452. [DOI] [PubMed] [Google Scholar]
- 4. Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 5. van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM, Kok WE, Aleman BM, van Leeuwen FE. Cardiovascular disease after Hodgkin lymphoma treatment: 40‐year disease risk. JAMA Intern Med. 2015;175:1007–1017. [DOI] [PubMed] [Google Scholar]
- 6. Tamura A, Takahara Y, Mogi K, Katsumata M. Radiation‐induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg. 2007;55:53–56. [DOI] [PubMed] [Google Scholar]
- 7. Wu W, Masri A, Popovic ZB, Smedira NG, Lytle BW, Marwick TH, Griffin BP, Desai MY. Long‐term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–1485. [DOI] [PubMed] [Google Scholar]
- 8. Parikh R, Goodman AL, Barr T, Sabik JF, Svensson LG, Rodriguez LL, Lytle BW, Grimm RA, Griffin BP, Desai MY. Outcomes of surgical aortic valve replacement for severe aortic stenosis: incorporation of left ventricular systolic function and stroke volume index. J Thorac Cardiovasc Surg. 2015;149:1558–1566.e1551. [DOI] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 11. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101‐102. [DOI] [PubMed] [Google Scholar]
- 12. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–688. [DOI] [PubMed] [Google Scholar]
- 13. Minners J, Allgeier M, Gohlke‐Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. [DOI] [PubMed] [Google Scholar]
- 14. Pibarot P, Dumesnil JG. Low‐flow, low‐gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 15. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamdar AR, Meadows TA, Roselli EE, Gorodeski EZ, Curtin RJ, Sabik JF, Schoenhagen P, White RD, Lytle BW, Flamm SD, Desai MY. Multidetector computed tomographic angiography in planning of reoperative cardiothoracic surgery. Ann Thorac Surg. 2008;85:1239–1245. [DOI] [PubMed] [Google Scholar]
- 17. Roselli EE, Pettersson GB, Blackstone EH, Brizzio ME, Houghtaling PL, Hauck R, Burke JM, Lytle BW. Adverse events during reoperative cardiac surgery: frequency, characterization, and rescue. J Thorac Cardiovasc Surg. 2008;135:316–323, 323.e311‐316. [DOI] [PubMed] [Google Scholar]
- 18. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL; American College of Cardiology, American Heart Association . 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (writing committee to develop cardiovascular endpoints data standards). Circulation. 2015;132:302–361. [DOI] [PubMed] [Google Scholar]
- 19. Reed GW, Masri A, Griffin BP, Kapadia SR, Ellis SG, Desai MY. Long‐term mortality in patients with radiation‐associated coronary artery disease treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2016;9(6). [DOI] [PubMed] [Google Scholar]
- 20. Handa N, McGregor CG, Danielson GK, Daly RC, Dearani JA, Mullany CJ, Orszulak TA, Schaff HV, Zehr KJ, Anderson BJ, Schomberg PJ, Puga FJ. Valvular heart operation in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2001;71:1880–1884. [DOI] [PubMed] [Google Scholar]
- 21. Veeragandham RS, Goldin MD. Surgical management of radiation‐induced heart disease. Ann Thorac Surg. 1998;65:1014–1019. [DOI] [PubMed] [Google Scholar]
- 22. Handa N, McGregor CG, Danielson GK, Orszulak TA, Mullany CJ, Daly RC, Dearani JA, Anderson BJ, Puga FJ. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg. 1999;117:1136–1142. [DOI] [PubMed] [Google Scholar]
- 23. Chang AS, Smedira NG, Chang CL, Benavides MM, Myhre U, Feng J, Blackstone EH, Lytle BW. Cardiac surgery after mediastinal radiation: extent of exposure influences outcome. J Thorac Cardiovasc Surg. 2007;133:404–413. [DOI] [PubMed] [Google Scholar]
- 24. Desai MY, Karunakaravel K, Wu W, Agarwal S, Smedira NG, Lytle BW, Griffin BP. Pulmonary fibrosis on multidetector computed tomography and mortality in patients with radiation‐associated cardiac disease undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2014;148:475–481.e473. [DOI] [PubMed] [Google Scholar]
- 25. Desai MY, Wu W, Masri A, Popovic ZB, Agarwal S, Smedira NG, Lytle BW, Griffin BP. Increased aorto‐mitral curtain thickness independently predicts mortality in patients with radiation‐associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg. 2014;97:1348–1355. [DOI] [PubMed] [Google Scholar]
- 26. Chirakarnjanakorn S, Popovic ZB, Wu W, Masri A, Smedira NG, Lytle BW, Griffin BP, Desai MY. Impact of long‐axis function on cardiac surgical outcomes in patients with radiation‐associated heart disease. J Thorac Cardiovasc Surg. 2015;149:1643–1651. e1641–1642. [DOI] [PubMed] [Google Scholar]
- 27. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 28. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG. 5‐year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. [DOI] [PubMed] [Google Scholar]
- 29. Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD, Williams MR, Fontana GP, Miller DC, Anderson WN, Akin JJ, Davidson MJ, Smith CR. 5‐year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. [DOI] [PubMed] [Google Scholar]
- 30. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallis SJ, Wall J, Biram RW, Romero‐Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943–949. [DOI] [PubMed] [Google Scholar]
- 32. Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–620. [DOI] [PubMed] [Google Scholar]


