Abstract
Background
Patients with unprovoked venous thromboembolism (VTE) are at an increased risk of mortality, but whether their cardiovascular risks also increase remains to be determined. We aimed to investigate the factors associated with overall mortality and major adverse cardiovascular events in patients with unprovoked VTE.
Methods and Results
We identified 2154 patients newly diagnosed with unprovoked VTE from Taiwan's National Health Insurance Database between 2000 and 2013, excluding those with reversible etiologies, underlying cancer, or autoimmune diseases. These patients with VTE were compared with an age‐, sex‐, and cardiovascular risk‐matched cohort of 4308 controls. The risk of mortality and major adverse cardiovascular events in patients with VTE was 2.23 (CI, 1.93–2.57; P<0.0001) and 1.86 (CI, 1.65–2.09; P<0.0001) times, respectively, higher than that of the conditions in controls. These events mostly occurred during the first year after the diagnosis of unprovoked VTE. Among patients with VTE, advanced age, male sex, and comorbid diabetes mellitus indicated a higher incidence of mortality and major adverse cardiovascular events. Conversely, comorbid hyperlipidemia attenuated these risks.
Conclusions
This nation‐wide cohort study revealed that patients with unprovoked VTE, particularly older males with diabetes mellitus, had an elevated risk of both mortality and cardiovascular events. Risk of mortality and major adverse cardiovascular events were highest within the first year after diagnosis and persisted during the 10 years of follow‐up.
Keywords: cardiovascular events, mortality, thrombosis
Subject Categories: Epidemiology, Thrombosis, Embolism
Introduction
Venous thromboembolism (VTE) is a common condition1, 2, 3, 4 comprising deep venous thrombosis and pulmonary embolism (PE). The long‐term survival of patients with VTE varies.5 A recent study reported an 8‐year mortality risk of 12%,6 whereas an earlier study reported a mortality risk of 50% after 8 years of follow‐up.7 Among the various etiologies of VTE, which include major surgery, previous VTE, autoimmune disease, and cancer, mortality is higher in patients with cancer.3, 8 The definition of provoked and unprovoked VTE was also released with guidance from the International Society on Thrombosis and Haemostasis.8 However, it is still unclear whether the mortality rate differs according to the VTE subtypes and number of comorbidities. In addition, studies on unprovoked VTE indicate that the exact etiologies of VTE remain mostly unknown at the time of diagnosis.4, 9, 10 It was reported that patients with unprovoked PE had a 1.4‐fold higher mortality risk compared with those with other subtypes.11 Through the occurrence of unprovoked VTE, it may imply the possibility of occult cancer whereas the routine screening fails to provide a clinically benefit of validation.9 Currently, guidelines for VTE therapy state that a 3‐month course of anticoagulants is sufficient for treating unprovoked VTE.12, 13 Nevertheless, it was reported that after a 6‐month course of anticoagulants, there remained an echogenic presence of residual thrombus in most patients, which correlated with an increased risk of thromboembolic recurrence.14 More studies are needed to determine the long‐term clinical significance of unprovoked VTE. Although venous and arterial thromboses have traditionally been considered as distinct pathophysiological entities, the 2 disorders have many features in common.15 Also, there is increasing evidence that patients with VTE may be at higher risk for adverse arterial events.16, 17 Therefore, we propose that unprovoked VTE is associated with arterial diseases, and in this study, we aim to investigate the effects of VTE on both all‐cause mortality and major adverse cardiovascular events (MACEs).
Methods
Data Source
Taiwan launched a single‐payer National Health Insurance (NHI) program on March 1, 1995. The NHI database provides medical information for most of the residents in Taiwan (coverage rate over 98% in 2009), making it one of the largest and most complete population‐based data sets worldwide. The data used in this study came from the Longitudinal Health Insurance Database 2000, a subset of the NHI database containing all claims data from 1996 to 2013 for 1 million beneficiaries randomly selected in 2000. There were no significant differences in age, sex, and healthcare costs between the sample group and all enrollees at that time. The Longitudinal Health Insurance Database 2000 provides encrypted patient identification numbers, sex, date of birth, dates of admission and discharge, the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes of diagnoses and procedures, details of prescriptions, registry in the Catastrophic Illness Patient Database, and costs covered and paid for by the NHI. The National Health Insurance Research Database has been described in detail in previous studies.18, 19 The accuracy of diagnosis of major diseases in the National Health Insurance Research Database, such as stroke and acute coronary syndrome, has been validated.19 This study was exempt from full review by the institutional review board of Chi‐Mei Hospital (CV code: 10406‐E01).
Study Design
This nation‐wide, population‐based, retrospective cohort study was conducted to determine the association between VTE diagnosis and subsequent mortality. The VTE cohort comprised patients with a diagnosis of deep venous thrombosis (ICD‐9‐CM codes 453.8) and PE (ICD‐9‐CM codes 415.1) at discharge for the first time from January 2000 to December 2013 by the same subset of the NHI database described above. These codes were considered to be reliable for a diagnosis of VTE on the basis of the clinical symptoms and those patients were regarded to be free from VTE previously. Previous validation studies showed that using ICD‐9 codes to define VTE, a positive predictive value was ≈90%.20, 21 The date of first‐time VTE diagnosis is called the index date in this study. To specifically select patients with unprovoked VTE at the time of initial enrollment, we excluded patients diagnosed with malignant cancer (ICD‐9 codes 140–208, concomitantly registered with catastrophic illness), autoimmune diseases (ICD‐9 codes 696.0, 696.1, 696.8, 710.0, 710.1, 710.2, 710.3, 710.4, and 714.0), or iatrogenic pulmonary embolism and infarction (ICD‐9 codes 415.11). Notably, because the issue of catastrophic illness is associated with the payment of the NHI, the coding of cancer and autoimmune diseases are validated to achieve a high accuracy.19 We also excluded patients who were pregnant at the time of enrollment (ICD‐9 codes 671.3, 671.4, 673, and 673.2) or were immobilized anytime during the 3 months before diagnosis owing to major surgeries or systemic diseases (ICD‐9 codes 820–823, procedure code 81.51, 81.52, 81.53, and 81.54).
The control cohort comprised randomly selected patients who were not diagnosed with VTE any time before their assigned index date (2 control patients for every VTE patient; n=4308). The same exclusion criteria mentioned above was also applied in controls. Also, controls were matched to patients with VTE using the propensity score matching approach for age, sex, and chronic cardiovascular risk factors, including hypertension (ICD‐9‐CM codes 401–405), diabetes mellitus (ICD‐9‐CM codes 250), hyperlipidemia (ICD‐9‐CM codes 272), coronary artery disease (ICD‐9‐CM codes 410–414), stroke (ICD‐9‐CM codes 430–438), heart failure (ICD‐9 code 428), and atrial fibrillation (ICD‐9‐CM codes 427.31). Age at the time of participation in this study was separated into 5 groups: 20 to 34, 35 to 49, 50 to 64, 65 to 74, and over 75 years. Propensity scores were estimated using the multivariable logistic regression model with the above potential risk factors as dependent variables among patients received VTE treatment and those without. According to the estimated scores, patients with VTE had the similar probability compared with matched controls. The propensity score matching approach was used to reduce selection bias because it can group many confounding covariates that may be present in an observational study. The matched controls were assigned the same index date of the corresponding VTE patient.
Outcomes
The primary outcome was mortality and the secondary outcome was MACEs, which included acute coronary syndrome (ICD‐9‐CM codes 410) and stroke (ICD‐9‐CM codes 430–436). Cases ending in mortality were identified using the “in‐hospital death” code at discharge. However, many Taiwanese choose to “die at home”; therefore, the in‐hospital death code would underestimate the true mortality rate. Because enrollment in the NHI program is mandatory for everyone in Taiwan and registration must be withdrawn within 30 days after death, patients who were withdrawn from the NHI program within 30 days after discharge from the last hospitalization were presumed to have died. All patients were followed from the index date to either death, the loss of follow‐up, or December 31, 2013.
The Validation of the Accuracy of Unprovoked VTE Diagnosis
To validate the accuracy of the unprovoked VTE diagnosis, we reviewed the charts for all patients, including both in‐patients and out‐patients, with an ICD‐9‐CM diagnosis code of deep venous thrombosis (ICD‐9‐CM codes 453.8) or PE (ICD‐9‐CM codes 415.1) from 2010 to 2015 in Chi‐Mei Medical Center (Tainan, Taiwan). Our goal was to understand whether the codes had been used correctly. Also, after excluding patients with risk factors of VTE, such as malignant cancer (ICD‐9 codes 140–208), autoimmune diseases (ICD‐9 codes 696.0, 696.1, 696.8, 710.0, 710.1, 710.2, 710.3, 710.4, and 714.0), iatrogenic pulmonary embolism and infarction (ICD‐9 codes 415.11), pregnancy (ICD‐9 codes 671.3, 671.4, 673, and 673.2), or immobilization anytime during the 3 months because of major surgeries or systemic diseases (ICD‐9 codes 820–823, procedure code 81.51, 81.52, 81.53, and 81.54), we investigated the accuracy of VTE diagnosis in the remaining patients according to the guidance of International Society on Thrombosis and Haemostasis. A cardiologist reviewed all the clinic records.
Statistical Analysis
The categorical variables, such as age groups, sexes, and comorbidities, are presented at frequency with percentage. The McNemar–Bowker test for age groups and McNemar's test for sexes and comorbidities were used to compare the difference between patients with VTE and matched controls. Continuous variables are presented as mean±SD (age) or median with interquartile range (time to MACEs and death). The age difference between patients with VTE and matched controls was analyzed using the paired t test. The Cox proportional hazard regression analysis with aggregated sandwich estimator was used to calculate the adjusted hazard ratio of MACEs between the VTE patient and matched control groups. The Kaplan–Meier method was used to plot survival and MACE‐free rates with log‐rank test to analyze the differences. A 2‐tailed P value less than 0.05 was considered to be statistically significant. All analyses were performed with SAS software (version 9.4; SAS Institute Inc, Cary, NC). The Kaplan–Meier curves were plotted using STATA (version 12; Stata Corp, College Station, TX).
Results
Characteristics of the Study Population
From January 2000 to December 2013, a total of 2154 newly diagnosed patients with VTE were identified. Mean age of patients with VTE was 63.8±17.2 years and the majority were female (54.04%). Notably, some cases presented with cardiovascular risks, including diabetes mellitus (18.43%), hyperlipidemia (12.26%), coronary artery disease (13.09%), and a relatively high prevalence of hypertension (36.91%). Compared with the 4308 controls, which were age‐, sex‐, and cardiovascular risks–matched to patients with VTE, significantly higher incidences of all‐cause mortality (8.77% versus 17.36%; P<0.0001) and MACEs (15.09% versus 24.28%; P<0.0001) were observed in patients with VTE. Also, mortality often occurred during the first year after diagnosis, which was much earlier than the mortality events in the control cohort. The detailed characteristics of both cohorts are provided in Table 1.
Table 1.
Baseline Characteristics and Comorbidities in Patients With Unprovoked VTE and the Matched Control Cohort
| Characteristic | Patients With VTE (n=2154) | Patients Without VTEa (n=4308) | P Valueb |
|---|---|---|---|
| N (%) | N (%) | ||
| Age, y (mean±SD) | 63.8±17.2 | 63.5±16.4 | 0.1403 |
| 20 to 34 | 160 (7.43) | 311 (7.22) | 0.9661 |
| 35 to 49 | 331 (15.37) | 658 (15.27) | |
| 50 to 64 | 531 (24.65) | 1070 (24.84) | |
| 65 to 74 | 464 (21.54) | 938 (21.77) | |
| ≥75 | 668 (31.01) | 1331 (30.9) | |
| Sex | |||
| Female | 1164 (54.04) | 2343 (54.39) | 0.8957 |
| Male | 990 (45.96) | 1965 (45.61) | |
| Comorbidities | |||
| Hypertension | 795 (36.91) | 1608 (37.33) | 0.8976 |
| Diabetes mellitus | 397 (18.43) | 809 (18.78) | 0.3408 |
| Hyperlipidemia | 264 (12.26) | 530 (12.3) | 0.7639 |
| CAD | 282 (13.09) | 573 (13.3) | 0.5027 |
| Stroke | 111 (5.15) | 217 (5.04) | 0.9333 |
| Heart failure | 90 (4.18) | 175 (4.06) | 0.4467 |
| Af | 29 (1.35) | 61 (1.42) | 0.5314 |
Af indicates atrial fibrillation; CAD, coronary artery disease; MACE, major adverse cardiovascular events; VTE, venous thromboembolism.
Propensity score matched for sex, age group, and comorbidities.
Paired t test for continuous variable and McNemar test or McNemar–Bowker test for categorical variables.
Long‐Term Risk of Mortality
During the 10‐year follow‐up period, patients with VTE had a higher mortality risk than the controls (adjusted hazard ratio, 2.23; CI, 1.93–2.57; P<0.0001; Table 2). At 1, 3, 5, and 10 years after diagnosis of VTE, survival rates were 90.61%, 85.73%, 82.03%, and 70.41%, respectively. These survival rates were significantly lower than those of the controls after the same number of years, which were 99.08%, 95.6%, 92.7%, and 83.83%, respectively (FigureA). The difference in survival diverged as soon as in the first year after a diagnosis of VTE. Notably, the mortality rate was 18.06‐fold (CI, 8.01–40.73; P<0.0001) higher in the oldest age group (above 75 years) than that in the youngest age group (less than 34 years). Also, the mortality risk was 1.50‐fold higher in males than in females. As expected, patients with concomitant VTE and systemic diseases like diabetes mellitus and stroke presented increased incidences of mortality, whereas hyperlipidemia reduced the risk. Comorbid hypertension, coronary artery disease, heart failure, and atrial fibrillation failed to make a difference. Conversely, hyperlipidemia attenuated the risk of mortality in patients with VTE. These data show that male sex and older age increased the risk of mortality in patients with VTE. Diabetes mellitus showed a negative effect on survival, but hyperlipidemia showed a protective effect (Table 3).
Table 2.
Multivariable Analysis for All‐Cause Mortality and MACE in Patients With Unprovoked VTE and in the Matched Control Cohort
| Variables | N | All‐Cause Mortality | MACEa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Adjusted HR | 95% CI | P Value | Events | Adjusted HR | 95% CI | P Value | ||
| Non‐VTE | 4308 | 379 | 1.00 | ··· | ··· | 650 | 1.00 | ··· | ··· |
| VTE | 2154 | 374 | 2.23 | 1.93 to 2.57 | <0.0001 | 523 | 1.86 | 1.65 to 2.09 | <0.0001 |
| Age at entry, y | |||||||||
| 20 to 34 | 471 | 6 | 1.00 | ··· | ··· | 10 | 1.00 | ··· | ··· |
| 35 to 49 | 989 | 39 | 3.30 | 1.40 to 7.81 | 0.0065 | 58 | 2.87 | 1.47 to 5.63 | 0.0021 |
| 50 to 64 | 1601 | 115 | 5.61 | 2.46 to 12.78 | <0.0001 | 204 | 5.82 | 3.07 to 11.02 | <0.0001 |
| 65 to 74 | 1402 | 180 | 9.48 | 4.18 to 21.52 | <0.0001 | 316 | 10.00 | 5.29 to 18.89 | <0.0001 |
| ≥75 | 1999 | 413 | 18.06 | 8.01 to 40.73 | <0.0001 | 585 | 15.35 | 8.14 to 28.95 | <0.0001 |
| Sex | |||||||||
| Female | 3507 | 332 | 1.00 | ··· | ··· | 551 | 1.00 | ··· | ··· |
| Male | 2955 | 421 | 1.50 | 1.30 to 1.74 | <0.0001 | 622 | 1.35 | 1.20 to 1.52 | <0.0001 |
| Comorbidities | |||||||||
| Hypertension | 2403 | 368 | 1.16 | 0.99 to 1.35 | 0.0768 | 580 | 1.16 | 1.02 to 1.32 | 0.0248 |
| Diabetes mellitus | 1206 | 204 | 1.65 | 1.37 to 1.99 | <0.0001 | 323 | 1.59 | 1.37 to 1.85 | <0.0001 |
| Hyperlipidemia | 794 | 69 | 0.68 | 0.52 to 0.89 | 0.0050 | 133 | 0.84 | 0.69 to 1.02 | 0.0801 |
| CAD | 855 | 142 | 1.01 | 0.79 to 1.28 | 0.9572 | 242 | 1.07 | 0.88 to 1.31 | 0.4783 |
| Stroke | 328 | 72 | 1.30 | 1.00 to 1.69 | 0.0469 | 137 | 1.96 | 1.60 to 2.40 | <0.0001 |
| Heart failure | 265 | 62 | 2.30 | 0.74 to 7.17 | 0.1517 | 94 | 1.34 | 0.54 to 3.37 | 0.5293 |
| Af | 90 | 23 | 1.77 | 0.78 to 4.01 | 0.1690 | 33 | 1.26 | 0.64 to 2.45 | 0.5035 |
Af indicates atrial fibrillation; CAD, coronary artery disease; HR, hazard ratio; MACE, major adverse cardiovascular events; VTE, venous thromboembolism.
Propensity score matched for sex, age, and comorbidities.
Figure 1.
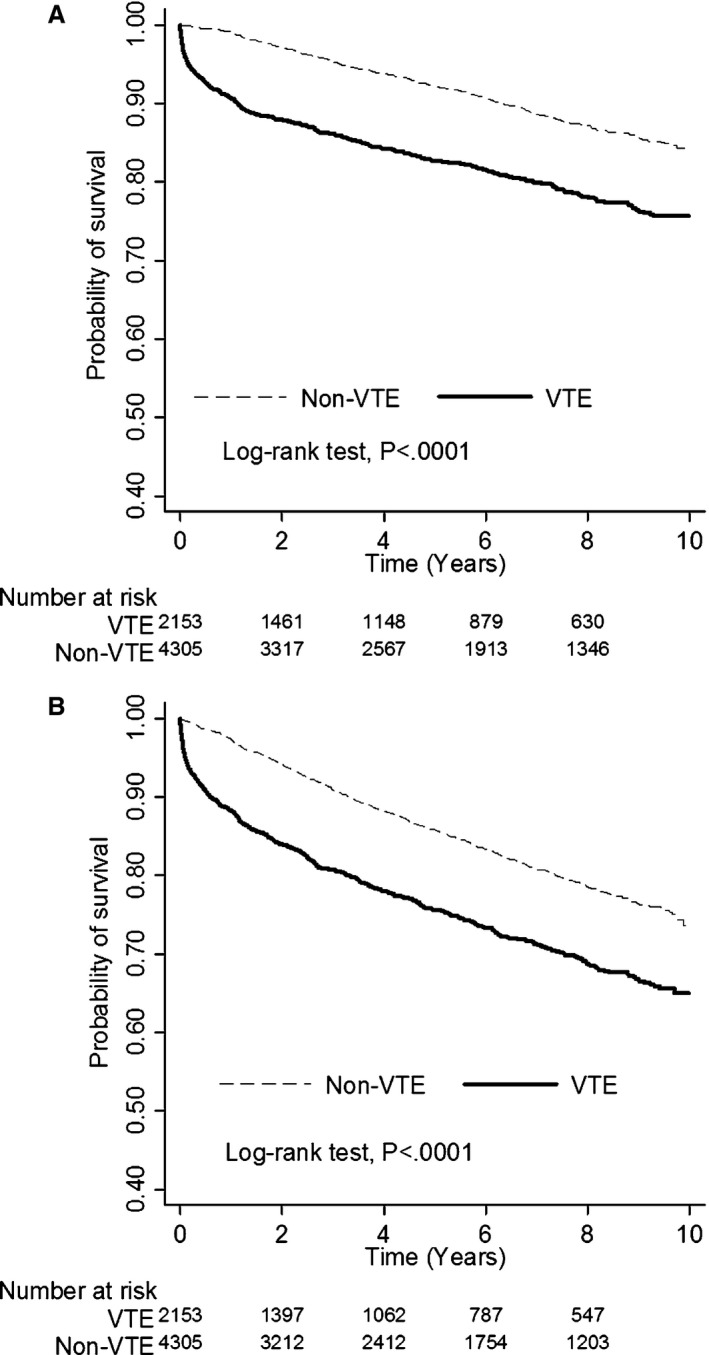
A, Differences of all‐cause mortality between patients with and without venous thromboembolism (VTE). B, Differences of major adverse cardiovascular diseases between patients with and without VTE. In the VTE group, median time to death was 1.0 year (interquartile range, 0.1–3.6), and median time to MACE was 1.2 years (interquartile range, 0.2–3.7). In the non‐VTE group, median time to death was 3.5 years (interquartile range, 1.8–6.0), and median time to MACE were 3.0 years (interquartile range, 1.5–5.4).
Table 3.
Multivariable Analysis for All‐Cause Mortality and MACE Among Patients With Unprovoked VTE
| Variables | N | All‐Cause Mortality | MACE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Adjusted HR | 95% CI | P Value | Events | Adjusted HRa | 95% CI | P Value | ||
| Age at entry, y | |||||||||
| 20 to 34 | 160 | 5 | 1.00 | ··· | ··· | 9 | 1.00 | ··· | ··· |
| 35 to 49 | 331 | 34 | 3.67 | 1.43 to 9.42 | 0.0070 | 44 | 2.59 | 1.26 to 5.35 | 0.0099 |
| 50 to 64 | 531 | 65 | 4.16 | 1.66 to 10.42 | 0.0024 | 93 | 3.26 | 1.63 to 6.54 | 0.0009 |
| 65 to 74 | 464 | 86 | 6.18 | 2.47 to 15.44 | <0.0001 | 128 | 5.13 | 2.57 to 10.23 | <0.0001 |
| ≥75 | 668 | 184 | 10.56 | 4.28 to 26.04 | <0.0001 | 249 | 8.21 | 4.14 to 16.28 | <0.0001 |
| Sex | |||||||||
| Female | 1164 | 160 | 1.00 | ··· | ··· | 245 | 1.00 | ··· | ··· |
| Male | 990 | 214 | 1.60 | 1.30 to 1.96 | <0.0001 | 278 | 1.35 | 1.14 to 1.61 | 0.0007 |
| Comorbidities | |||||||||
| Hypertension | 795 | 173 | 1.19 | 0.95 to 1.50 | 0.1291 | 237 | 1.13 | 0.93 to 1.36 | 0.2228 |
| Diabetes mellitus | 397 | 97 | 1.59 | 1.25 to 2.04 | 0.0002 | 135 | 1.62 | 1.31 to 2.00 | <0.0001 |
| Hyperlipidemia | 264 | 34 | 0.66 | 0.46 to 0.95 | 0.0270 | 55 | 0.74 | 0.55 to 0.99 | 0.0410 |
| CAD | 282 | 64 | 1.01 | 0.77 to 1.33 | 0.9438 | 94 | 1.10 | 0.87 to 1.40 | 0.4086 |
| Stroke | 111 | 25 | 0.90 | 0.60 to 1.36 | 0.6220 | 43 | 1.24 | 0.89 to 1.73 | 0.1968 |
| Heart failure | 90 | 22 | 1.00 | 0.62 to 1.62 | 0.9874 | 36 | 1.26 | 0.85 to 1.88 | 0.2504 |
| Af | 29 | 8 | 1.16 | 0.57 to 2.36 | 0.6789 | 11 | 1.03 | 0.54 to 1.96 | 0.9339 |
Af indicates atrial fibrillation; CAD, coronary artery disease; HR, hazard ratio; MACE, major adverse cardiovascular events; VTE, venous thromboembolism.
Propensity score matched for sex, age, and comorbidities.
Long‐Term Risk of MACE
Compared with the control cohort, patients with VTE, particularly males, suffered from a 1.86‐fold risk of MACEs (CI, 1.65–2.09; P<0.0001; Table 2). The risk continuously elevated with age and was up to 15.35‐fold higher (CI, 8.14–28.95; P<0.0001) in patients over 75 years of age. At 1, 3, 5, and 10 years, the free‐from‐MACE rates were 88.17%, 80.55%, 75.25%, and 60.93%, respectively, which were significantly decreased compared with the rates 97.45%, 91.30%, 86.42%, and 73.78%, respectively, that were observed in the control group (FigureB). Risk of MACEs was significantly higher during the first year after VTE diagnosis and continuously increased over time. Also, comorbid diabetes mellitus, hypertension, or stroke increased the risk of MACEs. Similarly, among patients with VTE, male sex and advanced age increased the risk of MACEs. Comorbid diabetes mellitus exacerbated, but hyperlipidemia reduced the risk of MACEs (Table 3).
The Validation of the Accuracy of Unprovoked VTE Diagnosis Based on the ICD‐9‐CM Coding
From 2010 to 2015, a total of 188 patients were diagnosed as unprovoked VTE in Chi‐Mei Medical Center. All of the VTE diagnoses were correct whereas 12 of them should be defined as provoked VTE, including 6 patients with cancer, 3 with autoimmune disease, and 3 experiencing immobilization in the previous 3 months (Table 4). Generally, the accuracy of ICD‐9‐CM derived unprovoked VTE diagnosis was up to 93.6%.
Table 4.
ICD‐9 Codes Vs. Chart Review Diagnosis Among Patients With Unprovoked VTE
| Code | No. | Clinical Diagnosis | No. |
|---|---|---|---|
| Unprovoked VTE (ICD‐9‐CM codes 453.8 or 415.1) | 188 | Unprovoked VTE | 176 |
| Provoked VTE (cancer:6 auto‐immune:3 immobilization:3) | 12 | ||
| Not VTE | 0 |
ICD‐9‐CM indicates International Classification of Diseases, Ninth Revision, Clinical Modification; VTE, venous thromboembolism.
Discussion
The following are the main findings of the present study: (1) The rates of mortality and MACE significantly increased in patients with unprovoked VTE compared with that in the matched controls; (2) mortality and MACEs occurred most frequently during the first year after the diagnosis of VTE; and (3) advanced age, male sex, and accompanying cardiovascular risks, except for hyperlipidemia, increased the risks of mortality and MACEs in patients with unprovoked VTE. To the best of our knowledge, this is the first large‐scale, nation‐wide, population‐based study with long‐term follow‐up, comprehensively describing the risks of unprovoked VTE in Asia.
In the past, VTE was regarded as a rare disease in Asia.22, 23 However, emerging evidences from India, Thailand, and Singapore indicated that VTE is no longer uncommon.24, 25, 26 Although the incidence of VTE in Asian populations remains lower than that in the Western populations, the rates of recurrence and mortality are increasing.22 One cohort study in Taiwan revealed a 1‐month mortality rate of 8.8% in patients diagnosed with VTE.22 Cancer, advanced age, longer hospital stays, and recent major surgeries preceding a diagnosis of VTE were independent predictors of mortality.20 Given the heterogeneous etiologies of VTE resulting in varied survival rates, the key determinants of survival are mainly associated with the underlying diseases.6 For example, mortality was markedly higher among patients with cancer than that in those without, but the mortality in those without cancer was still twice that in the controls.5 In addition, patients with VTE and an active malignancy or unprovoked PE had a worse prognosis than those with other subtypes of PE.6 Nevertheless, the effect of unprovoked VTE on overall survival remains unknown. In this study, we excluded most patients with a reversible etiology, autoimmune disease, or malignancy and specifically focused on the population with unprovoked VTE. We found a noteworthy 2.23‐fold risk of all‐cause mortality in the patients with unprovoked VTE. Survival significantly dropped during the first year after diagnosis and consistently decreased over time. Therefore, the potential treats of unprovoked VTE should be highly evaluated.
Besides mortality, patients with VTE had a 1.86‐fold higher risk of MACEs than controls. VTE may be regarded as part of the cardiovascular disease continuum and shared common risk factors, including cigarette smoking, obesity, hypertension, and diabetes mellitus, with arterial disease.1, 15 Also, the acceleration of atherosclerosis caused by VTE‐derived inflammatory processes is considered to be another hypothesis.27 It has been observed that myocardial infarction and heart failure increase the risk of thromboembolism.27 Conversely, another study indicated that patients with unprovoked VTE had a 4‐fold higher risk of acute myocardial infarction.28 Patients diagnosed with their first episode of PE showed a 3% increased risk of arterial thrombosis annually.28 Likewise, Kiryu et al reported a higher risk for coronary artery disease in patients undergoing computed tomography for suspicious PE.29 All of these data demonstrate a higher risk of subsequent arterial thrombosis after the diagnosis of VTE. Nevertheless, the actual relationship between VTE and arterial disease needs further researches with controlled, clinical trials.
Among patients with VTE, we found that the mortality rate was significantly increased in the population above 75 years, particularly in males. Despite certain specific risks of VTE identified in women, including estrogen and childbearing,2, 30 studies have shown that male sex is independently associated with a late recurrence of VTE.1, 31 Also, the pulmonary embolism severity index counts male sex as one of the major risk factors.32 The role of sex in the long‐term effects of VTE requires further study. In our study, patients with concomitant VTE and systemic diseases like diabetes mellitus, stroke, heart failure, and hypertension had increased mortality and MACEs; however, those with concomitant coronary artery disease and atrial fibrillation did not. If arterial diseases and VTEs share common characteristics in their pathophysiology, it may not be surprising that classic risk factors of arterial disease were also found in high risk VTE populations.1, 15 Of note, patients with coronary artery disease and atrial fibrillation may have been taking aspirin or anticoagulants before the diagnosis of VTE, resulting in protective effects. Similarly, the lower risk of mortality and MACEs in patients with VTE with comorbid hyperlipidemia may be owing to the benefits of statins. Statin use is associated with significantly reduced odds of developing VTE (32%) and decreases the risk of recurrent PE, irrespective of vitamin K antagonist treatment.33 Statins not only aid in lowering cholesterol but also have other effects such as decreasing inflammation.34 One study reported that endothelial dysfunction is associated with increased levels of oxidative stress in the venous systems of patients with coronary artery diseases; therefore, intensive lipid lowering is suggested as a treatment after the analysis of results of recent clinical trials.35 However, further investigations into the use of statins in the prevention of VTE complications are needed.
This study has several strengths. First, it involved a large number of unprovoked patients with VTE from a nation‐wide population. By analyzing data collected over a 13‐year period from 2154 cases, this study provided adequate statistical power for the analysis of the long‐term effects of unprovoked VTE. Second, we compared data from the case population with data from a matched control cohort with no VTE diagnosis. It helps distinguish the characteristics of unprovoked VTE population in the perspectives of survivals and outcomes. Also, among patients with unprovoked VTE, the effects of sex, age, and underlying comorbidities on mortality and MACEs were revealed, whereas the impact of these factors may have been obscured in studies on cancer‐related VTE. In addition, our findings illustrate the relationship between unprovoked VTE and the development of arterial diseases. This result indicates that the potential effects of VTE are serious and use of statins may serve as a safe adjunctive pharmacological therapy in select patients with VTE.
There are several limitations to this study. First, it is possible that VTE was miscoded, but previous studies indicated an adequate reliability of ICD‐9 coding.20, 21 To overcome the inherent limitations, we verified the accuracy of the diagnosis of unprovoked VTEs by the validation method of chart reviewing. Second, the incidence of VTE seems relatively lower compared with previous reports.2, 3, 4 One major reason was that only patients with unprovoked VTE were counted and also outpatients were not enrolled. It may underestimate the total number of patients with VTE. Third, although we excluded most of the cases with recent hospitalization, major surgeries, cancer, or autoimmune diseases, patients receiving self‐paid contraceptive pills may be missing in this database. Fourth, the diversities of medical or interventional therapies for VTE may have affected the outcomes that we were investigating. Nevertheless, because of the high prevalence of NHI in Taiwan, the anticoagulant therapy achieved a high coverage in patients with VTE36 and therefore we believed most of the patients received optimal therapies. Further investigations will be required to determine the effects of various therapies on long‐term VTE outcomes. Finally, although we used the propensity score matching approach to reduce the potential selection bias, the unobserved factors in the National Health Insurance Research Database, such as smoking, drinking, body mass index, race/ethnicity, and the severity of comorbidities, may affect the results. However, because of the large sample size and small selection bias in our study, we may have enough statistical power to judge the risk of mortality and MACEs compared with other small‐sized studies.
Conclusions
Compared with the control cohort, the risks of mortality and MACEs were significantly higher in patients with unprovoked VTEs. These risks were present since the first year after diagnosis. Among patients with VTE, advanced age, male sex, and comorbid cardiovascular risks contributed to increased risk of mortality and MACEs. These results indicate that the dangers of unprovoked VTE are significant and require further investigations.
Sources of Funding
This work was supported by a research grant from the Chi‐Mei Medical Center.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005466 DOI: 10.1161/JAHA.117.005466.)28468786
References
- 1. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs J, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. [DOI] [PubMed] [Google Scholar]
- 2. Robertson L, Evans C, Fowkes FG. Epidemiology of chronic venous disease. Phlebology. 2008;23:103–111. [DOI] [PubMed] [Google Scholar]
- 3. Tran NT, Meissner MH. The epidemiology, pathophysiology, and natural history of chronic venous disease. Semin Vasc Surg. 2002;15:5–12. [PubMed] [Google Scholar]
- 4. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sogaard KK, Schmidt M, Pedersen L, Horvath‐Puho E, Sorensen HT. 30‐year mortality after venous thromboembolism: a population‐based cohort study. Circulation. 2014;130:829–836. [DOI] [PubMed] [Google Scholar]
- 6. Flinterman LE, van Hylckama Vlieg A, Cannegieter SC, Rosendaal FR. Long‐term survival in a large cohort of patients with venous thrombosis: incidence and predictors. PLoS Med. 2012;9:e1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn SR, Solymoss S, Lamping DL, Abenhaim L. Long‐term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. J Gen Intern Med. 2000;15:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing G‐J, Kyrle PA; for the Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease . Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14:1480–1483. [DOI] [PubMed] [Google Scholar]
- 9. Carrier M, Lazo‐Langner A, Shivakumar S, Tagalakis V, Zarychanski R, Solymoss S, Routhier N, Douketis J, Danovitch K, Lee AY, Le Gal G, Wells PS, Corsi DJ, Ramsay T, Coyle D, Chagnon I, Kassam Z, Tao H, Rodger MA. Screening for occult cancer in unprovoked venous thromboembolism. N Engl J Med. 2015;373:697–704. [DOI] [PubMed] [Google Scholar]
- 10. Ageno W, Dentali F, Donadini MP, Squizzato A. Optimal treatment duration of venous thrombosis. J Thromb Haemost. 2013;11(suppl 1):151–160. [DOI] [PubMed] [Google Scholar]
- 11. Kearon C. Long‐term management of patients after venous thromboembolism. Circulation. 2004;110:I10–I18. [DOI] [PubMed] [Google Scholar]
- 12. Howard LS, Hughes RJ. Nice guideline: management of venous thromboembolic diseases and role of thrombophilia testing. Thorax. 2013;68:391–393. [DOI] [PubMed] [Google Scholar]
- 13. Wang KL, Chu PH, Lee CH, Pai PY, Lin PY, Shyu KG, Chang WT, Chiu KM, Huang CL, Lee CY, Lin YH, Wang CC, Yen HW, Yin WH, Yeh HI, Chiang CE, Lin SJ, Yeh SJ. Management of venous thromboembolisms: Part I. The consensus for deep vein thrombosis. Acta Cardiol Sin. 2016;32:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Rio Sola ML, Gonzalez Fajardo JA, Vaquero Puerta C. Identifying clinical risk factors in recurrent idiopathic deep venous thrombosis. Med Clin (Barc). 2016;146:254–257. [DOI] [PubMed] [Google Scholar]
- 15. Green D. Risk of future arterial cardiovascular events in patients with idiopathic venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2009;1:259–266. [DOI] [PubMed] [Google Scholar]
- 16. Piazza G, Goldhaber SZ. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010;121:2146–2150. [DOI] [PubMed] [Google Scholar]
- 17. Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, Prins MH, Girolami A. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–1441. [DOI] [PubMed] [Google Scholar]
- 18. Cheng CL, Chien HC, Lee CH, Lin SJ, Yang YH. Validity of in‐hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol. 2015;201:96–101. [DOI] [PubMed] [Google Scholar]
- 19. Nan‐Ping Y, Yi‐Hui L, Chi‐Yu C, Jin‐Chyr H, I‐Liang Y, Nien‐Tzu C, Chien‐Lung C. Comparisons of medical utilizations and categorical diagnoses of emergency visits between the elderly with catastrophic illness certificates and those without. BMC Health Serv Res. 2013;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158:1525–1531. [DOI] [PubMed] [Google Scholar]
- 21. White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med. 2000;343:1758–1764. [DOI] [PubMed] [Google Scholar]
- 22. Lee CH, Cheng CL, Lin LJ, Tsai LM, Yang YH. Epidemiology and predictors of short‐term mortality in symptomatic venous thromboembolism. Circ J. 2011;75:1998–2004. [DOI] [PubMed] [Google Scholar]
- 23. White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(suppl 4):S11–S17. [DOI] [PubMed] [Google Scholar]
- 24. Kamerkar DR, John MJ, Desai SC, Dsilva LC, Joglekar SJ. Arrive: a retrospective registry of Indian patients with venous thromboembolism. Indian J Crit Care Med. 2016;20:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee LH. Clinical update on deep vein thrombosis in Singapore. Ann Acad Med Singapore. 2002;31:248–252. [PubMed] [Google Scholar]
- 26. Angchaisuksiri P, Atichartakarn V, Aryurachai K, Archararit N, Rachakom B, Atamasirikul K, Tiraganjana A. Risk factors of venous thromboembolism in Thai patients. Int J Hematol. 2007;86:397–402. [DOI] [PubMed] [Google Scholar]
- 27. Previtali E, Bucciarelli P, Passamonti SM, Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011;9:120–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spencer FA, Ginsberg JS, Chong A, Alter DA. The relationship between unprovoked venous thromboembolism, age, and acute myocardial infarction. J Thromb Haemost. 2008;6:1507–1513. [DOI] [PubMed] [Google Scholar]
- 29. Kiryu S, Raptopoulos V, Baptista J, Hatabu H. Increased prevalence of coronary artery calcification in patients with suspected pulmonary embolism. Acad Radiol. 2003;10:840–845. [DOI] [PubMed] [Google Scholar]
- 30. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–I16. [DOI] [PubMed] [Google Scholar]
- 31. Douketis J, Tosetto A, Marcucci M, Baglin T, Cosmi B, Cushman M, Kyrle P, Poli D, Tait RC, Iorio A. Risk of recurrence after venous thromboembolism in men and women: patient level meta‐analysis. BMJ. 2011;342:d813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan CM, Woods C, Shorr AF. The validation and reproducibility of the pulmonary embolism severity index. J Thromb Haemost. 2010;8:1509–1514. [DOI] [PubMed] [Google Scholar]
- 33. Nguyen CD, Andersson C, Jensen TB, Gjesing A, Schjerning Olsen AM, Malta Hansen C, Büller H, Torp‐Pedersen C, Gislason GH. Statin treatment and risk of recurrent venous thromboembolism: a nationwide cohort study. BMJ Open. 2013;3:e003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid in atherosclerosis. Atherosclerosis. 2015;242:357–366. [DOI] [PubMed] [Google Scholar]
- 35. Al‐Benna S, Hamilton CA, McClure JD, Rogers PN, Berg GA, Ford I, Delles C, Dominiczak AF. Low‐density lipoprotein cholesterol determines oxidative stress and endothelial dysfunction in saphenous veins from patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:218–223. [DOI] [PubMed] [Google Scholar]
- 36. Chew TW, Gau CS, Wen YW, Shen LJ, Mullins CD, Hsiao FY. Epidemiology, clinical profile and treatment patterns of venous thromboembolism in cancer patients in Taiwan: a population‐based study. BMC Cancer. 2015;15:298. [DOI] [PMC free article] [PubMed] [Google Scholar]


