Abstract
Background
An individual's perceived need to improve their physical health (PNIPH) is an essential precursor to adopting healthy behaviors. Nine potentially modifiable risk factors (PMRFs) for myocardial infarction collectively account for ≥90% of the population attributable risk. Though widely recognized, their impact on individuals’ health perceptions is unclear.
Methods and Results
Residents from 6 provinces were administered a module on changes to improve health as part of the 2011–2012 Canadian Community Health Survey, yielding relevant data for 8 of the 9 PMRFs sought. The potential effects of PMRFs individually and cumulatively on PNIPH were examined using modified Poisson regression. In total, 45 443 respondents were included, representing 11 006 123 individuals and corresponding to 96.8% of the adult population of the sampled provinces. The sum of PMRFs was positively associated with PNIPH (adjusted prevalence ratio, 1.08; 95% CI, 1.07–1.09 per additional PMRF) with 82.3% of individuals with ≥5 PMRFs reporting this perception. Smoking, obesity, and low physical activity were most strongly associated with PNIPH, whereas hypertension and diabetes mellitus exhibited no association with this outcome after adjusting for potential confounders. Barriers to adopting healthy behaviors were reported by 55.9% of individuals endorsing PNIPH.
Conclusions
The cumulative burden of PMRFs is positively associated with PNIPH; however, individual PMRFs differentially contribute to this perception. Among those at highest cardiac risk, ≈1 in 5 denied PNIPH. A better understanding of factors underlying health perceptions and behaviors is needed to capitalize on cardiovascular preventive efforts.
Keywords: epidemiology, lifestyle, myocardial infarction, prevention, risk factors
Subject Categories: Cardiovascular Disease, Lifestyle, Risk Factors, Epidemiology
Introduction
Despite decades of steady progress, ischemic heart disease remains among the leading causes of morbidity and mortality.1, 2 Much of the progress made thus far has been attributed to advances in acute interventions and secondary preventive cardiovascular therapies; however, changes in risk factor trends are estimated to account for roughly half to two thirds of the observed improvements in developed countries.3, 4, 5 Emerging increases in prevalence of certain cardiovascular risk factors (particularly obesity, diabetes mellitus, and hypertension) therefore threaten to halt or potentially reverse these public health gains.3, 6 Indeed, disproportionately slower improvements or increases in mortality from ischemic heart disease among younger demographics, particularly among women, have recently been reported, collectively underscoring the need to bolster primary preventive strategies.7, 8, 9, 10, 11
INTERHEART identified 9 potentially modifiable risk factors (PMRFs) that collectively account for ≥90% of the population attributable risk for acute myocardial infarction (MI) worldwide: smoking, history of hypertension, diabetes mellitus, abdominal obesity, psychosocial factors, daily consumption of fruits and vegetables, regular alcohol consumption, regular physical activity, and a raised apolipoprotein (Apo)B/ApoA1 ratio. These PMRFs are associated with MI irrespective of sex or age and exhibited a cumulative effect.12 Though they have been well described in the medical literature and are broadly well known among laypersons, their impact on individuals’ health perceptions and behaviors is less clear. Given that an individual's perceived need to change is regarded as an essential precursor to intending and committing to behavioral changes,13, 14, 15 these associations have important implications for preventive health care strategies. We therefore sought to examine the association between individual and cumulative PRMFs with overall physical health perceptions using data from the 2011–2012 Canadian Community Health Survey (CCHS). We hypothesized that PMRFs would be positively associated with a perceived need to improve physical health (PNIPH) both individually and cumulatively.
Methods
Data Source and Sample
The 2011–2012 CCHS was a cross‐sectional survey of Canadians aged 12 years or older conducted by Statistics Canada (Ottawa, Ontario, Canada). Persons living on Indian Reserves or Crown lands, full‐time members of the Canadian Forces, institutionalized persons, and persons living in the Quebec health regions of Région du Nunavik and Région des Terres‐Cries‐de‐la‐Baie‐James were excluded from the survey (collectively accounting for <3% of potential respondents). The national combined household‐ and person‐level response rate was 68.4%.16 Residents from 6 provinces were administered a survey module on changes to improve health and formed the sample of our study. All analyses were restricted to individuals aged 18 years or older. Individuals who refused or otherwise did not provide an answer to the survey question relating to the outcome of interest were also excluded.
Public Use Microdata Files were used for this study, which contain anonymized data, underwent formal review and approval by an executive committee of Statistics Canada, and were made publicly available for statistical and research purposes through the Data Liberation Initiative.16, 17 Consent was obtained from all respondents at the time of survey administration.
Variable and Outcome Definitions
Eight PMRFs were dichotomized as present or absent. Cigarette smoking was defined as current smoking or having quit smoking within the preceding 12 months. Obesity was defined by a body mass index (BMI) ≥30 kg/m2 as calculated from self‐reported height and weight. Low physical activity was defined as a reported mean total daily energy expenditure of <1.7 kcal/kg per day on transportation and leisure time activities, which corresponds to less than moderate exercise for 4 hours weekly (the criterion used in the INTERHEART study12). High stress was defined as a perceived life and/or work stress score of ≥4 on a 5‐point scale (corresponding to “quite a bit stressful” or “extremely stressful”). Hypertension was deemed present if respondents reported that they had “high blood pressure” as diagnosed by a health professional, if they were “ever diagnosed with high blood pressure” by a health professional, or if they reported having taken “any medicine for high blood pressure” in the past month. Diabetes mellitus was self‐reported as having been diagnosed by a health professional. Fruit and vegetable consumption was measured as a frequency with 5 times/day used as the cutoff.18, 19 Low or moderate alcohol intake was defined as <4 drinks/week and having consumed alcohol within the preceding 12 months.20 Abstinence from alcohol and ≥4 drinks/week were considered separately and as a combined variable, when appropriate. Apo levels or other measures of dyslipidemia were not included because only indirect data on this PMRF were obtained in only a subset of survey respondents.16
Important covariates (potential confounders or effect modifiers of the association of interest) selected a priori included age, sex, marital status, culture or racial origin, highest level of education achieved, total yearly household income, and having a regular medical doctor. Age was categorized into 3 groups: 18 to 39, 40 to 59, and ≥60 years. Marital status was classified as single, never married; married or in a common‐law relationship; or widowed, separated, or divorced. Culture or racial origin was categorized as white or visible minority. Highest level of education was grouped into 3 levels: less than secondary school graduation; secondary school graduation without postsecondary education; and some postsecondary education (with or without program completion). Total yearly household income was divided into 3 levels: <$40 000, $40 000 to $79 999, and ≥$80 000 (Canadian dollars). Canadian dollars were comparable to US dollars during the 2011–2012 period.
Data on smoking were missing in 0.7%, BMI in 3.4%, physical activity in 0.7%, stress in 1.2%, hypertension in <0.1%, diabetes mellitus in 0.1%, fruit and vegetable consumption in 5.7%, alcohol intake in 1.7%, marital status in 0.2%, culture or racial origin in 3.1%, highest education level achieved in 3.4%, household income in <0.1%, and having a regular medical doctor in <0.1%. Data were complete for age and sex. For all variables with ≥1% missing data, an additional category (“unknown”) was created; otherwise, respondents with missing data were excluded from analyses. The number of self‐reported PMRFs per respondent was calculated as their sum, each based on responses to relevant survey questions.
The outcome of interest was PNIPH as defined by an affirmative response to the question, “Do you think there is anything you should do to improve your physical health?”. Responses to follow‐up questions on specific behavior changes were used to confirm the relevance of affirmative responses to cardiac risk. Analyses of responses to the questions, “Is there anything stopping you from making this improvement?”, and “What is [stopping you from making this improvement]?” among individuals endorsing PNIPH were also undertaken to gain insight into barriers to behavioral change.
Statistical Analyses
The CCHS uses a complex survey design with stratification, multiple stages of selection, and unequal probability sampling,16 which was taken into consideration in point and variance estimations.21 First, relative weights were calculated by dividing survey weights by average weights for responses from all respondents. Adjusted weights were then calculated by dividing relative weights by the square root of the average design effect, which is a mean of the coefficients of variation for the study variables (provided by Statistics Canada22). The use of this approximate method of incorporating the design effect tends to yield conservative tests of significance.23
Chi‐square and t tests were used to examine associations between all variables of interest and the outcome. The associations of individual PMRFs and their sum with the outcome were assessed by modified Poisson regression using a robust error variance procedure (sandwich estimation).24 Multivariable models were fit by manual forward and backward selection using the change in the exposure‐outcome association and clinical judgment to guide covariate inclusion. Adjusted prevalence ratios for combinations of PMRFs were derived by summation of their respective model coefficients.12 Effect measure modification was assessed by interaction terms and comparison of stratified effect measures. All analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC). An alpha level of 0.05 was used to define statistical significance (2‐tailed analyses). All continuous variables are reported as mean±SD or median (interquartile range; IQR) and all categorical variables as number (%). All prevalence ratios (PRs) are provided with 95% CIs.
Sensitivity analyses were performed to evaluate the impact of missing values for variables with overall ≥1% missing data. Additionally, the data were reanalyzed using a BMI criterion of 25 kg/m2 (to include overweight status as a PMRF) and a cutoff for mean total daily energy expenditure of <1.1 kcal/kg per day on transportation and leisure time activities (≈150 min/week of moderate exercise) to define low physical activity as per the American Heart Association definitions for ideal cardiovascular health.6 Last, all prespecified analyses were repeated using data from the 2013–2014 CCHS, which sampled fewer, but different, provinces.17
Results
Respondent Characteristics and Relevance of the Outcome to Cardiac Risk
A total of 45 443 respondents were included in the analyses after excluding 992 (2.1%) from whom the outcome of interest was not provided and 834 (1.8%) because of missing values for variables with <1% total missing data (Figure 1). This sample represented 11 006 123 individuals, corresponding to 96.8% of the adult population of the 6 provinces included and 40.9% of the entire country. Respondents were from Alberta (25.6%), Manitoba (8.0%), Quebec (55.4%), Nova Scotia (6.6%), Prince Edward Island (1.0%), and Newfoundland and Labrador (3.6%). The mean number of PMRFs was 2.5±1.0 with a median of 2 (IQR, 1–3). Mean BMI was 26.1±3.7 kg/m2.
Figure 1.
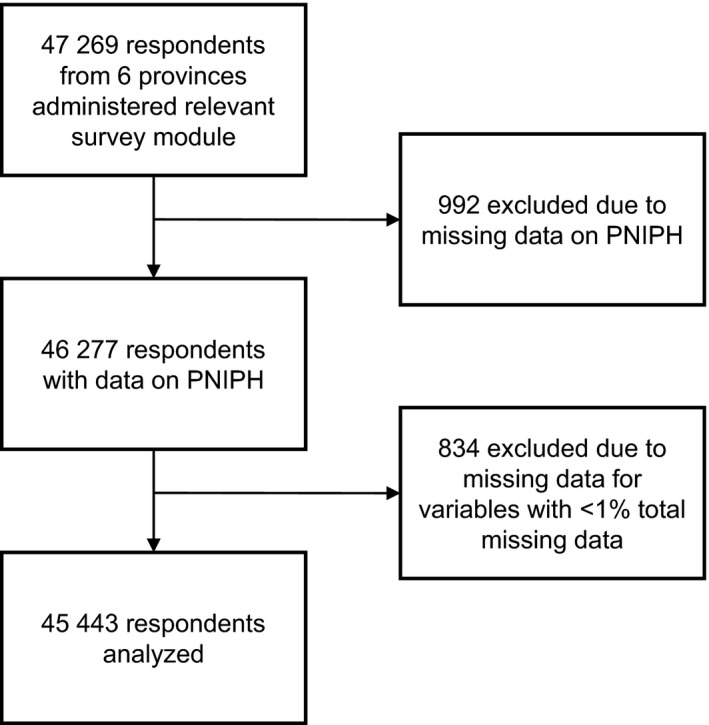
Study sample selection. PNIPH indicates perceived need to improve physical health.
Overall, 73.6% of individuals reported PNIPH, of which 81.1% reported an intention to improve their health in the upcoming year. Among individuals who endorsed PNIPH, 99.8% identified a specific behavioral change as being most important, of which increasing exercise, losing weight, improving dietary habits, and quitting/reducing smoking accounted for 90.7% (Figure 2A). These behaviors were also the most commonly reported changes planned among individuals who intended to improve their health within the year (Figure 2B).
Figure 2.
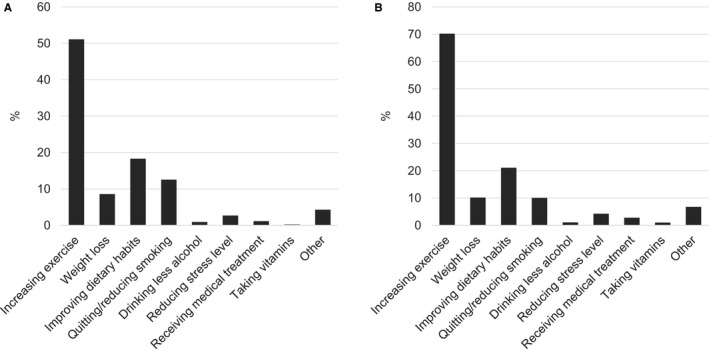
Relevance of a perceived need to improve physical health on cardiac risk. A, Most important behavioral change to improve health reported by individuals endorsing a perceived need to improve their physical health. B, Health behavior changes planned among individuals who reported intending to improve their physical health in the upcoming year.
Exploratory Analyses
Level of education was positively associated with PNIPH in unadjusted analyses. In contrast, older age; being widowed, separated, or divorced; low total yearly household income; and having a regular medical doctor were associated with a reduced likelihood of this perception. Men and women expressed this opinion in comparable proportions, as did whites and those of a visible minority. Smoking, obesity, low physical activity, high stress, and low fruit and vegetable consumption were positively associated with PNIPH; however, hypertension, diabetes mellitus, and lack of or excessive alcohol intake were not. The sum of self‐reported PMRFs was nevertheless significantly associated with PNIPH (Table 1).
Table 1.
Prevalence of PNIPH According to Respondent Demographics and PMRF
| Overall Sample | No. Reporting PNIPH | Weighted % Reporting PNIPHa | P Valueb | |
|---|---|---|---|---|
| Age, y | ||||
| 18 to 39 | 13 862 | 10 853 | 78.4 | <0.001 |
| 40 to 59 | 14 642 | 11 249 | 78.4 | |
| ≥60 | 16 939 | 9629 | 59.3 | |
| Sex | ||||
| Men | 19 933 | 13 927 | 73.6 | 0.989 |
| Women | 25 510 | 17 804 | 73.6 | |
| Marital status | ||||
| Married/common‐law | 25 311 | 18 190 | 74.4 | <0.001 |
| Widowed/separated/divorced | 9621 | 5762 | 66.0 | |
| Single, never married | 10 511 | 7779 | 75.9 | |
| Cultural or racial origin | ||||
| White | 39 685 | 27 687 | 73.9 | <0.001 |
| Visible minority | 4429 | 3274 | 74.3 | |
| Unknown | 1329 | 770 | 63.7 | |
| Education | ||||
| Less than sec. school graduate | 8801 | 5002 | 62.0 | <0.001 |
| Sec. school grad, no post‐sec | 7448 | 5314 | 72.5 | |
| Post‐sec. educationc | 27 715 | 20 580 | 77.0 | |
| Unknown | 1479 | 835 | 64.1 | |
| Annual household income | ||||
| ≤$39 999 | 16 166 | 9817 | 65.4 | <0.001 |
| $40 000 to $79 999 | 15 581 | 11 329 | 75.5 | |
| ≥$80 000 | 13 696 | 10 585 | 77.9 | |
| Having a regular medical doctor | ||||
| Yes | 37 892 | 26 094 | 73.0 | <0.001 |
| No | 7551 | 5637 | 76.1 | |
| Smokerd | ||||
| Yes | 11 220 | 8973 | 83.0 | <0.001 |
| No | 34 223 | 22 758 | 70.4 | |
| Obesitye | ||||
| Yes | 9356 | 7590 | 83.7 | <0.001 |
| No | 34 525 | 23 108 | 71.4 | |
| Unknown | 1562 | 1033 | 71.3 | |
| Physical activityf | ||||
| Low | 25 211 | 18 506 | 77.7 | <0.001 |
| High | 20 232 | 13 225 | 69.0 | |
| Stress | ||||
| High | 12 770 | 10 065 | 81.4 | <0.001 |
| Low | 32 114 | 21 325 | 69.8 | |
| Unknown | 559 | 341 | 63.9 | |
| Hypertension | ||||
| Yes | 13 713 | 9066 | 70.5 | <0.001 |
| No | 31 730 | 22 665 | 74.6 | |
| Diabetes mellitus | ||||
| Yes | 3945 | 2623 | 69.9 | <0.001 |
| No | 41 498 | 29 108 | 73.9 | |
| Fruit and vegetable consumption | ||||
| <5 times/day | 25 613 | 18 663 | 76.4 | <0.001 |
| ≥5 times/day | 17 332 | 11 652 | 70.9 | |
| Unknown | 2498 | 1416 | 62.2 | |
| Alcohol intake | ||||
| Abstinent | 8840 | 5236 | 63.8 | <0.001 |
| Excessive (≥4 drinks/week) | 5889 | 4200 | 75.9 | |
| Low/mod. (<4 drinks/week) | 30 049 | 21 912 | 75.8 | |
| Unknown | 665 | 383 | 63.3 | |
| No. of PMRFs | ||||
| 0 | 2706 | 1514 | 59.1 | <0.001 |
| 1 | 8081 | 5131 | 66.8 | |
| 2 | 12 068 | 8249 | 72.9 | |
| 3 | 11 327 | 8230 | 76.7 | |
| 4 | 7185 | 5431 | 80.6 | |
| ≥5 | 4076 | 3176 | 82.3 | |
| No. of PMRFs | ||||
| <3 | 22 855 | 14 894 | 69.1 | <0.001 |
| ≥3 | 22 588 | 16 837 | 78.8 | |
PMRF indicates potentially modifiable cardiac risk factor; PNIPH, perceived need to improve physical health; sec., secondary.
Weighted to the general population.
Chi‐square test of independence between variable and PNIPH.
With or without obtaining a postsecondary certificate/diploma or university degree.
Defined as current smoker or having quit smoking within the preceding 12 months.
Defined as body mass index ≥30 kg/m2.
Reported mean total daily energy expenditure <1.7 kcal/kg per day on transportation and leisure time activities (approximating <4 hours of moderate exercise/week).
Associations Between Individual and Cumulative PMRFs and PNIPH
Modified Poisson regression models for both individual and the sum number of PMRFs were fit. Multivariable models including all 8 PMRFs identified smoking, obesity, and low physical activity as most strongly associated with PNIPH. The association with the outcome was less marked for high stress and low fruit and vegetable consumption. Self‐reported hypertension, diabetes mellitus, and excessive alcohol consumption were not associated with PNIPH whereas abstinence from alcohol was negatively associated with this perception (Table 2).
Table 2.
Unadjusted and Adjusted Prevalence Ratios for PNIPH Associated With Individual PMRF
| Unadjusted PR (95% CI) | Adjusted PR (95% CI)a | P Valuea | |
|---|---|---|---|
| Smoking | 1.14 (1.11, 1.18) | 1.14 (1.10, 1.18) | <0.001 |
| Obesity | 1.18 (1.14, 1.22) | 1.17 (1.13, 1.22) | <0.001 |
| Low physical activity | 1.12 (1.08, 1.15) | 1.13 (1.10, 1.17) | <0.001 |
| High stress | 1.14 (1.10, 1.17) | 1.09 (1.05, 1.12) | <0.001 |
| Hypertension | 0.94 (0.91, 0.98) | 1.03 (0.99, 1.08) | 0.111 |
| Diabetes mellitus | 0.97 (0.91, 1.03) | 1.04 (0.97, 1.10) | 0.286 |
| Low fruit/vegetable consumption | 1.04 (1.01, 1.08) | 1.06 (1.03, 1.09) | <0.001 |
| Abstinence from alcoholb | 0.85 (0.81, 0.97) | 0.89 (0.85, 0.93) | <0.001 |
| Excessive alcohol intakeb | 1.01 (0.96, 1.05) | 1.03 (0.99, 1.08) | 0.141 |
PR indicates prevalence ratio.
Adjusted for age, sex, marital status, culture or racial origin, highest level of education achieved, total yearly household income, and having a regular medical doctor.
Reference: low/moderate alcohol consumption (<4 drinks/week).
Overall, each additional PMRF was associated with a PR of 1.06 (95% CI, 1.05–1.07) for PNIPH. Controlling for potential confounders increased this PR to 1.08 (95% CI, 1.07–1.09), with all terms in the multivariable model reaching statistical significance except for culture or racial origin and having a regular doctor, which did not change the adjusted PR when removed (Table 3). All variables were kept in the final model to generate adjusted and stratified PRs because of their impact on the effect measure and/or clinical relevance. Interaction terms suggested that age, culture or racial origin, and total yearly household income modified this association (P interaction≤0.025). Older individuals and those self‐identifying as white were more likely to endorse PNIPH relative to their younger counterparts and to those self‐identifying as being of visible minorities, respectively (Table 4). Given the nonuniform contribution of individual PMRFs to PNIPH, specific combinations of PMRFs were examined (Figure 3). The combination of all PMRFs (including excessive alcohol consumption) was associated with a PR of 1.92 (95% CI, 1.71–2.16).
Table 3.
Factors Other Than PMRF Associated With PNIPH
| PR (95% CI) | P Value | |
|---|---|---|
| Age 18 to 39 ya | 1.30 (1.24–1.36) | <0.001 |
| Age 40 to 59 ya | 1.26 (1.21–1.32) | <0.001 |
| Male sexb | 0.96 (0.94–0.99) | 0.001 |
| Married or common‐lawc | 0.99 (0.96–1.03) | 0.685 |
| Widowed, separated, or divorcedc | 0.95 (0.90–1.01) | 0.008 |
| Sec. school graduate, no post‐sec.d | 1.11 (1.04–1.17) | <0.001 |
| Postsecondary educationd | 1.17 (1.12–1.23) | <0.001 |
| Education level unknownd | 1.11 (0.95–1.28) | 0.015 |
| Visible minoritye | 0.97 (0.93–1.01) | 0.086 |
| Culture/racial origin unknowne | 0.94 (0.81–1.10) | 0.188 |
| Household income $40 000 to $79 999/yf | 1.10 (1.06–1.15) | <0.001 |
| Household income ≥$80 000/yf | 1.10 (1.06–1.15) | <0.001 |
| Having a regular medical doctor | 1.00 (0.96–1.03) | 0.806 |
sec. indicates secondary.
Reference: age ≥60 years.
Reference: female sex.
Reference: single, never married.
Reference: less than secondary school graduation.
Reference: self‐identified white.
Reference: income ≤$39 999/year.
Table 4.
Unadjusted and Adjusted Prevalence Ratios for PNIPH Associated With Each Additional PMRF and With a High Burden of PMRFs (≥3) According to Important Covariates
| PR Per PMRF (95% CI) | PR for ≥3 PMRFs (95% CI)a | |
|---|---|---|
| Unadjusted | 1.06 (1.05, 1.07) | 1.14 (1.12, 1.16) |
| Adjustedb | 1.08 (1.07, 1.09) | 1.19 (1.17, 1.22) |
| Men | 1.09 (1.07, 1.10) | 1.20 (1.16, 1.24) |
| Women | 1.08 (1.07, 1.09) | 1.18 (1.15, 1.21) |
| Age 18 to 39 y | 1.08 (1.07, 1.10) | 1.18 (1.15, 1.22) |
| Age 40 to 59 y | 1.07 (1.05, 1.08) | 1.15 (1.12, 1.19) |
| Age ≥60 y | 1.10 (1.09, 1.12) | 1.27 (1.21, 1.32) |
| White | 1.09 (1.08, 1.10) | 1.20 (1.18, 1.23) |
| Visible minority | 1.06 (1.03, 1.09) | 1.13 (1.06, 1.21) |
| Income ≤$39 999/y | 1.08 (1.06, 1.09) | 1.21 (1.16, 1.27) |
| Income $40 000 to $79 999/y | 1.07 (1.06, 1.09) | 1.16 (1.13, 1.20) |
| Income ≥$80 000/y | 1.09 (1.08, 1.10) | 1.20 (1.16, 1.23) |
P<0.001 for all PRs. PMRF indicates potentially modifiable cardiac risk factor; PR, prevalence ratio.
Reference: <3 PMRFs.
Adjusted for age, sex, marital status, culture or racial origin, highest level of education achieved, total yearly household income, and having a regular medical doctor.
Figure 3.
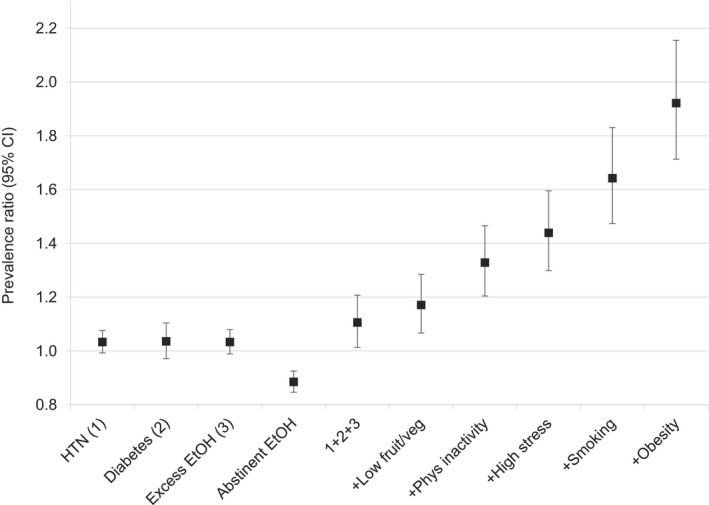
Perceived need to improve physical health associated with multiple potentially modifiable cardiac risk factors. Prevalence ratios adjusted for age, sex, marital status, culture or racial origin, highest level of education achieved, total yearly household income, and having regular medical doctor. Presented in manner analogous to INTERHEART study report.12 EtOH indicates alcohol; fruit/veg, fruit and vegetables; HTN, hypertension; phys, physical.
Perceived Barriers to Improving Physical Health
Barriers to adopting positive health changes were reported by 55.9% of individuals with PNIPH. The most frequently cited barriers were a lack of will power or self‐discipline, work schedule, and family responsibilities. Cost, stress, lack of available resources in an individual's area, and problems with transportation were each identified by fewer than 5% (Figure 4).
Figure 4.
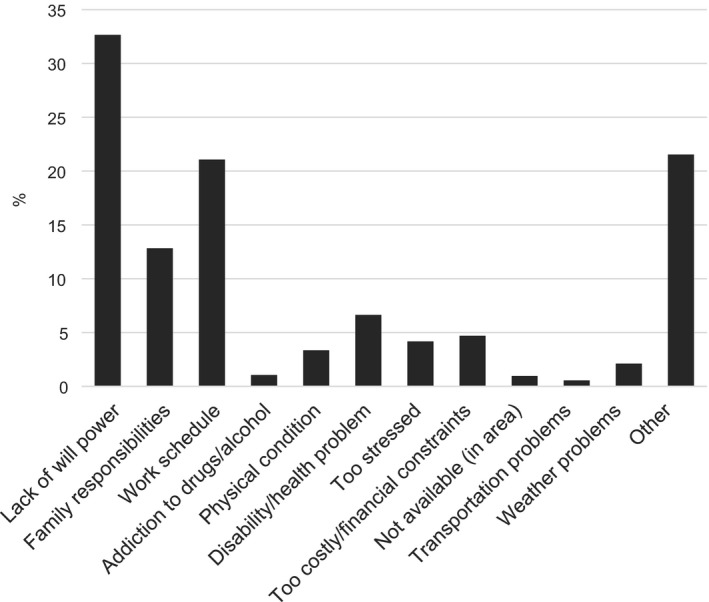
Perceived barriers to adopting positive health behaviors among individuals endorsing a perceived need to improve their physical health. Respondents could identify more than 1 barrier (mean, 1.1±0.4; range, 1–7).
Sensitivity Analyses
Excluding all respondents with missing data for any PMRF or demographic variable yielded a sample size of 39 772. Unadjusted and adjusted PRs for individual PMRFs were comparable with the preceding analyses, with all point estimates remaining within 0.01 of those presented in Table 2. Similarly, point estimates of the unadjusted, overall adjusted, and strata‐specific adjusted effect measures for the sum of PMRFs and for a high burden of PMRFs (≥3) remained within 0.01 and 0.02 of those presented in Table 4, respectively. Redefining low physical activity as mean total daily energy expenditure of <1.1 kcal/kg per day on transportation and leisure time activities and differentiating normal weight from overweight/obesity by using a BMI cutoff of 25 kg/m2 resulted in an additional 2626 (5.8%) respondents having ≥3 PMRFs. The weighted proportions of individuals with ≥3 and ≥5 PMRFs reporting PNIPH were similar at 78.5% and 80.1%, respectively, however. Adjusted PRs of individual PMRFs for the outcome of interest remained within 0.02 of those presented in Table 2. Similarly, the point estimates of adjusted and strata‐specific PRs for the sum of PMRFs and for a high burden of PMRFs (≥3) again remained within 0.01 and 0.02 of those presented in Table 4, respectively.
The 2013–2014 CCHS had a national combined household‐ and person‐level response rate of 66.2%.17 It administered the relevant survey module on changes to improve health to 4 provinces: British Columbia, Manitoba, Nova Scotia, and Prince Edward Island, resulting in a smaller sample size (n=26 315) after excluding those in whom the outcome of interest was not provided and with missing values for variables with <1% total missing data. This represented 5 227 803 individuals, which is 47.5% of the population that was covered in the 2011–2012 version used in our main analyses. The mean number of PMRFs was 2.4±1.0 with a median of 2 (IQR, 1–3). Mean BMI was 26.1±3.8 kg/m2. Weighted associations between respondent demographics or PMRFs and PNIPH were comparable to those identified in the 2011–2012 CCHS (Table S1). Weighted crude and adjusted PRs for individual PMRFs as well as for the sum of PMRFs and a high burden of PMRFs were also similar (Tables 5 and 6).
Table 5.
Unadjusted and Adjusted Prevalence Ratios for PNIPH Associated With Individual PMRFs From the 2013–2014 Canadian Community Health Survey
| Unadjusted PR (95% CI) | Adjusted PR (95% CI)a | P Valuea | |
|---|---|---|---|
| Smoking | 1.15 (1.10, 1.20) | 1.16 (1.11, 1.22) | <0.001 |
| Obesity | 1.21 (1.15, 1.27) | 1.20 (1.14, 1.26) | <0.001 |
| Low physical activity | 1.10 (1.05, 1.14) | 1.12 (1.07, 1.16) | <0.001 |
| High stress | 1.15 (1.10, 1.20) | 1.11 (1.06, 1.16) | <0.001 |
| Hypertension | 0.92 (0.88, 0.97) | 1.00 (0.95, 1.05) | 0.903 |
| Diabetes mellitus | 0.96 (0.88, 1.05) | 1.03 (0.94, 1.12) | 0.530 |
| Low fruit/vegetable consumption | 1.07 (1.03, 1.12) | 1.08 (1.04, 1.13) | <0.001 |
| Abstinence from alcoholb | 0.86 (0.81, 0.90) | 0.90 (0.85, 0.95) | <0.001 |
| Excessive alcohol intakeb | 1.01 (0.95, 1.07) | 1.04 (0.98, 1.10) | 0.239 |
PR indicates prevalence ratio.
Adjusted for age, sex, marital status, culture or racial origin, highest level of education achieved, total yearly household income, and having a regular medical doctor.
Reference: low/moderate alcohol consumption (<4 drinks/week).
Table 6.
Unadjusted and Adjusted Prevalence Ratios for PNIPH Associated With Each Additional PMRF and With a High Burden of PMRFs (≥3) According to Important Covariates From the 2013–2014 Canadian Community Health Survey
| PR Per PMRF (95% CI) | PR for ≥3 PMRFs (95% CI)a | |
|---|---|---|
| Unadjusted | 1.06 (1.05, 1.07) | 1.13 (1.10, 1.16) |
| Adjustedb | 1.09 (1.08, 1.10) | 1.20 (1.16, 1.23) |
| Men | 1.08 (1.07, 1.10) | 1.18 (1.13, 1.23) |
| Women | 1.09 (1.08, 1.10) | 1.21 (1.16, 1.26) |
| Age 18 to 39 y | 1.10 (1.08, 1.11) | 1.19 (1.14, 1.23) |
| Age 40 to 59 y | 1.08 (1.06, 1.10) | 1.19 (1.13, 1.26) |
| Age ≥60 y | 1.09 (1.07, 1.11) | 1.22 (1.17, 1.29) |
| White | 1.09 (1.08, 1.10) | 1.21 (1.18, 1.25) |
| Visible minority | 1.08 (1.05, 1.11) | 1.14 (1.06, 1.23) |
| Income ≤$39 999/y | 1.10 (1.08, 1.13) | 1.22 (1.14, 1.31) |
| Income $40 000 to $79 999/y | 1.08 (1.06, 1.10) | 1.17 (1.11, 1.24) |
| Income ≥$80 000/y | 1.08 (1.07, 1.10) | 1.20 (1.15, 1.24) |
P<0.001 for all PRs. PMRF indicates potentially modifiable cardiac risk factor; PR, prevalence ratio.
Reference: <3 PMRFs.
Adjusted for age, sex, marital status, culture or racial origin, highest level of education achieved, total yearly household income, and having a regular medical doctor.
Discussion
Ischemic heart disease is a leading cause of morbidity and mortality in North America and abroad, yet it is largely preventable with most of the risk attributed to a small number of potentially modifiable, lifestyle‐related factors.12 Though the importance of primary preventive strategies is well recognized, little is known about the association between PMRFs and individuals’ health perceptions and behaviors. Our study suggests (1) that the burden of PMRFs is positively associated with PNIPH, but (2) that individual PMRFs unequally contribute to this perception, and (3) that even among those at highest risk (those with ≥5 PMRFs), nearly 1 in 5 do not feel that they need to improve their physical health.
Among the PMRFs examined, smoking, obesity, and low physical activity were most strongly associated with PNIPH. Commensurate with these associations, the population attributable risks for MI of these PMRFs are substantial, reportedly ranging from 26.1% to 59.5% for North American men and women.12 Low fruit and vegetable consumption and high levels of stress were modestly associated with PNIPH, whereas this association was absent for diabetes mellitus, hypertension, and excessive alcohol consumption and was negative for abstinence from alcohol. Similar discrepancies in health perceptions associated with cardiovascular risk factors have previously been reported by Vähäsarja et al in their study of Finnish individuals at high risk of type 2 diabetes mellitus, finding that larger waist circumference and low physical activity were associated with a perceived need to increase physical activity levels, but that smoking, hypertension, dyslipidemia, or a family history of diabetes mellitus were not.13
Several points are worth noting when interpreting our results and their implications. Though beliefs about potential harms (ie, risk perceptions) play a fundamental role in shaping health behaviors,25 the relationship is complex26 and may be influenced by individual dimensions of the perceived risk (perceived likelihood, susceptibility, or severity), ease or cost of carrying out the behavior,25 the value ascribed to the outcome of the behavior,13 and sociocultural norms or attitudes.27 For instance, though both smoking and obesity were most strongly associated with PNIPH, these associations have not equally translated into positive behavioral changes: A continuous decline in the prevalence of smoking has been observed in North America,28 whereas the prevalence of obesity remains markedly high,29 may be increasing,28 and is projected to increase further.30 The implications of these latter trends are considerable given that elevated BMI is associated with further weight gain over the long term, compounding its associated risk.31 It is likely that such disparities are a product of the above influences on the risk perception/health behavior relationship. In effect, PNIPH is essential, but alone may not be sufficient to bring about health behavior change.32, 33 This is consistent with the psychological theories of behavioral change, including the theory of planned behavior, the transtheoretical model of change, and principles underlying motivational interviewing.13, 14, 15 It is also supported by smaller studies examining the association between PNIPH and specific intended health changes relevant to cardiac risk.34, 35, 36 In our study, nearly all individuals endorsing PNIPH also identified a health behavior change that was perceived as important to improving their health; however, nearly 19% reported that they did not intend to improve their health within the following year. Furthermore, though more than one half of individuals with PNIPH reported that barriers to adopting positive behavior changes existed, the most frequently cited barrier was a lack of willpower or self‐discipline.
Current North American guidelines on physical activity recommend considerably less exercise than the cutoff used in the INTERHEART study (150 versus 240 minutes of moderate exercise weekly).6, 37 Using this less‐demanding criterion for high physical activity resulted in an additional 13.4% being categorized as lacking this PMRF, but did not appreciably change its effect measure for the outcome of interest, suggesting that physical health perceptions in this group were comparable to those of individuals exercising more intensely and/or frequently. In fact, though increasing amounts of exercise generally confer increasing health benefits, most cardiovascular gains occur with at least 150 minutes—a finding that has formed the basis of current recommendations.6
Though fruit and vegetable consumption is known to confer cardiovascular benefits18, 19 and pertinent dietary recommendations exist,38, 39 quantifying the relationship between amount consumed and cardiovascular benefit is difficult, in part because fruits and vegetables typically form only part of an individual's diet.6 Cereals, meats, dairy products, and the fat and glycemic content of foods selected can contribute to cardiovascular risk or benefit.40, 41, 42, 43, 44 Therefore, though a diet rich in fruits and vegetables is more likely to be cardioprotective and reflective of more health‐conscious behavior, this association is imperfect. Similarly, though life and work stress were combined into 1 PMRF in our study (analogous to the home, work, and financial stress components of the combined psychosocial index used in the INTERHEART study12), data on other components of that index (depression, locus of control, and stressful life events)45 were incomplete and therefore not incorporated, potentially resulting in an underestimation of the prevalence and in a less‐precise estimate of the effect of the more broadly defined psychosocial PMRF. As well, data on respondents’ medication regimens (particularly antihypertensive and glycemic agents) or treatment effectiveness were not available or deemed sufficiently reliable to include in the study. Though self‐reported diabetes mellitus has been validated in the INTERHEART modifiable risk score model for MI,46 the inability to adjust for this potential confounder may account for the respective effect measures observed given that individuals being treated for hypertension or diabetes mellitus may have felt that these conditions were controlled. This could have led to under‐reporting of either or both conditions while still perceiving a need to improve physical health or incorrectly reporting the conditions, but perceiving that their health impacts were minimized. Notably, however, the importance of lifestyle modification irrespective of pharmacological treatment has been emphasized, particularly for diabetes mellitus given a lack of evidence that glycemic control alone improves macrovascular outcomes.47 Last, though low or moderate alcohol consumption has been associated with cardiovascular benefits,48 the link is complicated20, 49 and the benefits of alcohol are offset by its well‐known potential harms, which may affect its perceived health impact. Differing interpretations or estimations of this balance of risk and benefit may account for the negative association between abstinence from alcohol and PNIPH as well as for the lack of association between excessive alcohol consumption and PNIPH.
The sum of PMRFs was positively and significantly associated with PNIPH; however, individual PMRFs differentially contributed to this perception, with some not contributing at all. Though these associations may suggest a degree of public awareness of the health implications of PMRFs in general, our study suggests that it is modest and inconsistent, with a sizeable proportion of the public reporting that they do not feel that they should improve their health even among those at highest cardiovascular risk. Moreover, this association was attenuated among younger age groups and persons identifying as being of visible minorities—findings that warrant further investigation. Numerous explanations may account for these findings, including increased contact with health care providers at older ages and cultural influences on health and/or health care use50; however, these remain speculative given the limitations of the data set. The statistical power afforded by our sample size may have also identified minimal differences in certain cases (as in our exploratory analysis). A greater understanding of factors underlying health perceptions and behaviors, including among these subgroups, may yield considerable benefits.
There are several important limitations of our study. The outcome variable selected is inherently imperfect and likely failed to capture important nuances in health perceptions. However, perceived need to change cardiovascular health behavior has been assessed in analogous fashions by others given a lack of accepted measure for this or similar latent variables.13, 34, 35, 36 Furthermore, among those with PNIPH as defined by this variable, nearly all identified a specific lifestyle change as being most important for improving their health, the majority reported an intention to improve their physical health in the next year, and nearly all the changes planned are known to modify cardiac risk, arguing for the value of the outcome selected. Nevertheless, a panel of questions targeting different aspects of health perceptions and determinants of lifestyle behavioral change (eg, the validated Determinants of Lifestyle Behavior Questionnaire51) would have allowed for a more‐robust analysis. Our regression models examining the relationship between the overall sum of PMRFs and PNIPH assume that each PMRF contributes uniformly to the outcome, which we show to not be the case and which render our effect estimates less precise. However, this analysis is relevant to clinical practice given that cumulative PMRFs elicit greatest concern. Data on individual and specific combinations of PMRFs are also provided. Data were collected by self‐reports and are therefore subject to measurement error, particularly recall bias. Standardized interviewer questionnaires and the sampling strategy used16 render interviewer and selection bias less likely. In addition, as mentioned above, only 8 of the 9 PMRFs identified in the INTERHEART study were assessed because a robust measure of dyslipidemia was not collected. Given the strong association between dyslipidemia and other PMRFs,52, 53 it is likely that its inclusion would have attenuated the exposure‐outcome associations identified. Abdominal obesity was not included in the survey; therefore, BMI was used as a surrogate measure, which may not optimally reflect the importance of fat distribution on cardiovascular risk.54, 55 However, BMI is recommended and routinely used to detect and monitor weight in clinical practice.56, 57 Knowledge regarding additional comorbidities that may independently influence individuals’ opinions on the need to improve their physical health were unaccounted for. Last, culture or racial origin was grouped into white, visible minority, and not stated, which limits detailed analyses of potential sociocultural influences on health perceptions.
Conclusions
The cumulative burden of PMRFs is positively associated with PNIPH; however, individually, PMRFs are differentially associated with this perception. A substantial proportion of individuals at risk for cardiovascular events do not feel a need to improve their physical health, indicating an urgent need to identify means to modify public health perceptions and behaviors.
Disclosures
None.
Supporting information
Table S1. Prevalence of Perceiving a Need to Improve Physical Health According to Respondent Demographics and Potentially Modifiable Cardiac Risk Factors From the 2013–2014 Canadian Community Health Survey
Acknowledgments
This analysis is based on the Statistics Canada Community Health Survey Microdata Files, which contain anonymized data collected in the 2011–2012 and 2013–2014 Canadian Community Health Surveys. All computations, use, and interpretation of these data are entirely that of Ramirez et al.
(J Am Heart Assoc. 2017;6:e005491 DOI: 10.1161/JAHA.117.005491.)28468783
References
- 1. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2013 DALYs and HALE Collaborators . Global, regional, and national disability‐adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 4. Wijeysundera HC, Machado M, Farahati F, Wang X, Witteman W, van der Velde G, Tu JV, Lee DS, Goodman SG, Petrella R, O'Flaherty M, Krahn M, Capewell S. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA. 2010;303:1841–1847. [DOI] [PubMed] [Google Scholar]
- 5. Guzman‐Castillo M, Ahmed R, Hawkins N, Scholes S, Wilkinson E, Lucy J, Capewell S, O'Flaherty M. The contribution of primary prevention medication and dietary change in coronary mortality reduction in England between 2000 and 2007: a modelling study. BMJ Open. 2015;5:e006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 7. Wilmot KA, O'Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Flaherty M, Ford E, Allender S, Scarborough P, Capewell S. Coronary heart disease trends in England and Wales from 1984 to 2004: concealed levelling of mortality rates among young adults. Heart. 2008;94:178–181. [DOI] [PubMed] [Google Scholar]
- 9. O'Flaherty M, Allender S, Taylor R, Stevenson C, Peeters A, Capewell S. The decline in coronary heart disease mortality is slowing in young adults (Australia 1976–2006): a time trend analysis. Int J Cardiol. 2012;158:193–198. [DOI] [PubMed] [Google Scholar]
- 10. Nedkoff LJ, Briffa TG, Preen DB, Sanfilippo FM, Hung J, Ridout SC, Knuiman M, Hobbs M. Age‐ and sex‐specific trends in the incidence of hospitalized acute coronary syndromes in Western Australia. Circ Cardiovasc Qual Outcomes. 2011;4:557–564. [DOI] [PubMed] [Google Scholar]
- 11. Izadnegahdar M, Singer J, Lee MK, Gao M, Thompson CR, Kopec J, Humphries KH. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Womens Health (Larchmt). 2014;23:10–17. [DOI] [PubMed] [Google Scholar]
- 12. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 13. Vähäsarja K, Salmela S, Villberg J, Rintala P, Vanhala M, Saaristo T, Peltonen M, Keinanen‐Kiukaanniemi S, Korpi‐Hyovalti E, Kujala UM, Moilanen L, Niskanen L, Oksa H, Poskiparta M. Perceived need to increase physical activity levels among adults at high risk of type 2 diabetes: a cross‐sectional analysis within a community‐based diabetes prevention project FIN‐D2D. BMC Public Health. 2012;12:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64:527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. [DOI] [PubMed] [Google Scholar]
- 16. Statistics Canada . Canadian Community Health Survey (CCHS) annual component. User guide 2012 and 2011‐2012 microdata files. 2013.
- 17. Statistics Canada . Canadian Community Health Survey (CCHS) annual component. User guide 2014 and 2013‐2014 microdata files. 2015.
- 18. He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta‐analysis of cohort studies. J Hum Hypertens. 2007;21:717–728. [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose‐response meta‐analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leong DP, Smyth A, Teo KK, McKee M, Rangarajan S, Pais P, Liu L, Anand SS, Yusuf S; INTERHEART Study Investigators . Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case‐control study. Circulation. 2014;130:390–398. [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Dales R, Krewski D, Breithaupt K. Increased effects of smoking and obesity on asthma among female Canadians: the National Population Health Survey, 1994–1995. Am J Epidemiol. 1999;150:255–262. [DOI] [PubMed] [Google Scholar]
- 22. Statistics Canada . Canadian Community Health Survey (CCHS) 2011‐2012 public use microdata file. Approximate sampling variability tables. 2013.
- 23. Moses LE. Graphical methods in statistical analysis. Annu Rev Public Health. 1987;8:309–353. [DOI] [PubMed] [Google Scholar]
- 24. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 25. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta‐analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26:136–145. [DOI] [PubMed] [Google Scholar]
- 26. Ferrer R, Klein WM. Risk perceptions and health behavior. Curr Opin Psychol. 2015;5:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamers SL, Allen J, Yang M, Stoddard A, Harley A, Sorensen G. Does concern motivate behavior change? Exploring the relationship between physical activity and body mass index among low‐income housing residents. Health Educ Behav. 2014;41:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–570. [DOI] [PubMed] [Google Scholar]
- 31. Lloyd‐Jones DM, Liu K, Colangelo LA, Yan LL, Klein L, Loria CM, Lewis CE, Savage P. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults study. Circulation. 2007;115:1004–1011. [DOI] [PubMed] [Google Scholar]
- 32. Pellmar TC, Brandt EN Jr, Baird MA. Health and behavior: the interplay of biological, behavioral, and social influences: summary of an Institute of Medicine report. Am J Health Promot. 2002;16:206–219. [DOI] [PubMed] [Google Scholar]
- 33. Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28:413–433. [DOI] [PubMed] [Google Scholar]
- 34. Povey R, Conner R, Sparks P, James R, Shepherd R. Application of the theory of planned behaviour to two dietary behaviours: roles of perceived control and self‐efficacy. Br J Health Psychol. 2000;5:121–139. [Google Scholar]
- 35. Paisley CM, Sparks P. Expectations of reducing fat intake: the role of perceived need within the theory of planned behaviour. Psychol Health. 1998;13:341–353. [Google Scholar]
- 36. Payne N, Jones F, Harris PR. The role of perceived need within the theory of planned behaviour: a comparison of exercise and healthy eating. Br J Health Psychol. 2004;9:489–504. [DOI] [PubMed] [Google Scholar]
- 37. Canadian Society for Exercise Physiology . Canadian physical activity guidelines. Canadian sedentary behaviour guidelines. 2011. Available at: http://www.csep.ca/CMFiles/Guidelines/CSEP_Guidelines_Handbook.pdf. Accessed December 15, 2016.
- 38. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans. 8th ed 2015. Available at: http://health.gov/dietaryguidelines/2015/guidelines/. Accessed December 15, 2016. [Google Scholar]
- 39. Health Canada . Eating well with Canada's food guide. 2007. Available at: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php. Accessed December 15, 2016.
- 40. Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996;275:447–451. [DOI] [PubMed] [Google Scholar]
- 41. Wolk A, Manson JE, Stampfer MJ, Colditz GA, Hu FB, Speizer FE, Hennekens CH, Willett WC. Long‐term intake of dietary fiber and decreased risk of coronary heart disease among women. JAMA. 1999;281:1998–2004. [DOI] [PubMed] [Google Scholar]
- 42. Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose‐response meta‐analysis of prospective cohort studies. Public Health Nutr. 2016;19:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mirrahimi A, de Souza RJ, Chiavaroli L, Sievenpiper JL, Beyene J, Hanley AJ, Augustin LS, Kendall CW, Jenkins DJ. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta‐analysis of prospective cohorts. J Am Heart Assoc. 2012;1:e000752 DOI: 10.1161/JAHA.112.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. [DOI] [PubMed] [Google Scholar]
- 45. Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐amorn C, Sato H, Yusuf S; INTERHEART Study Investigators . Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
- 46. McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A, Prabhakaran D, Almahmeed W, Rumboldt Z, Budaj A, Dans AL, Gerstein HC, Teo K, Anand SS; INTERHEART Study Investigators . Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32:581–589. [DOI] [PubMed] [Google Scholar]
- 47. Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. [DOI] [PubMed] [Google Scholar]
- 48. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta‐analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Neill D, Britton A, Brunner EJ, Bell S. Twenty‐five‐year alcohol consumption trajectories and their association with arterial aging: a prospective cohort study. J Am Heart Assoc. 2017;6:e005288 DOI: 10.1161/JAHA.116.005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bennett GG, Wolin KY. Satisfied or unaware? Racial differences in perceived weight status. Int J Behav Nutr Phys Act. 2006;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lakerveld J, Bot SD, Chinapaw MJ, Knol DL, de Vet HC, Nijpels G. Measuring pathways towards a healthier lifestyle in the Hoorn prevention study: the Determinants of Lifestyle Behavior Questionnaire (DLBQ). Patient Educ Couns. 2011;85:e53–e58. [DOI] [PubMed] [Google Scholar]
- 52. Lindsay RS, Howard BV. Cardiovascular risk associated with the metabolic syndrome. Curr Diab Rep. 2004;4:63–68. [DOI] [PubMed] [Google Scholar]
- 53. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 54. Koster A, Leitzmann MF, Schatzkin A, Mouw T, Adams KF, van Eijk JT, Hollenbeck AR, Harris TB. Waist circumference and mortality. Am J Epidemiol. 2008;167:1465–1475. [DOI] [PubMed] [Google Scholar]
- 55. Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, Thun MJ, Gapstur SM. Waist circumference and all‐cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–1301. [DOI] [PubMed] [Google Scholar]
- 56. Brauer P, Connor Gorber S, Shaw E, Singh H, Bell N, Shane AR, Jaramillo A, Tonelli M; Canadian Task Force on Preventive Health Care . Recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. CMAJ. 2015;187:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prevalence of Perceiving a Need to Improve Physical Health According to Respondent Demographics and Potentially Modifiable Cardiac Risk Factors From the 2013–2014 Canadian Community Health Survey


