Abstract
Background
Heart rate recovery (HRR) is a noninvasive assessment of autonomic dysfunction and has been implicated with risk of cardiovascular events and all‐cause mortality. However, evidence has not been systematically assessed. We performed a meta‐analysis of prospective cohort studies to quantify these associations in the general population.
Methods and Results
A literature search using 3 databases up to August 2016 was conducted for studies that reported hazard ratios with 95% CIs for the association between baseline HRR and outcomes of interest. The overall hazard ratios were calculated using a random‐effects model. There were 9 eligible studies in total, with 5 for cardiovascular events enrolling 1061 cases from 34 267 participants, and 9 for all‐cause mortality enrolling 2082 cases from 41 600 participants. The pooled hazard ratios associated with attenuated HRR versus fast HRR that served as the referent were 1.69 (95% CI 1.05–2.71) for cardiovascular events and 1.68 (95% CI 1.51–1.88) for all‐cause mortality. For every 10 beats per minute decrements in HRR, the hazard ratios were 1.13 (95% CI 1.05–1.21) and 1.09 (95% CI 1.01–1.19), respectively. Further analyses suggested that the associations observed between attenuated HRR and risk of fatal cardiovascular events and all‐cause mortality were independent of traditional metabolic factors for cardiovascular disease (all P<0.05).
Conclusions
Attenuated HRR is associated with increased risk of cardiovascular events and all‐cause mortality, which supports the recommendation of recording HRR for risk assessment in clinical practice as a routine.
Keywords: cardiovascular events, heart rate recovery, mortality
Subject Categories: Cardiovascular Disease, Epidemiology, Exercise, Electrophysiology
Introduction
Heart rate recovery (HRR) is defined as the rate at which heart rate decreases within the following minutes after the cessation of physical exercise,1, 2 and reflects the dynamic balance and coordinated interplay between parasympathetic reactivation and sympathetic withdrawal.2, 3, 4 As a simple and noninvasive assessment of autonomic nervous system function that is capable of indicating one's ability to get adaptation to exercise stimuli, HRR has received substantial interest and is widely used as a guide to monitor changes in physical fitness and training status.4, 5, 6
In recent years, there has been a large body of epidemiological evidence that HRR might also be a potential prognostic marker for predicting health outcomes including cardiovascular disease (CVD),7, 8, 9, 10, 11 since autonomic dysfunction as signified by attenuated HRR has been suggested to be a precursor to hyperglycemia as well as an indicator of cardiovascular dysfunction.12, 13, 14, 15 In addition, evidence has also indicated that HRR may assist in predicting the risk of all‐cause mortality.1, 16, 17, 18, 19, 20, 21 However, findings regarding its prognostic power from current available studies varied substantially among the studies. For instance, some studies observed that attenuated HRR might be associated with an increased risk of incident CVD and all‐cause mortality,7, 20 whereas others stated that the strength of the associations was diluted or even became no longer statistically significant after controlling for confounders.8, 20 Furthermore, differences in the target populations (eg, healthy versus diseased adults) and variations in the recovery time points following the cessation of exercise (eg, 1‐minute versus 2‐minute HRR) might also contribute to the inconsistent findings.2 Although previous narrative reviews have provided important insights into the understanding of these issues and highlighted the clinical significance of HRR,2, 22, 23 their conclusions are somehow largely subjective and importantly, they did not systematically or comprehensively quantify the magnitude of the association between HRR and the risk of the aforementioned related health outcomes.
In order to enrich the understanding of the prognostic value of HRR for future clinical decision making, we conducted a meta‐analysis of prospective cohort studies with dose–response analysis to determine the relationship between attenuated HRR and the risk of cardiovascular events and all‐cause mortality in the general population, as well as to identify the potential modifiers.
Methods
This meta‐analysis was conducted using a preregistered protocol (PROSPERO CRD42016048172) and reported in accordance with the MOOSE (Meta‐analysis Of Observational Studies in Epidemiology) guideline (Table S1).24
Data Sources and Search Strategy
A comprehensive literature search of PubMed, Cochrane Library, and Web of Science for English‐language studies was performed through its inception to August 2016 with the use of search terms modified from published systematic review or meta‐analysis25, 26 as follows: (“heart rate recovery”) and (risk or cox or hazard or “survival analysis” or odds) (Table S2). Bibliographies of selected articles and related reviews were manually checked to identify additional studies.
Study Selection
Studies were included if they met the following criteria: (1) the study design was a prospective cohort; (2) the study group was the general population with a mean age >18 years at entry; (3) the exposure of interest was HRR, which was assessed using an exercise stress test and expressed as 1‐ or 2‐minute HRR (representing fast‐phase and slow‐phase of HRR, respectively)2; (4) the outcome of interest was cardiovascular events (include coronary heart disease, myocardial infarction, and stroke) or all‐cause mortality; and (5) the study reported hazard ratio (HR) with the corresponding 95% CIs or provided sufficient data to calculate them. The reasons for choosing only 1‐ or 2‐minute HRR for analysis are because of the fact that they are most widely used compared with other time points of HRR, and to minimize the heterogeneity across studies. To avoid double‐counting of a single observational cohort (eg, the Lipid Research Clinics Prevalence Study) that had generated multiple publications and reported the same outcome of interest, the first priority of selection was given to the study with the longest follow‐up duration, and then to the study that provided the most completely analyzed outcome or covered the largest number of participants.
Studies were excluded if they limited the target populations to those with confirmed pre‐existing diseases such as diabetes mellitus, had overlaps in the target populations (eg, studies conducted in the Cleveland Clinic Foundation), or enrolled patients who were referred to exercise stress tests mainly for clinical reasons such as chest pain or other symptoms suspected to be attributable to coronary heart disease.
Data Extraction and Quality Assessment
Data extraction was performed by 2 independent authors and disagreements were resolved by discussion. The following information was collected: name of first author, year of publication, country of origin, mean age of participants, proportion of men, number of participants and outcome of cases, duration of follow‐up, number of person‐years (for dose–response analysis), assessment of HRR, adjusted covariates, and HR with the 95% CIs. For studies that provided several adjustment models for potential confounders, the most completely adjusted HR was used.
Study quality was assessed using the validated 9‐star Newcastle‐Ottawa Scale,27 which assigns a maximum of 4 stars to the selection category, 2 stars for the comparability category, and 3 stars for the outcome category. Studies with Newcastle‐Ottawa Scale scores ≥7 were considered to be of high quality; otherwise, they were of low quality.
Data Synthesis and Statistical Analysis
HR was chosen as the major effect size. For studies that reported odds ratio instead of HR, HR was computed using the odds ratio directly, because the incidence of the outcome of interest is very low. For studies that provided results stratified by sex, they were analyzed as separate studies; and for studies that utilized both 1‐ and 2‐minute HRR as different exposures, 1‐minute HRR was chosen for the primary analyses. However, sensitivity analysis was performed to assess the potential influences on the overall HRs by replacing 1‐minute HRR with 2‐minute HRR.
In order to obtain the summary HRs with 95% CIs regarding the association of HRR with the risk of the outcome of interest, 2 approaches that used a random‐effects model were applied. First, categorical analysis was conducted between the HRs for attenuated HRR versus fast HRR. For this, if studies reported stratum‐specific HRs from at least 2 different attenuated HRR groups compared with the same fastest HRR category that serves as the referent, these groups were combined into a single one and a combined HR was then calculated using a fixed‐effects model for the primary analysis as previously suggested.28, 29 In this case, the newly generated group was assigned as the attenuated HRR group, whereas the fastest one was assigned as the fast HRR group. For studies reporting HRs using the attenuated HRR group as the referent, the HRs and 95% CIs were recalculated as inverses. Secondly, dose–response analysis was performed by computing the HRs with 95% CIs for every 10‐beats‐per‐minute (bpm) decrement in HRR, with the assumption that there exists a linear relationship of the natural logarithm of HR with decreasing HRR and 95% CIs. For studies that reported the association with categories of HRR instead, the method described by Greenland and Longnecker was applied to estimate the HRs with 95% CIs for 10‐bpm decrements in HRR.30 For studies that reported categories of HRR with the upper and lower boundaries, the medians were imputed using their midpoint values. For studies that provided open‐ended highest or lowest categories of HRR, the widths of these categories were assumed to be the same as their closest adjacent categories.26
Statistical heterogeneity between studies was evaluated with the I2 statistic. To test the robustness of the results, sensitivity analyses were conducted by removing a single study each time.31 To assess the potential sources of heterogeneity, subgroup analyses on the basis of adjustment for smoking, maximal oxygen consumption, or at least 3 metabolic factors related to obesity, diabetes mellitus, hypertension, or dyslipidemia, exercise stress testing, and testing equipment, as well as the meta‐regression analyses of baseline BMI, age, sex, resting heart rate, and follow‐up duration were performed if possible. Publication bias was assessed using the Egger test with P<0.10 indicative of significance. All the statistical analyses were performed using STATA software (V. 12.0, College Station, TX), and the statistical significance was defined as 2‐sided P<0.05 unless otherwise stated.
Results
Literature Search
Our initial search identified 1049 potentially relevant citations, and a total of 9 articles met the inclusion criteria and were included in the analysis (Figure 1).7, 8, 9, 10, 11, 18, 19, 20, 21 Among these articles, 5 provided results for cardiovascular events.7, 8, 9, 10, 11 Since 1 article reported sex‐stratified results on all‐cause mortality,21 the number of studies for all‐cause mortality was 9 (from 8 articles).7, 8, 9, 11, 18, 19, 20, 21
Figure 1.
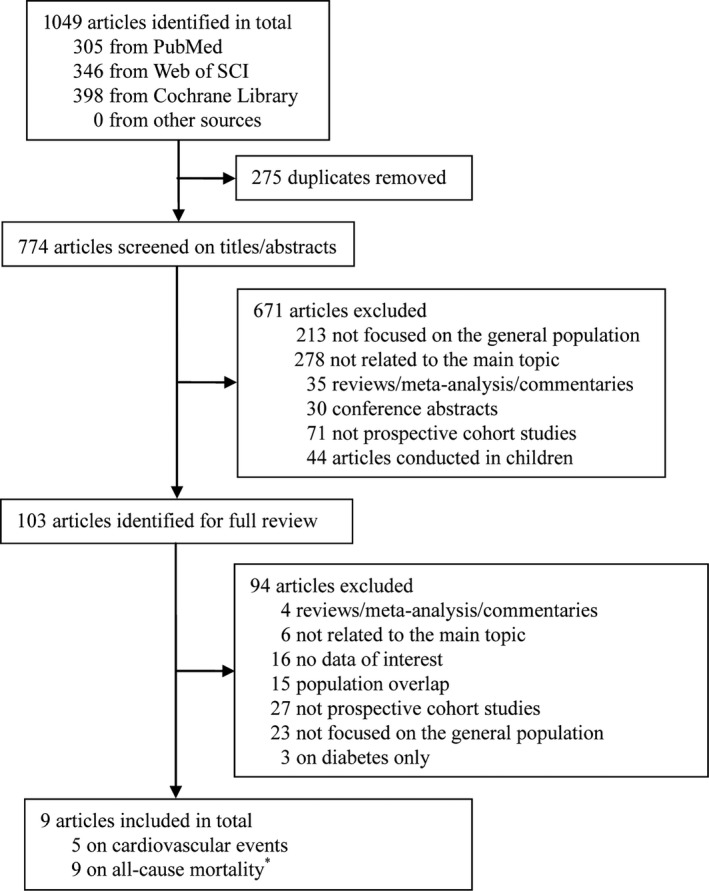
Flow diagram of literature search. *The study by Wandell et al21 had results stratified by sex, and were treated as separate studies.
Study Characteristics
The main characteristics of included studies are summarized in Table 1. The large majority of them were conducted in the United States, enrolling middle‐aged populations. For cardiovascular events, a total of 1061 incident cases were identified from 34 267 participants during a mean follow‐up period of 16.5 years. For all‐cause mortality, a total of 2082 incident cases were identified from 41 600 participants during a mean follow‐up period of 15 years.
Table 1.
Characteristics of Studies Included in the Meta‐Analysis
| Source and Location | Age (y) and Men (%) | Follow‐up (y) | Exercise and Recovery Protocol | HRR‐Phase (Minute) | Outcome Assessment | Covariates Adjusted | Study Quality |
|---|---|---|---|---|---|---|---|
| Cardiovascular events | |||||||
| Ho et al 20107; USA | 51.0; 70.2 | 7.2 | Modified Balke protocol (maximal workload); NS | 1 | Registry (for CVD death) | Age, sex, abnormal/equivocal ST segment response, hypertension, hyperlipidemia, diabetes mellitus, and current tobacco use | 9 |
| Mora et al 20038; USA | 46.3; 0 | 20 | Bruce protocol (maximal workload); No | 2 | Death certificates and medical records (for CVD death) | Age, current smoking, diabetes mellitus, family history of premature coronary heart disease, obesity, HDL‐C, LDL‐C, high TG, and hypertension | 9 |
| Morshedi‐Meibodi et al 20029; USA | 43.0; 47.2 | 15 | Bruce protocol (submaximal workload); No | 1/2 | Medical records (for CVD events) | Age, BMI, smoking, systolic and DBP, antihypertensive treatment, diabetes mellitus, TC, HDL‐C, resting HR and peak HR | 9 |
| Park et al 201510; USA | 55.0; 46.4 | 27 | NS (submaximal workload); NS | 2 | Death certificates (for Mis, and silent Mis) | Age, sex, TC, diabetes mellitus history, and smoking history | 9 |
| Newman et al 200611; USA | 73.5; 48 | 4.9 | 400‐m walk component (NS); NS | 2 | Death certificates and records (for CVD events) | Age and sex | 9 |
| All‐cause mortality | |||||||
| Ho et al 20107; USA | 51.0; 70.2 | 7.2 | Modified Balke protocol (maximal workload); NS | 1 | Registry | The other non‐ST segment exercise parameters, sex, abnormal/equivocal stress test, resting HR, presence of diabetes mellitus, hypertension, hyperlipidemia, and current tobacco use | 9 |
| Mora et al 20038; USA | 46.3; 0 | 20 | Bruce protocol (maximal workload); No | 2 | Death certificates and medical records | Age, current smoking, diabetes mellitus, family history of premature coronary heart disease, obesity, HDL‐C, LDL‐C, high TG level, and hypertension | 9 |
| Morshedi‐Meibodi et al 20029; USA | 43.0; 47.2 | 15 | Bruce protocol (submaximal workload); No | 2 | Medical records | Age, BMI, smoking, systolic and DBP, antihypertensive treatment, diabetes mellitus, TC, HDL‐C, resting HR and peak HR | 9 |
| Newman et al 200611; USA | 73.5; 48 | 4.9 | 400‐m walk component (NS); NS | 2 | Death certificates and medical records | Age, sex, race, smoking history, pack‐years of smoking, SBP, TC, fasting glucose, BMI, physical activity, coronary heart disease, intermittent claudication, stroke, depression symptoms, ankle–brachial index, major ECG abnormalities, forced expiratory volume in first second/forced vital capacity, and use of digoxin, blockers, or calcium channel blockers | 9 |
| Aktas et al 200418; USA | 57.0; 80.8 | 8 | Bruce or modified Bruce protocol (symptom‐limited workload); Active recovery protocol | 1 | Registry | Diabetes mellitus, BMI, levels of TG and HDL‐C, black race, and use of medications including blockers, aspirin, angiotensin‐converting enzyme inhibitors, nondihydropyridine calcium blockers, statins, and thiazide diuretics | 9 |
| Johnson et al 201219; USA | 55.0; 57.0 | 9.9 | Bruce protocol (maximal workload); No | 1 | Registry | Categorical analysis: none; Dose–response analysis: all variables except peak HR and late HRR | 8 |
| Savonen et al 201120; Finland | 51.0; 100 | 18 | NS (maximal workload); No | 1/2 | Registry | Categorical analysis: none; Dose–response analysis: age, alcohol consumption, BMI, cigarette smoking, plasma fibrinogen, serum LDL‐C, resting SBP and serum CRP, chronotropic incompetence | 9 |
| Wandell et al 2010—Men21; Sweden | 37.6; 100 | 26 | NS (maximal workload); No | 1 | Registry | Age, high HR, hypertension, diabetes mellitus, BMI, smoking, physical fitness and care need group | 8 |
| Wandell et al 2010—Women21; Sweden | 33.0; 0 | 26 | NS (maximal workload); No | 1 | Registry | Age, high HR, hypertension, diabetes mellitus, BMI, smoking, physical fitness and care need group | 8 |
CRP indicates C‐reactive protein; CVD, cardiovascular diseases; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; HRR, heart rate recovery; LDL‐C, low‐density lipoprotein cholesterol; Mis, myocardial ischemia; NS, not specified; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Almost all studies utilized Bruce or modified Balke protocol for HRR assessment, yet only 1 study clearly indicated the use of an active cool‐down protocol.18 None of the studies specified the position (eg, supine or standing) for counting heart rate during the recovery periods following the cessation of exercise. The reference categories of HRR and the number of adjusted confounding factors generally varied across studies. Yet most studies included age, smoking, maximal oxygen consumption, and a group of conventional metabolic factors related to obesity, diabetes mellitus, or dyslipidemia in their multivariable‐adjusted models. All studies were judged to be of high quality (Table 1).
Heart Rate Recovery and Risk of Cardiovascular Events
The random‐effects meta‐analyses revealed that pooled HR of cardiovascular events related to attenuated HRR was 1.69 (95% CI 1.05–2.71; I2=60.9%, P heterogeneity=0.05; Figure 2) in comparison to the referent, and the HR was 1.13 (95% CI 1.05–1.21; I2=4.0%, P heterogeneity=0.35; Figure 3) for every 10‐bpm decrement in HRR.
Figure 2.
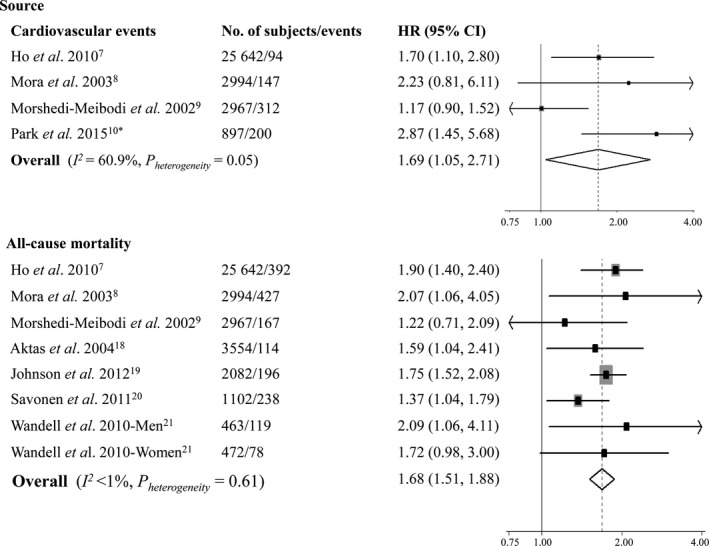
Meta‐analysis of cardiovascular events and all‐cause mortality for attenuated vs fast heart rate recovery. HR indicates hazard ratio. *The study by Park et al10 reported separate data on ischemia and silent myocardial ischemia, which were combined together using a fixed‐effects model.
Figure 3.
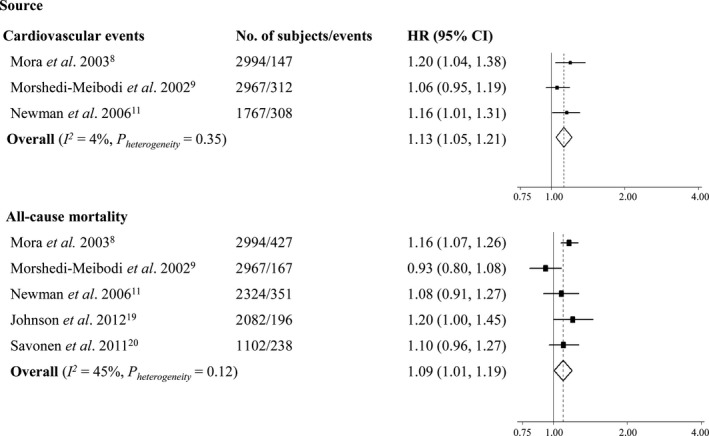
Meta‐analysis of cardiovascular events and all‐cause mortality for every 10‐beats‐per‐minute decrement in heart rate recovery. HR indicates hazard ratio.
Results from the subgroup or meta‐regression analysis were generally consistent between categorical and dose–response analyses (Table 2). There was a trend that the magnitude of the associations became smaller among studies using 1‐minute HRR as exposure compared with those using 2‐minute HRR (P for interaction=0.07 for categorical analysis, and 0.16 for dose–response analysis). Although the overall HRs were attenuated after controlling for multiple metabolic factors, results for risk of fatal cardiovascular events remained significant (HR 1.78, 95% CI 1.17–2.72 for categorical analysis; and HR 1.20, 95% CI 1.04–1.38 for dose–response analysis). Studies employing maximal exercise testing protocols resulted in significant outcomes. Meta‐regression analysis did not identify any significant moderator for the associations (Table 3).
Table 2.
Subgroup Analysis of Attenuated HRR and Risk of Cardiovascular Events
| Variable | Attenuated HRR vs Fast HRR | Per 10 bpm Decrement in HRR | ||||||
|---|---|---|---|---|---|---|---|---|
| N | HR (95% CI) | I2 (%) | P heterogeneity | N | HR (95% CI) | I2 (%) | P heterogeneity | |
| Recovery time point | ||||||||
| 2 minutes | 2 | 2.65 (1.51, 4.67) | <1 | 0.69 | 2 | 1.18 (1.07, 1.30) | <1 | 0.73 |
| 1 minute | 2 | 1.30 (0.78, 2.16) | 61.8 | 0.11 | 1 | 1.06 (0.95, 1.19) | NA | NA |
| Exercise stress testing workloads | ||||||||
| Maximal | 2 | 1.78 (1.17, 2.72) | <1 | 0.63 | 1 | 1.20 (1.04, 1.38) | NA | NA |
| Submaximal | 2 | 1.64 (0.59, 4.56) | 84.6 | 0.01 | 1 | 1.06 (0.95, 1.19) | NA | NA |
| Other | 0 | NA | NA | NA | 1 | 1.16 (1.01, 1.31) | NA | NA |
| Country | ||||||||
| USA | 4 | 1.69 (1.05, 2.71) | 60.9 | 0.05 | 3 | 1.13 (1.05, 1.21) | 4.0 | 0.45 |
| Others | 0 | NA | NA | NA | 0 | NA | NA | NA |
| Cardiovascular events | ||||||||
| Fatal | 2 | 1.78 (1.17, 2.72) | <1 | 0.63 | 1 | 1.20 (1.04, 1.38) | NA | NA |
| Nonfatal | 2 | 1.64 (0.59, 4.56) | 84.6 | 0.01 | 2 | 1.10 (1.01, 1.20) | 5.2 | 0.3 |
| Adjusting for: | ||||||||
| Smoking | ||||||||
| Yes | 4 | 1.69 (1.05, 2.71) | 60.9 | 0.05 | 3 | 1.13 (1.05, 1.21) | 4.0 | 0.45 |
| No | 0 | NA | NA | NA | 0 | NA | NA | NA |
| VO2max | ||||||||
| Yes | 0 | NA | NA | NA | 0 | NA | NA | NA |
| No | 4 | 1.69 (1.05, 2.71) | 60.9 | 0.05 | 3 | 1.13 (1.05, 1.21) | 4.0 | 0.45 |
| At least 3 metabolic factors | ||||||||
| Yes | 3 | 1.41 (0.90, 2.19) | 45.7 | 0.16 | 2 | 1.12 (0.99, 1.26) | 44.7 | 0.18 |
| No | 1 | 2.87 (1.45, 5.68) | NA | NA | 1 | 1.16 (1.02, 1.32) | NA | NA |
bpm indicates beats per minute; HR, hazard ratio; HRR, heart rate recovery; NA, not applicable; VO2max, maximal oxygen consumption.
Table 3.
Meta‐Regression Analysis of Attenuated HRR and Risk of Cardiovascular Events
| Variable | Attenuated HRR vs Fast HRR | Per 10 bpm Decrement in HRR | ||||
|---|---|---|---|---|---|---|
| N | Beta‐Coefficient | P Value | N | Beta‐Coefficient | P Value | |
| BMI | 4 | 0.91 | 0.79 | 3 | 1.01 | 0.94 |
| Agea | 4 | 37.84 | 0.13 | 3 | 1.10 | 0.71 |
| Proportion of men | 4 | 1.00 | 0.76 | 3 | 1.00 | 0.51 |
| Follow‐up | 4 | 1.03 | 0.48 | 3 | 1.00 | 0.98 |
| Resting heart rate | 3 | 1.05 | 0.23 | NA | NA | NA |
bpm indicates beats per minute; HRR, heart rate recovery; NA, not applicable.
Age data were log‐transformed for analysis.
Heart Rate Recovery and Risk of All‐Cause Mortality
Results from the meta‐analyses using a random‐effects model indicated that the overall HR of all‐cause mortality associated with attenuated HRR was 1.68 (95% CI 1.51–1.88; I2<1%, P heterogeneity=0.61; Figure 2) compared with the referent, and it was pooled to be 1.09 (95% CI 1.01–1.19; I2=44.8%, P heterogeneity=0.12; Figure 3) per every 10‐bpm decrement in HRR.
Subgroup analysis showed that 2‐minute HRR did not differ substantially from 1‐minute HRR in the observed associations (P for interaction=0.41 for categorical analysis, and 0.38 for dose–response analysis, Table 4). The strength of the associations remained statistically significant with adjustment for potential confounders such as smoking and metabolic factors in the categorical analyses. Yet only studies utilizing maximal exercise testing protocols yielded significant results for predicting the risk of all‐cause mortality (HR 1.72, 95% CI 1.53–1.93 for categorical analysis; and HR 1.15, 95% CI 1.08–1.23 for dose–response analysis). Meta‐regression analysis showed consistently that none of the prespecified variables predicted the changes in the observed associations (all P>0.30, Table 5).
Table 4.
Subgroup Analysis of Attenuated HRR and Risk of All‐Cause Mortality
| Variable | Attenuated HRR vs Fast HRR | Per 10 bpm Decrement in HRR | ||||||
|---|---|---|---|---|---|---|---|---|
| N | HR (95% CI) | I2 (%) | P heterogeneity | N | HR (95% CI) | I2 (%) | P heterogeneity | |
| Recovery time point | ||||||||
| 2 minutes | 2 | 1.50 (1.07, 2.08) | 20.1 | 0.26 | 2 | 1.14 (1.06, 1.23) | <1 | 0.45 |
| 1 minute | 6 | 1.74 (1.54, 1.96) | <1 | 0.77 | 3 | 1.06 (0.92, 1.23) | 59.2 | 0.09 |
| Exercise stress testing workloads | ||||||||
| Maximal | 6 | 1.72 (1.53, 1.93) | <1 | 0.57 | 3 | 1.15 (1.08, 1.23) | <1 | 0.73 |
| Submaximal | 1 | 1.22 (0.71, 2.09) | NA | NA | 1 | 0.93 (0.80, 1.08) | NA | NA |
| Other | 1 | 1.59 (1.04, 2.42) | NA | NA | 1 | 1.08 (0.91, 1.27) | NA | NA |
| Treadmill | ||||||||
| Yes | 5 | 1.74 (1.54, 1.97) | <1 | 0.64 | 3 | 1.09 (0.94, 1.27) | 71.9 | 0.03 |
| No | 3 | 1.49 (1.19, 1.88) | <1 | 0.45 | 2 | 1.09 (0.98, 1.22) | <1 | 0.87 |
| Country | ||||||||
| USA | 5 | 1.74 (1.54, 1.97) | <1 | 0.64 | 4 | 1.09 (0.98, 1.22) | 58.6 | 0.07 |
| Others | 3 | 1.49 (1.19, 1.88) | <1 | 0.45 | 1 | 1.10 (0.96, 1.27) | NA | NA |
| Sex | ||||||||
| Men | 2 | 1.50 (1.07, 2.12) | 22.2 | 0.26 | 1 | 1.10 (0.96, 1.27) | NA | NA |
| Women | 2 | 1.86 (1.21, 2.85) | <1 | 0.68 | 1 | 1.16 (1.07, 1.26) | NA | NA |
| Combined | 4 | 1.73 (1.53, 1.96) | <1 | 0.52 | 3 | 1.06 (0.91, 1.22) | 56.5 | 0.10 |
| Adjusting for: | ||||||||
| Smoking | ||||||||
| Yes | 5 | 1.79 (1.47, 2.19) | <1 | 0.64 | 3 | 1.06 (0.93, 1.22) | 69.1 | 0.04 |
| No | 3 | 1.62 (1.39, 1.89) | 15.4 | 0.31 | 2 | 1.14 (1.02, 1.27) | <1 | 0.46 |
| VO2max | ||||||||
| Yes | 2 | 1.86 (1.21, 2.87) | <1 | 0.66 | 1 | 1.08 (0.91, 1.28) | NA | NA |
| No | 6 | 1.67 (1.49, 1.87) | <1 | 0.41 | 4 | 1.10 (0.99, 1.21) | 58 | 0.07 |
| At least 3 metabolic factors | ||||||||
| Yes | 6 | 1.75 (1.46, 2.10) | <1 | 0.73 | 3 | 1.06 (0.93, 1.22) | 69.1 | 0.04 |
| No | 2 | 1.59 (1.26, 2.01) | 57.3 | 0.13 | 2 | 1.14 (1.02, 1.27) | <1 | 0.46 |
HR indicates hazard ratio; HRR, heart rate recovery; NA, not applicable; VO2max, maximal oxygen consumption.
Table 5.
Meta‐Regression Analysis of Attenuated HRR and Risk of All‐Cause Mortality
| Variable | Attenuated HRR vs Fast HRR | Per 10 bpm Decrement in HRR | ||||
|---|---|---|---|---|---|---|
| N | Beta‐Coefficient | P Value | N | Beta‐Coefficient | P Value | |
| BMI | 6 | 1.08 | 0.47 | 5 | 1.02 | 0.79 |
| Agea | 8 | 1.03 | 0.96 | 5 | 1.13 | 0.68 |
| Proportion of men | 8 | 1.00 | 0.38 | 5 | 1.00 | 0.75 |
| Follow‐up | 8 | 0.99 | 0.47 | 5 | 1.00 | 0.90 |
| Resting heart rate | 3 | 1.03 | 0.46 | 3 | 1.01 | 0.49 |
bpm indicates beats per minute; HRR, heart rate recovery.
Age data were log‐transformed for analysis.
Sensitivity Analysis and Publication Bias
Sensitivity analyses by omitting a single study at a time or re‐analyzing the data upon the replacement of 1‐minute HRR with 2‐minute HRR9, 20 did not substantially affect the overall HRs of all the outcomes of interest in the categorical or dose–response analyses. No significant evidence of publication bias was detected for all‐cause mortality (P=0.85 for categorical analysis and 0.49 for dose–response analysis), except for cardiovascular events (P=0.29 for categorical analysis and 0.08 for dose–response analysis).
Discussion
Our meta‐analysis of prospective cohort studies showed that attenuated HRR was associated with a higher risk of cardiovascular events and all‐cause mortality in comparison to the references among the general population. For every 10‐bpm decrement in HRR, the risk was increased by 13% and 9%, respectively. Our meta‐analysis also showed that 2‐minute HRR made no significant difference from 1‐minute HRR in predicting the risk of all‐cause mortality, but it seems likely that 2‐minute HRR was more sensitive in predicting the risk of cardiovascular events. Our meta‐analysis further showed that the inverse associations observed between attenuated HRR and risk of fatal cardiovascular events and all‐cause mortality were independent of traditional metabolic factors for CVD.
Interpretations
It is biologically plausible that attenuated HRR may lead to a spectrum of poor health outcomes as shown in our study, although the exact underlying mechanisms are unclear. First, evidence suggests that there is a strong and direct dose–response association between HRR and cardiorespiratory fitness,1 while the latter was demonstrated to be closely related to the incident risk of cardiovascular events as well as all‐cause mortality.32 Secondly, it is well recognized that the autonomic nervous system is essential in the maintenance of glycemic homeostasis, in which parasympathetic fibers stimulate the β cells to release insulin in response to elevated glucose levels, and sympathetic activation inhibits insulin secretion.33 Dysfunction of the autonomic nervous system that is signified by attenuated HRR would then result in declined insulin secretion but raised glucose levels, leading subsequently to the development of diabetes mellitus and disorders such as CVD through multiple mechanisms including glucose toxicity,34 inflammation,35 and endothelial dysfunction.36 Thirdly, a recent cross‐sectional study by Kuo et al noted that chronic inflammation and insulin resistance were inversely related to HRR,37 while both factors are believed to be hallmarks of CVD. Finally, since HRR may reflect parasympathetic nervous system function, and given that increased parasympathetic tone has antiarrhythmic effects,38 it is conceivable that attenuated HRR would predict death because of the possibly increased risk of cardiac arrhythmias.
In agreement with some results from our meta‐analysis, several narrative reviews pointed out that attenuated HRR has great promise in predicting cardiac events or all‐cause mortality.2, 22, 39 However, the enrolled participants varied substantially from healthy populations to patients with type 2 diabetes mellitus, myocardial infarction, or heart failure among these reviews, making their conclusions difficult to apply to the general population. Moreover, the lack of critical analyses such as subgroup or dose–response analyses further limits their depths in investigating the prognostic value of HRR. Our current meta‐analysis therefore provides more robust findings on these issues.
However, one should be aware of the fact that measurement of HRR has not been recommended as a routine examination in the guidelines for risk stratification of CVD or mortality40, 41 until recently, when an updated clinical recommendation was proposed.42 Yet the concern still remains regarding the validation issues of HRR, which primarily include the standardization of exercise or recovery protocol and the criterion for abnormality.2, 43 Noteworthy, the results from our meta‐analysis might assist at least partly in addressing these uncertainties for the following reasons. First, as also suggested by Peçanha et al,2 it seems likely that the maximal workloads using the Bruce or Balke protocol might be superior to the submaximal workloads in predicting the risk of cardiovascular events and all‐cause mortality as evidenced by our subgroup analyses. Secondly, the HRs of all‐cause mortality related to attenuated HRR were comparable for studies involving active cool‐down protocols versus others employing passive ones (P for interaction=0.78), although this comparison was indirect and the number of included studies was limited. Thirdly, the criteria of abnormal HRR (or attenuated HRR) varied from each individual study in general, which might limit the interpretation of such findings. Yet the dose–response analysis using HRR as a continuous variable in our meta‐analysis would help to overcome this limitation, which suggests the possibility of “the faster the HRR, the better the outcome.” Moreover, our results showed that both 1‐ and 2‐minute HRR were effective in predicting cardiovascular events and all‐cause mortality, indicating that the efficacy of HRR in predicting health outcomes might be independent of recovery time points, which are recognized to contribute to the different criteria of abnormal HRR.
There is evidence that attenuated HRR coexists with metabolic risk factors such as obesity and dyslipidemia,16 and individuals with attenuated HRR are prone to unhealthy lifestyle behaviors such as smoking,16 which raises the concern of whether attenuated HRR is an independent predictor. As a minimum response, our results indicated that attenuated HRR was able to predict the risk of fatal cardiovascular events and all‐cause mortality when adjusting for these risk factors. On the other hand, given that Jolly et al observed that patients with attenuated HRR at baseline but normalized HRR after rehabilitation exhibited similar survival rates to those with normal HRR at baseline and after rehabilitation,44 it might be safe to conclude that attenuated HRR is an independent risk predictor, at least for all‐cause mortality.
Strengths and Limitations
Our study has several strengths. First, it is to date the most comprehensive meta‐analysis on this topic. Secondly, it included only prospective cohort studies, which were found to be of high quality with long periods of follow‐up. Thirdly, it applied strict selection criteria to enroll only a general population and employed dose–response analyses to complement the results from categorical analyses.
Our study has also some limitations. First, although most of the included studies reported outcomes controlling for various known risk factors, we cannot exclude the possibility of residual confounding by dietary or behavioral factors. Secondly, the measurement of HRR may be affected by some factors in addition to the aforementioned ones, such as the position adopted (eg, supine)2 and the use of heart rate–lowering medication (eg, β‐blockers).43 However, the majority of included studies failed to specify them and might not have had proper controls. Thirdly, because of the relatively small number of studies, we were unable to identify a particular population (eg, participants with poor physical fitness, or not taking β‐blockers45) who might be more susceptible to the observed associations, or to employ the restricted cubic splines that have been widely used in the epidemiology literature to account for any potential nonlinearity in these relationships.46, 47 Fourthly, some evidence of heterogeneity and publication bias was detected in our meta‐analysis. Yet the generally consistent results across subgroups or from the sensitivity analyses indicated the robustness of our main findings. Fifthly, it should be mentioned that cardiovascular events cover a wide spectrum of diseases such as coronary heart disease, myocardial infarction, and stroke. Although subgroup analyses restricting to fatal or nonfatal cardiovascular events were performed in our study to lower the chance of existing heterogeneity by definition, the generalization of such results to a certain cardiovascular event like stroke or heart failure might be somehow limited. Moreover, none of the included studies had specifically assessed the value of HRR in predicting such outcomes. Further studies are required to address these issues. Finally, our meta‐analysis did not assess the efficacy or cut‐off point of HRR in diagnosing CVD, which also call for further investigations.
Conclusions
In conclusion, findings from our meta‐analysis provided evidence that attenuated HRR is consistently associated with increased risk of cardiovascular events and all‐cause mortality in the general population. These results support the recommendation of recording HRR for risk assessment in routine clinical practice, which would enable the implementation of timely preventive interventions. Future studies are required to determine the normal reference range of HRR across different recovery time points among ethnic‐specified populations, to evaluate the cost‐effectiveness of exercise stress testing for assessing HRR with regard to the primary prevention of chronic disease morbidity and mortality, and to assess whether HRR has any therapeutic implications.
Author Contributions
Qiu conducted the study, collected and analyzed the data, and wrote the manuscript. Cai collected the data. Zuegel and Steinacker contributed to the introduction, and reviewed/edited the manuscript. Sun, Li, and Schumann designed the study, contributed to the discussion, and edited the manuscript. All authors read and approved the final manuscript.
Disclosures
None.
Supporting information
Table S1. Meta‐Analysis of Observational Studies in Epidemiology Checklist
Table S2. Search Strategy
(J Am Heart Assoc. 2017;6:e005505 DOI: 10.1161/JAHA.117.005505.)28487388
References
- 1. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. [DOI] [PubMed] [Google Scholar]
- 2. Peçanha T, Silva‐Junior ND, Forjaz CL. Heart rate recovery: autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin Physiol Funct Imaging. 2014;34:327–339. [DOI] [PubMed] [Google Scholar]
- 3. Coote JH. Recovery of heart rate following intense dynamic exercise. Exp Physiol. 2010;95:431–440. [DOI] [PubMed] [Google Scholar]
- 4. Borresen J, Lambert MI. Autonomic control of heart rate during and after exercise: measurements and implications for monitoring training status. Sports Med. 2008;38:633–646. [DOI] [PubMed] [Google Scholar]
- 5. Bellenger CR, Fuller JT, Thomson RL, Davison K, Robertson EY, Buckley JD. Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta‐analysis. Sports Med. 2016;46:1461–1486. [DOI] [PubMed] [Google Scholar]
- 6. Daanen HA, Lamberts RP, Kallen VL, Jin A, Van Meeteren NL. A systematic review on heart‐rate recovery to monitor changes in training status in athletes. Int J Sports Physiol Perform. 2012;7:251–260. [DOI] [PubMed] [Google Scholar]
- 7. Ho JS, Fitzgerald SJ, Barlow CE, Cannaday JJ, Kohl HW III, Haskell WL, Cooper KH. Risk of mortality increases with increasing number of abnormal non‐ST parameters recorded during exercise testing. Eur J Cardiovasc Prev Rehabil. 2010;17:462–468. [DOI] [PubMed] [Google Scholar]
- 8. Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all‐cause death in asymptomatic women: a 20‐year follow‐up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–1607. [DOI] [PubMed] [Google Scholar]
- 9. Morshedi‐Meibodi A, Larson MG, Levy D, O'Donnell CJ, Vasan RS. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (the Framingham Heart Study). Am J Cardiol. 2002;90:848–852. [DOI] [PubMed] [Google Scholar]
- 10. Park JI, Shin SY, Park SK, Barrett‐Connor E. Usefulness of the integrated scoring model of treadmill tests to predict myocardial ischemia and silent myocardial ischemia in community‐dwelling adults (from the Rancho Bernardo study). Am J Cardiol. 2015;115:1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long‐distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. [DOI] [PubMed] [Google Scholar]
- 12. Carnethon MR, Jacobs DR Jr, Sidney S, Liu K; Study C . Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care. 2003;26:3035–3041. [DOI] [PubMed] [Google Scholar]
- 13. Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population‐based study. Diabetes. 2002;51:803–807. [DOI] [PubMed] [Google Scholar]
- 14. Kannankeril PJ, Le FK, Kadish AH, Goldberger JJ. Parasympathetic effects on heart rate recovery after exercise. J Investig Med. 2004;52:394–401. [DOI] [PubMed] [Google Scholar]
- 15. Buchheit M, Papelier Y, Laursen PB, Ahmaidi S. Noninvasive assessment of cardiac parasympathetic function: postexercise heart rate recovery or heart rate variability? Am J Physiol Heart Circ Physiol. 2007;293:H8–H10. [DOI] [PubMed] [Google Scholar]
- 16. Carnethon MR, Sternfeld B, Liu K, Jacobs DR Jr, Schreiner PJ, Williams OD, Lewis CE, Sidney S. Correlates of heart rate recovery over 20 years in a healthy population sample. Med Sci Sports Exerc. 2012;44:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. [DOI] [PubMed] [Google Scholar]
- 18. Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all‐cause mortality in a preventive medicine program. JAMA. 2004;292:1462–1468. [DOI] [PubMed] [Google Scholar]
- 19. Johnson NP, Goldberger JJ. Prognostic value of late heart rate recovery after treadmill exercise. Am J Cardiol. 2012;110:45–49. [DOI] [PubMed] [Google Scholar]
- 20. Savonen KP, Kiviniemi V, Laaksonen DE, Lakka TA, Laukkanen JA, Tuomainen TP, Rauramaa R. Two‐minute heart rate recovery after cycle ergometer exercise and all‐cause mortality in middle‐aged men. J Intern Med. 2011;270:589–596. [DOI] [PubMed] [Google Scholar]
- 21. Wandell PE, Carlsson AC, Theobald H. Effect of heart‐rate recovery on long‐term mortality among men and women. Int J Cardiol. 2010;144:276–279. [DOI] [PubMed] [Google Scholar]
- 22. Huang PH, Leu HB, Chen JW, Lin SJ. Heart rate recovery after exercise and endothelial function–two important factors to predict cardiovascular events. Prev Cardiol. 2005;8:167–170; quiz 171. [DOI] [PubMed] [Google Scholar]
- 23. Okutucu S, Karakulak UN, Aytemir K, Oto A. Heart rate recovery: a practical clinical indicator of abnormal cardiac autonomic function. Expert Rev Cardiovasc Ther. 2011;9:1417–1430. [DOI] [PubMed] [Google Scholar]
- 24. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 25. Snoek JA, van Berkel S, van Meeteren N, Backx FJ, Daanen HA. Effect of aerobic training on heart rate recovery in patients with established heart disease: a systematic review. PLoS One. 2013;8:e83907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all‐cause mortality: a meta‐analysis. JAMA. 2011;305:2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 28. Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta‐analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378–1385. [DOI] [PubMed] [Google Scholar]
- 29. Manzoli L, Villari P, M Pirone G, Boccia A. Marital status and mortality in the elderly: a systematic review and meta‐analysis. Soc Sci Med. 2007;64:77–94. [DOI] [PubMed] [Google Scholar]
- 30. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 31. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011. Available at: http://handbook.cochrane.org/. Accessed February 11, 2017. [Google Scholar]
- 32. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 33. Kiba T. Relationships between the autonomic nervous system and the pancreas including regulation of regeneration and apoptosis: recent developments. Pancreas. 2004;29:e51–e58. [DOI] [PubMed] [Google Scholar]
- 34. Campos C. Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med. 2012;124:90–97. [DOI] [PubMed] [Google Scholar]
- 35. Hevener AL, Febbraio MA; Stock Conference Working G . The 2009 stock conference report: inflammation, obesity and metabolic disease. Obes Rev. 2010;11:635–644. [DOI] [PubMed] [Google Scholar]
- 36. Davel AP, Wenceslau CF, Akamine EH, Xavier FE, Couto GK, Oliveira HT, Rossoni LV. Endothelial dysfunction in cardiovascular and endocrine‐metabolic diseases: an update. Braz J Med Biol Res. 2011;44:920–932. [DOI] [PubMed] [Google Scholar]
- 37. Kuo HK, Gore JM. Relation of heart rate recovery after exercise to insulin resistance and chronic inflammation in otherwise healthy adolescents and adults: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Clin Res Cardiol. 2015;104:764–772. [DOI] [PubMed] [Google Scholar]
- 38. Lauer MS. Autonomic function and prognosis. Cleve Clin J Med. 2009;76(suppl 2):S18–S22. [DOI] [PubMed] [Google Scholar]
- 39. Chou R, Arora B, Dana T, Fu R, Walker M, Humphrey L. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive services Task Force. Ann Intern Med. 2011;155:375–385. [DOI] [PubMed] [Google Scholar]
- 40. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr; American College of Cardiology/American Heart Association Task Force on Practice G . ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to update the 1997 exercise testing guidelines). Circulation. 2002;106:1883–1892. [DOI] [PubMed] [Google Scholar]
- 41. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J; EACPR, AHA . EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012;33:2917–2927. [DOI] [PubMed] [Google Scholar]
- 42. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016;133:e694–e711. [DOI] [PubMed] [Google Scholar]
- 43. Gibbons RJ. Abnormal heart‐rate recovery after exercise. Lancet. 2002;359:1536–1537. [DOI] [PubMed] [Google Scholar]
- 44. Jolly MA, Brennan DM, Cho L. Impact of exercise on heart rate recovery. Circulation. 2011;124:1520–1526. [DOI] [PubMed] [Google Scholar]
- 45. Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- 46. Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta‐analysis of prospective cohort studies. Circulation. 2014;130:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang D, Shen X, Qi X. Resting heart rate and all‐cause and cardiovascular mortality in the general population: a meta‐analysis. CMAJ. 2016;188:E53–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Meta‐Analysis of Observational Studies in Epidemiology Checklist
Table S2. Search Strategy


