Abstract
Background
Chronic hypertension complicates around 3% of all pregnancies. There is evidence that treating severe hypertension reduces maternal morbidity. This study aimed to systematically review randomized controlled trials of antihypertensive agents treating chronic hypertension in pregnancy to determine the effect of this intervention.
Methods and Results
Medline (via OVID), Embase (via OVID) and the Cochrane Trials Register were searched from their earliest entries until November 30, 2016. All randomized controlled trials evaluating antihypertensive treatments for chronic hypertension in pregnancy were included. Data were extracted and analyzed in Stata (version 14.1). Fifteen randomized controlled trials (1166 women) were identified for meta‐analysis. A clinically important reduction in the incidence of severe hypertension was seen with antihypertensive treatment versus no antihypertensive treatment/placebo (5 studies, 446 women; risk ratio 0.33, 95%CI 0.19‐0.56; I2 0.0%). There was no difference in the incidence of superimposed pre‐eclampsia (7 studies, 727 women; risk ratio 0.74, 95%CI 0.49‐1.11; I2 28.1%), stillbirth/neonatal death (4 studies, 667 women; risk ratio 0.37, 95%CI 0.11‐1.26; I2 0.0%), birth weight (7 studies, 802 women; weighted mean difference −60 g, 95%CI −200 to 80 g; I2 0.0%), or small for gestational age (4 studies, 369 women; risk ratio 1.01, 95%CI 0.53‐1.94; I2 0.0%) with antihypertensive treatment versus no treatment/placebo.
Conclusions
Antihypertensive treatment reduces the risk of severe hypertension in pregnant women with chronic hypertension. A considerable paucity of data exists to guide choice of antihypertensive agent. Adequately powered head‐to‐head randomized controlled trials of commonly used antihypertensive agents are required to inform prescribing.
Keywords: antihypertensive agent, hypertension, meta‐analysis, pregnancy, systematic review
Subject Categories: Pregnancy, Hypertension, Treatment, Meta Analysis
Introduction
Chronic hypertension complicates around 3% of all pregnancies.1, 2 There is growing evidence that the incidence is rising with increasing maternal age and obesity.2, 3, 4, 5 The increased risks of adverse perinatal outcomes for pregnant women with chronic hypertension are well established.6, 7 In addition, controlling severe systolic hypertension has been recommended repeatedly by national and international guidance to reduce the risks of maternal morbidity and mortality.8, 9, 10
There remains some debate regarding the efficacy of treating chronic hypertension in pregnancy before it reaches severe levels due to concerns for fetal growth.11, 12, 13, 14, 15, 16 Internationally, guidelines vary for the management of chronic hypertension in pregnancy.17 However, the Control of Hypertension in Pregnancy Study, published in 2015, reported that there was no effect of tight blood pressure control (target diastolic 85 mm Hg) compared to less tight control (target diastolic 105 mm Hg) on a composite outcome of pregnancy loss and high‐level neonatal care within the first 48 hours of infant life (31.4% versus 30.7%) and the overall risk of small‐for‐gestational‐age infants (birth weight <10th centile) was not different between groups (16.1% versus 19.7%; odds ratio 0.78, 95%CI 0.56‐1.08). The frequency of severe hypertension was significantly higher with less‐tight control compared with tight control (40.6% versus 27.5%; odds ratio 1.8, 95%CI 1.3‐2.4).18 There are likely to be additional benefits of reducing the incidence of severe hypertension through a decrease in short‐ and long‐term maternal morbidity and mortality from stroke and other end‐organ damage9, 19, 20, 21, 22 and potential cost savings with a reduction in healthcare resource use.23, 24
Given the physiological demands of pregnancy, duration of treatment and potential impacts on maternal and perinatal outcomes, there is a need for evidence on efficacy and safety of antihypertensive treatment specifically in pregnancy complicated by chronic hypertension. Current international guidance points to the lack of evidence for antihypertensive agent prescribing in chronic hypertension in pregnancy.8, 17 Because the benefits of tight‐control blood pressure targets have now been demonstrated in women with hypertension in pregnancy, this study aimed to systematically review and meta‐analyze available data from randomized controlled trials specifically in chronic hypertension to establish the efficacy and safety of antihypertensive agents or class of agents.
Methods
The study protocol for this systematic review was developed in line with the PRISMA‐P statement25 and registered on the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO/ reference number CRD42015020733). No ethical approval was required.
Literature Search
A comprehensive literature review using Medline (via Ovid), Embase (via Ovid), and the Cochrane Trials Register from their earliest entries until the November 30, 2016 was performed. Search strategies were adapted to each database. Searches of exploded MeSH terms “pregnancy,” “hypertension,” and “antihypertensive” (Embase) or “cardiovascular agent” (Medline) were performed individually and then combined in each database. For Medline and Embase searches, a search filter for randomized controlled trials was then applied as recommended in the Cochrane Handbook for Systematic Reviews of Interventions.26 Relevant unpublished data were sought by searching for trials registered on clinicaltrials.gov and ISRCTN (www.isrctn.com) and reviewing thesis titles from the World Cat dissertations and theses database. References of retrieved studies and relevant review articles were also searched using the snowballing approach. No language restrictions were applied. The study protocol (including the literature search strategy) is detailed in Data S1.
Study Selection Criteria
All randomized controlled trials of pregnant women with chronic hypertension comparing an antihypertensive agent with another treatment arm as long‐term antepartum management were included. No blood pressure cutoffs were utilized in the eligibility criteria for inclusion, but studies examining acute treatment of severe hypertension via intravenous/fast‐acting routes were excluded. Comparisons with other antihypertensive drug(s), placebo, no treatment, or an alternative such as bed rest were eligible for inclusion. Studies that included participants with gestational hypertension and chronic hypertension were only eligible for inclusion if the data for the women with chronic hypertension were reported separately to allow fair comparison. Studies that compared management strategies only but did not include a randomized comparison of drug treatments were not eligible for inclusion. Trials that did not report any of the predefined outcomes were excluded. Trials that did not include sufficient information on the outcomes (eg, standard deviations) could not be included in the meta‐analysis. No other restrictions were applied to the study search.
Data Extraction
The titles, abstracts and selected full texts generated from the literature search were independently screened by authors L.M.W. and F.C.R. Data from the trials that met all inclusion criteria were manually extracted and entered into a standard extraction table independently from full texts by L.M.W. and F.C.R. The authors were not masked to the results of the study or authors. Where 2 articles published results from the same study, individual pertinent outcomes were extracted from both articles without repetition of data extraction. The following outcome measures were recorded for each study: maternal, severe hypertension (definitions used in each study documented), superimposed pre‐eclampsia (definitions used in each study documented), cesarean section delivery, abruption; perinatal, stillbirth/neonatal death, birth weight, small‐for‐gestational‐age infants (within trial definition), preterm birth (defined as less than 37 completed weeks’ gestation), and Apgar score less than 7 at 5 minutes. Details of potential confounders (maternal age, body mass index, ethnicity) were recorded wherever provided in the manuscripts. The PRISMA statement was considered and observed for all procedures and reporting.27
Study Quality Assessment
Each trial was independently quality assessed using the Cochrane Collaboration Risk of Bias tool by L.M.W. and F.C.R.26 The risk of bias in each of the following domains was assessed: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias.
Statistical Methods
Data were analyzed in the statistical package Stata (version 14.1, StataCorp, College Station, TX), using the metan suite of commands.28 All outcomes were analyzed on an intention‐to‐treat basis. Per‐protocol data for an end point were excluded from the analysis. Meta‐analysis was performed using a fixed‐effects model where there was more than 1 study with analyzable data. If there was evidence of significant heterogeneity, the meta‐analysis was repeated using the random‐effects model for comparison; however, the results presented are from the fixed‐effects analysis. Initial analysis of treatment effects was performed by class of antihypertensive agent and subsequently by active versus nonactive treatment. Treatment effects are presented as estimated differences in mean or risk ratios with 95% confidence intervals. Heterogeneity was quantified via the τ‐squared and I‐squared statistics.29
Results
Description of Studies
The study selection process is illustrated in the flowchart (Figure 1). After removal of duplicates, the initial search generated 501 titles and abstracts for review. Following screening, 39 articles underwent full‐text assessment. Sixteen articles met inclusion criteria, reporting on 15 trials that recruited a total of 1166 women, with a median of 20 participants per trial (interquartile range 12‐60 participants per trial). The characteristics of the studies meeting entry criteria are presented in Table 1.
Figure 1.
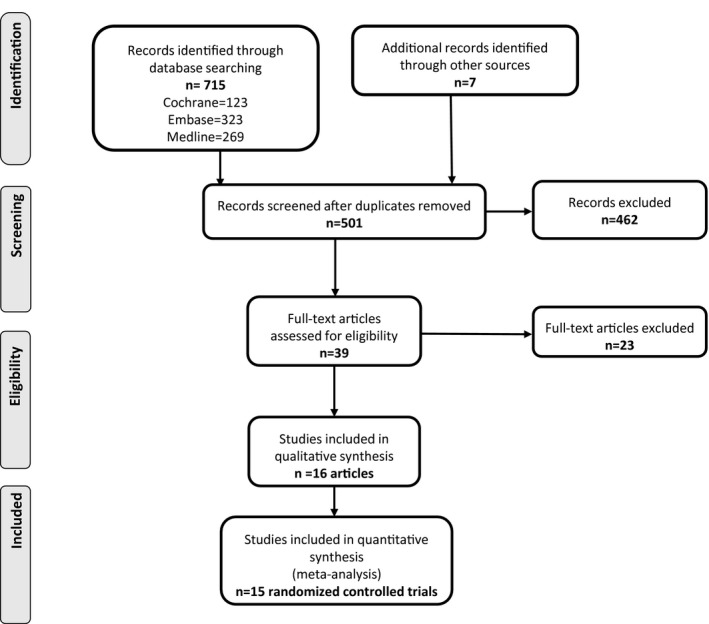
Flowchart of articles identified reporting randomized controlled trials of antihypertensive agents for the treatment of chronic hypertension in pregnancy.
Table 1.
Characteristics of the Studies Included in the Meta‐Analysis
| Study First Author, Country, Year | Methods | Participants With Chronic Hypertension | Intervention | Outcomes Included in Meta‐Analysis |
|---|---|---|---|---|
| Arias, USA, 197930, a |
|
Excluded:
|
Active:
A varied combination of:
Vs nonactive:
|
Maternal:
Perinatal:
|
| Butters, UK, 199031 |
|
Excluded:
|
Active:
Vs nonactive:
|
Perinatal:
|
| Fiddler, UK, 198332 |
|
Excluded:
|
Active:
Vs active:
|
Maternal:
Perinatal:
|
| Freire, Brazil, 198833 |
|
Excluded:
|
Active:
Vs active:
|
Maternal:
Perinatal:
|
| Hirsch, Israel, 199634 |
|
Excluded:
|
Active:
Vs non‐active:
|
Maternal:
Perinatal:
|
| Horvath, Australia, 198535 |
|
|
Active:
Vs nonactive:
|
Perinatal:
|
| Kahhale, Brazil, 198536 |
|
Excluded:
|
Active:
Vs nonactive:
|
Perinatal:
|
| Mutch, UK, 197737, b |
|
Excluded:
|
Active:
Vs nonactive:
|
Maternal:
|
| Parazzini, Italy, 199838 |
|
Excluded:
|
Active:
Vs nonactive:
|
Perinatal:
|
| Redman, UK, 197639, b |
|
Excluded:
|
Active:
Vs nonactive:
|
Perinatal:
|
| Sibai, USA, 198440 |
|
|
Active:
Vs nonactive:
|
Maternal:
Perinatal:
|
| Sibai, USA, 199041 |
|
Excluded:
|
Active:
Vs active:
Vs nonactive:
|
Maternal:
Perinatal:
|
| Steyn, South Africa, 199742 |
|
Excluded:
|
Active:
Vs nonactive:
|
Maternal:
Perinatal:
|
| Voto, Argentina, 199043 |
|
Excluded:
|
Active:
Vs active:
Vs active:
|
Maternal:
|
| Weitz, USA, 198744 |
|
|
Active:
Vs nonactive:
|
Maternal:
Perinatal:
|
| Welt, USA, 198145 |
|
Excluded:
|
Active:
Vs active:
Vs nonactive:
|
Maternal:
Perinatal:
|
BP indicates blood pressure.
Participants randomized to antihypertensive treatment (not to an agent) vs no antihypertensive treatment.30
Articles reporting on the same study population.
All studies included in the meta‐analysis were completed before 1998. Ten of the trials were conducted in a predefined chronic hypertension cohort alone,1 and the remaining 5 reported outcomes for a subgroup of women with chronic hypertension.32, 35, 37, 38, 39, 43 Six studies were head‐to‐head comparisons of 2 or more antihypertensive agents (435 women),32, 33, 35, 41, 43, 45 4 were placebo‐controlled studies of a single antihypertensive agent (219 women),31, 34, 42, 44 and 5 were studies of a single antihypertensive agent compared to no treatment (714 women).30, 36, 37, 38, 39, 40
Of the 23 articles that were excluded, 14 studies included a mixed population of gestational and chronic hypertension and did not report outcomes separately, 6 studies included only gestational hypertension, 1 article reported no additional outcomes for a trial already included in the meta‐analysis (Table 2).46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 In addition, Leather and colleagues reported a randomized controlled trial in 1968 that recruited 47 chronic hypertensive participants randomized to bendroflumethiazide and methyldopa versus no treatment. This article could not be included due to inadequate reporting of the statistical information relating to the outcomes, prohibiting inclusion of the data in the meta‐analysis.58 Leather and colleagues concluded that the treatment of “early hypertension” (present before 20 weeks’ gestation) resulted in a longer pregnancy, increased birth weight and reduced perinatal mortality. A pilot study by Vigil‐De Gracia and colleagues in 2014 compared furosemide, amlodipine, and aspirin in a 3‐arm randomized controlled trial and found no significant difference in outcomes among all treatment arms.65 These data could not be included in the active versus nonactive treatment meta‐analysis, as the third arm of aspirin was considered active treatment given that the other arms did not receive this agent. In addition, the data from the amlodipine and furosemide arms could not be included in the antihypertensive treatment versus antihypertensive treatment meta‐analysis as there are no other head‐to‐head trials evaluating calcium‐channel blockers or diuretics for comparison.
Table 2.
Studies Excluded From the Meta‐Analysis and Rationale
| Study (First Author, Country, Year Published) | Reason for Exclusion and Study Details |
|---|---|
| Antony, South Africa, 199046 |
Study participants had gestational and not chronic hypertension Methods: Prospective, randomized block design, no further details given Participants: 60 women at 28 to 36 weeks’ gestation with mean 24‐h diastolic BP 100 to 120 mm Hg±proteinuria Intervention: Indoramin 50 mg twice daily vs methyldopa 1 g twice daily vs placebo 1 tablet daily |
| Bolte, Netherlands, 199847 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized, open‐label multicenter trial Participants: 31 women, 26 to 32 weeks’ gestation with diastolic BP >110 mm Hg and previously normotensive or in women with chronic hypertension: diastolic BP >20 mm Hg compared to BP at <20 weeks. Intervention: IV ketanserin 5 mg bolus then 4 mg/h vs IV dihydralazine 1 mg/h |
| Bott‐Kanner, Israel, 199248 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized, double‐blind trial. Women randomized in blocks of 6 using serial numbers Participants: 60 women before 35 weeks’ gestation with diastolic BP 85 to 99 mm Hg Intervention: Pindolol 5 mg twice daily or placebo 1 tablet twice daily |
| Cruickshank, UK, 199149 |
Study participants had gestational and not chronic hypertension Methods: Randomized open‐label trial, using numbered sealed envelopes Participants: 114 women with singleton pregnancies between 24 and 39 weeks’ gestation, diastolic BP >90 mm Hg for >24 h in absence of proteinuria Intervention: Labetalol 100 mg twice daily vs no treatment |
| Faneite, Venezuela, 198850 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized trial Participants: 31 women >14 weeks’ gestation, with BP >140/90 and <170/110 mm Hg on 2 occasions Intervention: Mepindolol 5 mg once daily vs methyldopa 250 mg twice daily |
| Gallery, Australia, 197951 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized comparison study Participants: 56 women at any gestation with sitting diastolic BP >95 mm Hg on 2 occasions at least 24 h apart or 100 mm Hg on 2 occasions at least 8 h apart Intervention: Oxprenolol vs methyldopa. Doses not specified |
| Gallery, Australia, 198552 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized open study, allocation by random number series Participants: 183 women with singleton pregnancies and sitting diastolic BP of >90 mm Hg on 2 occasions at least 24 h apart or >95 mm Hg on 2 occasions 12 h apart or >100 mm Hg on 2 occasions 8 h apart Intervention: Oxprenolol 40 mg twice daily vs methyldopa 250 mg twice daily |
| Hall, South Africa, 200053 |
Study participants had pre‐eclampsia or gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized single‐blind controlled trial. Computer‐generated balanced blocks of 50 numbers. Women allocated using consecutive numbered, opaque envelopes containing medication Participants: 150 women with severe early‐onset pre‐eclampsia or hypertension and BP not controlled with methyldopa 2 mg daily Intervention: Nifedipine 10 mg 3 times daily vs prazosin 1 mg 3 times daily |
| Henderson‐Smart, Australia, 198454 |
Details of participants with chronic hypertension not stated Methods: Reporting neonatal outcomes of infants born to women with hypertension in pregnancy who were entered in a prospective randomized double‐blind trial Participants: 95 infants born to mothers treated with clonidine hydrochloride and methyldopa Intervention: Clonidine hydrochloride 150 to 1200 μg/day vs methyldopa 250 to 2000 mg/day |
| Högstedt, Sweden, 198555 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized open controlled trial Participants: 161 women in antenatal care with diastolic BP >90 mm Hg on 2 occasions at least 6 h apart, confirmed the following day with diastolic BP >90 mm Hg for at least 2 out of 4 BP readings Intervention: 50 mg metoprolol and 25 mg hydralazine twice daily vs no treatment |
| Jannet, France, 199456 |
Study participants had gestational or chronic hypertension or pre‐eclampsia. Outcomes for those with chronic hypertension not reported separately Methods: Randomized comparative trial. Computer‐generated random numbers, allocated using sealed envelopes Participants: 100 women with singleton pregnancies at >20 weeks’ gestation with systolic BP >140 mm Hg and/or diastolic BP >90 mm Hg on 2 successive measurements Intervention: Nicardipine 20 mg 3 times daily vs slow‐release metoprolol 200 mg once daily |
| Lardoux, France, 198857 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized open comparative trial Participants: 63 women between 7 and 36 weeks’ gestation with diastolic BP >90 mm Hg on 2 occasions at least 8 days apart Intervention: Methyldopa 500 mg/day vs labetalol 400 mg/day vs acebutolol 400 mg/day |
| Leather, UK, 196858 |
Outcome data not presented with adequate statistical information to allow inclusion in the meta‐analysis Methods: Randomized controlled trial Participants: 100 women with diastolic BP >90 mm Hg on 2 occasions at least 48 h apart Intervention: Bendroflumethiazide 5 to 10 mg daily and methyldopa 400 to 2000 mg daily vs no treatment |
| Livingstone, Australia, 198359 |
Study participants had gestational and not chronic hypertension Methods: Randomized prospective study, no further details given Participants: 28 women with BP >140/90 mm Hg on 2 consecutive readings at least 24 h apart Intervention: Propranolol vs methyldopa. Doses not specified |
| Moore, UK, 198260 |
Study participants had gestational or chronic hypertension. Outcomes for women with chronic hypertension not reported separately Methods: Randomized trial, no further details given Participants: 74 women at <36 weeks’ gestation with systolic BP >170 mm Hg and/or diastolic BP >110 mm Hg Intervention: Labetalol 100 mg 4 times daily vs 250 mg methyldopa 4 times daily |
| Plouin, France, 198861 |
Study participants had gestational or chronic hypertension. Outcomes for women with chronic hypertension not reported separately Methods: Randomized open controlled trial. Stratified randomization using blinded envelopes Participants: 176 women with a singleton pregnancy, gestational age between 12 and 34 weeks and diastolic BP >89 mm Hg on 2 separate occasions Intervention: Labetalol 400 mg in 2 doses vs methyldopa 500 mg in 2 doses |
| Rosenfeld, Israel, 198662 |
Study participants had gestational or chronic hypertension. Outcomes for those with chronic hypertension not reported separately Methods: Randomized study, no further details given Participants: 44 women at <36 weeks’ gestation with systolic BP >150 mm Hg or diastolic BP >90 mm Hg on 2 separate occasions at least 24 h apart Intervention: Hydralazine 25 mg twice daily vs hydralazine 25 mg twice daily and pindolol 5 mg twice daily |
| Steyn, South Africa, 200163 |
Reporting data from same trial as Steyn 1997,42 reported no additional outcomes Methods: Randomized double blind controlled trial. Computer‐generated balanced‐block structure Participants: 102 women between 12 and 20 weeks’ gestation with diastolic BP >80 mm Hg without proteinuria Intervention: Ketanserin 20 mg twice daily and aspirin 75 mg once daily vs placebo 1 tablet twice daily and aspirin 75 mg once daily |
| Tuimala, Finland, 198864 |
Study participants had gestational and not chronic hypertension Methods: Randomized trial, no further details given Participants: 51 women with BP >149/94 mm Hg measured twice in sitting position after 2 days’ bed rest in hospital Intervention: Atenolol 50 to 100 mg/day vs pindolol 10 to 20 mg/day. If needed, hydralazine 150 mg/day added |
| Vigil‐De Gracia, Panama, 201465 |
3‐arm pilot study including a third active treatment arm of aspirin Methods: Randomized open‐label pilot trial. Computer‐generated code with block size of 6, allocation through sealed envelopes Participants: 63 women at <20 weeks’ gestation with systolic BP 90 to 109 mm Hg Intervention: Furosemide 20 mg once daily vs amlodipine 5 mg once daily vs aspirin 75 mg once daily |
| Voto, Argentina, 198766 |
Study participants had gestational or chronic hypertension or pre‐eclampsia. Outcomes for those with chronic hypertension not reported separately Methods: Randomized open study, no further details given Participants: 20 women with systolic BP >159 mm Hg and/or diastolic BP >99 mm Hg recorded twice 24 h apart Intervention: Ketanserin 20 to 80 mg daily vs methyldopa 500 to 2000 mg daily |
| Wichman, Sweden, 198467 |
Study participants had gestational and not chronic hypertension Methods: Randomized placebo‐controlled trial. Selective allocation Participants: 52 women at <37 weeks’ gestation with systolic BP >140 mm Hg or diastolic BP >90 mm Hg or if there was an elevation of >30 mm Hg systolic or >15 mm Hg diastolic from previous readings Intervention: Metoprolol 50 mg twice daily vs placebo 1 tablet twice daily |
| Wide‐Swensson, Sweden, 199568 |
Study participants had gestational and not chronic hypertension Methods: Randomized parallel double‐blind multicenter trial. Block randomization by numbers, allocation by sealed envelope Participants: 118 women with singleton pregnancy, gestational age between 25 and 37 weeks and diastolic BP between >95 and <110 mm Hg Intervention: Isradipine slow‐release 5 mg twice daily vs placebo 1 tablet twice daily |
BP indicates blood pressure; IV, intravenous.
Definitions of severe hypertension and superimposed pre‐eclampsia for each included study are listed in Table 3. Minimum diastolic and systolic blood pressure eligibility cutoffs ranged from 80 to 99 and 140 to 160 mm Hg, respectively. Two studies excluded women with proteinuria,33, 44 3 studies included women with proteinuria at study entry,32, 35, 43 and the remainder of studies did not specify presence or absence of proteinuria in their methods. Six studies excluded multifetal pregnancies30, 32, 40, 41, 44, 45; the remainder either included women with multifetal pregnancies or did not specify inclusion or exclusion in their methods. Maternal age was the only potential confounding baseline characteristic consistently reported. This ranged from 28 to 33 years, and no adjustment was deemed pertinent to this analysis. Body mass index was not reported in any of the trials, but 6 studies reported maternal weight at trial entry. Ethnicity of the participants was not considered or recorded in any of the trials.
Table 3.
Definitions of Severe Hypertension and Superimposed Pre‐Eclampsia for Each Included Study
| Study (First Author, Country, Year) | Definition of Severe Hypertension | Definition of Superimposed Pre‐Eclampsia |
|---|---|---|
| Arias, USA, 197930 | “Pregnancy‐aggravated hypertension”: >28 weeks’ gestation diastolic BP >100 mm Hg in 2 consecutive readings 6 or more h apart | >1+ proteinuria or more than 300 mg/L protein in 24‐h collection with “pregnancy‐aggravated hypertension” (see definition of severe hypertension) |
| Butters, UK, 199031 | Not reported | Not reported |
| Fiddler, UK, 198332 | Admitted to hospital for hypertension: diastolic BP >110 mm Hg | Not reported |
| Freire, Brazil, 198833 | Diastolic BP persistently >110 mm Hg | Systolic BP increased by 30 mm Hg or diastolic BP increased by 20 mm Hg for 2 consecutive readings at least 6 h apart OR proteinuria OR edema |
| Hirsch, Israel, 199634 | Uncontrolled elevation of diastolic BP >100 mm Hg | Not reported |
| Horvath, Australia, 198535 | Not reported | Not reported |
| Kahhale, Brazil, 198536 | Not reported | BP >170/110 mm Hg or proteinuria <37 weeks’ gestation |
| Mutch, UK, 197737, a | Systolic BP >170 mm Hg or diastolic BP >110 mm Hg on 2 occasions >4 h apart | Edema, proteinuria from midstream urine in absence of infection and raised plasma urate |
| Parazzini, Italy, 199838 | Not reported | Not reported |
| Redman, UK, 197639, a | Systolic BP >170 mm Hg or diastolic BP >110 mm Hg on 2 occasions >4 h apart | Edema, proteinuria from midstream urine in absence of infection and raised plasma urate |
| Sibai, USA, 198440 | Not reported | Not defined but reported as confirmed superimposed pre‐eclampsia |
| Sibai, USA, 199041 | Systolic BP >160 mm Hg or diastolic BP >100 mm Hg | Proteinuria (>1 g/24 h) or elevated uric acid (≥6 mg/dL) during second half of pregnancy |
| Steyn, South Africa, 199742 | Single diastolic BP >120 mm Hg OR 2 consecutive readings of 110 mm Hg at least 4 h apart | Single diastolic BP >110 mm Hg or 2 consecutive measurements of 90 mm Hg or more at least 4 h apart with proteinuria 300 mg/L on 24‐h collection OR 2+ proteinuria on dipstick |
| Voto, Argentina, 199043 | Not reported | Additional proteinuria |
| Weitz, USA, 198744 | Not reported | Sudden rise in systolic BP >30 mm Hg or diastolic BP >15 mm Hg and sudden weight gain >2 lb per week OR proteinuria 2+ or more on dipstick |
| Welt, USA, 198145 | Diastolic BP >100 torr on 2 occasions 6 or more h apart | Proteinuria >trace on dipstick or >300 mg/L in 24 h, edema, or both |
BP indicates blood pressure.
Articles reporting the same study population.
Risk of Bias in Included Studies
All studies were assessed to be at high risk of bias apart from Steyn and colleagues,42 which was assigned unclear risk of bias. Full details of the allocated risk‐of‐bias scoring are displayed in Figure 2. No formal assessment of socioeconomic settings of the studies was made given the small number of studies, but all were from middle‐ or high‐income countries (see Table 1).
Figure 2.
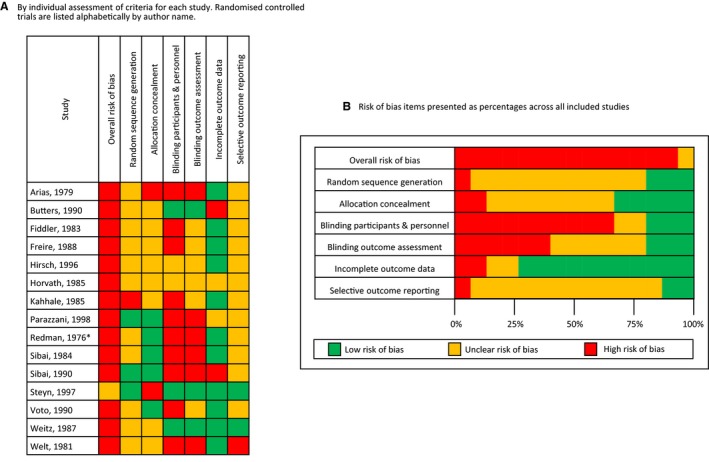
Risk‐of‐bias assessment of each study included in the meta‐analysis. A, Risk‐of‐bias assessment by individual assessment of criteria for each study. Randomized controlled trials are listed alphabetically by author name. B, Risk‐of‐bias items presented as percentages across all included studies. *Redman et al39 and Mutch et al37 both publish data from the same study; only the Redman article has been assessed for risk of bias. Risk‐of‐bias summary shows review authors’ judgments about each risk‐of‐bias domain in randomized controlled trials on efficacy of antihypertensive treatment for chronic hypertension in pregnancy.
Effects of Intervention: Active Versus Nonactive Treatment
Antihypertensive treatment reduces the incidence of severe hypertension in pregnancy complicated by chronic hypertension compared with no antihypertensive or placebo, with a risk ratio of 0.33 (95%CI 0.19‐0.56), based on 446 women from 5 studies. The risk of superimposed pre‐eclampsia was not significantly different between those randomized to active versus nonactive treatment; risk ratio 0.74 (95%CI 0.49‐1.11: 727 women, 7 studies) (Figure 3).
Figure 3.
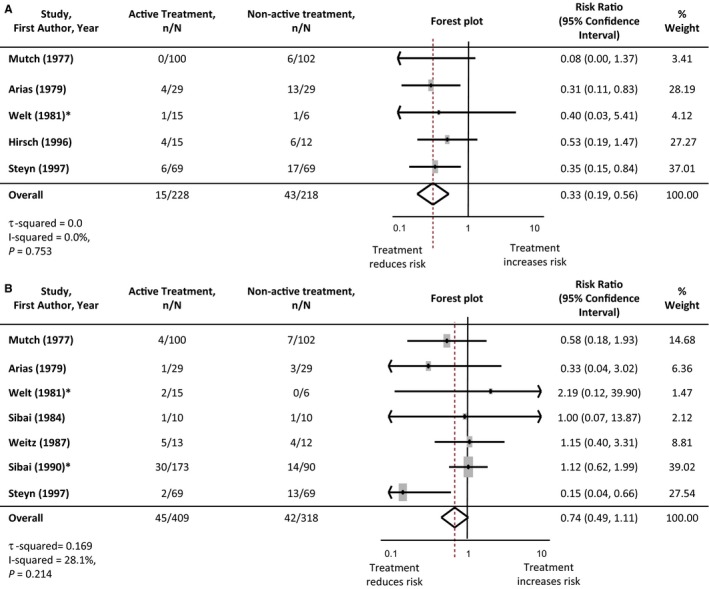
Maternal outcomes: active vs nonactive treatment. A, Severe hypertension. B, Superimposed pre‐eclampsia. *Where studies had more than 1 active treatment arm, the data from the active treatment arms were pooled and compared with the non‐active‐treatment data. Studies are listed in order of the year they were published. Antihypertensive agents used in each study are listed in Table 1. The numbers of participants experiencing severe hypertension or superimposed pre‐eclampsia in each treatment group are denoted as “n,” with the total number of participants with chronic hypertension in each study arm denoted as “N.” Forest plots of the meta‐analysis for each maternal outcome: active vs nonactive treatment. The gray rectangles represent the risk ratio for each study and are sized in proportion to the weight assigned to the study within the analysis. The red dotted line represents to overall risk ratio for each outcome and the lateral tips of the diamond represent the 95% confidence interval for the summary measure.
Perinatal outcomes were assessed to determine the potential fetal and neonatal risks associated with antihypertensive use when compared to nonactive treatment. The analysis of stillbirth and neonatal death demonstrated a nonsignificant reduction with the use of antihypertensive treatment: risk ratio 0.37 (95%CI 0.11‐1.26: 667 women, 4 studies). Birth weight was not significantly different when active versus nonactive treatments were compared (−60 g weighted mean difference, 95%CI −200 to 80 g: 802 women, 7 studies). There was no difference in small‐for‐gestational‐age infants with the use of antihypertensive agents (risk ratio 1.01, 95%CI 0.53‐1.94: 369 women, 4 studies) (Figure 4). A single study by Butters and colleagues comparing atenolol to placebo found a significant reduction in birth weight and increase in small‐for‐gestational‐age infants in the active treatment arm.31 Given the degree of heterogeneity, these results were explored further with the Egger test. This demonstrated the Butters study31 to be an outlier (Figure 5). When this study was included in the meta‐analysis, weighted mean difference in birth weight did not reach significance, −100 g (95%CI −240 to 40 g; I2 49.6%) and similarly although the risk of small‐for‐gestational‐age birth weight increased, it was not significant (risk ratio 1.58, 95%CI l 0.88‐2.85; I2 38.6%).
Figure 4.
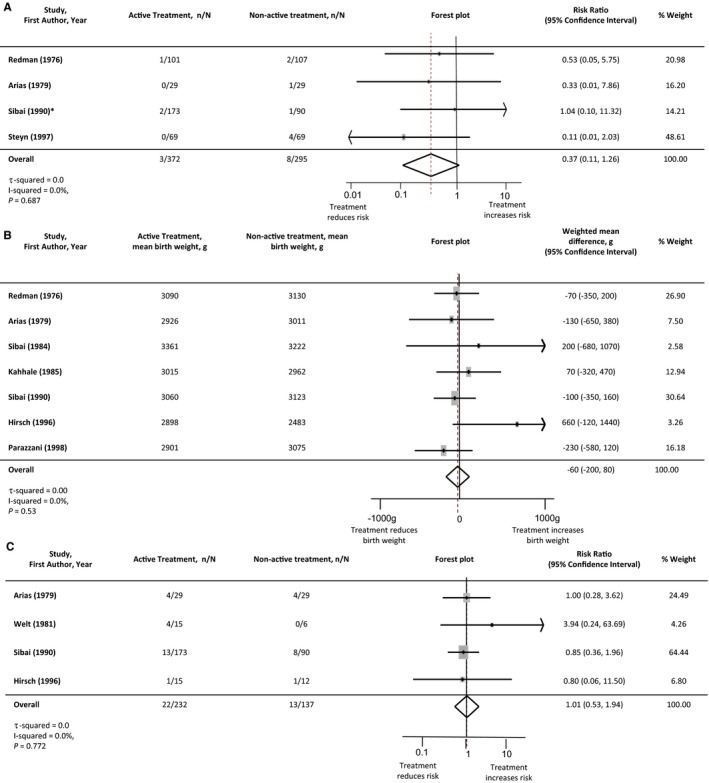
Perinatal outcomes: active vs nonactive treatment. A, Stillbirth or neonatal death. B, Birth weight. C, Small‐for‐gestational‐age infants. *Where studies had more than 1 active treatment arm, the data from the active treatment arms were pooled and compared with the nonactive treatment data. Studies are listed in order of the year they were published. Antihypertensive agents used in each study are listed in Table 1. The numbers of participants experiencing a stillbirth/neonatal death or small‐for‐gestational‐age infant in each treatment group are denoted as “n,” with the total number of participants with chronic hypertension in each study arm denoted as “N.” Forest plots of the meta‐analysis for each perinatal outcome: active vs nonactive treatment. The gray rectangles represent the risk ratio for each study and are sized in proportion to the weight assigned to the study within the analysis. The red dotted line represents to overall risk ratio for each outcome and the lateral tips of the diamond represent the 95% confidence interval for the summary measure.
Figure 5.
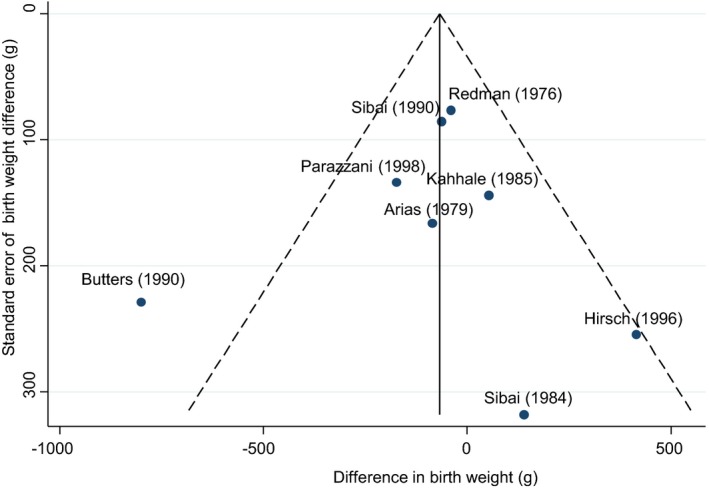
Funnel plot comparing birth‐weight difference between studies. Funnel plot demonstrates that Butters and colleagues31 (atenolol vs placebo) is an outlier within the meta‐analysis of birth weight when comparing active and nonactive treatment. Antihypertensive agents used in each study are listed in Table 1.
The additional maternal and perinatal outcomes meta‐analyzed between active and nonactive arms are listed in Table 4. There were no additional significant differences.
Table 4.
Summary of Meta‐Analysis Findings Comparing Active With Nonactive Treatment and the Effect on Maternal and Perinatal Outcomes in Pregnancy Complicated by Chronic Hypertension
| Outcome | Number of Studies Reporting Outcome | Total Participants | Risk Ratio/Weighted Mean Difference | 95%CI | Degree of Heterogeneity, I2 |
|---|---|---|---|---|---|
| Maternal | |||||
| Severe hypertension | 530, 34, 37, 42, 45 | 446 | 0.33 | 0.19 to 0.56 | 0.0% |
| Superimposed pre‐eclampsia | 730, 37, 40, 41, 42, 44, 45 | 727 | 0.74 | 0.49 to 1.11 | 28.1% |
| Cesarean section delivery | 430, 37, 40, 41 | 543 | 1.23 | 0.92 to 1.63 | 0.0% |
| Abruption | 241, 42 | 401 | 0.35 | 0.10 to 1.27 | 20.9% |
| Perinatal | |||||
| Stillbirth/neonatal death | 430, 39, 41, 42 | 667 | 0.37 | 0.11 to 1.26 | 0.0% |
| Birth weight, g | 730, 34, 36, 38, 39, 40, 41 | 802 | −60 | −200 to 80 g | 0.0% |
| Small for gestational age | 430, 34, 41, 45 | 369 | 1.01 | 0.53 to 1.94 | 0.0% |
| Gestation at delivery, weeks | 730, 34, 39, 40, 41, 42, 44 | 785 | 0.10 | −0.05 to 0.24 | 83.7% |
| Preterm birth | 330, 40, 41 | 341 | 1.23 | 0.58 to 2.54 | 0.0% |
| Apgar score <7 at 5 min | 434, 36, 40, 41 | 410 | 1.13 | 0.40 to 3.20 | 0.0% |
Risk ratios provided where binary data were analyzed, and weighted mean difference given for continuous outcomes.
Effects of Intervention: Antihypertensive Agent Versus Antihypertensive Agent
Due to the small number of studies, comparison of antihypertensive agents was restricted to methyldopa versus other classes of antihypertensive, and where possible methyldopa versus β‐blockers (Table 5). There was no difference in incidence of severe hypertension between agents when methyldopa was compared with other antihypertensive treatments. Two head‐to‐head studies (86 women) reported incidence of severe hypertension comparing methyldopa and β‐blocker antihypertensive treatment: risk ratio 0.85 (95%CI 0.57‐1.37). There was no difference in the incidence of superimposed pre‐eclampsia when methyldopa was compared with other antihypertensive agents. There were additionally no significant differences in perinatal outcomes between antihypertensive agents. Forest plots of these meta‐analyses are presented in Figures 6 and 7.
Table 5.
Summary of Meta‐Analysis Findings Comparing Methyldopa With Other Antihypertensive Agents and the Effect on Maternal and Perinatal Outcomes in Pregnancy Complicated by Chronic Hypertension
| Outcome | Number of Studies Reporting Outcome | Total Participants | Risk Ratio/Weighted Mean Difference | 95%CI | Degree of Heterogeneity, I2 |
|---|---|---|---|---|---|
| Maternal | |||||
| Severe hypertension | 332, 33, 45 | 101 | 1.13 | 0.71 to 1.81 | 36.4% |
| Superimposed pre‐eclampsia | 433, 41, 43, 45 | 277 | 0.99 | 0.62 to 1.58 | 0.0% |
| Perinatal | |||||
| Stillbirth/neonatal death | 235, 41 | 186 | 2.24 | 0.35 to 14.28 | 0.0% |
| Birth weight, g | 332, 33, 41 | 259 | 50 | −200 to 290 | 0.0% |
Risk ratios provided where binary data were analyzed, and weighted mean difference given for continuous outcomes.
Figure 6.
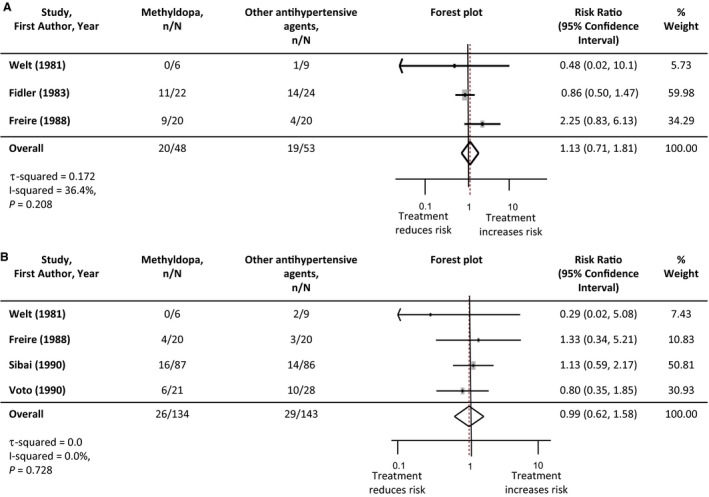
Maternal outcomes: comparison of methyldopa vs other antihypertensive agents. A, Severe hypertension. B, Superimposed pre‐eclampsia. Studies are listed in order of the year they were published. Antihypertensive agents used in each study are listed in Table 1. The number of participants experiencing severe hypertension or superimposed pre‐eclampsia in each treatment group are denoted as “n,” with the total number of participants with chronic hypertension in each study arm denoted as “N.” Forest plots of the meta‐analysis for each maternal outcome: comparison of methyldopa vs other antihypertensive agents. The gray rectangles represent the risk ratio for each study and are sized in proportion to the weight assigned to the study within the analysis. The red dotted line represents to overall risk ratio for each outcome and the lateral tips of the diamond represent the 95% confidence interval for the summary measure.
Figure 7.
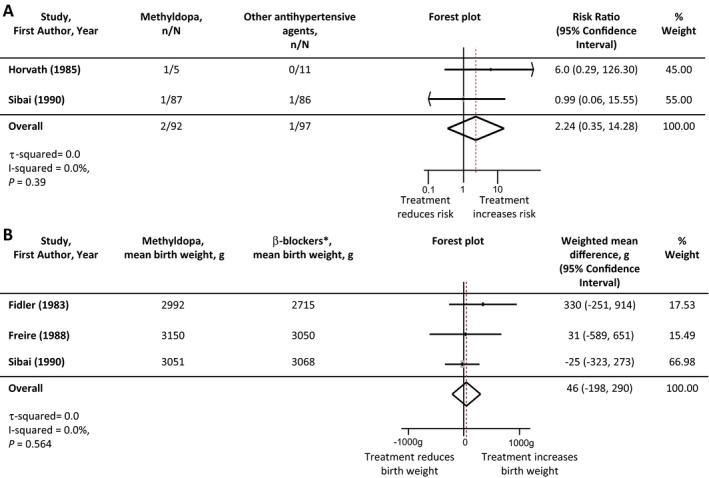
Perinatal outcomes: comparison of methyldopa vs other antihypertensive agents. A, Stillbirth and neonatal death. B, Birth weight. *Comparison made between methyldopa and beta‐blockers as these were the only agents used in head‐to‐head trials reporting birth weight. Studies are listed in order of the year they were published. Antihypertensive agents used in each study are listed in Table 1. The number of participants experiencing a stillbirth/neonatal death in each treatment group are denoted as “n,” with the total number of participants with chronic hypertension in each study arm denoted as “N.” Forest plots of the meta‐analysis for each perinatal outcome: comparison of methyldopa vs other antihypertensive agents. The gray rectangles represent the risk ratio for each study and are sized in proportion to the weight assigned to the study within the analysis. The red dotted line represents to overall risk ratio for each outcome and the lateral tips of the diamond represent the 95% confidence interval for the summary measure.
Discussion
This is the largest systematic review of the evidence from randomized controlled trials to guide antihypertensive treatment specifically for chronic hypertension in pregnancy. Other systematic reviews have pooled results for chronic and gestational hypertension, but given the different etiology and duration of treatment, there are concerns with this approach. The reduction in the incidence of severe hypertension in pregnant women with chronic hypertension with the use of antihypertensive treatment is clinically important given the short‐ and long‐term associated maternal morbidity and mortality.8, 9, 10, 19, 20, 21 It is not possible at this time to recommend one agent over another for optimal blood pressure control, as there have only been 3 head‐to‐head randomized controlled trials enrolling 101 women that have examined this outcome.32, 33, 45 No overall reduction in the risk of pre‐eclampsia was seen with the use of antihypertensive treatment.
The paucity of data to guide selection of antihypertensive treatment for chronic hypertension in pregnancy is also highlighted. Only 15 randomized controlled trials totaling 1166 pregnancies meeting study eligibility criteria were identified, and many of these were too small to address whether antihypertensive treatments reduce the risk of superimposed pre‐eclampsia or influence other measures of perinatal morbidity. The only study published in the last 18 years by Vigil and colleagues compared 3 active treatment arms and antihypertensive treatments not recommended by most international guidelines as first‐line agents (amlodipine, furosemide, and aspirin).65 It could not be included in the meta‐analysis given this design. This compares with many more trials and participants outside pregnancy; a recent systematic review of antihypertensive treatment (excluding pregnant participants) for the prevention of cardiovascular disease identified 123 randomized controlled trials including 613 815 participants.69 Of the 15 studies reported here, only 10 focused on chronic hypertension in pregnancy, and the other 5 enrolled a mixed population of chronic and gestational hypertension, from which data for the participants with chronic hypertension were extracted. Given the changes in management of hypertension both inside and outside pregnancy and that all of these trials were published between 1976 and 1998, optimal antihypertensive therapy for treating chronic hypertension in pregnancy warrants further investigation through large randomized controlled trials.
Antihypertensive use in pregnancy complicated by chronic hypertension does not increase the risk of stillbirth or neonatal death. No reduction in birth weight or increase in small‐for‐gestational‐age infants was seen, although heterogeneity was evident. This strengthens the finding that antihypertensive agents do not significantly affect perinatal morbidity; agent selection and higher than recommended dose are likely to account for the evidence from Butters and colleagues, who published data from a study of 29 participants randomized in the second trimester to atenolol or placebo.31 Although it is evident that the results of this study have influenced clinical practice,8, 17 this appears to be specific to this agent or to the very high doses that were used (up to 200 mg daily). Doses above 50 mg atenolol daily are not recommended and infrequently used nowadays for hypertension, as above this, the dose‐response curve is typically quite flat for blood‐pressure lowering, with the maximum licensed dose for other indications being 100 mg daily. The primary results for this analysis have been presented without the inclusion of this study for these reasons. Von Dadelszen and colleagues also analyzed with and without the data from the Butters study when examining the impact of antihypertensive treatment on the risk of small‐for‐gestational‐age newborns due to concerns over trial reporting.16, 70
Ten of the 15 studies included in the meta‐analysis evaluated agents that are no longer used for the routine management of hypertension in pregnancy in many countries (atenolol, acebutalol, oxprenolol, pindolol, bendroflumethiazide, hydrochlorothiazide, furosemide) or in the general nonpregnant population (ketanserin), accounting for about 45% of the participants studied. Although labetalol is commonly used in pregnancy, not all β‐blockers can be considered equivalent. Labetalol is a racemate with α‐ and nonselective β‐antagonist activity (in a ratio of around 1 to 3) for oral labetalol.71, 72 Oxprenolol, acebutalol, and pindolol are more selective for β1 receptors than β2 receptors but are additionally partial agonists, possessing intrinsic sympathomimetic activity (resulting in less effect on reducing heart rate). Although licensed for hypertension, β‐blockers are no longer recommended as first‐line antihypertensive treatment, but are now regarded as fourth line agents for resistant hypertension in the general (nonpregnant) population.73 The dose of bendroflumethiazide used (5‐10 mg daily) is higher than that currently used for hypertension (2.5 mg daily). Therefore, a substantial proportion of the evidence for treatment of hypertension in pregnancy is based on outdated drugs and outdated doses. It is difficult to draw conclusions over the effect of antihypertensive agents on other maternal and perinatal outcomes. Meta‐analysis of many maternal and fetal secondary outcomes was not possible due to a lack of reporting in the trials conducted to date. In addition, the planned adjustment for potential confounders such as body mass index was not possible due to inconsistent or absent reporting in the trial manuscripts. Further studies are needed to answer these questions and assess the potential impact of maternal characteristics such as obesity and other medical comorbidities.
The Cochrane risk‐of‐bias assessment was high or unclear for all the studies included. This is primarily due to assignment of unclear risk of bias to many areas of study conduct and restrictions in the Cochrane tool. Many studies were open‐label, assigning them high risk of bias, which reflects the difficulties in blinding medication within pregnancy when blood pressure is dynamic and multiple dosing changes are required over a short time period. Additionally, the studies are not uniform in their reported outcome measures, which reflect the large time frame and variation in geographical setting of the studies. All studies included are at least 18 years old, and given the improvements in standards of clinical care in addition to standards of study conduct, there is the potential for substantial bias to be introduced.
Previous meta‐analyses of the antihypertensive treatment of chronic hypertension in pregnancy are smaller than this study and have focused on other interventions and outcomes.14, 74 The most recent of these was published in 2000. This study aimed to assess long‐term treatment of chronic hypertension in pregnancy, and the majority of trials did not provide sufficient detail to allow categorization into mild or severe hypertension. In addition, a considerable portion of women will cross over from 1 group to the other, making analysis problematic. A Cochrane review has been conducted on the use of antihypertensive treatment for “mild to moderate” hypertension in pregnancy.12 The authors conclude “whether the reduction in the risk of severe hypertension is considered sufficient to warrant treatment is a decision that should be made by women in consultation with their obstetrician” and classed “mild to moderate” hypertension as a systolic blood pressure up to and including 169 mm Hg. In contrast, the Control of Hypertension in Pregnancy Study concludes that “tight control” of blood pressure should be recommended to reduce the risk of short‐ and long‐term maternal morbidity given that this does not affect fetal or neonatal outcome adversely.18, 22 Subgroup analyses of those with chronic hypertension suggest a possible trend toward small for gestational age, birth weight <10th centile (13.9% versus 19.7%; adjusted odds ratio 0.66, 95%CI 0.44‐1.00); however, it is notable that in this subgroup the primary perinatal outcome was no different (odds ratio 1.08, 95%CI 0.78‐1.51). A post hoc analysis of the Control of Hypertension in Pregnancy Study found that severe hypertension occurring in either intervention group (tight versus less‐tight control) was associated with higher rates of pregnancy loss, neonatal unit admission, and birth weight <10th centile,22 suggesting a perinatal benefit to reducing the risk of severe hypertension. Additionally, those with severe hypertension in the less‐tight control group were found to have an increased risk of serious maternal morbidity/mortality (odds ratio 3.74, 95%CI 1.25‐11.22).22 Although some still question the need to treat hypertension before it reaches severe levels, the American Heart Association and the American Stroke Association recommend systolic blood pressure should be treated above the level of 150 mm Hg to reduce the risk of stroke.75 This recommendation is echoed in the findings of the UK triennial enquiry into maternal death, which found severe hypertension to be a factor in a significant proportion of hypertension‐related deaths.9 Of note, since this recommendation, deaths from pre‐eclampsia have fallen to less than 1 per million in the UK.76
The potential effects of “less‐tight control” on long‐term maternal morbidity and mortality have recently been highlighted.19, 20 The Systolic Blood Pressure Intervention Trial stopped recruitment early due to the significant 25% reduction seen in a composite cardiovascular outcome (stroke, myocardial infarction, and cardiac failure) with tighter control of systolic hypertension to a target of 120 mm Hg rather than the standard treatment target of 140 mm Hg; however, this was coupled with a significant increase in serious adverse events such as hypotension, syncope, and acute kidney injury.77 Women of reproductive age with chronic hypertension are at substantially increased risk of cardiovascular morbidity and mortality.78 Reducing the incidence of severe hypertension and maintaining tighter blood pressure control in pregnancy might contribute to lowering their long‐term cardiovascular risk and warrant further investigation.
Earlier systematic reviews have focused on magnitude of initial hypertension rather than the underlying condition causing the hypertension. However, separating chronic and gestational hypertension, given the differing pathophysiological pathways and implications of treatment, allows focus on optimizing treatment for each condition and is much more relevant to clinical practice.12, 13 Advances in the understanding of the mechanisms behind the exacerbation of hypertension in pregnancy and the associated increased risk of superimposed pre‐eclampsia should be complemented with randomized controlled trials that examine how antihypertensive treatment may need to be tailored to the underlying pathophysiology. The International Society for the Study of Hypertension in Pregnancy guidelines classifying subtypes of hypertension in pregnancy have been refined over time, and head‐to‐head randomized controlled trials comparing antihypertensive agents specifically for the treatment of chronic hypertension in pregnancy using these definitions are urgently needed.79
There is emerging evidence that tighter control of hypertension outside pregnancy reduces risks of long‐term cardiovascular morbidity and mortality.77 In light of the Control of Hypertension In Pregnancy Study data suggesting fetal safety with tighter control of hypertension, future research should focus on head‐to‐head randomized controlled trials of the most commonly used antihypertensive agents in current practice; this should include smaller trials to evaluate efficacy and larger trials to assess effectiveness of agent(s) for control of chronic hypertension in pregnancy. In addition, further consideration of the impact of maternal demographic factors should be considered such as body mass index and ethnicity. Outside pregnancy, calcium‐channel blockers are recommended as first‐line antihypertensive therapy for those of African or Caribbean family origin73; this is due to differing pathophysiological pathways causing hypertension that vary with ethnic origin.80 It is possible that the efficacy of antihypertensive treatment is similarly affected by maternal ethnic background. This systematic review provides evidence to recommend that women with chronic hypertension in pregnancy should receive antihypertensive treatment to reduce the incidence of severe hypertension and its associated maternal morbidity without adversely affecting perinatal outcome.
Conclusions
Antihypertensive treatment reduces the risk of severe hypertension in pregnant women with chronic hypertension. A considerable paucity of data exists from randomized controlled trials to guide choice of antihypertensive agent for chronic hypertension in pregnancy. Adequately powered head‐to‐head randomized controlled trials of the commonly used antihypertensive agents are required to inform prescribing.
Author Contributions
Protocol was written by Webster and Conti‐Ramsden and reviewed by Chappell, Seed, Webb, and Nelson‐Piercy. Data were extracted and tabulated independently by Webster and Conti‐Ramsden. Data were analyzed by Seed with contributions from other authors. The first draft of the manuscript was written by Webster and Conti‐Ramsden and subsequently edited by Chappell, Webb, Seed, and Nelson‐Piercy. The guarantor of the review is Professor Lucy Chappell. All authors had full access to all of the data including statistical reports and take responsibility for the integrity of the data and accuracy of the data analysis.
Sources of Funding
This is independent research supported by the National Institute for Health Research Professorship of Lucy Chappell (RP‐2014‐05‐019). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. Paul Seed is partly funded by Tommy's (Registered charity no. 1060508) and Collaborations for Leadership in Applied Health Research and Care South London (National Institute for Health Research). Open access for this article was funded by King's College London.
Disclosures
Professor Nelson‐Piercy reports personal fees from Alliance Pharmaceuticals, personal fees from UCB Pharmaceuticals, LEO Pharmaceuticals, Sanofi Aventis, and Warner Chilcott outside the submitted work. The other authors report no disclosures.
Supporting information
Data S1. Study Protocol.
(J Am Heart Assoc. 2017;6:e005526 DOI: 10.1161/JAHA.117.005526.)28515115
Note
References
- 1. Roberts CL, Bell JC, Ford JB, Hadfield RM, Algert CS, Morris JM. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy. 2008;27:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bateman BT, Bansil P, Hernandez‐Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134.e1–134.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seely EW, Ecker J. Chronic hypertension in pregnancy. N Engl J Med. 2011;365:439–446. [DOI] [PubMed] [Google Scholar]
- 4. Matthews T, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;21:1–8. [PubMed] [Google Scholar]
- 5. Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115:989–997. [DOI] [PubMed] [Google Scholar]
- 6. Bramham K, Parnell B, Nelson‐Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta‐analysis. BMJ. 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chappell LC, Enye S, Seed P, Briley AL, Poston L, Shennan AH. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension a prospective study. Hypertension. 2008;51:1002–1009. [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health and Clinical Excellence . Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. Vol (Clinical Guideline 107). Available at: https://www.nice.org.uk/guidance/cg107/evidence/full-guideline-134794333. Accessed March 1, 2015.
- 9. Centre for Maternal and Child Enquiries (CMACE) . Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. BJOG. 2011;118:1–203. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . WHO Recommendations for Prevention and Treatment of Pre‐Eclampsia and Eclampsia. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 11. American College of Obstetricians and Gynecologists . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122. [DOI] [PubMed] [Google Scholar]
- 12. Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;2:CD002252. [DOI] [PubMed] [Google Scholar]
- 13. Magee LA, Duley L. Oral beta‐blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003;3:CD002863. [DOI] [PubMed] [Google Scholar]
- 14. Ferrer RL, Sibai BM, Mulrow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96(5 Pt 2):849–860. [DOI] [PubMed] [Google Scholar]
- 15. Society for Maternal and Fetal Medicine . SMFM Statement: benefit of antihypertensive therapy for mild‐to‐moderate chronic hypertension during pregnancy remains uncertain. Am J Obstet Gynecol. 2015;213:3–4. [DOI] [PubMed] [Google Scholar]
- 16. Von Dadelszen P, Ornstein M, Bull S, Logan A, Koren G, Magee L. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta‐analysis. Lancet. 2000;355:87–92. [DOI] [PubMed] [Google Scholar]
- 17. Gillon TE, Pels A, von Dadelszen P, MacDonell K, Magee LA. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One. 2014;9:e113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, Gruslin A. Less‐tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. [DOI] [PubMed] [Google Scholar]
- 19. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 20. Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 21. Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy‐related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol. 2015;125:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Gafni A Helewa M, Hutton E, Koren G, Lee SK, Logan AG, Ganzevoort W, Welch R, Thornton JG, Moutquin JM; CHIPS Study Group . The CHIPS randomized controlled trial (Control of Hypertension in Pregnancy Study) is severe hypertension just an elevated blood pressure? Hypertension. 2016;68:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu SY, Bachman DJ, Callaghan WM, Whitlock EP, Dietz PM, Berg CJ, O'Keeffe‐Rosetti M, Bruce FC, Hornbrook MC. Association between obesity during pregnancy and increased use of health care. N Engl J Med. 2008;358:1444–1453. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed RJ, Gafni A, Hutton EK, Hu ZJ, Pullenayegum E, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez JJ, Ganzevoort W, Helewa M, Lee SK, Lee T, Logan AG, Moutquin JM, Singer J, Thornton JG, Welch R, Magee LA. The cost implications of less tight versus tight control of hypertension in pregnancy (CHIPS Trial). Hypertension. 2016;68:1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available at: http://handbook.cochrane.org/. Accessed March 1, 2015. [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 30. Arias F, Zamora J. Antihypertensive treatment and pregnancy outcome in patients with mild chronic hypertension. Obstet Gynecol. 1979;53:489–494. [PubMed] [Google Scholar]
- 31. Butters L, Kennedy S, Rubin PC. Atenolol in essential hypertension during pregnancy. BMJ. 1990;301:587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fidler J, Smith V, Fayers P, De Swiet M. Randomised controlled comparative study of methyldopa and oxprenolol in treatment of hypertension in pregnancy. BMJ. 1983;286:1927–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freire S, França LAD, Callou MRDA, Oliveira JEAD, Barbosa Filho J. Estudo comparativo com pindolol e metildopa em gestantes com hipertensäo arterial crônica. J Bras Ginecol. 1988;98:157–160. [Google Scholar]
- 34. Hirsch M, Bar J, Bott KG, Kaplan B, Fuchs J, Ovadia J. Effect of the beta‐adrenergic blocker pindolol on platelet function in chronic hypertensive pregnancy. Hypertens Pregnancy. 1996;15:193–202. [Google Scholar]
- 35. Horvath JS, Phippard A, Korda A. Clonidine hydrochloride—a safe and effective antihypertensive agent in pregnancy. Obstet Gynecol. 1985;66:634–638. [PubMed] [Google Scholar]
- 36. Kahhale S, Zuaib M, Carrara W, Jota de Paula F, Sabbaga E, Neme B. Comparative study of chronic hypertensive pregnant women treated and non‐treated with pindolol [in Portuguese]. Ginecol Obstet Bras. 1985;8:85–89. [Google Scholar]
- 37. Mutch LM, Moar VA, Ounsted MK, Redman CW. Hypertension during pregnancy, with and without specific hypotensive treatment. I. Perinatal factors and neonatal morbidity. Early Hum Dev. 1977;1:47–57. [DOI] [PubMed] [Google Scholar]
- 38. Parazzini F, Benedetto C, Bortolus R, Ricci E, Marozio L, Donvito V, Tibaldi C, Alberico S, Guaschino S, Remuzzi G, Massobrio M. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. BJOG. 1998;105:718–722. [DOI] [PubMed] [Google Scholar]
- 39. Redman C, Beilin L, Bonnar J, Ounsted M. Fetal outcome in trial of antihypertensive treatment in pregnancy. Lancet. 1976;2:753–756. [DOI] [PubMed] [Google Scholar]
- 40. Sibai BM, Grossman RA, Grossman HG. Effects of diuretics on plasma volume in pregnancies with long‐term hypertension. Am J Obstet Gynecol. 1984;150:831–835. [DOI] [PubMed] [Google Scholar]
- 41. Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960–967. [DOI] [PubMed] [Google Scholar]
- 42. Steyn DW, Odendaal HJ. Randomised controlled trial of ketanserin and aspirin in prevention of pre‐eclampsia. Lancet. 1997;350:1267–1271. [DOI] [PubMed] [Google Scholar]
- 43. Voto LS, Quiroga CA, Lapidus AM, Catuzzi P, Imaz FU, Margulies M. Effectiveness of antihypertensive. Hypertens Pregnancy. 1990;9:339–348. [Google Scholar]
- 44. Weitz C, Khouzami V, Maxwell K, Johnson JWC. Treatment of hypertension in pregnancy with methyldopa: a randomized double blind study. Int J Gynaecol Obstet. 1987;25:35–40. [DOI] [PubMed] [Google Scholar]
- 45. Welt SI, Dorminy JH, Jelovsek FR, Crenshaw MC, Gall SA. The effects of prophylactic management and therapeutics on hypertensive disease in pregnancy: preliminary studies. Obstet Gynecol. 1981;57:557–565. [PubMed] [Google Scholar]
- 46. Anthony J, Rees AE, Davey DA. Indoramin in the treatment of pregnancy hypertension. A placebo‐controlled trial comparing the efficacy of indoramin with alpha‐methyldopa. S Afr Med J. 1990;78:458–461. [PubMed] [Google Scholar]
- 47. Bolte AC, van Eyck J, Strack van Schijndel RJ, van Geijn HP, Dekker GA. The haemodynamic effects of ketanserin versus dihydralazine in severe early‐onset hypertension in pregnancy. BJOG. 1998;105:723–731. [DOI] [PubMed] [Google Scholar]
- 48. Bott‐Kanner G, Hirsch M, Friedman S, Boner G, Ovadia J, Merlob P, Mor N, Modan M, Galai N, Rosenfeld JB. Antihypertensive therapy in the management of hypertension in pregnancy—a clinical double‐blind study of pindolol. Clin Exp Hypertens B. 1992;11:207–220. [Google Scholar]
- 49. Cruickshank DJ, Robertson AA, Campbell DM, MacGillivray I. Maternal obstetric outcome measures in a randomised controlled study of labetalol in the treatment of hypertension in pregnancy. Clin Exp Hypertens B. 1991;10:333–344. [Google Scholar]
- 50. Faneite A, Pedro J, González de Chirivella X, Salazar de Dugarte G. Evaluación de antihipertensivos en embarazadas: mepindolol y alfametildopa. Estudio prospectivo y randomizado. Rev Obstet Ginecol Venez. 1988;48:139–143. [Google Scholar]
- 51. Gallery EDM, Saunders DM, Hunyor SN, Gyory AZ. Randomised comparison of methyldopa and oxprenolol for treatment of hypertension in pregnancy. BMJ. 1979;1:1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gallery ED, Ross MR, Gyory AZ. Antihypertensive treatment in pregnancy: analysis of different responses to oxprenolol and methyldopa. BMJ. 1985;291:563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hall DR, Odendaal HJ, Steyn DW, Smith M. Nifedipine or prazosin as a second agent to control early severe hypertension in pregnancy: a randomised controlled trial. BJOG. 2000;107:759–765. [DOI] [PubMed] [Google Scholar]
- 54. Henderson‐Smart DJ, Horvath JS, Phippard A, Korda A, Child A, Duggin GG, Hall BM, Storey B, Tiller DJ. Effect of antihypertensive drugs on neonatal blood pressure. Clin Exp Pharmacol Physiol. 1984;11:351–354. [DOI] [PubMed] [Google Scholar]
- 55. Högstedt S, Lindeberg S, Axelsson O, Lindmark G, Rane A, Sandström B, Lindberg BS. A prospective controlled trial of metoprolol‐hydralazine treatment in hypertension during pregnancy. Acta Obstet Gynecol Scand. 1985;64:505–510. [DOI] [PubMed] [Google Scholar]
- 56. Jannet D, Carbonne B, Sebban E, Milliez J. Nicardipine versus metoprolol in the treatment of hypertension during pregnancy: a randomized comparative trial. Obstet Gynecol. 1994;84:354–359. [PubMed] [Google Scholar]
- 57. Lardoux H, Blazquez G, Leperlier E, Gerard J. Randomized and comparative study of methyldopa (MD), acebutolol (ACE) and labetalol for the treatment of moderate hypertension during pregnancy (HDP). Arch Mal Coeur Vaiss. 1988;81(suppl):137–140. [PubMed] [Google Scholar]
- 58. Leather HM, Humphreys DM, Baker P, Chadd MA. A controlled trial of hypotensive agents in hypertension in pregnancy. Lancet. 1968;2:488–490. [DOI] [PubMed] [Google Scholar]
- 59. Livingstone I, Craswell PW, Bevan EB, Smith MT, Eadie MJ. Propranolol in pregnancy three year prospective study. Clin Exp Hypertens B. 1983;2:341–350. [DOI] [PubMed] [Google Scholar]
- 60. Moore MP, Redman CWG. The treatment of hypertension in pregnancy. Curr Med Res Opin. 1982;8(suppl 1):39–46.7049584 [Google Scholar]
- 61. Plouin PF, Breart G, Maillard F, Papiernik E, Relier JP. Comparison of antihypertensive efficacy and perinatal safety of labetalol and methyldopa in the treatment of hypertension in pregnancy: a randomized controlled trial. BJOG. 1988;95:868–876. [DOI] [PubMed] [Google Scholar]
- 62. Rosenfeld J, Bott‐Kanner G, Boner G, Nissenkorn A, Friedman S, Ovadia J, Merlob P, Reisner S, Paran E, Zmora E, Biale Y. Treatment of hypertension during pregnancy with hydralazine monotherapy or with combined therapy with hydralazine and pindolol. Eur J Obstet Gynecol Reprod Biol. 1986;22:197–204. [DOI] [PubMed] [Google Scholar]
- 63. Steyn DW, Odendaal HJ. Blood pressure patterns in pregnant patients on oral ketanserin. Cardiovasc J S Afr. 2001;12:82–87. [PubMed] [Google Scholar]
- 64. Tuimala R, Hartikainen‐Sorri AL. Randomized comparison of atenolol and pindolol for treatment of hypertension in pregnancy. Curr Ther Res Clin Exp. 1988;44:579–584. [Google Scholar]
- 65. Vigil‐De Gracia P, Dominguez L, Solis A. Management of chronic hypertension during pregnancy with furosemide, amlodipine or aspirin: a pilot clinical trial. J Matern Fetal Neonatal Med. 2014;27:1291–1294. [DOI] [PubMed] [Google Scholar]
- 66. Voto LS, Zin C, Neira J, Lapidus AM, Margulies M. Ketanserin versus alpha‐methyldopa in the treatment of hypertension during pregnancy: a preliminary report. J Cardiovasc Pharmacol. 1987;10(suppl 3):S101–S103. [PubMed] [Google Scholar]
- 67. Wichman K, Ryden G, Karlberg BE. A placebo controlled trial of metoprolol in the treatment of hypertension in pregnancy. Scand J Clin Lab Invest. 1984;44(suppl 169):90–95. [DOI] [PubMed] [Google Scholar]
- 68. Wide‐Swensson DH, Ingemarsson I, Lunell NO, Forman A, Skajaa K, Lindberg B, Lindeberg S, Marsàl K, Andersson KE. Calcium channel blockade (isradipine) in treatment of hypertension in pregnancy: a randomized placebo‐controlled study. Am J Obstet Gynecol. 1995;173:872–878. [DOI] [PubMed] [Google Scholar]
- 69. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 70. Collins R, Duley L. Any antihypertensive therapy for pregnancy hypertension. Pregnancy and childbirth module In Cochrane Library review. 1995. (04426).
- 71. Richards D, Tuckman J, Prichard B. Assessment of alpha‐ and beta‐adrenoceptor blocking actions of labetalol. Br J Clin Pharmacol. 1976;3:849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics of beta‐adrenergic blockers in humans. J Pharm Pharm Sci. 2001;4:185–200. [PubMed] [Google Scholar]
- 73. National Institute for Health and Clinical Excellence . Hypertension: clinical management of primary hypertension in adults 2011. Vol 127. Available at: https://www.nice.org.uk/guidance/cg127/evidence/full-guideline-8949179413. Accessed March 1, 2015.
- 74. Magee L, Ornstein M, Von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ. Guidelines for the prevention of stroke in women. Stroke. 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Knight M, Nair M, Tuffnell D, Kenyon S, Shakespeare J, Brocklehurst P, Kurinczuk JJ, eds; on behalf of MBRRACE‐UK . Saving Lives, Improving Mothers’ Care—Surveillance of Maternal Deaths in the UK 2012–14 and Lessons Learned to Inform Maternity Care From the UK and Ireland Confidential Enquiries Into Maternal Deaths and Morbidity 2009–14. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2016. [Google Scholar]
- 77. SPRINT Research Group . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46:280–286. [DOI] [PubMed] [Google Scholar]
- 79. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. [DOI] [PubMed] [Google Scholar]
- 80. Leenen FH, Nwachuku CE, Black HR, Cushman WC, Davis BR, Simpson LM, Alderman MH, Atlas SA, Basile JN, Cuyjet AB, Dart R. Clinical events in high‐risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin‐converting enzyme inhibitor in the antihypertensive and lipid‐lowering treatment to prevent heart attack trial. Hypertension. 2006;48:374–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Study Protocol.


