Abstract
Background
Bioprosthetic heart valves (BHVs), fabricated from glutaraldehyde‐pretreated bovine pericardium or porcine aortic valves, are widely used for the surgical or interventional treatment of heart valve disease. Reoperation becomes increasingly necessary over time because of BHV dysfunction.
Methods and Results
Forty‐seven explanted BHV aortic valve replacements were retrieved at reoperation for clinically severe BHV dysfunction over the period 2010–2016. Clinical explant analyses of BHV leaflets for calcium (atomic absorption spectroscopy) and oxidized amino acids, per mass spectroscopy, were primary end points. Comorbidities for earlier BHV explant included diabetes mellitus and coronary artery bypass grafting. Mean calcium levels in BHV leaflets were significantly increased compared with unimplanted BHV (P<0.001); however, time to reoperation did not differ comparing calcified and noncalcified BHV. BHV dityrosine, an oxidized amino acid cross‐link, was significantly increased in the explants (227.55±33.27 μmol/mol [dityrosine/tyrosine]) but was undetectable in unimplanted leaflets (P<0.001). BHV regional analyses revealed that dityrosine, ranging from 57.5 to 227.8 μmol/mol (dityrosine/tyrosine), was detectable only in the midleaflet samples, indicating the site‐specific nature of dityrosine formation. 3‐Chlorotyrosine, an oxidized amino acid formed by myeloperoxidase‐catalyzed chlorinating oxidants, correlated with BHV calcium content in leaflet explant analyses from coronary artery bypass graft patients (r=0.62, P=0.01) but was not significantly correlated with calcification in non–coronary artery bypass graft explanted BHV.
Conclusions
Both increased BHV leaflet calcium levels and elevated oxidized amino acids were associated with bioprosthesis dysfunction necessitating reoperation; however, BHV calcium levels were not a determinant of implant duration, indicating a potentially important role for oxidized amino acid formation in BHV dysfunction.
Keywords: bioprosthesis, calcification, oxidation, oxidative stress
Subject Categories: Valvular Heart Disease, Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cardiovascular Surgery
Introduction
Bioprosthetic heart valves (BHVs) composed of glutaraldehyde‐pretreated heterograft tissue are increasingly used for cardiac valve replacements instead of mechanical prostheses. BHVs will likely have an even more important role in the treatment of cardiac valve disease given their almost exclusive use in transcatheter valve prostheses. The advantages of BHVs include a reduced risk of thrombosis compared with mechanical valve prostheses1, 2, 3 and their suitability for use with interventional transcatheter valve delivery systems.4 BHVs, however, have been observed to have a limited functional life because of what has been termed structural valve degeneration (SVD).5, 6, 7, 8, 9, 10, 11, 12 SVD may be defined as BHV dysfunction, resulting in symptomatic stenosis, regurgitation, or both, that necessitates reoperation; SVD exclusion criteria include endocarditis, perivalvar leakage, and pannus overgrowth. The mechanistic basis of SVD has been reported to be associated with leaflet calcium deposits in the majority of cases,13 although ≥25% of BHV reoperations due to SVD occur without leaflet mineralization being a contributing factor.6, 13 BHV calcification has been shown to be clinically associated with young age; children and younger adults have diminished implant durations over time, with a time‐dependent increased occurrence of BHV calcification.10, 14, 15 Renal failure is an important comorbidity of SVD via calcification,16, 17 and earlier BHV failure due to SVD has been observed in diabetic patients.16, 18, 19 The mechanisms responsible for SVD in the absence of calcification have been studied to a limited extent.20, 21, 22
Our group reported a series of 15 bovine pericardial (BP) BHV clinical explants with increased levels of 3 posttranslational modifications of proteins via oxidative modifications to specific amino acids via distinct pathways, namely, dityrosine, an oxidative cross‐link generated via tyrosyl radical mediated oxidation, and both orthotyrosine and metatyrosine, phenylalanine oxidative modifications generated via redox active transition metal ion catalyzed hydroxyl radical‐dependent oxidative modifications.21 This publication also documented with in vitro methodology, using controlled oxidation conditions, the susceptibility of BHVs to oxidative damage.21
The present study sought to examine in depth a larger clinical series of explanted BP and porcine aortic valve (PAV) BHVs with primary end points including both analyses for BHV leaflet calcium content and a larger panel of distinct oxidized amino acids (OxAAs) formed via specific oxidative processes. We also investigated the association of comorbidities with the chemical end points of interest. The goals of our study were to investigate the occurrence of increased leaflet calcium levels and oxidative modifications in BHV retrievals that may be associated with BHV dysfunction and deterioration over time. Our approach was to compile and analyze the clinical characteristics and comorbidities and to compare this information with our primary end points: explant analyses of BHV leaflets for both calcium content and various OxAA levels that serve as molecular fingerprints of distinct oxidative processes.23, 24
Materials and Methods
Reoperation and Retrieval of BHVs
BHV retrieval analyses were performed on explants from BHV aortic valve replacement patients requiring reoperation for symptomatic stenosis, regurgitation, or both. All patients were enrolled preoperatively with informed consent at the Hospital of the University of Pennsylvania according to the approved institutional review board protocol no. 809349. Exclusion criteria for the present study included BHV endocarditis, perivalvular regurgitation, subannular pannus and aortic wall segment dehiscence in PAV BHV with an aortic root segment. BHV specimens were retrieved at the time of surgery and were immediately transported to the investigators' laboratory in a fresh state. Deidentified medical records, including all laboratory tests, echocardiographic data, and medication listings, were available for all patients undergoing BHV reoperative surgery. A total of 47 patients met the exclusion criteria, and their BHV specimens were retrieved over the study period from July 1, 2010, to February 29, 2016. A detailed description of the demographics of the patient population is provided in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics of Bioprosthetic Aortic Valve Explants
| Total number of patients | 47 | ||
| Male, n (%) | 31 (66.0) | ||
| Female, n (%) | 16 (34.0) | ||
| Age at bioprosthetic valve implant, y | Age at bioprosthesis explant, y | ||
| Mean±SE | 55.7±2.3 | Mean±SE | 64.6±2.1 |
| Range | 24–78 | Range | 31–86 |
| New York Heart Association, n (%) | Comorbidity, n (%) | ||
| Class I | 2 (4.3) | Bicuspid aortic valve | 20 (42.6) |
| Class II | 14 (29.8) | Coronary artery disease | 16 (34.0) |
| Class III | 22 (46.8) | Peripheral arterial disease | 3 (6.4) |
| Class IV | 9 (19.1) | Diabetes mellitus | 11 (23.4) |
| Diabetes mellitus and CABG | 7 (14.9) | ||
| Bioprosthetic diagnosis at explant, n (%) | Hyperlipidemia | 24 (51.1) | |
| Stenosis | 20 (42.6) | Creatinine >2 mg/dL | 4 (8.5) |
| Regurgitation and stenosis | 20 (42.6) | Statin therapy | 22 (46.8) |
| Regurgitation | 7 (14.9) | ||
| Aortic gradient per ultrasound, mm Hg | Bioprosthetic valve size (mean duration, years), n (%) | ||
| Peak (mean±SE) | 68.0±4.1 | 19 mm (8.0±1.8) | 5 (10.6) |
| Mean (mean±SE) | 39.5±2.5 | 21 mm (8.8±1.1) | 9 (19.1) |
| 23 mm (6.9±0.7) | 12 (25.5) | ||
| Previous and concomitant surgery, n (%) | 25 mm (10.1±1.0) | 9 (19.1) | |
| None | 25 (53.2) | 27 mm (8.7±0.7) | 7 (14.9) |
| CABG | 16 (34.0) | 29 mm (8.6±0.9) | 5 (10.6) |
| CABG with bicuspid aortic valve | 5 (10.6) | ||
| CABG with tricuspid aortic valve | 11 (23.4) | ||
| Mitral valve repair | 4 (8.5) | Bioprostheses used, n (%) | |
| Mitral valve replacement | 6 (12.8) | Carpentier‐Edwards (bovine pericardial) | 32 (68.1) |
| Ascending aorta replacement | 9 (19.1) | Carpentier‐Edwards (porcine aortic valve) | 3 (6.4) |
| St. Jude (porcine aortic valve) | 7 (14.9) | ||
| Duration of implant, y | Sorin (bovine pericardial) | 3 (6.4) | |
| Mean±SE | 8.4±0.4 | Medtronic (porcine aortic valve) | 1 (2.1) |
| Range | 1.5 to 14.8 | Carbomedics (bovine pericardial) | 1 (2.1) |
CABG indicates coronary artery bypass grafting.
BHV Explant Sample Preparation
Following visual inspection, each explant was fixed in neutral buffered formalin and dehydrated in graded ethanol. A representative BHV leaflet was removed and divided midleaflet. Half was used for calcium analyses (see below), and the remaining half was processed through a paraffin‐embedding procedure, followed by thick sectioning to create samples for OxAA analyses (see below).
Quantification of OxAA in the BHV Explants
Thick paraffin sections (50 μm) were used for OxAA analyses, as published previously.22 For all specimens analyzed, a cross‐section for OxAA analyses was taken from the free edge of the cusp, the annular margin. The content of each OxAA in paraffin‐embedded BHV leaflet samples was determined by stable isotope dilution liquid chromatography tandem mass spectrometry methods with an AB SCIEX API 5000 triple quadrupole mass spectrometer interfaced with an Aria LX Series HPLC multiplexing system (Cohesive Technologies Inc) in the Cleveland Clinic laboratories, as described.22 Briefly, samples were deparaffinized with xylene and mixed with a single‐phase mixture of H2O/methanol/H2O‐saturated diethylether (1:3:8 vol/vol/vol) after protein delipidation and desalting. The mixture was then supplemented with synthetic [13C6]‐labeled OxAA standards and isotope‐labeled precursor amino acids ([13C9, 15N1]‐tyrosine and [13C9, 15N1]‐phenylalanine). The mixtures were then hydrolyzed under argon gas using methane sulfonic acid and then passed through a C18 solid‐phase extraction column (Discovery DSC18 minicolumn, 3 mL; Supelco). During liquid chromatography tandem mass spectrometry, each OxAA and its precursor, tyrosine or phenylalanine, were quantitated, with results presented as a ratio of OxAA:tyrosine, the precursor amino acid.23, 24 A subgroup of leaflets, with sufficient leaflet material, were analyzed in detail for dityrosine content, with paraffin sections from samples of the free edge, sewing cushion attachment, commissural attachment, and midcusp.
Quantification of BHV Leaflet Calcium Levels
Atomic absorption spectrometry was used to quantify tissue content of calcium in acid hydrolysates of the BHV cusp samples (half leaflet, as above), as follows25: Samples of BHV leaflets were lyophilized, weighed, and then transferred to acid‐resistant tubes. After adding 1 mL of 6 N HCl per sample and sealing, samples were incubated at 95°C overnight, followed by evaporation using a heating block at 65°C for 24 hours until each sample became completely dehydrated. The residue was reconstituted with 1 mL of 0.01 N HCl and stored at −20°C. The hydrolyzed samples were diluted with atomic absorption spectrometry matrix (0.3 N HCl with 0.5% La2O3), followed by quantification of calcium using a Perkin Elmer 2380 atomic absorption spectrophotometer with standard curves, as previously published.25
Statistical Methods
Quantitative data including age at the initial surgery, calcium levels, and OxAA levels in BHV leaflets were presented as mean±SE. Depending on equality of variances within the data and the number of levels within the independent variable, these data were analyzed by Student t test, Welch's t test, or ANOVA. Differences in implant duration, referred to as freedom of reoperation, of BHV were assessed by Kaplan–Meier survival curve analysis with a log‐rank test. One‐way ANOVAs combined with Bonferroni or Games‐Howell post hoc tests were used to test differences of calcium and OxAA levels between the different groups. Pearson correlation coefficients and linear regressions were analyzed to assess relationships between OxAA and calcification or implanted duration of BHV explants. Statistical analyses were performed with Prism 6 (GraphPad Software, Inc), SigmaPlot 11 (Systat Software, Inc), and SPSS (IBM Corp). P≤0.05 was deemed significant.
Results
Patient Demographics for BHV Explants
Enrolled participants (n=47) had a mean age of 55.7±2.27 years at the time of the original BHV aortic valve replacement, with a range between 24 and 78 years (Table 1). The mean duration of BHV explant after operation was 8.63±0.42 years, with a range of 1.5 to 14.8 years. Echocardiography assessment before BHV removal revealed BHV aortic stenosis in 20 patients, stenosis and regurgitation in 20, and regurgitation without stenosis in 7. Comorbidities were bicuspid aortic valve (n=21); coronary artery disease (n=16), with all patients requiring coronary artery bypass grafting (CABG); type 2 diabetes mellitus (DM; n=11), hyperlipidemia (n=25); statin use (n=23); and creatinine >2 mg/dL (n=4). Other past or concomitant surgical procedures included mitral valve repair (n=4), mitral valve replacement (n=6), and ascending aorta replacement (n=10).
Factors Affecting Freedom From Reoperation
Reoperation in this population was required in >50% of patients by 8 years after operation (Figure 1A). Time to reoperation did not significantly differ between male and female participants (Figure 1B). The type of BHV heterograft material used, BP or PAV, was not associated with a difference in freedom from reoperation in this series (Figure 1C); however, DM (Figure 1D) and CABG (Figure 1E) were significantly associated with earlier reoperation. Of importance, 7 of the 16 CABG patients had DM (Table 1).
Figure 1.
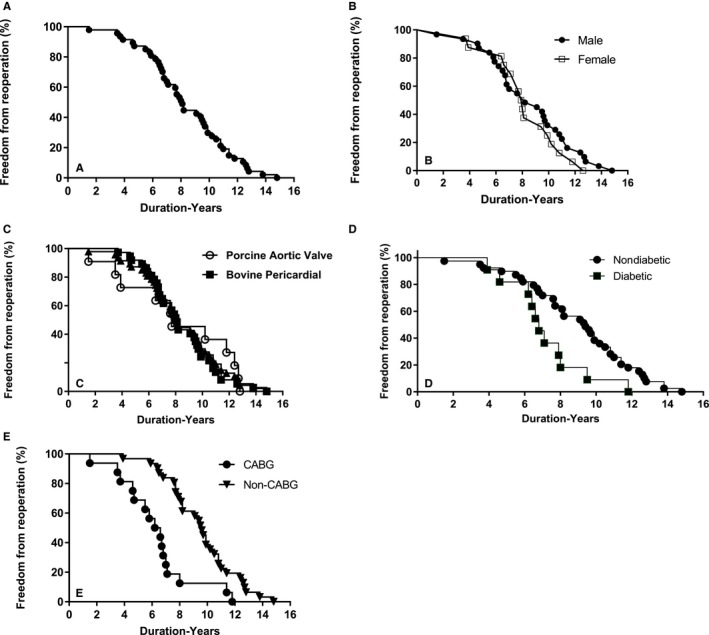
Implant duration of bioprosthetic heart valve explants from 2010 to 2016. A, Freedom from reoperation in the entire population. B, Freedom from reoperation comparing male and female participants, P value not significant (NS). C, Comparisons between bovine pericardial (BP) and porcine aortic valve, P=NS. D, Significantly shorter implant duration in diabetic patients compared with nondiabetic patients (P=0.0269). E, Significantly earlier failure of BP bioprosthetic heart valve from coronary artery bypass grafting (CABG) patients than non‐CABG patients (P<0.001).
Clinical Factors and Calcium Content of BHV Leaflets From Explants
BHV leaflet calcium levels in the explants were significantly elevated compared with unimplanted leaflets of both types of heterograft materials (Table 2, Figure 2). Age at initial surgery was significantly inversely correlated with freedom from reoperation (Figure 2A). Older participants tended to have shorter implant duration (Figure 2A); however, no correlation was noted between leaflet calcium content and age (Figure 2B). In accord with this observation, BHV leaflet calcium content also was not correlated with implant duration (Figure 2C). In addition, a subanalysis was carried out to learn if the higher calcium values shown in Figure 2C (>30 μg/mg) demonstrated a correlation with implant duration; there was not a significant correlation (n=30, P=0.0686, r=0.33) in this subgroup, as described (data not shown). Leaflet calcium levels did not differ significantly between explants of male and female participants (Figure 2D).
Table 2.
Calcification and BHV Structural Valve Degeneration
| Group | Number Analyzed | Implant Duration (Y, Mean±SE) | Ca Content of Explant (μg/mg, Mean±SE) |
|---|---|---|---|
| Unimplanted bovine pericardial leaflets | 5 | N/A | 7.07±0.30 |
| Unimplanted porcine aortic valve cusps | 5 | N/A | 0.60±0.08 |
| Entire explants population | 47 | 8.63±0.42 | 124.24±15.30 |
| BP explants | 36 | 8.52±0.43 | 142.00±16.95 |
| PAV explants | 11 | 8.94±1.24 | 66.28±29.49* |
| Stenosis | 20 | 7.77±0.62 | 153.64±23.23 |
| Regurgitation plus stenosis | 20 | 8.81±0.68 | 123.14±22.93 |
| Regurgitation only | 7 | 10.31±0.87 | 43.63±30.22† |
| DM | 11 | 7.16±0.66 | 168.79±30.99 |
| No DM | 36 | 9.05±0.49 | 110.68±17.18 |
| CABG | 16 | 6.89±0.73 | 124.81±27.75 |
| With DM | 7 | 7.30±0.85 | 169.19±37.98 |
| Non‐CABG | 31 | 9.61±0.44 | 124.00±18.58 |
| With DM | 4 | 6.93±1.19 | 168.09±61.29 |
BHV indicates bioprosthetic heart valve; BP, bovine pericardial; CABG, coronary artery bypass grafting; DM, diabetes mellitus; N/A, not assessed; PAV, porcine aortic valve.
Statistically significant differences between calcium content are shown comparing all explants and unimplanted (P<0.001, Welch t test), BP (bovine pericardial) and PAV (porcine aortic valve) explants (*P=0.035, Welch t test), and regurgitation only and stenosis only († P=0.049, 1‐way ANOVA with Bonferroni post hoc correction).
Figure 2.
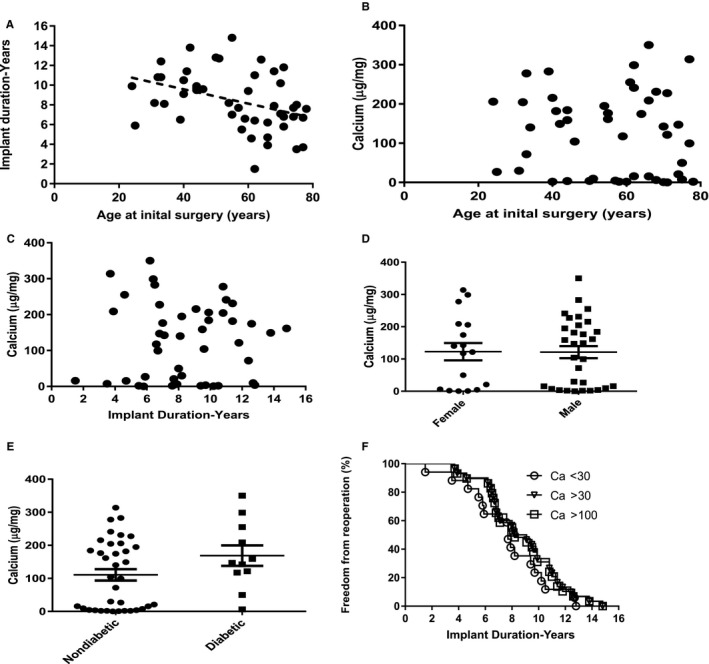
Age effects, bioprosthetic heart valve (BHV) pathophysiology, and implant duration in BHV explants with SVD. A, A significant inverse correlation (r=−0.348, P=0.008) was noted between age at initial surgery and implant duration. B, Leaflet calcium levels were not related to age at initial BHV surgery, P value not significant (NS). C, No trends were observed between implant duration and leaflet calcium levels, P=NS. D, BHV calcium (Ca) content comparing explant results from male and female participants, P=NS. E, Although mean Ca content of explanted BHVs from patients with and without diabetes mellitus (DM) did not differ significantly, only 1 DM BHV had minimal Ca content compared with 14 of 36 non‐DM explants. F, No significant differences were noted in duration of bioprosthetic implants comparing participants with leaflet Ca levels >30 and >100 μg/mg and those with Ca <30 μg/mg, P=NS.
BP leaflet calcium content was significantly higher than PAV (Table 2), and roughly one third of 47 BHV explants had calcium levels near zero (Figure 2B). In addition, stenotic BHVs had significantly higher calcium leaflet content than regurgitant BHVs (Table 2). DM and CABG groups did not differ in BHV calcium content compared with the overall population; although it was observed that non‐DM explants demonstrated little to nondetectable BHV calcium content in 14 of 36 specimens, only 1 of the 11 DM BHV explants was not calcified (Figure 2E).
It is noteworthy that Kaplan–Meier curves plotting duration of implants comparing those with low BHV leaflet calcium (<30 μg/mg, n=17) and BHV patient groups with higher levels of calcium (>30 and >100 μg/mg) demonstrated no significant differences in freedom from reoperation related to elevated BHV calcium levels (Figure 2F).
BHV Leaflet OxAA Content
BHV leaflet samples were subjected to quantitative analyses for a panel of OxAAs (Table 3). Although unimplanted BP and PAV leaflet samples had no detectable dityrosine, both BP and PAV explants demonstrated comparable overall significant accumulation of dityrosine (Table 3). Dityrosine was quantified in leaflet cross‐section analyses in 39 of 47 BHV leaflets analyzed (Table 3), and dityrosine was significantly increased in all explant groups compared with unimplanted (Table 3). Unimplanted PAV had significantly higher 3‐chlorotyrosine than BP. It was observed that unimplanted PAV had significantly higher levels of metatyrosine and orthotyrosine than did BP (Table 3). In addition, unimplanted BP had significantly higher 3‐bromotyrosine than PAV (Table 3). Significant increases were noted in stenotic BHV explants in both metatyrosine and orthotyrosine (Table 3). PAV explants demonstrated significantly lower levels of metatyrosine and orthotyrosine than unimplanted samples, and BP explants had significantly lower levels of 3‐bromotyrosine than unimplanted BHV leaflets (Table 3). However, 3‐bromotyrosine was also significantly elevated in BHV explants from CABG patients compared with non‐CABG (Table 3). Nitrotyrosine was present in both unimplanted and explanted PAV and BP leaflets and was significantly decreased in BP explants compared with unimplanted BP (Table 3).
Table 3.
Oxidized Amino Acid Content of BHV Aortic Valve Explants
| BHV Subgroup | Number Analyzed | Oxidized Amino Acid Levels (μmol/mol Tyr, Mean±SE) | |||||
|---|---|---|---|---|---|---|---|
| di‐Tyra | Cl‐Tyr | m‐Tyr | o‐Tyr | NO2‐Tyr | Br‐Tyr | ||
| Unimplanted BP | 10 | 0.00±0.00 | 55.46±11.70b | 170.42±26.15a | 816.69±50.07a | 82.01±10.43a | 1334.41±131.10a |
| Unimplanted PAV | 5 | 0.00±0.00 | 223.49±50.49b | 1764.25±90.26a | 3715.67±188.35a | 66.65±18.02 | 541.58±47.68a |
| All explants | 47 | 227.55±33.27 | 70.56±10.65 | 254.46±29.04 | 771.33±70.55 | 31.56±5.75 | 800.13±65.41 |
| BP | 36 | 228.22±36.85 | 70.78±13.25 | 291.38±35.09c | 835.24±84.83 | 29.20±6.44a | 778.13±74.74 |
| PAV | 11 | 225.36±78.78 | 69.84±14.82 | 133.64±23.98a , c | 562.14±98.41a | 39.29±12.93 | 872.14±139.36 |
| Stenosis | 20 | 278.16±66.86 | 95.48±22.94 | 307.18±52.58d | 976.85±115.83e | 38.67±12.25 | 733.38±108.72 |
| Regurgitation plus stenosis | 20 | 199.60±37.82 | 53.14±7.55 | 248.18±39.16f | 662.73±101.37 | 26.27±5.69 | 880.60±99.46 |
| Regurgitation only | 7 | 162.83±36.30 | 49.12±7.04 | 121.80±16.60d , f | 494.41±57.77e | 26.36±3.15 | 760.91±135.27 |
| DM | 11 | 221.48±59.89 | 96.22±26.76 | 325.58±65.71 | 1207.18±115.15a | 27.19±7.73 | 938.01±143.41 |
| No DM | 36 | 229.41±39.83 | 62.72±11.17 | 231.81±31.75 | 638.15±72.23a | 32.89±7.16 | 758.00±72.99 |
| CABG | 16 | 284.12±49.20 | 100.78±25.71 | 259.63±54.90 | 880.76±123.01 | 28.08±6.79 | 1012.51±124.46g |
| With DM | 7 | 308.30±73.93 | 110.13±35.20 | 355.54±92.50 | 1279.19±153.11 | 24.39±10.66 | 1048.9±217.49 |
| Non‐CABG | 31 | 198.36±43.19 | 54.96±8.33 | 251.79±34.39 | 714.84±85.75 | 33.36±8.04 | 690.51±69.07g |
| With DM | 4 | 69.55±40.71 | 71.88±43.77 | 281.40±92.01 | 1081.16±176.55 | 69.55±40.71 | 745.36±59.32 |
BHV indicates bioprosthetic heart valve; BP, bovine pericardial; Br‐Tyr, 3‐bromotyrosine; CABG, coronary artery bypass grafting; Cl‐Tyr, 3‐chlorotyrosine; di‐Tyr, dityrosine; DM, diabetes mellitus; m‐Tyr, metatyrosine; NO2‐Tyr, nitrotyrosine; o‐Tyr, orthotyrosine; PAV, porcine aortic valve; Tyr, tyrosine.
P<0.001 for unimplanted comparisons (Welch t test), PAV explants vs unimplanted (Welch t test), and unimplanted bovine NO2‐Tyr vs implanted bovine NO2‐Tyr (Student t test).
P=0.028 (Welch t test).
P=0.001 (Welch t test).
P=0.007 (ANOVA with Games‐Howell).
P=0.003 (ANOVA with Games‐Howell).
P=0.017 (ANOVA with Games‐Howell).
P=0.17 (Student t test).
To assess possible differences in spatial distribution of dityrosine in BHV leaflets, analyses were carried out for dityrosine on a subset of representative BHV explants with sufficient material for sampling different BHV regions. As described in Methods, 7 BHVs were analyzed in detail with paraffin sections from samples taken from the free edge, sewing cushion attachment, commissure, and midcusp (Figure 3A). The 7 BHV consisted of 6 BP and 1 PAV bioprostheses explanted from patients with CABG in 3 BP cases and DM in 2 BP cases. The single PAV bioprosthesis analyzed and 1 of the BP cases had neither CABG nor DM as comorbidities. Dityrosine, ranging from 57.5 to 227.8 μmol/mol tyrosine, was detectable only in the midleaflet samples (Figure 3B).
Figure 3.
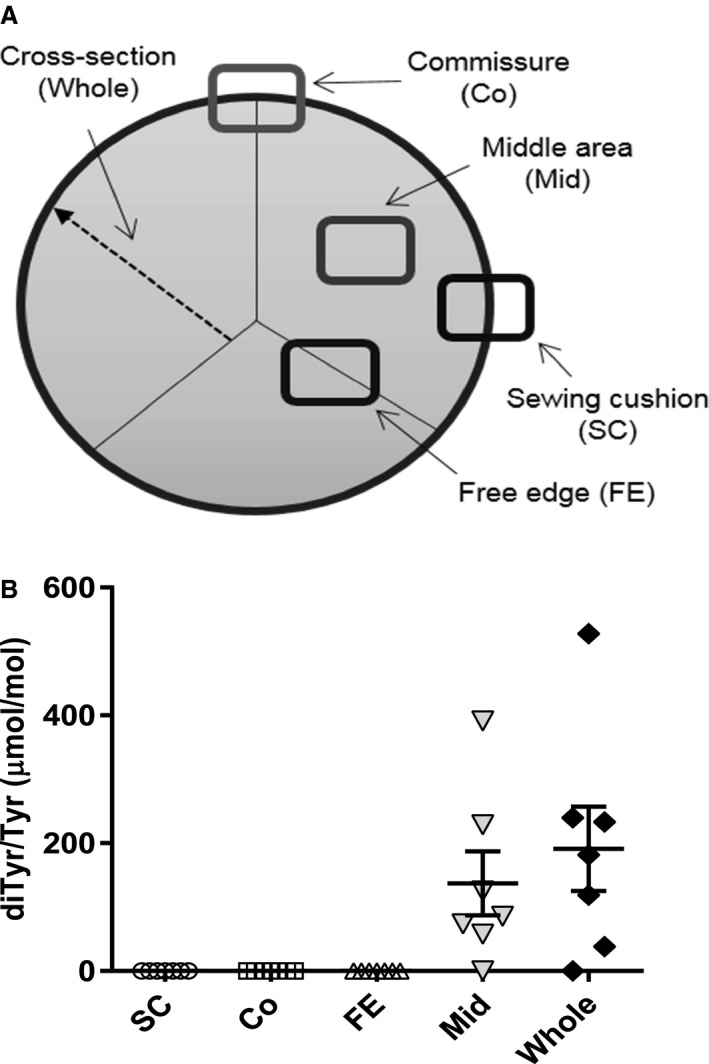
Regional distribution of dityrosine (diTyr) in explanted bioprosthetic heart valves (BHVs). A, Sample preparation scheme from sewing cushion (SC), commissure (Co), free edge (FE), and middle area (Mid), as well as cross‐section of the leaflets. B, Dityrosine formation was significantly elevated in the midleaflet region (P=0.033, 1‐sample t test) and the cross‐section (P=0.027, 1‐sample t test) compared with the sections in which dityrosine was not detected, with no significant differences when compared with each other.
The relationship between OxAA and calcium levels in BHVs was investigated in the explant data overall and in clinically relevant subgroups. These comparisons revealed no correlation between calcium levels and OxAA in the explants (data not shown); in particular, dityrosine was not correlated with BHV calcium levels (data not shown). Furthermore, although BHV explant results overall and in non‐CABG explants demonstrated no correlation between BHV calcium content and 3‐chlorotyrosine (Figure 4A and 4B), BHV explants from patients with CABG demonstrated a significant correlation between calcium and 3‐chlorotyrosine (Figure 4C).
Figure 4.
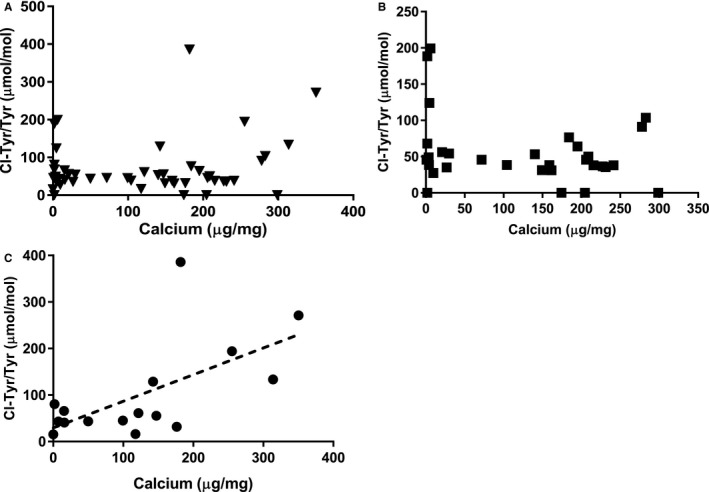
Bioprosthetic heart valve (BHV) calcium (Ca) and 3‐chlorotyrosine (Cl‐Tyr) relationships. A, BHV leaflet Ca vs Cl‐Tyr in the entire sample set. B, BHV leaflet Ca vs Cl‐Tyr in patients without coronary artery bypass grafting (CABG) only. C, The correlation between leaflet calcium level and Cl‐Tyr in clinical BHV explants from CABG patients (r=0.62, P=0.01).
Discussion
This study reports novel clinical BHV explant analyses demonstrating that both calcification and oxidative modifications are associated with progressive BHV dysfunction. Prior observational studies of BHV aortic valve replacements have demonstrated, in general, freedom from SVD requiring reoperation in ≥95% of cases by 10 years after operation,6, 10, 15, 26 unlike our results that constitute a selected group with relatively early BHV dysfunction. Several important comorbidities leading to reoperation are evident in our results: DM and coronary artery disease that was severe enough to require CABG. Both comorbidities were associated with significantly earlier reoperation than the rest of the present study population. Explant analyses of BHV leaflets in this population revealed the presence of both calcification and OxAA in the BHV leaflets. In addition, one third of the explants in the present studies had little to no detectable BHV leaflet calcium content; however, these relatively noncalcified BHV explants had implant durations that did not differ significantly from calcified explants (Figure 2E). These results emphasize the need to further understand noncalcific mechanisms, such as oxidation, that lead to BHV dysfunction.
BHV aortic valve replacements with progressive dysfunction requiring reoperation have been reported in a number of observational studies over the past 2 decades with comparable outcomes between these investigations.6, 10, 15, 26 These prior results differ from the present study that focuses on BHVs retrieved in a registry. Although our population in the present study was relatively young, with a mean age of 55 years at initial surgery, young age was not associated with earlier BHV dysfunction in our population (Figure 2), unlike observations in larger population studies, in which patients receiving BHVs at a younger age required reoperation sooner than older patients.10 In fact, the present results showed an inverse correlation with age, with earlier BHV dysfunction in older patients (Figure 2A), and this is mostly likely due to our observation of earlier reoperation in CABG patients (Figure 1D) and the older age distribution of this group (Table 1). Similarly, younger patients in the present study group did not demonstrate increased BHV calcification (Figure 2), also differing from clinical observations in studies of populations of patients undergoing BHV aortic valve replacement.6, 10, 15, 26
Prior studies have also reported the association of DM with BHV dysfunction, with an increased risk of earlier reoperation in DM patients, in agreement with the present results (Table 1, Figure 1C). Lorusso et al18 reported not only earlier BHV dysfunction in DM patients but also significantly reduced long‐term survival in this patient group. In their study, reoperation was required in <5% of patients in the non‐DM population after 5 years but in 20% of diabetic patients.18 In addition, in their study, DM with metabolic syndrome had even worse outcomes, with >40% requiring reoperation after 5 years.18 Briand et al also observed more rapidly progressive BHV dysfunction requiring reoperation in BHV patients with DM or metabolic syndrome or both.16 In the present study, calcium analyses of the BHVs retrieved from DM patients demonstrated only 1 noncalcified sample from the sample group of 11 specimens compared with one third of the non‐DM BHV retrievals with little to no measurable leaflet calcium, indicating increased BHV calcification in the DM group (Figure 2E). It is noteworthy that a quarter or more of BHVs removed for SVD have been reported by other studies not to be calcified6, 13; this is in keeping with the present observations concerning the non‐DM explants (Figure 2E).
Our study noted significantly earlier reoperation in BHV patients with either prior or concurrent CABG. Prior studies have reported concurrent CABG and BHV aortic valve replacement in ≥35% of BHV aortic valve replacement patients.2, 10, 27 However, the impact of this association on subsequent freedom from reoperation after BHV surgery has not been described in other observational studies. Nevertheless, Naji et al28 reported a large series of patients with severe BHV aortic stenosis requiring reoperation, with 95% incidence of SVD, and noted that 33% of these patients required CABG at the time of BHV reoperation. Their results suggest an association of BHV dysfunction with severe coronary disease, in agreement with the present observations. In addition, in the study of Naji et al, DM was present in 27% of this study population.28 In the present study, 7 of 16 patients with CABG also had DM (Table 1). The presence of oxidized low‐density lipoprotein in BHV leaflets with SVD associated hyperlipidemia with SVD, and related lipoprotein abnormalities as comorbidities with SVD leading to BHV reoperation have been observed by others.29, 30, 31, 32, 33, 34 This may be of importance concerning our observation of increased SVD in CABG patients. In addition, retrospective studies have reported a reduced incidence of BHV with SVD in patients receiving statin therapy,35 although there is not agreement concerning statins having a beneficial effect for mitigating SVD.36 In the present study, 51.1% of our patients with BHV requiring reoperation had hyperlipidemia, and 46.8% were receiving statins. These past and present results, taken together, support the view that atherosclerotic cardiovascular disease may have an impact on progressive BHV dysfunction associated with both calcification and OxAA formation.
Oxidative modifications of the proteins in BHVs have been reported recently by our group in a series of 15 BP BHV explants, with failed BHVs demonstrating increased levels of OxAAs (ie, dityrosine, orthotyrosine, and metatyrosine) that serve as molecular fingerprints of distinct pathways. 22 It is of interest that in the present study, which included an expanded series of BP BHVs and PAV BHVs, similar results with dityrosine were observed, that is, while not present in unimplanted BHV, dityrosine was significantly increased in both PAV and BP explants (Table 3). Dityrosine is formed via an oxidative cross‐link mediated by an intermediate tyrosyl radical. This can be formed by a variety of oxidation systems, especially the enzymatic myeloperoxidase pathway arising from activated inflammatory cells, either leukocytes or monocytes (and selected monocyte‐derived macrophages).37 Of the OxAA monitored, dityrosine is the only one that is also a protein cross‐link,37, 38 and thus its unique presence in BHV explants suggests a possible contribution of dityrosine cross‐linking to altered mechanical properties of BHV leaflets. Furthermore, in the present studies, the localization of dityrosine exclusively in the central cusp region of explanted BHV leaflets (Figure 3) is also noteworthy. Clinical explant studies have demonstrated in BHV analyses that the midcusp region in clinically failed BHVs with SVD showed collagen disruption that was most often spatially localized apart from x‐ray–detectable calcifications.20
It is also noteworthy that in BP BHVs, orthotyrosine and metatyrosine in the present studies were not significantly different than levels in unimplanted BHV leaflets (Table 3), unlike our prior series of BP BHV analyses.22 The present results also showed that for PAVs, unimplanted orthotyrosine and metatyrosine levels were significantly higher than those in unimplanted BP (Table 3). However, explant BHV orthotyrosine and metatyrosine were significantly reduced in PAV BHVs compared with unimplanted levels (Table 3). Orthotyrosine and metatyrosine are oxidation products that are not formed enzymatically37, 39 and are thought to be the result of protein modifications by reactive oxygen species arising from redox active metal ions and hydroxyl radical species reacting with the precursor amino acid phenylalanine in extracellular matrix proteins forming these nonphysiological tyrosine isomers.23 The presence of OxAA (Table 3), such as 3‐bromotyrosine and nitrotyrosine, in the unimplanted BHV materials analyzed is noteworthy because it is unknown if these posttranslational modifications23, 24 were present in the heterograft materials before harvest or if they resulted from oxidative conditions during processing. For example, 3‐bromotyrosine has been reported to be a specific eosinophil‐mediated oxidation product in the setting of allergen‐induced asthma,40 and eosinophilic infiltrates have not been reported in BHV pathology studies.9, 13 The heterograft materials used in bioprostheses are harvested in abattoirs under nonsterile conditions where various disinfectants and bleaching materials are used per US Department of Agriculture and US Food and Drug Administration guidelines. These agents could be responsible for oxidative modifications, and this has not been investigated. Nevertheless, the presence of these OxAAs and the significant differences observed indicate that oxidative events affect heterograft materials used in BHVs both before and after clinical use.
Antioxidant strategies for BHV have been investigated to a limited extent and were not the subject of the present study. The clinical BHV explants analyzed in the present study were not prepared with antioxidant modifications. Our group demonstrated the susceptibility of BHVs to oxidative damage in a series of in vitro studies.22 Oxidative damage to BHVs in vitro, causing structural deterioration, used exposures to dilute FeCl3 and H2O2. 22 This model system resulted in a net loss of extracellular matrix proteins, destruction of glutaraldehyde cross‐links, and increased susceptibility of BP BHV leaflets to collagenase digestion.22 All of these oxidation‐related end points were mitigated by the use of a polyphenol antioxidant that was covalently attached to the BP BHV leaflets in these in vitro studies; this indicated a possible therapeutic direction that could be explored for mitigating BHV oxidative stress.22 However, a subsequent investigation by our group of rat subdermal implants of BHVs,21 while demonstrating explant calcification comparable to the clinical explants in the present article (Table 1), showed less overall OxAA formation21 than that observed in the present study (Table 3). Thus, subdermal implants of BHV leaflets may not be an optimal experimental system to study oxidative mechanisms that affect BHVs in clinical use.
The present results also demonstrated significantly increased calcium levels in BP explants compared with PAVs (Figure 2D); however, freedom from reoperation did not differ in comparing the 2 types of heterograft BHV implants (Figure 1B). The relatively small PAV sample size, 11 explants, precluded demonstrating any statistically significant different associations in other subgroupings comparing PAV and BP BHV in terms of either comorbidities or OxAA levels. In addition, others have reported an association of earlier BHV reoperation due to SVD with a smaller BHV annulus,26, 41 but this was not observed in the present series (Table 1).
Both BHV aortic stenosis and regurgitation were observed in the present study. Stenotic BHV had significantly higher calcium levels than quantified in BHV explants from regurgitant cases (Table 2); however, the BHV OxAA levels were not specifically associated with one or the other BHV pathophysiology. Of importance is the observation that subpopulations with high BHV calcium levels compared with explants with low calcium levels were not associated with any significant differences in freedom from reoperation (Figure 2E). These results indicate that the level of leaflet calcium deposition is not a determinant of the progression of BHV dysfunction but may contribute to the specific pathophysiology; in other words, higher leaflet calcium levels could result in BHV stenosis rather than regurgitation.
Are oxidative and calcification mechanisms interrelated in the pathogenesis of BHV dysfunction? The present results were analyzed seeking correlations between specific OxAAs and calcification, and in general, no significant relationships were identified except for a subanalysis of the CABG patients. In the CABG patients, leaflet analyses of retrieved BHVs demonstrated a significant correlation between BHV leaflet Ca++ and 3‐chlorotyrosine (Figure 4), an OxAA formed by myeloperoxidase42; however, the small sample size and the presence of several higher values that influence the correlation limit the strength of this observation. Importantly, dityrosine is also formed by myeloperoxidase43 and was uniquely present in BHV with SVD and was not detectable in unimplanted BHV leaflet samples. Interestingly, BHV dityrosine levels did not correlate with BHV calcium levels (data not shown).
The study presented in this article has a number of limitations. The bioregistry approach used has a selection bias favoring early BHV failure due to SVD. Although our study included analyses of 47 explants, the sample sizes of the subgroups precluded both adjusting for covariates and detecting statistically significant differences for a number of questions of interest. In addition, the primary end points of these studies, calcium levels and OxAAs, required sample preparation for analyses utilizing an entire explanted BHV leaflet (see Methods) for each specimen to attain a representative set of assays; therefore, BHV morphology studies of the same leaflet sample were beyond the scope of the investigations.
Conclusions
Both BHV calcification and OxAAs are present in bioprostheses requiring reoperation. Dityrosine, an OxAA cross‐link, was uniquely present in failed BHVs and was localized to the central region of BHV leaflets. 3‐Chlorotyrosine, another oxidative product specific for myeloperoxidase‐catalyzed oxidative modification, was the only OxAA that correlated with BHV leaflet calcium levels but only in CABG patients. Taken together, these results indicate that both calcification and oxidative modifications of BHV leaflets may contribute to BHV dysfunction. Furthermore, BHV calcium levels were not a determinant of implant duration, indicating a potentially important role for OxAA formation in BHV dysfunction.
Sources of Funding
This work was supported in part by: NIH R01 HL131872 (Ferrari and Levy), NIH R01 HL122805 (Ferrari), NIH 5T32 HL007915 (Lee), The Children's Heart Foundation (Levy), The Kibel Fund for Aortic Valve Research (Ferrari and Levy), The Valley Hospital Foundation “Marjorie C Bunnel” charitable fund (Ferrari), The Children's Hospital of Philadelphia‐Drexel University‐Hebrew University of Jerusalem Pediatric Research Program (Levy), Erin's Fund of the Children's Hospital of Philadelphia (Levy), and The William J. Rashkind Endowment of the Children's Hospital of Philadelphia (Levy). Hazen was partially supported by NIH grant P01 HL076491.
Disclosures
Dr Levy has been a consultant for the Sorin Group and for Symetis in the past 3 years; these relationships do not have a conflict of interest with this publication.
Acknowledgments
The authors thank Susan Kerns for assistance in the preparation of this manuscript. We also thank Professor Abba Krieger, The Wharton School, The University of Pennsylvania, for reviewing the statistical methodology.
(J Am Heart Assoc. 2017;6:e005648 DOI: 10.1161/JAHA.117.005648.)28483776
References
- 1. Akins CW, Hilgenberg AD, Vlahakes GJ, MacGillivray TE, Torchiana DF, Madsen JC. Results of bioprosthetic versus mechanical aortic valve replacement performed with concomitant coronary artery bypass grafting. Ann Thorac Surg. 2002;74:1098–1106. [DOI] [PubMed] [Google Scholar]
- 2. Isaacs AJ, Shuhaiber J, Salemi A, Isom OW, Sedrakyan A. National trends in utilization and in‐hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg. 2015;149:1262–1269.e3. [DOI] [PubMed] [Google Scholar]
- 3. McClure RS, McGurk S, Cevasco M, Maloney A, Gosev I, Wiegerinck EM, Salvio G, Tokmaji G, Borstlap W, Nauta F, Cohn LH. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity‐matched analysis. J Thorac Cardiovasc Surg. 2014;148:1931–1939. [DOI] [PubMed] [Google Scholar]
- 4. Doss M, Buhr EB, Martens S, Moritz A, Zierer A. Transcatheter‐based aortic valve implantations at midterm: what happened to our initial patients? Ann Thorac Surg. 2012;94:1400–1406. [DOI] [PubMed] [Google Scholar]
- 5. Cohn LH, Collins JJ Jr, Rizzo RJ, Adams DH, Couper GS, Aranki SF. Twenty‐year follow‐up of the Hancock modified orifice porcine aortic valve. Ann Thorac Surg. 1998;66:S30–S34. [DOI] [PubMed] [Google Scholar]
- 6. Roselli EE, Smedira NG, Blackstone EH. Failure modes of the Carpentier‐Edwards pericardial bioprosthesis in the aortic position. J Heart Valve Dis. 2006;15:421–427; discussion 427–8. [PubMed] [Google Scholar]
- 7. Alvarez JR, Sierra J, Vega M, Adrio B, Martinez‐Comendador J, Gude F, Martinez‐Cereijo J, Garcia J. Early calcification of the aortic Mitroflow pericardial bioprosthesis in the elderly. Interact Cardiovasc Thorac Surg. 2009;9:842–846. [DOI] [PubMed] [Google Scholar]
- 8. David TE, Armstrong S, Maganti M. Hancock II bioprosthesis for aortic valve replacement: the gold standard of bioprosthetic valves durability? Ann Thorac Surg. 2010;90:775–781. [DOI] [PubMed] [Google Scholar]
- 9. Butany J, Feng T, Luk A, Law K, Suri R, Nair V. Modes of failure in explanted mitroflow pericardial valves. Ann Thorac Surg. 2011;92:1621–1627. [DOI] [PubMed] [Google Scholar]
- 10. Johnston DR, Soltesz EG, Vakil N, Rajeswaran J, Roselli EE, Sabik JF III, Smedira NG, Svensson LG, Lytle BW, Blackstone EH. Long‐term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eichinger WB, Hettich IM, Ruzicka DJ, Holper K, Schricker C, Bleiziffer S, Lange R. Twenty‐year experience with the St. Jude medical Biocor bioprosthesis in the aortic position. Ann Thorac Surg. 2008;86:1204–1210. [DOI] [PubMed] [Google Scholar]
- 12. Hickey GL, Grant SW, Bridgewater B, Kendall S, Bryan AJ, Kuo J, Dunning J. A comparison of outcomes between bovine pericardial and porcine valves in 38,040 patients in England and Wales over 10 years. Eur J Cardiothorac Surg. 2015;47:1067–1074. [DOI] [PubMed] [Google Scholar]
- 13. Schoen FJ, Kujovich JL, Webb CL, Levy RJ. Chemically determined mineral content of explanted porcine aortic valve bioprostheses: correlation with radiographic assessment of calcification and clinical data. Circulation. 1987;76:1061–1066. [DOI] [PubMed] [Google Scholar]
- 14. Sanders SP, Levy RJ, Freed MD, Norwood WI, Castaneda AR. Use of Hancock porcine xenografts in children and adolescents. Am J Cardiol. 1980;46:429–438. [DOI] [PubMed] [Google Scholar]
- 15. Saleeb SF, Newburger JW, Geva T, Baird CW, Gauvreau K, Padera RF, Del Nido PJ, Borisuk MJ, Sanders SP, Mayer JE. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation. 2014;130:51–60. [DOI] [PubMed] [Google Scholar]
- 16. Briand M, Pibarot P, Despres JP, Voisine P, Dumesnil JG, Dagenais F, Mathieu P. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation. 2006;114:I512–I517. [DOI] [PubMed] [Google Scholar]
- 17. Washiyama N, Shiiya N, Yamashita K, Terada H, Ohkura K, Takahashi K. Etiology of renal failure influences the outcome of heart valve replacement in chronic dialysis patients. J Artif Organs. 2011;14:39–42. [DOI] [PubMed] [Google Scholar]
- 18. Lorusso R, Gelsomino S, Luca F, De Cicco G, Bille G, Carella R, Villa E, Troise G, Vigano M, Banfi C, Gazzaruso C, Gagliardotto P, Menicanti L, Formica F, Paolini G, Benussi S, Alfieri O, Pastore M, Ferrarese S, Mariscalco G, Di Credico G, Leva C, Russo C, Cannata A, Trevisan R, Livi U, Scrofani R, Antona C, Sala A, Gensini GF, Maessen J, Giustina A. Type 2 diabetes mellitus is associated with faster degeneration of bioprosthetic valve: results from a propensity score‐matched Italian multicenter study. Circulation. 2012;125:604–614. [DOI] [PubMed] [Google Scholar]
- 19. Nollert G, Miksch J, Kreuzer E, Reichart B. Risk factors for atherosclerosis and the degeneration of pericardial valves after aortic valve replacement. J Thorac Cardiovasc Surg. 2003;126:965–968. [DOI] [PubMed] [Google Scholar]
- 20. Sacks MS, Schoen FJ. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J Biomed Mater Res. 2002;62:359–371. [DOI] [PubMed] [Google Scholar]
- 21. Christian AJ, Alferiev IS, Connolly JM, Ischiropoulos H, Levy RJ. The effects of the covalent attachment of 3‐(4‐hydroxy‐3,5‐di‐tert‐butylphenyl) propyl amine to glutaraldehyde pretreated bovine pericardium on structural degeneration, oxidative modification, and calcification of rat subdermal implants. J Biomed Mater Res A. 2015;103:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christian AJ, Lin H, Alferiev IS, Connolly JM, Ferrari G, Hazen SL, Ischiropoulos H, Levy RJ. The susceptibility of bioprosthetic heart valve leaflets to oxidation. Biomaterials. 2014;35:2097–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brennan ML, Hazen SL. Amino acid and protein oxidation in cardiovascular disease. Amino Acids. 2003;25:365–374. [DOI] [PubMed] [Google Scholar]
- 24. Heinecke JW, Hsu FF, Crowley JR, Hazen SL, Leeuwenburgh C, Mueller DM, Rasmussen JE, Turk J. Detecting oxidative modification of biomolecules with isotope dilution mass spectrometry: sensitive and quantitative assays for oxidized amino acids in proteins and tissues. Methods Enzymol. 1999;300:124–144. [DOI] [PubMed] [Google Scholar]
- 25. Levy RJ, Schoen FJ, Levy JT, Nelson AC, Howard SL, Oshry LJ. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde‐preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol. 1983;113:143–155. [PMC free article] [PubMed] [Google Scholar]
- 26. Flameng W, Rega F, Vercalsteren M, Herijgers P, Meuris B. Antimineralization treatment and patient‐prosthesis mismatch are major determinants of the onset and incidence of structural valve degeneration in bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2014;147:1219–1224. [DOI] [PubMed] [Google Scholar]
- 27. Hickey GL, Bridgewater B, Grant SW, Deanfield J, Parkinson J, Bryan AJ, Dalrymple‐Hay M, Moat N, Buchan I, Dunning J. National registry data and record linkage to inform postmarket surveillance of prosthetic aortic valve models over 15 years. JAMA Intern Med. 2017;177:79–86. [DOI] [PubMed] [Google Scholar]
- 28. Naji P, Griffin BP, Sabik JF, Kusunose K, Asfahan F, Popovic ZB, Rodriguez LL, Lytle BW, Grimm RA, Svensson LG, Desai MY. Characteristics and outcomes of patients with severe bioprosthetic aortic valve stenosis undergoing redo surgical aortic valve replacement. Circulation. 2015;132:1953–1960. [DOI] [PubMed] [Google Scholar]
- 29. Mahjoub H, Mathieu P, Larose E, Dahou A, Senechal M, Dumesnil JG, Despres JP, Pibarot P. Determinants of aortic bioprosthetic valve calcification assessed by multidetector CT. Heart. 2015;101:472–477. [DOI] [PubMed] [Google Scholar]
- 30. Mahjoub H, Mathieu P, Senechal M, Larose E, Dumesnil J, Despres JP, Pibarot P. ApoB/ApoA‐I ratio is associated with increased risk of bioprosthetic valve degeneration. J Am Coll Cardiol. 2013;61:752–761. [DOI] [PubMed] [Google Scholar]
- 31. Mahmut A, Mahjoub H, Boulanger MC, Fournier D, Despres JP, Pibarot P, Mathieu P. Lp‐PLA2 is associated with structural valve degeneration of bioprostheses. Eur J Clin Invest. 2014;44:136–145. [DOI] [PubMed] [Google Scholar]
- 32. Nsaibia MJ, Mahmut A, Mahjoub H, Dahou A, Bouchareb R, Boulanger MC, Despres JP, Bosse Y, Arsenault BJ, Larose E, Pibarot P, Mathieu P. Association between plasma lipoprotein levels and bioprosthetic valve structural degeneration. Heart. 2016;102:1915–1921. [DOI] [PubMed] [Google Scholar]
- 33. Shetty R, Girerd N, Cote N, Arsenault B, Despres JP, Pibarot P, Mathieu P. Elevated proportion of small, dense low‐density lipoprotein particles and lower adiponectin blood levels predict early structural valve degeneration of bioprostheses. Cardiology. 2012;121:20–26. [DOI] [PubMed] [Google Scholar]
- 34. Shetty R, Pibarot P, Audet A, Janvier R, Dagenais F, Perron J, Couture C, Voisine P, Despres JP, Mathieu P. Lipid‐mediated inflammation and degeneration of bioprosthetic heart valves. Eur J Clin Invest. 2009;39:471–480. [DOI] [PubMed] [Google Scholar]
- 35. Antonini‐Canterin F, Popescu BA, Zuppiroli A, Nicolosi GL. Are statins effective in preventing bioprosthetic aortic valve failure? A need for a prospective, randomized trial. Ital Heart J. 2004;5:85–88. [PubMed] [Google Scholar]
- 36. Chacko J, Harling L, Ashrafian H, Athanasiou T. Can statins improve outcomes after isolated cardiac valve surgery? A systematic literature review Clin Cardiol. 2013;36:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Citardi MJ, Song W, Batra PS, Lanza DC, Hazen SL. Characterization of oxidative pathways in chronic rhinosinusitis and sinonasal polyposis. Am J Rhinol. 2006;20:353–359. [DOI] [PubMed] [Google Scholar]
- 38. Ganapathi AM, Englum BR, Keenan JE, Schechter MA, Wang H, Smith PK, Glower DD, Hughes GC. Long‐term survival after bovine pericardial versus porcine stented bioprosthetic aortic valve replacement: does valve choice matter? Ann Thorac Surg. 2015;100:550–559. [DOI] [PubMed] [Google Scholar]
- 39. Dhayal SK, Sforza S, Wierenga PA, Gruppen H. Peroxidase induced oligo‐tyrosine cross‐links during polymerization of alpha‐lactalbumin. Biochim Biophys Acta. 2015;1854:1898–1905. [DOI] [PubMed] [Google Scholar]
- 40. Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. Eosinophils generate brominating oxidants in allergen‐induced asthma. J Clin Invest. 2000;105:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis‐patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–2129. [DOI] [PubMed] [Google Scholar]
- 42. Hazen SL, Heinecke JW. 3‐Chlorotyrosine, a specific marker of myeloperoxidase‐catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heinecke JW. Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross‐linking of proteins. Toxicology. 2002;177:11–22. [DOI] [PubMed] [Google Scholar]


