Abstract
Background
Impaired orthostatic blood pressure (BP) stabilization is highly prevalent in older adults and is a predictor of end‐organ injury, falls, and mortality. We sought to characterize the relationship between postural BP responses and the kidney.
Methods and Results
We performed a cross‐sectional analysis of 4204 participants from The Irish Longitudinal Study on Ageing, a national cohort of community‐dwelling adults aged ≥50 years. Beat‐to‐beat systolic and diastolic BP were measured during a 2‐minute active stand test. The primary predictor was cystatin C estimated glomerular filtration rate (eGFR) categorized as follows (mL/min per 1.73 m2): ≥90 (reference, n=1414); 75 to 89 (n=1379); 60 to 74 (n=942); 45 to 59 (n=337); <45 (n=132). We examined the association between eGFR categories and (1) sustained orthostatic hypotension, defined as a BP drop exceeding consensus thresholds (systolic BP drop ≥20 mm Hg±diastolic BP drop ≥10 mm Hg) at each 10‐second interval from 60 to 110 seconds inclusive; (2) pattern of BP stabilization, characterized as the difference from baseline in mean systolic BP/diastolic BP at 10‐second intervals. The mean age of subjects was 61.6 years; 47% of subjects were male, and the median eGFR was 82 mL/min per 1.73 m2. After multivariable adjustment, participants with eGFR <60 mL/min per 1.73 m2 were approximately twice as likely to have sustained orthostatic hypotension (P=0.008 for trend across eGFR categories). We observed a graded association between eGFR categories and impaired orthostatic BP stabilization, particularly within the first minute of standing.
Conclusions
We report a novel, graded relationship between diminished eGFR and impaired orthostatic BP stabilization. Mapping the postural BP response merits further study in kidney disease as a potential means of identifying those at risk of hypotension‐related events.
Keywords: blood pressure measurement/monitoring, cystatin C, glomerular filtration rate, hypotension, kidney, low blood pressure, orthostatic hypotension
Subject Categories: Blood Pressure, Nephrology and Kidney, Epidemiology, Autonomic Nervous System
Introduction
Recent large‐scale observational studies have demonstrated a J‐shaped relationship between blood pressure (BP) and all‐cause mortality in individuals with chronic kidney disease (CKD),1, 2 raising concern about the potential harms associated with hypotension in this population. In the general population, orthostatic hypotension (OH) has been shown to be an independent predictor of cardiovascular outcomes including coronary artery disease, heart failure, and stroke.3, 4, 5 Older individuals have the highest burden of both OH and CKD, yet the relationship between postural BP responses and kidney function has rarely been examined in this demographic. Furthermore, our tools for detecting OH are insensitive in clinical practice. OH is conventionally defined as a sustained drop in BP that exceeds consensus thresholds within 3 minutes of standing.6 However, what constitutes a sustained change in postural BP is not clearly defined. The current classification of OH is based on a small number of data points, which do not capture the full dynamic BP response to standing.
Beat‐to‐beat BP measurements offer the potential to refine our understanding of the relationship between postural BP behavior and the kidney by mapping the postural BP response, including the initial drop in BP and the slope of BP recovery, or stabilization, toward baseline values. Older adults demonstrate progressively delayed BP recovery (hereafter referred to as impaired BP stabilization),7 and this has important consequences such as an increased risk of falls8 and all‐cause mortality.9 Impaired BP stabilization is associated with end‐organ dysfunction in areas of the body with high perfusion demand such as the eye10 and the brain.11 A consistent finding among these studies is that the degree of BP stabilization, rather than the absolute initial fall in BP, appears to be the main predictor of outcome, suggesting that sustained reductions in BP result in relative hypoperfusion to vital organs. As a highly vascular organ that relies on precise autoregulation of blood flow to maintain function, the kidney may be susceptible to injury following repeated episodes of sustained hypotension. Kidney disease is also associated with the development of autonomic dysfunction, which itself could contribute to a greater likelihood of OH.
A more granular assessment of the postural BP response could inform clinicians attempting to balance the benefits of treating hypertension with attendant risks of hypotension in the clinically heterogeneous population of older adults with diminished kidney function. We sought to characterize the postural BP response, in particular sustained reductions in BP, among a large sample of older community‐dwelling individuals across the spectrum of estimated glomerular filtration rate (eGFR).
Methods
Study Population
Data from the first wave of The Irish Longitudinal Study on Ageing were analyzed. The Irish Longitudinal Study on Ageing is a nationally representative cohort of community‐dwelling Irish adults aged 50 years and over. Details of the sampling procedure and study design have been described elsewhere.12, 13 Wave 1 was performed between June 2009 and June 2011. All participants undertook a computer‐assisted personal interview in their homes and were subsequently invited to take part in a comprehensive health assessment in a dedicated health center, carried out by trained nurses. Details of the health assessment have been described previously.14 This analysis included all participants who completed an active stand test at the wave 1 health assessment and had a simultaneous measurement of kidney function. All participants provided informed signed consent. Ethical approval for the study was granted by the Research Ethics Committee of Trinity College Dublin. All experimental procedures adhered to the Declaration of Helsinki.
Measurement of Orthostatic Blood Pressure
The continuous BP response to postural change was recorded using the volume‐clamp method combined with Physiocal and brachial artery waveform reconstruction (Finometer®, Finapres Medical Systems, Amsterdam, The Netherlands). Recordings were obtained in a comfortably lit quiet room at an ambient temperature of 21°C to 23°C. Participants were asked to rest in the supine position for 10 minutes. Throughout this time the Physiocal (recalibration) function was enabled. The variables “baseline SBP” and “baseline DBP” were calculated as the mean systolic (SBP) and diastolic BP (DBP) values recorded between 60 and 30 seconds prior to the participant standing. Physiocal was switched off immediately prior to standing and remained off until completion of the test to ensure that no data were lost during this time period due to the recalibration process. Participants were asked to stand in a timely manner (<5 seconds) with or without assistance from the research nurse. The participant's arm was resting by his or her side during the period of supine rest and during standing. Upon the participants' standing, SBP and DBP were recorded for 2 minutes, during which time the participants stood quietly. The participants' BP was estimated at 10‐second intervals using 5‐second moving averages around each time point. The steps involved in data processing have been described in detail elsewhere.15
Measurement of Renal Biomarkers
At wave 1, a venous blood sample (25 mL) was collected from each consenting participant. The blood samples were transported to a central laboratory in temperature‐controlled shipping boxes, where they were centrifuged, aliquoted into cryovials, and stored at −80°C. Cystatin C and creatinine were measured simultaneously from frozen plasma. Cystatin C was measured using a second‐generation particle enhanced immunoturbidimetric assay (Roche Tina‐quant™) on a Roche (Pleasanton, CA) Cobas 701 analyzer. This assay has a measuring range of 0.40 to 6.80 mg/L and is traceable to the European reference standard material (ERM‐DA471/IFCC) for cystatin C. Creatinine was measured on the same analyzer using an enzymatic method traceable to isotope dilution mass spectrometry.
Predictor Variables
For all analyses eGFR was calculated primarily from cystatin C (eGFRcys), and in secondary analyses from creatinine (eGFRcreat), according to the CKD Epidemiology Collaboration equations16, 17 and categorized as follows: ≥90 mL/min per 1.73 m2 (reference category); 75 to 89 mL/min per 1.73 m2; 60 to 74 mL/min per 1.73 m2; 45 to 59 mL/min per 1.73 m2; or <45 mL/min per 1.73 m2. These categories are consistent with Kidney Disease Improving Global Outcomes guidelines for staging of eGFR. Due to relatively small numbers of participants with eGFR <30 mL/min per 1.73 m2 we created a single category for participants with eGFR <45 mL/min per 1.73 m2.
Study Outcomes
The primary outcome was “sustained OH,” which we defined as a postural drop in BP that exceeded consensus BP thresholds for OH (SBP drop ≥20 mm Hg and/or DBP drop ≥10 mm Hg)6 at each 10‐second interval from 60 to 110 seconds inclusive.7 Only participants who met consensus OH criteria at all of these time points were considered to have sustained OH. In an exploratory analysis we examined the pattern of postural BP responses during the active stand test, characterized as the difference in mean SBP and DBP values from baseline (resting supine) at each 10‐second interval after standing, up to and including 110 seconds. The presence of “impaired BP stabilization” was inferred when the mean deficit in SBP or DBP for a given eGFR category was statistically significantly greater than that of the reference group.
Covariates
Participant characteristics included age, sex, smoking history, and self‐reported physician‐diagnosed conditions. Medication use was recorded during the interview, cross‐checked with medication labels, and coded according to the World Health Organization Anatomical Therapeutic Chemical Classification.18 Low‐ and high‐density lipoprotein were measured from each of the blood samples prior to freezing. We defined the presence of diabetes mellitus as a self‐reported physician's diagnosis, or receiving insulin or oral hypoglycemic medications. We defined the presence of cardiovascular disease as any 1 of the following self‐reported physician‐diagnosed conditions: angina, heart failure, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, transient ischemic attack, or stroke. Height and waist circumference were measured at the health assessment.
Statistical Analysis
Continuous variables are described as mean (SD) or median (interquartile range) as appropriate. Categorical variables are described as count (%). We used ANOVA for the comparison of normally distributed continuous variables and the chi‐squared test for the comparison of categorical variables. We examined the relationship between eGFRcys categories and sustained OH using logistic regression, with estimates reported as odds ratios and 95%CI. These models were adjusted for the following covariates: age, sex, height, baseline SBP, number of antihypertensive medications (0, 1, 2, 3, or more), cardiovascular disease, diabetes mellitus, smoking (current/former/never), waist circumference, and high‐ and low‐density lipoprotein cholesterol. The models included a quadratic term for age to account for nonlinearity in the relationships between age and both postural BP responses7 and prevalence of reduced eGFR.19 In a stratified analysis we examined the differential associations between eGFRcys categories and sustained OH under antihypertensive therapy, using the likelihood ratio test.
For the exploratory analysis, we estimated the mean differences in SBP and DBP from baseline at each 10‐second interval after standing for each category of eGFRcys versus the reference group (eGFRcys ≥90 mL/min per 1.73 m2). To account for the fact that the repeated BP measurements are nested within the individual and therefore correlated with each other, we ran mixed‐effects linear regression models. We reparameterized the “time” variables from 11 discrete time variables (10 to 110 seconds inclusive) to a set of linear spline variables with 3 knots as follows: 10 to 20 seconds; 20 to 30 seconds; 30 to 110 seconds. We chose these knots because, at a population level, most of the absolute mean change in BP from baseline occurs within the first 30 seconds of standing, beyond which time the mean BP change from baseline is uniform. These reparameterized time variables were included in the models as fixed effects and as random effects (random intercept). Because BP measurements closer together had stronger correlations than those further apart, conditional (residual) variance was modeled using an autoregressive variance‐covariance matrix across all 11 time points per individual. Each independent variable was included in the fixed part of the model, both as a main effect and as an interaction term with the time variables. Based on the fitted models, for each eGFR category, we estimated the conditional mean differences in SBP and DBP from baseline for each 10‐second interval of the active stand test and plotted these. These conditional mean estimates are conditional at the means of other covariates in the model.
In a secondary analysis we estimated and plotted the conditional mean differences in SBP and DBP from baseline at each 10‐second interval after standing for each category of eGFRcreat, using the same mixed‐effects linear regression model as that described for eGFRcys above.
Only complete cases were analyzed. All analyses were performed using Stata version 14.1 (StataCorp, College Station, TX).
Results
Participant Characteristics
A total of 5035 participants attended the wave 1 health assessment. The majority (94.1%) of these participants had a venous blood sample taken for measurement of cystatin C and creatinine. Of these, 533 participants had incomplete active stand data, resulting in a study population of 4204 participants with nonmissing eGFR and complete active stand data (Figure 1). Participant characteristics, by eGFRcys category, are displayed in Table 1. Mean (SD) age of participants was 61.6 (8.2) years, 46.8% were male, and median (interquartile range) eGFRcys was 82 (70‐94) mL/min per 1.73 m2. Participants with diminishing eGFR tended to be older and to have a higher prevalence of diabetes mellitus and cardiovascular disease, higher baseline SBP and waist circumference, and a greater burden of antihypertensive therapy. Table 2 provides a description of study participants who did not attend for a health assessment and participants who attended a health assessment but who were missing data for either eGFR or the active stand test.
Figure 1.

Flowchart of case ascertainment. eGFR indicates estimated glomerular filtration rate.
Table 1.
Participant Characteristics
| Cystatin C eGFR (mL/min per 1.73 m2) | P Valuea | |||||
|---|---|---|---|---|---|---|
| >90 (n=1414) | 75 to 89 (n=1379) | 60 to 74 (n=942) | 45 to 59 (n=337) | <45 (n=132) | ||
| Age, y | 57.1 (6.0) | 60.8 (6.6) | 64.9 (8.1) | 69.8 (7.8) | 73.4 (8.6) | <0.001 |
| Male sex, n (%) | 692 (48.9) | 640 (46.4) | 436 (46.3) | 134 (39.8) | 64 (48.5) | 0.05 |
| Height, cm | 167.3 (9.0) | 166.5 (9.3) | 166.1 (9.3) | 163.9 (9.0) | 164.0 (8.4) | <0.001 |
| Diabetes mellitus, n (%) | 74 (5.2) | 69 (5.0) | 67 (7.1) | 43 (12.8) | 26 (19.7) | <0.001 |
| CVD, n (%) | 74 (5.2) | 99 (7.2) | 118 (12.5) | 63 (18.7) | 42 (31.8) | <0.001 |
| Waist, cm | 91.9 (13.0) | 93.8 (12.8) | 97.6 (13.8) | 99.8 (13.9) | 101.3 (15.6) | <0.001 |
| LDL, mmol/L | 3.01 (0.93) | 3.02 (0.92) | 2.89 (0.97) | 2.65 (0.95) | 2.55 (0.91) | <0.001 |
| HDL, mmol/L | 1.61 (0.45) | 1.57 (0.43) | 1.50 (0.42) | 1.42 (0.41) | 1.38 (0.42) | <0.001 |
| Smoking, n (%) | <0.001 | |||||
| Current | 159 (11.2) | 209 (15.2) | 181 (19.2) | 63 (18.7) | 11 (8.3) | |
| Former | 571 (40.4) | 532 (38.6) | 350 (37.2) | 145 (43.0) | 67 (50.8) | |
| Never | 684 (48.4) | 638 (46.3) | 411 (43.6) | 129 (38.3) | 54 (40.9) | |
| SBP, mm Hg | 133.7 (21.3) | 136.3 (22.1) | 138.3 (22.8) | 140.3 (22.6) | 138.8 (28.8) | <0.001 |
| DBP, mm Hg | 73.5 (11.2) | 73.8 (10.9) | 73.3 (11.1) | 71.6 (10.8) | 69.9 (13.9) | <0.001 |
| BP agents, n (%) | <0.001 | |||||
| None | 1109 (78.4) | 1022 (74.1) | 555 (58.9) | 132 (39.2) | 30 (22.7) | |
| 1 agent | 186 (13.2) | 183 (13.3) | 182 (19.3) | 81 (24.0) | 31 (23.5) | |
| 2 agents | 86 (6.1) | 124 (9.0) | 142 (15.1) | 79 (23.4) | 40 (30.3) | |
| 3 or more | 33 (2.3) | 50 (3.6) | 63 (6.7) | 45 (13.4) | 31 (23.5) | |
| β‐Blocker, n (%) | 94 (6.7) | 131 (9.5) | 133 (14.1) | 93 (27.6) | 44 (33.3) | <0.001 |
| RAASi, n (%) | 197 (13.9) | 232 (16.8) | 274 (29.1) | 138 (41.0) | 75 (56.8) | <0.001 |
| CCB, n (%) | 79 (5.6) | 94 (6.8) | 94 (10.0) | 53 (15.7) | 35 (26.5) | <0.001 |
| Diuretic, n (%) | 31 (2.2) | 61 (4.4) | 65 (6.9) | 51 (15.1) | 32 (24.2) | <0.001 |
Numbers are expressed as count (%) or mean (SD). Data missing for height (n=2), waist circumference (n=11), and cholesterol (n=7). BP indicates blood pressure; CCB, calcium channel blocker; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RAASi, renin‐angiotensin‐aldosterone‐system inhibitor; SBP, systolic blood pressure.
For differences across eGFR categories.
Table 2.
Characteristics of Participants Who Did Not Attend a Health Assessment and Participants With Missing Data for Either Kidney Function or the Active Stand Test, Compared to the Final Study Population
| No Assessment (n=3140) | Missing Data (n=831) | Final Cohort (n=4204) | |
|---|---|---|---|
| Age, y | 66.8 (10.9) | 63.8 (9.4) | 61.6 (8.2) |
| Female sex | 1702 (54.2) | 491 (59.1) | 2238 (53.2) |
| Smoking | |||
| Current | 735 (23.4) | 133 (16.0) | 623 (14.8) |
| Former | 1137 (36.2) | 315 (37.9) | 1665 (39.6) |
| Never | 1267 (40.4) | 383 (46.1) | 1916 (45.6) |
| Diabetes mellitus | 335 (10.7) | 58 (7.0) | 279 (6.6) |
| Hypertension | 1660 (52.9) | 389 (46.8) | 1621 (38.6) |
| Cardiovascular disease | 450 (14.3) | 110 (13.2) | 396 (9.4) |
| Antihypertensives | |||
| None | 1738 (55.4) | 503 (60.5) | 2848 (67.8) |
| 1 agent | 646 (20.6) | 165 (19.9) | 663 (15.8) |
| 2 agents | 457 (14.6) | 107 (12.9) | 471 (11.2) |
| 3 or more agents | 297 (9.5) | 56 (6.7) | 222 (5.3) |
Numbers are expressed as count (%) or mean (SD).
Sustained Orthostatic Hypotension
The results of logistic regression models for the outcome sustained OH are provided in Table 3. The overall frequency of sustained OH was 6.0% (n=252). In unadjusted analyses the likelihood of sustained OH increased steadily with diminishing eGFRcys (P for trend <0.001). A similar pattern was observed after multivariable adjustment; however, the relatively low number of events contributed to wide error bounds for these estimates. In adjusted analyses, participants with eGFRcys <60 mL/min per 1.73 m2 were approximately twice as likely to have sustained OH compared with those with preserved kidney function: odds ratio 2.25 (95%CI 1.34‐3.78) for eGFRcys 45 to 59 mL/min per 1.73 m2; odds ratio 1.77 (95%CI 0.85‐3.72) for eGFRcys <45 mL/min per 1.73 m2. We stratified the sample by the presence or absence of CKD (eGFRcys <60 mL/min per 1.73 m2). After multivariable adjustment, participants with CKD had a 67% increased likelihood of having sustained OH compared with those without CKD (odds ratio 1.67 [95%CI 1.13‐2.47]). The association between eGFRcys categories and sustained OH did not vary significantly by the presence or absence of antihypertensive therapy (likelihood ratio P=0.42).
Table 3.
Likelihood of Sustained Orthostatic Hypotension Per Category of Cystatin C eGFR
| eGFR Stage | Sustained OH Odds Ratio (95%CI) | ||
|---|---|---|---|
| Count (%) | Model 1 | Model 2 | |
| ≥90 | 58 (4.1) | Ref | Ref |
| 75 to 89 | 76 (5.5) | 1.36 (0.96‐1.94) | 1.23 (0.85‐1.78) |
| 60 to 74 | 64 (6.8) | 1.70a (1.18‐2.46) | 1.38 (0.91‐2.09) |
| 45 to 59 | 39 (11.6) | 3.06b (2.00‐4.68) | 2.25a (1.34‐3.78) |
| <45 | 15 (11.4) | 3.00b (1.65‐5.45) | 1.77 (0.85‐3.72) |
| P trend | <0.001 | 0.008 | |
Model 1 (n=4204), unadjusted. Model 2 (n=4185), adjusted for age, age2, sex, height, baseline systolic blood pressure, cardiovascular disease, diabetes mellitus, smoking, waist circumference, LDL/HDL cholesterol, number of antihypertensive medications. eGFR indicates estimated glomerular filtration rate (mL/min per 1.73 m2); LDL/HDL, low‐density/high‐density lipoprotein cholesterol; OH, orthostatic hypotension.
P<0.01.
P<0.001.
Pattern of Postural Blood Pressure Change
The unadjusted and multivariable adjusted relationships between eGFRcys and postural SBP responses during the active stand test are illustrated graphically in Figure 2. The pattern of SBP responses differed by eGFRcys category. In unadjusted analyses there was a graded association between lower eGFRcys categories and impaired SBP stabilization, which was particularly marked below an eGFRcys of 45 mL/min per 1.73 m2. This pattern was evident from 20 seconds after standing and was most pronounced within the first minute. The graded nature of the association was consistent in the multivariable adjusted models. The results of the multivariable adjusted regression model for the SBP response at each 10‐second interval during the active stand test are summarized graphically in Figure 3. Each eGFRcys category is compared to the reference group (eGFRcys ≥90 mL/min per 1.73 m2, represented by a horizontal line extending from the Y axis). Differences in the SBP response were most marked below an eGFRcys of 60 mL/min per 1.73 m2.
Figure 2.

Unadjusted (left panel) and multivariable‐adjusted (right panel) estimate of the conditional mean difference (with corresponding 95%CI) in systolic blood pressure from baseline (y axis) at each 10‐second interval during the active stand (x axis) among categories of estimated glomerular filtration rate (eGFR) derived from cystatin C. SBP indicates systolic blood pressure.
Figure 3.

Multivariable‐adjusted differences across categories of cystatin C–estimated glomerular filtration rate in the change from baseline in systolic blood pressure (y axis) at each 10‐second interval during the active stand (x axis). Each estimated glomerular filtration rate (eGFR) category is compared to the reference group (eGFR >90 mL/min per 1.73 m2, represented by a horizontal red line): eGFR 75 to 89 mL/min per 1.73 m2 (top left), eGFR 60 to 74 mL/min per 1.73 m2 (top right), eGFR 45 to 59 mL/min per 1.73 m2 (bottom left), eGFR <45 mL/min per 1.73 m2 (bottom right). SBP indicates systolic blood pressure.
The relationship between eGFRcys categories and postural DBP responses during the active stand test is illustrated in Figure 4. Although we acknowledge the difference in BP scale, the adjusted association between eGFRcys categories and the DBP response was overall less pronounced than the adjusted association between eGFRcys categories and the SBP response.
Figure 4.
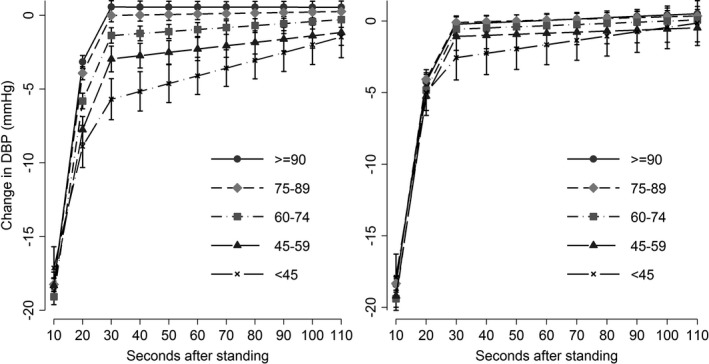
Unadjusted (left panel) and multivariable‐adjusted (right panel) estimate of the conditional mean difference (with corresponding 95%CI) in diastolic blood pressure from baseline (y axis) at each 10‐second interval during the active stand (x axis) among categories of estimated glomerular filtration rate derived from cystatin C. DBP indicates diastolic blood pressure.
Secondary Analyses
The unadjusted relationship between eGFRcreat categories and the SBP response was similar to the unadjusted association between eGFRcys categories and the SBP response (Figure 5). After multivariable adjustment, the differences between eGFRcreat groups in mean postural SBP were consistently smaller than those for eGFRcys groups. A similar pattern was observed for the association between eGFRcreat and the postural DBP response (Figure 6).
Figure 5.

Unadjusted (left panel) and multivariable‐adjusted (right panel) estimate of the conditional mean difference (with corresponding 95%CI) in systolic blood pressure from baseline (y axis) at each 10‐second interval during the active stand (x axis) among categories of estimated glomerular filtration rate derived from creatinine. SBP indicates systolic blood pressure.
Figure 6.

Unadjusted (left panel) and multivariable‐adjusted (right panel) estimate of the conditional mean difference (with corresponding 95%CI) in diastolic blood pressure from baseline (y axis) at each 10‐second interval during the active stand (x axis) among categories of estimated glomerular filtration rate derived from creatinine. DBP indicates diastolic blood pressure.
Discussion
In this study of postural blood pressure responses among older community‐dwelling adults, we observed an increased likelihood of sustained OH with greater reductions in kidney function. This relationship was evident in individuals with relatively modest declines in eGFRcys and was independent of age, cardiovascular risk factors, resting mean BP, and antihypertensive therapy. By mapping the postural BP response across the range of eGFRcys, we observed a graded association between eGFRcys and impaired BP stabilization. This pattern was particularly marked within the first minute of standing, a time window not routinely captured in clinical practice.
There is a paucity of data describing the relationship between the postural BP response and the kidney. A study examining determinants of OH in middle‐aged men found a cross‐sectional association between postural SBP impairment and lower eGFR.20 A longitudinal study of middle‐aged community‐dwelling adults demonstrated an increased risk of incident kidney dysfunction with OH, defined by consensus criteria using oscillometric measurements.21 The magnitude of this risk was modest in whites using a creatinine‐based eGFR threshold (<60 mL/min per 1.73 m2) to define CKD. Creatinine generation tends to be unstable in older individuals due to changes in muscle mass, often resulting in nonlinear relationships with clinical outcomes. Cystatin C has gained traction as an alternative filtration marker in older individuals because, unlike creatinine, it is not influenced by dietary protein intake22 or muscle mass.23 Cystatin C has demonstrated stronger and more linear risk relationships with clinical outcomes than creatinine.24 Our study advances the literature by providing a granular description of the postural BP response in a large sample of older community‐dwelling adults using novel beat‐to‐beat measurements across the spectrum of eGFR calculated from cystatin C.
The cross‐sectional design of our study limits a discussion regarding a causal relationship between kidney function and postural BP responses. Nevertheless, the association was strong and should generate some hypotheses regarding this relationship. The differential postural BP response across eGFR categories occurred early and was pronounced within the first 40 seconds of standing. Short‐term regulation of BP is governed principally by the baroreceptor reflex arc. CKD has been shown to be associated with reduced baroreflex sensitivity,25 which could explain wider fluctuations in early postural BP responses with diminished eGFR. Reductions in baroreflex sensitivity have been linked to stiffness or lower compliance of large arteries.26 Vascular stiffness is predominantly a complication of advanced CKD but has also been demonstrated in earlier stages of CKD.27 We also observed an increased likelihood of sustained OH in individuals with eGFRcys <60 mL/min per 1.73 m2. This suggests a greater degree of autonomic dysfunction in this population, which in theory could affect several components of the circulatory reflex including reduced vasomotor responsiveness or impaired central control of BP. Studies in patients with advanced CKD and those receiving dialysis have proposed that autonomic dysfunction is an important factor in the progression of kidney disease and its cardiovascular complications.28 Our findings suggest that autonomic dysfunction may be occurring at much earlier stages of kidney disease, contributing to a greater likelihood of OH.
The presence of impaired orthostatic BP stabilization may be a surrogate marker for vascular disease. Although GFR is known to decrease with age, the underlying mechanisms and natural history of this process are poorly understood. Aging is associated with a number of structural and functional changes in the kidney that might predispose to an increased susceptibility to injury from a vascular insult. Renal blood flow declines with age, particularly in the cortex, and the kidney's ability to preserve glomerular hydrostatic pressure via autoregulation also diminishes.29 The number of functioning glomeruli reduces with advancing age,30 further hindering the compensatory response. Glomerulosclerosis increases in a linear fashion across the age spectrum in healthy kidney donors,31 and the ischemic pattern of this age‐related glomerulosclerosis suggests an underlying vascular etiology.32 It is thus plausible that repeated episodes of sustained hypotension, as a consequence of impaired orthostatic BP stabilization, could overwhelm the already diminished autoregulatory capacity of the aging kidney.
The association between eGFR and postural BP responses could also be explained by a greater level of cardiovascular comorbidity among participants with diminished kidney function. Individuals with lower eGFR tend to have a greater burden of cardiovascular disease, which could also account for an exaggerated orthostatic fall in BP due to reduced stroke volume. The prevalence of self‐reported physician‐diagnosed cardiovascular disease was low (<10%) in our study population. We adjusted for cardiovascular disease in our analysis as well as for cardiovascular risk factors including diabetes mellitus, central adiposity, smoking, and dyslipidemia, although it remains possible that unidentified cardiovascular disease may have contributed to our findings. Participants with lower eGFR also tended to have higher baseline SBP and proportionately greater use of antihypertensive medications. Adjusting for baseline SBP or the burden of antihypertensive therapy did not attenuate the strength of the association between eGFR and postural BP responses. Furthermore, the estimates of association in multivariable adjusted models did not vary by the presence or absence of antihypertensive therapy.
Our study has several strengths. We measured beat‐to‐beat BP during an active stand test in a large sample of older individuals. The Finapres device has been used extensively as both a clinical and research tool, and its methodology is well validated.33, 34 We estimated kidney function from a standardized measurement of cystatin C, a potentially preferable filtration marker to creatinine in older adults. The Irish Longitudinal Study on Ageing data set is comprehensive, facilitating a robust appraisal of potential confounders including medications. The findings should be interpreted in the context of the study's exploratory nature and potential limitations. Our ability to infer any causal relationship between BP instability and eGFR is limited by the study's cross‐sectional design. There remains the possibility of residual measured or unmeasured confounding. We included self‐reported physician‐diagnosed conditions as covariates, which are subject to measurement error. No explicit adjustment for multiple testing was performed, although the actual confidence intervals are provided to allow interpretation of the results. Cystatin C and creatinine were measured at a single time point, and we did not measure urinary albumin excretion. Data were missing for both kidney function and the active stand test, which may have introduced a selection bias. The degree of this bias is likely to be smaller in the estimates from the multivariable adjusted models, as the covariates in these models are predictive of “missingness” as well as predictive of exposure and outcome. Nevertheless, the potential for bias remains, and our findings are only generalizable to older community‐dwelling white adults who would have been healthy enough to attend our health center assessment.14
We report a novel association between postural BP responses and kidney function in community‐dwelling older adults using beat‐to‐beat BP measurements. We identified an independent and graded association between impaired orthostatic BP stabilization and reductions in eGFR, which was stronger for eGFRcys than for eGFRcreat. This pattern of impaired BP stabilization was evident at early stages of kidney disease and was particularly marked within the first minute of standing, a time window not usually captured by conventional oscillometric BP measurements. Although further studies are warranted to determine the clinical implications of these findings, our data suggest that there is a need for heightened awareness of postural BP behavior in those with diminished kidney function. Assessment of the postural BP response merits further study in the CKD population as a potential means of identifying individuals at higher risk of developing hypotension‐related events such as falls, an important outcome not predicted by kidney function alone.35
Sources of Funding
This research was sponsored by the Health Research Board (HPF/2014/540 [to Canney] and HRB/RL/2013/7). M.D.L.O is supported by an Ageing Research Leadership Fellowship awarded from the Centre for Ageing Research and Development in Ireland, which became the Ageing Research and Development Division within the Institute of Public Health in Ireland (IPH) in September 2015, sponsored by the American Federation for Aging Research Paul B Beeson Career Development Awards in Aging Research for the Island of Ireland. The Irish Longitudinal Study on Ageing is funded by the Irish Department of Health, Irish Life and The Atlantic Philanthropies.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005661 DOI: 10.1161/JAHA.117.005661.)28473404
References
- 1. Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, Quarles LD, Kalantar‐Zadeh K. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss JW, Peters D, Yang X, Petrik A, Smith DH, Johnson ES, Thorp ML, Morris C, O'Hare AM. Systolic BP and mortality in older adults with CKD. Clin J Am Soc Nephrol. 2015;10:1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all‐cause mortality and coronary events in middle‐aged individuals (The Malmo Preventive Project). Eur Heart J. 2010;31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fedorowski A, Engstrom G, Hedblad B, Melander O. Orthostatic hypotension predicts incidence of heart failure: the Malmo preventive project. Am J Hypertens. 2010;23:1209–1215. [DOI] [PubMed] [Google Scholar]
- 5. Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1996. Stroke. 2000;31:2307–2313. [DOI] [PubMed] [Google Scholar]
- 6. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 7. Finucane C, O'Connell MD, Fan CW, Savva GM, Soraghan CJ, Nolan H, Cronin H, Kenny RA. Age‐related normative changes in phasic orthostatic blood pressure in a large population study: findings from the Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130:1780–1789. [DOI] [PubMed] [Google Scholar]
- 8. van der Velde N, van den Meiracker AH, Stricker BH, van der Cammen TJ. Measuring orthostatic hypotension with the Finometer device: is a blood pressure drop of one heartbeat clinically relevant? Blood Press Monit. 2007;12:167–171. [DOI] [PubMed] [Google Scholar]
- 9. Lagro J, Schoon Y, Heerts I, Meel‐van den Abeelen AS, Schalk B, Wieling W, Olde Rikkert MG, Claassen JA. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J Gerontol A Biol Sci Med Sci. 2014;69:471–478. [DOI] [PubMed] [Google Scholar]
- 10. Ni Bhuachalla B, McGarrigle CA, Akuffo KO, Peto T, Beatty S, Kenny RA. Phenotypes of orthostatic blood pressure behaviour and association with visual acuity. Clin Auton Res. 2015;25:373–381. [DOI] [PubMed] [Google Scholar]
- 11. Frewen J, Finucane C, Savva GM, Boyle G, Kenny RA. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J Gerontol A Biol Sci Med Sci. 2014;69:878–885. [DOI] [PubMed] [Google Scholar]
- 12. Kearney PM, Cronin H, O'Regan C, Kamiya Y, Savva GM, Whelan B, Kenny R. Cohort profile: the Irish Longitudinal Study on Ageing. Int J Epidemiol. 2011;40:877–884. [DOI] [PubMed] [Google Scholar]
- 13. Whelan BJ, Savva GM. Design and methodology of the Irish Longitudinal Study on Ageing. J Am Geriatr Soc. 2013;61(suppl 2):S265–S268. [DOI] [PubMed] [Google Scholar]
- 14. Cronin H, O'Regan C, Finucane C, Kearney P, Kenny RA. Health and aging: development of the Irish Longitudinal Study on Ageing Health Assessment. J Am Geriatr Soc. 2013;61(suppl 2):S269–S278. [DOI] [PubMed] [Google Scholar]
- 15. Soraghan CJ, Fan CW, Hayakawa T, Cronin H, Foran T, Boyle G, Kenny RA, Finucane C. TILDA Signal Processing Framework (SPF) for the analysis of BP responses to standing in epidemiological and clinical studies. LEEE EMBS Int Conf Biomed Health Inform. 2014; 793–796. [Google Scholar]
- 16. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC Classification and DDD Assignment 2012. Oslo: 2011. [Google Scholar]
- 19. Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age‐ and gender‐specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72:632–637. [DOI] [PubMed] [Google Scholar]
- 20. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Consequences of orthostatic blood pressure variability in middle‐aged men (The Malmo Preventive Project). J Hypertens. 2010;28:551–559. [DOI] [PubMed] [Google Scholar]
- 21. Franceschini N, Rose KM, Astor BC, Couper D, Vupputuri S. Orthostatic hypotension and incident chronic kidney disease: the Atherosclerosis Risk in Communities Study. Hypertension. 2010;56:1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, Coresh J, Levey AS. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011;79:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johansson M, Gao SA, Friberg P, Annerstedt M, Bergstrom G, Carlstrom J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, Nielsen FD, Strombom U. Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens. 2005;18:995–1000. [DOI] [PubMed] [Google Scholar]
- 26. Mattace‐Raso FU, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias‐Smale S, Reneman RS, Hoeks AP, Hofman A, Witteman JC. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25:1421–1426. [DOI] [PubMed] [Google Scholar]
- 27. Madero M, Wassel CL, Peralta CA, Najjar SS, Sutton‐Tyrrell K, Fried L, Canada R, Newman A, Shlipak MG, Sarnak MJ. Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol. 2009;20:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salman IM. Cardiovascular autonomic dysfunction in chronic kidney disease: a comprehensive review. Curr Hypertens Rep. 2015;17:59. [DOI] [PubMed] [Google Scholar]
- 29. Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rule AD, Cornell LD, Poggio ED. Senile nephrosclerosis—does it explain the decline in glomerular filtration rate with aging? Nephron Physiol. 2011;119(suppl 1):p6–p11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012;82:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Imholz BPM, Wieling W, van Montfrans GA, Wesseling KH. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res. 1998;38:605–616. [DOI] [PubMed] [Google Scholar]
- 34. Imholz BPM, Settels JJ, Vandermeiracker AH, Wesseling KH, Wieling W. Noninvasive continuous finger blood‐pressure measurement during orthostatic stress compared to intraarterial pressure. Cardiovasc Res. 1990;24:214–221. [DOI] [PubMed] [Google Scholar]
- 35. Bowling CB, Bromfield SG, Colantonio LD, Gutierrez OM, Shimbo D, Reynolds K, Wright NC, Curtis JR, Judd SE, Franch H, Warnock DG, McClellan W, Muntner P. Association of reduced eGFR and albuminuria with serious fall injuries among older adults. Clin J Am Soc Nephrol. 2016;11:1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]


