Abstract
Background
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors are a novel class of antihyperglycemic agents that improve glycemic control by increasing glycosuria. Additional benefits beyond glucose lowering include significant improvements in seated clinic blood pressure (BP), partly attributed to their diuretic‐like actions. Less known are the effects of this class on 24‐hour ambulatory BP, which is a better predictor of cardiovascular risk than seated clinic BP.
Methods and Results
We performed a meta‐analysis of randomized, double‐blind, placebo‐controlled trials to investigate the effects of SGLT2 inhibitors on 24‐hour ambulatory BP. We searched all studies published before August 17, 2016, which reported 24‐hour ambulatory BP data. Mean differences in 24‐hour BP, daytime BP, and nighttime BP were calculated by a random‐effects model. SGLT2 inhibitors significantly reduce 24‐hour ambulatory systolic and diastolic BP by −3.76 mm Hg (95% CI, −4.23 to −2.34; I2=0.99) and −1.83 mm Hg (95% CI, −2.35 to −1.31; I2=0.76), respectively. Significant reductions in daytime and nighttime systolic and diastolic BP were also found. No association between baseline BP or change in body weight were observed.
Conclusions
This meta‐analysis shows that the reduction in 24‐hour ambulatory BP observed with SGLT2 inhibitors is a class effect. The diurnal effect of SGLT2 inhibitors on 24‐hour ambulatory BP may contribute to their favorable effects on cardiovascular outcomes.
Keywords: ambulatory blood pressure monitoring, diabetes mellitus, diabetic therapy/glitazones, high blood pressure, hypertension, metformin, sodium‐glucose cotransporter 2 inhibitors
Subject Categories: High Blood Pressure; Hypertension; Diabetes, Type 2; Meta Analysis
Clinical Perspective
What is New?
This meta‐analysis demonstrates that Sodium‐glucose cotransporter 2 inhibitors, as a class, significantly reduce 24‐hour ambulatory blood pressure, which further supports their favorable cardiovascular profile.
What are the Clinical Implications?
The decrease in 24‐hour ambulatory blood pressure observed with Sodium‐glucose cotransporter 2 inhibitors further emphasizes the need for close monitoring of blood pressure and volume status, especially in patients receiving concomitant diuretic therapy.
Introduction
Sodium‐glucose cotransporter 2 (SGLT2) mediates glucose uptake in the early proximal tubule of the kidney and is responsible for the majority (80–90%) of glucose reabsorption at the glomerulus level.1 The remaining 10% to 20% is then reabsorbed by SGLT1. In patients with diabetes mellitus, inhibitors of SGLT2 improve hyperglycemia by increasing glycosuria. Currently, 3 SGLT2 inhibitors have been approved by the US Food and Drug Administration for the treatment of type 2 diabetes mellitus as adjuncts to diet and exercise to improve glycemic control: canagliflozin, dapagliflozin, and empagliflozin. Notably, empagliflozin has the highest selectivity for SGLT2 compared with canagliflozin and dapagliflozin.2
In the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA‐REG OUTCOME) trial,3 empagliflozin reduced total and cardiovascular mortality in patients with type 2 diabetes mellitus compared with placebo, an effect that was mainly driven by the beneficial effects on heart failure. These impressive data have recently resulted in a label change for empagliflozin to highlight the reduction in cardiovascular mortality risk.4 However, the cardiovascular benefits of empagliflozin cannot be justified by the improvement in glycemic control alone, which suggests the presence of non‐glucose‐lowering effects of empagliflozin.
One of the most consistently reported non‐glucose‐lowering effects of SGLT2 inhibitors is the reduction of systemic arterial blood pressure (BP). A recent meta‐analysis of 27 randomized controlled trials (RCTs) concluded that, in patients with type 2 diabetes mellitus, SGLT2 inhibitors improve systolic BP by −4 mm Hg (95% CI, −4.4 to −3.5) and diastolic BP by −1.6 mm Hg (95% CI, −1.9 to −1.3).5 The exact BP reduction‐related mechanisms of SGLT2 inhibitors have not been fully elucidated. However, it has been suggested that inhibition of glucose‐sodium reabsorption in the proximal tubule results in an osmotic diuresis and decreased BP.6
Additional studies have also observed that SGLT2 inhibitors improve 24‐hour ambulatory BP. Importantly, 24‐hour ambulatory BP is a better predictor of cardiovascular risk and mortality than seated clinic BP and therefore, possibly, a more‐appropriate therapeutic target.7 Moreover, 24‐hour ambulatory BP can identify subjects with masked hypertension, which is associated with increased cardiovascular risk and is more prevalent in patients with diabetes mellitus.8, 9 Data on the effects of SGLT2 inhibition on 24‐hour ambulatory BP are confined to relatively small studies. The purpose of this meta‐analysis was to determine the magnitude of the effect SGLT2 inhibitors exert on 24‐hour ambulatory BP.
Methods
This meta‐analysis conforms to standard guidelines and was written in accord with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement.10
Literature Search
A systematic search of 3 databases was conducted along with a search of the reference lists of the retrieved publications, in order to identify published articles addressing the topic. Databases searched included PubMed/Medline, Web of Science, and The Cochrane Database of Clinical Trials. The articles included were limited to the English language and those published anytime from the inception of each database to present. The search was broken into 3 concept groups. One group encompassed the terminology used to describe “sodium‐glucose cotransporter 2 inhibitors,” another covered the terms relevant to “blood pressure,” and a third addressed “randomized controlled trials.” Medical Subject Headings (MeSH) and equivalent controlled vocabulary and keywords were utilized in each database. The last search of the databases was conducted on August 17, 2016.
Study Selection
Three authors (D.L.D., W.L.B., and L.F.B.) reviewed all potentially relevant articles in a parallel manner by using a priori defined criteria. Studies were eligible for inclusion in the meta‐analysis if they met the following criteria: (1) RCT in humans; (2) evaluated a SGLT2 inhibitor compared with either placebo or an active control; and (3) reported data on changes in 24‐hour ambulatory BP from baseline in a form suitable for pooling. Only data from peer‐reviewed publications were considered for inclusion in this analysis.
Data Abstraction and Validity Assessment
For each included study, 2 authors (D.L.D., L.F.B.) used a standardized abstraction tool to extract data for the analysis, with disagreements resolved by a third investigator (M.K.). Information collected from each study included author, year of publication, study design, duration of follow‐up, population and setting, sample size, SGLT2 inhibitor evaluated, and concomitant antihyperglycemic therapies. Data on changes in 24‐hour BP were obtained. Missing data were collected through a search of the study results reported on clinicaltrials.gov. Two reviewers (D.L.D., L.F.B.) assessed the quality of each study by answering “yes,” “no,” or “unclear” to 11 questions regarding similarity of baseline populations, randomization, allocation concealment, blinding of study participants and personnel, outcome adjudication, completeness of follow‐up, and conflicts of interest using the Methods Guide for Comparative Effectiveness Reviews.11 Studies were given an overall score of good, fair, or poor with disagreements resolved through discussion.
Statistical Analysis
Meta‐analyses were performed on the mean changes from baseline in 24‐hour systolic and diastolic BP, daytime systolic and diastolic BP, and nighttime systolic and diastolic BP. All continuous data are reported as a mean difference and accompanying 95% CI and was pooled using a Hartung‐Knapp method12 random‐effects model using the “meta” package in R (version 3.1.3; The R Project for Statistical Computing). We reported doses for each drug, regardless of its regulatory status. We assessed presence of statistical heterogeneity using the Cochrane P value (P<0.10 significant) and the degree of heterogeneity using the I2 statistic with a value >50% considered substantial.13 Contour plots were constructed as a visual representation of bias in the main results (24‐hour systolic and diastolic BP), and we used Egger's test of asymmetry of the plots.14
Subgroup analyses were performed by pooling data for each individual SGLT‐2 inhibitor separately and assessing the pooled between‐group variance. We also conducted random‐effects metaregression to evaluate whether the BP effects observed with the SGLT‐2 inhibitors were associated with either baseline 24‐hour ambulatory BP or changes in body weight during the trial. Additional variables, such as percent patients with hypertension at baseline and urinary sodium excretion, were not sufficiently reported to allow for metaregression to be performed.
Results
Study Selection and Characteristics
The literature search process and results are shown in Figure 1. A total of 6 randomized, double‐blind, placebo‐controlled studies were included in the analysis (Table 1).15, 16, 17, 18, 19, 20 Of these, 3 RCTs (n=952) evaluated dapagliflozin,16, 17, 18 1 RCT (n=823) evaluated empagliflozin,19 1 RCT (n=169) evaluated canagliflozin,15 and 1 RCT (n=154) evaluated ertugliflozin.20 The dose of dapagliflozin used in each study was 10 mg/day, and sample sizes ranged from 49 to 530 participants.16, 17, 18 Doses of empagliflozin, canagliflozin, and ertugliflozin ranged from 10 to 25, 100 to 300, and 1 to 25, respectively.15, 19, 20 Key clinical data for each of the included studies are provided in detail in Table 2.15, 16, 17, 18, 19, 20 The quality of each study was determined to be “good” with no disagreements between the reviewers.
Figure 1.
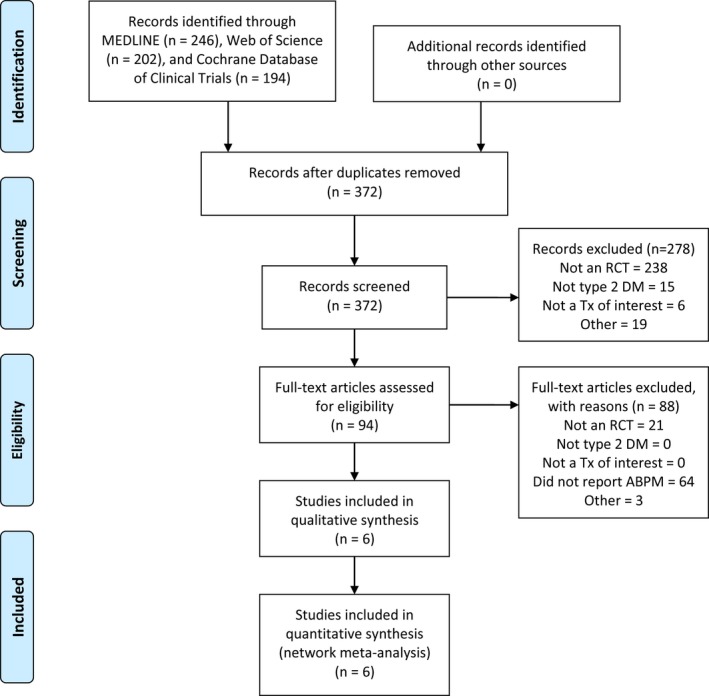
PRISMA diagram of study selection process. ABPM indicates ambulatory blood pressure monitoring; DM, type 2 diabetes mellitus; RCT, randomized, controlled trial; Tx, treatment.
Table 1.
Baseline Characteristics of Trials Evaluating SGLT2 Inhibitors and 24‐Hour Ambulatory Blood Pressure
| Study Name | Study Design | SGLT2 Group | n | Duration (Weeks) | Average Age (Y) | Baseline 24‐Hour SBP | Baseline 24‐Hour DBP | Baseline Daytime SBP | Baseline Nighttime SBP |
|---|---|---|---|---|---|---|---|---|---|
| Lambers‐Heerspink et al (2013)16 | R, DB, PC | DAPA 10 mg/d | 24 | 12 | 52.7±9.4 | 131±12 | 77±7 | 138±12 | 120±13 |
| Placebo | 25 | 58.0±9.5 | 127±12 | 74±7 | 131±12 | 117±15 | |||
| Amin et al (2015)20 | R, DB, PC | ERTU 1 mg/d | 39 | 4 | 54.4±7.0 | 133.1±1.7 | 78.6±1.3 | NR | NR |
| ERTU 5 mg/d | 38 | 53.8±9.9 | 135.0±1.9 | 80.1±1.4 | |||||
| ERTU 25 mg/d | 39 | 52.5±6.6 | 135.5±1.9 | 80.3±1.3 | |||||
| Placebo | 38 | 55.1±6.7 | 136.1±2.5 | 81.8±1.5 | |||||
| Tikkanen et al (2015)19 | R, DB, PC | EMPA 10 mg/d | 276 | 12 | 60.6±8.5 | 131.3±13.0 | 75.1±8.3 | NR | NR |
| EMPA 25 mg/d | 276 | 59.9±9.7 | 131.2±12.1 | 74.6±7.5 | |||||
| Placebo | 271 | 60.3±8.8 | 131.7±11.8 | 75.2±7.5 | |||||
| Townsend et al (2016)15 | R, DB, PC | CANA 100 mg/d | 57 | 6 | 57.8±8.7 | 136.5±11.5 | 78.0±8.1 | NR | NR |
| CANA 300 mg/d | 56 | 58.3±6.9 | 139.6±10.6 | 79.3±7.9 | |||||
| Placebo | 56 | 59.6±9.5 | 136.7±10.3 | 78.4±7.3 | |||||
| Weber et al (2016)18 | R, DB, PC | DAPA 10 mg/d | 187 | 12 | 56.0 (51–62) | 146.5±10.4 | NR | NR | NR |
| Placebo | 186 | 57.0 (50–62) | 149.2±12.7 | ||||||
| Weber et al (2016)17 | R, DB, PC | DAPA 10 mg/d | 267 | 12 | 55.6±8.4 | 145.9±11.7 | 87.0±6.9 | 149.1±11.6 | 139.7±14.7 |
| Placebo | 263 | 56.2±8.9 | 146.6±11.7 | 87.2±6.7 | 150.4±1.2 | 139.1±15.1 |
CANA indicates canagliflozin; DAPA, dapagliflozin; DB, double‐blind; DBP, diastolic blood pressure; EMPA, empagliflozin; ERTU, ertugliflozin; NR, not reported; PC, placebo‐controlled; R, randomized; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Table 2.
Reported Clinical End Point Data
| Study Name | SGLT2 Group | n | Change in 24‐Hour SBP From Baseline | Change in 24‐Hour DBP From Baseline | Change in Daytime SBP From Baseline | Change in Daytime DBP From Baseline | Change in Nighttime SBP From Baseline | Change in Nighttime DBP From Baseline |
|---|---|---|---|---|---|---|---|---|
| Lambers‐Heerspink et al (2013)16 | DAPA 10 mg/d | 24 | −5.6±11.62 | NR | −8.8±12.25 | NR | −1.9±12.5 | NR |
| Placebo | 25 | −0.7±9.18 | −0.8±9.31 | −0.6±11.48 | ||||
| Amin et al (2015)20 | ERTU 1 mg/d | 39 | −2.97±4.62 | −1.9±3.03 | −2.9±4.78 | −2.1±3.19 | −2.6±5.89 | −1.6±4.3 |
| ERTU 5 mg/d | 38 | −4±4.87 | −2.3±3.15 | −3.6±5.35 | −1.9±3.46 | −3.6±6.45 | −2.6±4.72 | |
| ERTU 25 mg/d | 39 | −3.69±4.62 | −1.5±3.03 | −4.2±4.64 | −1.7±3.19 | −2.4±6.05 | −0.9±4.46 | |
| Placebo | 38 | 0.1±4.4 | 0.8±3.30 | 0.8±5.19 | 0.9±3.3 | −0.4±6.29 | 0.9±4.40 | |
| Tikkanen et al (2015)19 | EMPA 10 mg/d | 276 | −2.99±8.86 | −1.1±4.96 | −3.4±9.55 | −1.28±5.41 | −2.22±10.21 | −0.8±6.21 |
| EMPA 25 mg/d | 276 | −3.59±9.30 | −1.32±4.96 | −4.12±9.55 | −1.58±5.35 | −2.47±11.09 | −0.75±6.32 | |
| Placebo | 271 | 0.42±8.25 | 0.3±5.06 | 0.38±8.74 | 0.26±5.36 | 0.51±10.22 | 0.36±6.8 | |
| Townsend et al (2016)15 | CANA 100 mg/d | 57 | −4.78±8.33 | −2.18±4.87 | −5.05±8.66 | −2.39±5.41 | −4.33±11.51 | −1.73±6.48 |
| CANA 300 mg/d | 56 | −7.31±11.41 | −3.27±6.46 | −7.36±11.66 | −3.23±6.56 | −6.98±12.88 | −3.42±8.06 | |
| Placebo | 56 | −1.26±9.86 | −0.26±5.35 | −0.65±9.87 | −0.2±5.26 | −3.49±12.47 | −0.98±7.75 | |
| Weber et al (2016)18 | DAPA 10 mg/d | 187 | −11.33±21.9 | −7.56±13.95 | NR | NR | NR | NR |
| Placebo | 186 | −6.88±21.5 | −5.57±13.64 | |||||
| Weber et al (2016)17 | DAPA 10 mg/d | 267 | −9.62±20.1 | −6.15±13.73 | −10±20.43 | NR | −8.8±22.88 | NR |
| Placebo | 263 | −6.73±20.1 | −5.53±13.78 | −6.9±20.69 | −6.5±23.17 |
CANA indicates canagliflozin; DAPA, dapagliflozin; DBP, diastolic blood pressure; EMPA, empagliflozin; ERTU, ertugliflozin; NR, not reported; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Quantitative Data Synthesis
24‐hour BP
When the 6 RCTs were pooled, SGLT2 inhibitor use was associated with a statistically significant 3.76 mm Hg reduction in 24‐hour systolic BP when compared with placebo (95% CI, −4.23 to −2.34; I2=0.99; Figure 2).15, 16, 17, 18, 19, 20 The mean differences observed with canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin were −4.65 mm Hg (95% CI, −20.63 to 11.33), −3.73 mm Hg (95% CI, −6.38 to −1.07), −3.70 mm Hg (95% CI, −7.52 to 0.11), and −3.65 mm Hg (95% CI, −4.96 to −2.34), respectively. No significant difference between individual SGLT‐2 inhibitors was noted (P=0.90). There was also no strong evidence of small‐study effects (Egger's test, P=0.25; Figure 3).
Figure 2.
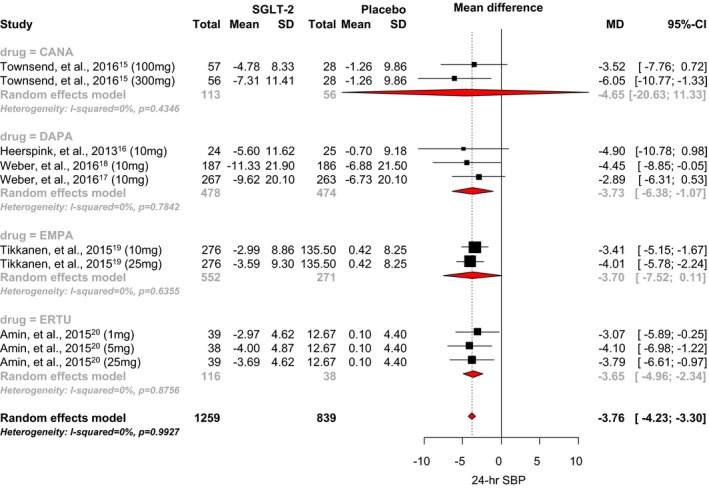
Effect of SGLT2 inhibitors on 24‐hour systolic blood pressure. CANA indicates canagliflozin; DAPA, dapagliflozin; EMPA, empagliflozin; ERTU, ertugliflozin; MD, mean difference; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Figure 3.
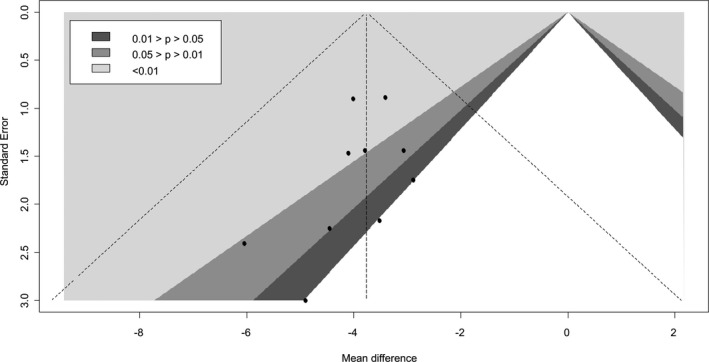
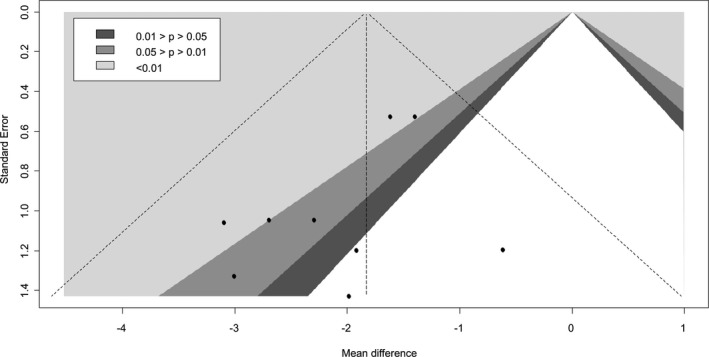
Contour‐enhanced funnel plots of 24‐hour systolic blood pressure.
Only 4 of the 6 RCTs reported data for 24‐hour diastolic BP. SGLT2 inhibitor use was associated with a statistically significant 1.83 mm Hg reduction in 24‐hour diastolic BP when compared with placebo (95% CI, −2.35 to −1.31; I2=0.76; Figure 4).15, 17, 18, 19, 20 The mean differences observed with canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin were −2.41 mm Hg (95% CI, −9.30 to 4.48), −1.18 mm Hg (95% CI, −9.75 to 7.38), −1.51 mm Hg (95% CI, −2.91 to −0.11), and −2.70 mm Hg (95% CI, −3.69 to −1.71), respectively. A significant difference between individual SGLT‐2 inhibitors was noted (P<0.0001). No strong evidence of small‐study effects was observed (Egger's test P=0.15; Figure 5).
Figure 4.
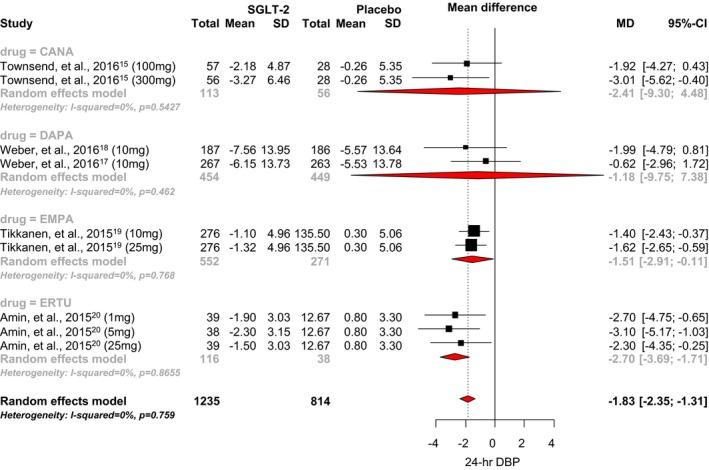
Effect of SGLT2 inhibitors on 24‐hour diastolic blood pressure. CANA indicates canagliflozin; DAPA, dapagliflozin; DBP, diastolic blood pressure; EMPA, empagliflozin; ERTU, ertugliflozin; MD, mean difference; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Figure 5.
Contour‐enhanced funnel plots of 24‐hour diastolic blood pressure.
Daytime BP
An analysis of the 5 RCTs that reported daytime systolic BP showed that SGLT2 inhibitor use was associated with a statistically significant 4.34 mm Hg reduction when compared with placebo (95% CI, −5.09 to −3.58; I2=0.90; Figure 6).15, 16, 17, 19, 20 The mean differences observed with canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin were −5.43 mm Hg (95% CI, −20.03 to 9.16), −4.31 mm Hg (95% CI, −31.17 to 22.55), −4.14 mm Hg (95% CI, −8.71 to 0.43), and −4.37 mm Hg (95% CI, −6.00 to −2.74), respectively. No significant difference between individual SGLT‐2 inhibitors was observed (P=0.75).
Figure 6.
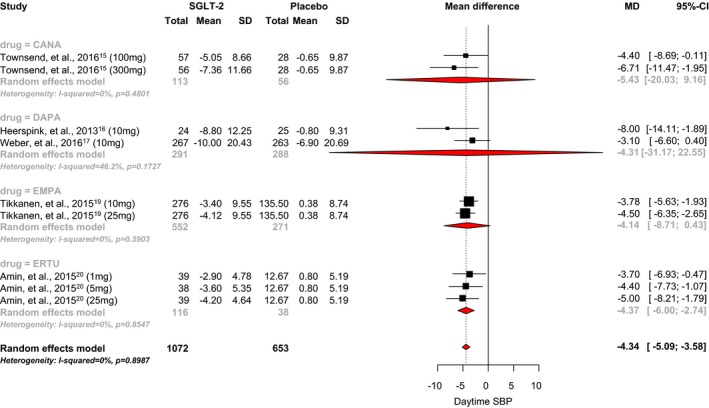
Effect of SGLT2 inhibitors on daytime systolic blood pressure. CANA indicates canagliflozin; DAPA, dapagliflozin; EMPA, empagliflozin; ERTU, ertugliflozin; MD, mean difference; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Only 3 RCTs reported data for daytime diastolic BP. We found no studies that evaluated the effects of dapagliflozin on daytime diastolic BP. SGLT2 inhibitor use was associated with a statistically significant 2.09 mm Hg reduction in daytime diastolic BP when compared with placebo (95% CI, −2.63 to −1.54; I2=0.80; Figure 7).15, 19, 20 The mean differences observed with canagliflozin, empagliflozin, and ertugliflozin were −2.58 mm Hg (95% CI, −7.90 to 2.74), −1.69 mm Hg (95% CI, −3.60 to 0.22), and −2.80 mm Hg (95% CI, −3.30 to −2.30), respectively. A significant difference between individual SGLT‐2 inhibitors was noted (P<0.0001).
Figure 7.
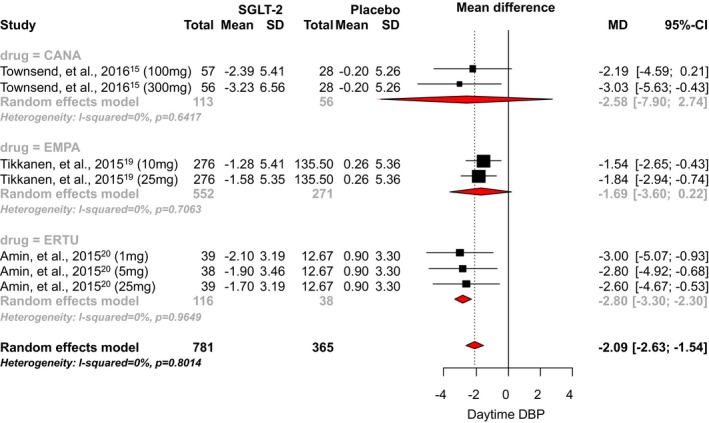
Effect of SGLT2 inhibitors on daytime diastolic blood pressure. CANA indicates canagliflozin; DAPA, dapagliflozin; DBP, diastolic blood pressure; EMPA, empagliflozin; ERTU, ertugliflozin; MD, mean difference; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Nighttime BP
An analysis of the 5 RCTs that reported nighttime systolic BP showed that SGLT2 inhibitor use was associated with a statistically significant 2.61 mm Hg reduction when compared with placebo (95% CI, −3.08 to −2.14; I2=0.99; Figure 8).15, 16, 17, 19, 20 The mean differences observed with canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin were −2.11 mm Hg (95% CI, −18.94 to 14.71), −2.05 mm Hg (95% CI, −7.58 to 3.48), −2.85 mm Hg (95% CI, −4.44 to −1.26), and −2.46 mm Hg (95% CI, −4.04 to −0.87), respectively. No significant difference between individual SGLT‐2 inhibitors was observed (P=0.25).
Figure 8.
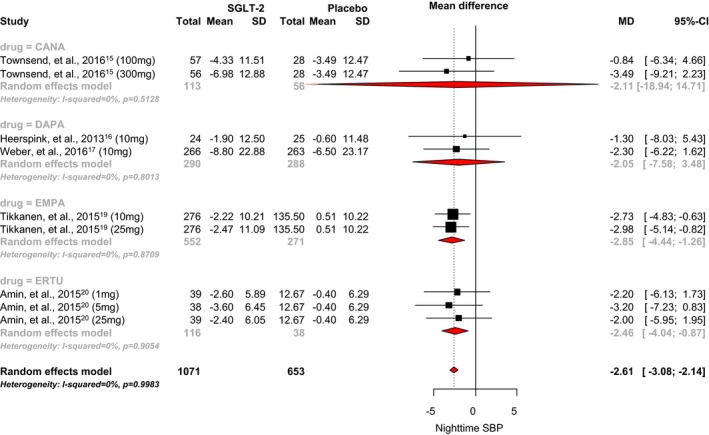
Effect of SGLT2 inhibitors on nighttime systolic blood pressure. CANA indicates canagliflozin; DAPA, dapagliflozin; EMPA, empagliflozin; ERTU, ertugliflozin; MD, mean difference; SBP, systolic blood pressure; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Only 3 RCTs reported data for nighttime diastolic BP. We found no studies that evaluated the effects of dapagliflozin on nighttime diastolic BP. SGLT2 inhibitor use was associated with a statistically significant 1.49 mm Hg reduction in nighttime diastolic BP when compared with placebo (95% CI, −2.20 to −0.78; I2=0.79; Figure 9).15, 19, 20 The mean differences observed with canagliflozin, empagliflozin, and ertugliflozin were −1.54 mm Hg (95% CI, −12.25 to 9.17), −1.14 mm Hg (95% CI, −1.45 to −0.82), and −2.49 mm Hg (95% CI, −4.60 to −0.38), respectively. A significant difference between individual SGLT‐2 inhibitors was observed (P=0.02).
Figure 9.
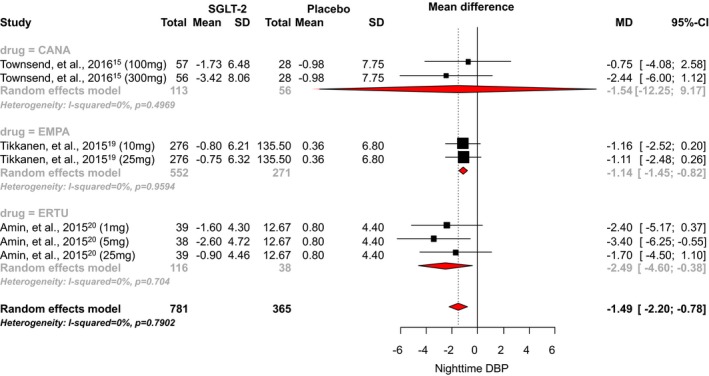
Effect of SGLT2 inhibitors on nighttime diastolic blood pressure. CANA indicates canagliflozin; DAPA, dapagliflozin; DBP, diastolic blood pressure; EMPA, empagliflozin; ERTU, ertugliflozin; MD, mean difference; SGLT2, sodium‐glucose cotransporter 2 inhibitor.
Metaregression
Random‐effects metaregression analysis showed no significant association between the 24‐hour ambulatory systolic or diastolic BP and either baseline BP (P=0.96, P=0.69, respectively) or changes in body weight (P=0.44; P=0.88, respectively). Because relatively few studies for each drug reported 24‐hour ambulatory BP data, we could not perform meta‐regression by individual drug dose.
Discussion
This meta‐analysis involving 2098 participants showed that SGLT2 inhibitors significantly reduce 24‐hour ambulatory systolic BP by −3.76 mm Hg and 24‐hour ambulatory diastolic BP by −1.83 mm Hg. These results are similar to a previous meta‐analysis of seated clinic BP, which showed a reduction in systolic BP of −3.8 mm Hg and diastolic BP of −1.6 mm Hg.5 Additionally, our results show that SGLT2 inhibitors significantly reduce daytime systolic and diastolic BP and, to a lesser extent, nighttime systolic and diastolic BP. The effects of SGLT2 inhibitors on 24‐hour ambulatory BP were consistent across the doses used in clinical trials. No association was found between baseline BP and change in body weight. Because direct comparisons between individual agents could not be made, the reduction in 24‐hour ambulatory BP observed with SGLT2 inhibitors should be considered a class effect.
The exact mechanism(s) through which SGLT2 inhibitors lower BP are not completely understood, but are likely multifactorial. SGLT2 inhibitors induce volume contraction because of natriuretic and osmotic diuretic effects.1 Because of chronic caloric deficit as a result of glucose excretion in the urine, SGLT2 inhibitors can also induce weight loss and, more important, body composition improvements by reduction of fat mass with minor changes in lean mass.1, 21 Fat mass reduction has been reported to improve BP, proposing this as an additional mechanism of BP reduction with SGLT2 inhibitors.1 Treatment with SGLT2 inhibitors also improves arterial compliance, which is associated with improved BP.1 Furthermore, SGLT2 inhibitors reduce sympathetic nervous system activity and, as recently suggested, renin‐angiotensin‐aldosterone system activity by increasing sodium delivery at the macula densa level in the kidney, both of which are important determinants of BP.1, 22 Further study is warranted to identify the underlying mechanism(s) that contribute to the apparent effects of SGLT2 inhibitors on BP.
In the current meta‐analysis, it appears that the observed BP reduction with SGLT2 inhibitors persists throughout a 24‐hour day with a greater percent reduction in BP during the daytime compared with nighttime. This may be, in part, attributed to the glucose‐dependent nature of how SGLT2 inhibitors improve glycemic control, considering that osmotic diuresis is likely increased during daytime periods of higher caloric and fluid intake. Reduced urine production at night attributed to circadian rhythms in kidney function may also explain the reduced BP‐lowering effect at nighttime. Of course, the BP‐lowering effect of SGLT2 inhibitors may also be independent of diuresis, and differences in diurnal and nocturnal BP regulation may reflect differences in sympathetic tone.
The effects of other antihyperglycemic therapies on 24‐hour ambulatory BP have also been reported. In a study of 220 patients, metformin was shown to have no significant effect on 24‐hour ambulatory BP.23 Sitagliptin, at doses of 50 mg twice‐daily and 100 mg twice‐daily, produced a small, but significant, reduction in 24‐hour ambulatory systolic and diastolic BP of −2.0 to −2.2 mm Hg and −1.6 to −1.8 mm Hg, respectively, when compared with placebo.24 Twice‐daily exenatide25 and once‐daily liraglutide26 have not been shown to significantly reduce 24‐hour ambulatory BP, whereas dulaglutide 1.5 mg once‐weekly significantly reduced 24‐hour systolic BP by −2.8 mm Hg (95% CI, −4.6 to −1.0, P≤0.001) compared with placebo.27 Interestingly, rosiglitazone significantly reduced 24‐hour ambulatory systolic BP (−3.8 mm Hg) at both 6 and 12 months, when compared with adding metformin (−1.2 to −1.3 mm Hg; 6 months, P=0.015; 12 months, P=0.031) to background sulfonylurea therapy.28 Similar reductions with rosiglitazone were also observed for 24‐hour ambulatory diastolic BP. It is well established that hyperinsulinemia resulting from insulin resistance is associated with elevated BP; however, the effects of exogenous insulin on BP are variable.29, 30 Despite the favorable effects of select antihyperglycemic therapies on 24‐hour ambulatory BP, none have been shown to improve cardiovascular outcomes. This is possibly attributed to other deleterious effects that may offset any benefit from a reduction in 24‐hour ambulatory BP, such as the increase in plasma volume observed with thiazolidinediones.31 Our analysis demonstrates that the SGLT2 inhibitors are the only known class to have a consistent and significant reduction in 24‐hour ambulatory BP regardless of which agent is used.
The EMPA‐REG OUTCOME trial3 was the first study to demonstrate that an antihyperglycemic therapy, empagliflozin, significantly reduces death from cardiovascular causes, death from any cause, and hospitalization for heart failure. Additionally, a prespecified analysis of the EMPA‐REG OUTCOME trial showed that empagliflozin also reduces progression of diabetic nephropathy.32 In the EMPA‐REG OUTCOME study, empagliflozin reduced seated clinic systolic BP by −4 to −6 mm Hg at week 16, whereas diastolic BP was similar to placebo.3, 33 Ongoing cardiovascular safety trials with canagliflozin (CANVAS [CANagliflozin cardioVascular Assessment Study], NCT01032629) and dapagliflozin DECLARE‐TIMI 58 ([Dapagliflozin Effect on CardiovascuLAR Events‐TIMI 58], NCT01730534) will determine whether the cardiovascular benefit of empagliflozin is a class effect. Much remains to be determined regarding the specific mechanism for how empagliflozin improves mortality and decreases heart failure hospitalizations, yet it seems feasible that the reductions in BP would contribute.
It is important to note the limitations of this analysis. Study protocols related to the measurement of 24‐hour ambulatory BP varied between studies, as did baseline BP. However, metaregression showed no effects of SGLT2 inhibitors on BP according to baseline BP. Additional heterogeneity in the study populations also exists, such as presence or absence of a hypertension diagnosis and use and duration of background antihyperglycemic and antihypertensive therapies. Moreover, we were unable to test whether the observed reduction in 24‐hour ambulatory BP would result in improvement in cardiovascular outcomes. The power of the statistical tests for funnel plot symmetry is likely to be low because of less than 10 trials being included.
Conclusions
This meta‐analysis shows that SGLT2 inhibitors significantly improve overall 24‐hour ambulatory BP and daytime and nighttime BP in a diurnal fashion. The linear relationship between 24‐hour ambulatory BP and risk of cardiovascular events, especially in patients with diabetes mellitus, suggests that the favorable effects of SGLT2 inhibitors on 24‐hour ambulatory BP may, at least in part, contribute to a reduction in cardiovascular risk.7, 34, 35 While additional study is warranted to investigate mechanisms to describe the BP‐lowering effects of SGLT2 inhibitors and the impact of these effects on clinical outcomes, these data highlight a unique characteristic of this class. It also emphasizes the need for monitoring BP outside of the clinic through the use of home BP monitoring or 24‐hour ambulatory BP monitoring, when available, to better monitor for hypotension. Caution is warranted in individuals with lower baseline BP and/or those already receiving diuretic therapies, whereas volume status should also be monitored closely.36 Together with cardiovascular outcomes data from EMPA‐REG and this meta‐analysis on 24‐hour ambulatory BP, the evidence continues to suggest a favorable effect of SGLT2 inhibitors on cardiovascular outcomes in patients with diabetes mellitus.
Sources of Funding
Mr Carbone, MS, is supported by a Mentored Clinical and Population Research Award from the American Heart Association. Dr Abbate has received research support from Janssen Pharmaceuticals.
Disclosures
Dr Abbate has served as a consultant for Janssen Pharmaceuticals. The other authors have no disclosures to report.
(J Am Heart Assoc. 2017;6:e005686 DOI: 10.1161/JAHA.117.005686.)28522675
References
- 1. DeFronzo RA, Norton L, Abdul‐Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2016;13:11–26. [DOI] [PubMed] [Google Scholar]
- 2. Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co‐transporter 2 inhibitor. Clin Pharmacokinet. 2014;53:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedi JC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Food and Drug Administration . FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed January 11, 2017.
- 5. Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium‐glucose co‐transporter 2 inhibitors on blood pressure: a systematic review and meta‐analysis. J Am Soc Hypertens. 2014;8:262–275. [DOI] [PubMed] [Google Scholar]
- 6. Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. [DOI] [PubMed] [Google Scholar]
- 7. Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 8. Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y, Asayama K, Bjorklund‐Bodegard K, Ohkubo T, Jeppesen J, Torp‐Pedersen C, Dolan E, Kuznetsova T, Stolarz‐Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka‐Jaszcz K, Filipovsky J, Imai Y, Wang J, Ibsen H, O'Brien E, Staessen JA; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes Investigators . Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz JE, Burg MM, Shimbo D, Broderick JE, Stone AA, Ishikawa J, Sloan R, Yurgel T, Grossman S, Pickering TG. Clinic blood pressure underestimates ambulatory blood pressure in an untreated employer‐based US population: results from the Masked Hypertension Study. Circulation. 2016;134:1794–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1 DOI: 10.1186/2046‐4053‐4‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Methods guide for effectiveness and comparative effectiveness reviews. AHRQ Publication No. 10(14)‐EHC063‐EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Chapters Available at: www.effectivehealthcare.ahrq.gov. Accessed April 12, 2017. [Google Scholar]
- 12. IntHout J, Ioannidis JPA, Borm GF. The Hartung‐Knapp‐Sidik‐Jonkman method for random effects meta‐analysis is straightforward and considerably outperforms the standard DerSimonian‐Laird method. BMC Med Res Methodol. 2014;14:25 DOI: 10.1186/1471‐2288‐14‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Townsend RR, Machin I, Ren J, Trujillo A, Kawaguchi M, Vijapurkar U, Damaraju CV, Pfeifer M. Reductions in mean 24‐hour ambulatory blood pressure after 6‐week Treatment with canagliflozin in patients with type 2 diabetes mellitus and hypertension. J Clin Hypertens (Greenwich). 2016;18:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lambers‐Heerspink HJ, De Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin‐angiotensin system blockade. Blood Press. 2016;25:93–103. [DOI] [PubMed] [Google Scholar]
- 18. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. [DOI] [PubMed] [Google Scholar]
- 19. Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. [DOI] [PubMed] [Google Scholar]
- 20. Amin NB, Wang X, Mitchell JR, Lee DS, Nucci G, Rusnak JM. Blood pressure lowering effect of the sodium glucose co‐transporter‐2 inhibitor, ertugliflozin, assessed via ambulatory blood pressure monitoring in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2015;17:805–808. [DOI] [PubMed] [Google Scholar]
- 21. Cefalu WT, Leiter LA, Yoon K‐H, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet. 2013;382:941–950. [DOI] [PubMed] [Google Scholar]
- 22. Škrtić M, Cherney DZI. Sodium–glucose cotransporter‐2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24:96–103. [DOI] [PubMed] [Google Scholar]
- 23. Wulffelé MG, Kooy A, Lehert P, Betst D, Donker AJM, Stehouwer CD. Does metformin decrease blood pressure in patients with type 2 diabetes intensively treated with insulin? Diabet Med. 2005;22:907–913. [DOI] [PubMed] [Google Scholar]
- 24. Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, Herman GA. Effect of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598. [DOI] [PubMed] [Google Scholar]
- 25. Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, Braun D, Giaconia J, Malone J. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double‐blind, placebo‐controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6 DOI: 10.1186/1475‐2840‐9‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38:132–139. [DOI] [PubMed] [Google Scholar]
- 27. Ferdinand KC, White WB, Calhoun DA, Lonn EM, Sager PT, Brunelle R, Jiang HH, Threlkeld RJ, Robertson KE, Geiger MJ. Effects of the once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–737. [DOI] [PubMed] [Google Scholar]
- 28. Komajda M, Curtis P, Hanefeld M, Beck‐Nielsen H, Pocock SJ, Zambanini A, Jones NP, Gomis R, Home PD. Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: a randomized controlled trial (the RECORD study). Cardiovasc Diabetol. 2008;7:10 DOI: 10.1186/1475‐2840‐7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol. 2007;293:974–984. [DOI] [PubMed] [Google Scholar]
- 30. Heise T, Magnusson K, Heinemann L, Sawicki PT. Insulin resistance and the effect of insulin on blood pressure in essential hypertension. Hypertension. 1998;32:243–248. [DOI] [PubMed] [Google Scholar]
- 31. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure. Circulation. 2003;108:2941–2948. [DOI] [PubMed] [Google Scholar]
- 32. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Matthewus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 33. Perseghin G, Solini A. The EMPA‐REG outcome study: critical appraisal and potential clinical implications. Cardiovasc Diabetol. 2016;15:85 DOI: 10.1186/s12933‐016‐0403‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Schwartz JE, Shimada K, Kario K. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause‐specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 36. Cherney DZ, Udell JA. Use of Sodium Glucose Cotransporter 2 Inhibitors in the Hands of Cardiologists: With Greater Power Comes Great Responsibility. Circulation. 2016;134:1915–1917. [DOI] [PubMed] [Google Scholar]


