Abstract
Background
There is controversy surrounding the risk of ischemic stroke associated with the use of calcium supplements either in monotherapy or in combination with vitamin D.
Methods and Results
A nested case‐control study was performed with patients aged 40 to 89 years old, among whom a total of 2690 patients had a first episode of nonfatal ischemic stroke and for which 19 538 controls were randomly selected from the source population and frequency‐matched with cases for age, sex, and calendar year. Logistic regression provided the odds ratios while adjusting for confounding factors. A sensitivity analysis was performed by restricting to patients who were new users of calcium supplements as either monotherapy or with vitamin D. Calcium supplementation with vitamin D was not associated with an increased risk of ischemic stroke (odds ratio 0.85; 95% confidence interval, 0.67–1.08) in the population as a whole or under any of the conditions examined (dose, duration, background cardiovascular risk, sex, or age). Calcium supplement monotherapy was not associated with an increased risk in the population as a whole (odds ratio 1.18; 95% confidence interval, 0.86–1.61), although a significant increased risk at high doses (≥1000 mg/day: odds ratio 2.09; 95% confidence interval, 1.25–3.49; <1000 mg: odds ratio 0.76; 95% confidence interval, 0.45–1.26) compared with nonuse was observed. The sensitivity analysis did not affect the inferences, with similar results observed among new users as to the overall study population.
Conclusions
This study suggests that calcium supplements given as monotherapy at high doses may increase the risk of ischemic stroke, whereas their combination with vitamin D seems to offset this hazard.
Keywords: calcium, stroke, vitamin D
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke
Introduction
Calcium supplements, alone or combined with vitamin D, represent the first‐line therapy for the treatment of osteoporosis, particularly among women.1 In 2010, Bolland et al2 reported a meta‐analysis of 8 randomized clinical trials in which calcium supplements in monotherapy (CaM) were associated with a significant increased risk of acute myocardial infarction (AMI; relative risk: 1.27; 95% confidence interval [CI], 1.01–1.59) and a marginally nonsignificant increased risk of stroke (relative risk: 1.12; 95% CI, 0.92–1.36). Two years later, the same authors extended the concern to the combination of calcium with vitamin D (CaD), after reanalysis of the WHI (Women's Health Initiative) trial and a pooled analysis with 2 other clinical trials, giving rise to a relative risk of 1.21 (95% CI, 1.01–1.44) for AMI and 1.20 (95% CI, 1.00–1.43) for stroke.3 Such meta‐analyses, however, have been criticized because the clinical trials were not primarily designed to evaluate cardiovascular events.4, 5 In addition, a reanalysis of the WHI trial performed by its own investigators6 and 2 more recent meta‐analyses showed no significant increased risk of AMI,7, 8 stroke,8 and all‐cause mortality.7
Although a randomized clinical trial specifically designed to detect cardiovascular events would be the best option to put an end to this controversy,9 it is a large endeavor that may not be feasible in the short to medium term. Consequently, the evidence provided by epidemiological studies may be the only data available to shed some light and help make important public health decisions. We recently performed a nested case‐control study to primarily assess the association of nonsteroidal anti‐inflammatory drugs with ischemic stroke (IS)10 using a primary care database in which all medicinal products prescribed by primary care practitioners (PCPs) are automatically recorded. We asked the database owner (the Spanish Agency for Medicines and Medical Devices, the drug regulatory body in Spain) for additional information concerning prescriptions of calcium supplements, as monotherapy or in fixed‐dose combination with vitamin D, and tested the hypothesis of an increased risk of IS associated with these drugs.
Observational studies may be affected by a prevalent‐user bias, which has been alleged to partly explain the differences found with the results from randomized clinical trials in the assessment of coronary events associated with hormone replacement therapy.11 This bias can be eliminated and, consequently, the validity of observational data can be improved if prevalent users are excluded from the analysis (the so‐called new user design).11 We applied this approach in a sensitivity analysis to check the impact of such a potential bias.
Methods
Data Source
The study was performed using BIFAP (Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria).12 This database has been validated for pharmacoepidemiological research, and results have been successfully compared with those from other well‐known European databases.13, 14, 15 Over the study period (January 1, 2001, to December 31, 2007), BIFAP included anonymized information on 2 410 942 patients. This cohort is comparable with the Spanish population with respect to age and sex distribution.12 Data recorded in BIFAP include demographic information, prescription details, clinical events, specialist referrals, and results from laboratory and other exploratory tests. Prescription data in BIFAP include product name, the date of prescription, quantity dispensed, dosage regimens, strength, and indication. The vast majority of patients get their prescriptions from their PCPs; even when they visit a specialist or are discharged from the hospital, they usually go to the PCP to get their prescriptions, especially for long‐term treatments. Patient complaints and diagnoses are coded according to the International Classification of Primary Care (ICPC).16 This information is often enriched with free text in clinical notes linked to the coded diagnosis.
Study Design
We performed a population‐based case‐control study nested in a primary cohort selected from BIFAP over the period 2001–2007. People entered the primary cohort (start date) once they fulfilled all of the following criteria: aged 40 to 89 years, registered with their PCP for at least 1 year, and no record of cancer. Patients were followed up until the earliest occurrence of one of the following end points: the event of interest, 90 years of age, a diagnosis of cancer, death, or the end of the study period.
Selection of Cases
An initial computer search was performed including all patients with an ICPC‐BIFAP code of either K90.2 (IS) or K90.1 (unspecific stroke). All potential cases were manually reviewed and considered valid if they had a diagnosis of IS (by code or free text) made in the hospital (eg, hospital discharge letter, record of hospitalization) or by a neurologist (eg, specialist report). We also accepted a case as valid if, in addition to the code and/or free‐text diagnosis of IS, the clinical records included a result from neuromaging techniques and/or clinical information (eg, neurological sequelae) supporting the diagnosis. In addition, no death should be recorded within 30 days after the index date. Patients with a transient ischemic attack were not considered as cases.
The index date was normally considered the date of the first record of the outcome (IS) in the database, unless the reviewers had evidence to support an earlier date based on clinical signs. In the original study, we did not exclude patients with a previous IS episode,10 but for the present analysis, we preferred to focus only on first events.
Selection of Controls
Eligible controls were randomly selected from the study population using an incidence density sampling.17 Briefly, all persons in the study cohort were randomly assigned a date within the study period, and study cohort members with their corresponding random date occurring within their observation period were considered eligible. In this way, we ensured that the probability of being sampled was proportional to the amount of person‐time each person contributed to the study period. Cohort members were then frequency‐matched to cases by age (within 1 year), sex, and calendar year, and from this pool, we selected a random sample of 20 000 controls. The random date assigned to controls in the process of selection was considered the index date.
Exposure Definitions
We categorized patients as current users when they had a recorded prescription of the drug of interest (either CaM or CaD) that ended within 30 days before the index date, as recent users when they had a recorded prescription that ended between 31 and 365 days before the index date, and as past users when they had a recorded prescription that ended >365 days before the index date. Patients with no recorded prescription of the drug of interest before the index date were categorized as nonusers. Among CaD users, we considered only those patients using a fixed‐dose combination of calcium and vitamin D. The number of patients using both calcium and vitamin D as separate medicinal products was negligible.
Among current users, we studied the effect of calcium daily dose and duration. Calcium daily dose was considered low when it was <1000 mg and high when it was ≥1000 mg. The duration was measured as “continuous duration” using only consecutive prescriptions (defined as those with a <60‐day gap between the end of supply of the previous prescription and the start of the next one).
Potential Confounding Variables
We considered as potential confounding variables the antecedents of the following diseases or risk factors at the time of index date: ischemic heart disease (including AMI), transient ischemic attack, peripheral artery disease, heart failure, atrial fibrillation, diabetes mellitus (recorded as such or when patients were using glucose‐lowering drugs), renal failure, rheumatoid arthritis, osteoarthritis, gout (recorded as such), hyperuricemia (when recorded as such and no record of gout), depression, chronic obstructive pulmonary disease, dyslipidemia (recorded as such or when patients were using lipid‐lowering drugs) and hypertension, smoking, alcohol abuse (defined as such by the general practitioner), and body mass index. In addition, we also included the use of the following drugs: nonsteroidal anti‐inflammatory drugs, metamizole, paracetamol (acetaminophen), corticosteroids, colchicine, allopurinol, α‐blockers, calcium channel blockers, β‐blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, nitrates, low‐dose aspirin, nonaspirin antiplatelet drugs, oral anticoagulants, and acid‐suppressing drugs.
Statistical Analysis
The association between the use of either CaM or CaD and nonfatal IS was analyzed using an unconditional logistic regression model including matching factors and all potentially confounding variables described above to compute full‐adjusted odds ratios (ORs) and their 95% confidence intervals (CIs). The level of statistical significance was set at <0.05.
Some covariates (smoking and body mass index) had missing values, and we applied multiple imputation by chained equations models, building 20 imputation data sets to account for random variability.18 The variables included in the imputation models were the same as those included in the full model plus the outcome variable (nonfatal IS).
We also studied whether the main effect was or was not modified by age (<70, and ≥70 years), sex, and background cardiovascular risk. For the latter, we divided patients in 3 categories: (1) High risk included those with records of peripheral artery disease, AMI, diabetes mellitus, transient ischemic attack, and atrial fibrillation; (2) intermediate risk included those with at least 1 cardiovascular risk factor (hypertension, dyslipidemia, current smoking, or renal failure) but none of the qualifying criteria for high risk, and (3) low risk included the remainder. We included patients with diabetes mellitus within the high‐risk group because it has been reported to have a risk equivalent to ischemic heart disease.19
For the statistical evaluation of the effect modification (or interaction), we ran fully adjusted logistic models across different categories of potential modifiers and computed the OR associated with current use of the drugs of interest compared with nonuse by each stratum. We compared the ORs using the test of interaction described by Altman and Bland.20
Statistical analyses were conducted using the software STATA version 12/SE.
New‐User Analyses
For the new‐user analyses, we excluded from both cases and controls all patients with a recorded prescription of either CaM or CaD before the start date of cohort entry, thus ensuring that all users of CaM or CaD initiated the treatment during their observation time.11
Ethics Review
The scientific committee of BIFAP granted a positive opinion to the study protocol. The investigators had access to only fully anonymized data, and under this condition, no specific ethics review was required according to Spanish law.
Results
Main Analysis
We identified 2690 cases with a first episode of IS and 19 538 controls (Figure). The characteristics of cases and controls are described in Table 1. As expected, cases presented a higher prevalence of cardiovascular diseases (in particular, history of atrial fibrillation, transient ischemic attack, diabetes mellitus, and peripheral artery disease), cardiovascular risk factors (renal failure, hypertension, dyslipidemia, and smoking), and use of cardiovascular drugs. Among cases, 87 participants (3.23%) were current users of CaD compared with 743 (3.80%) among controls, yielding an adjusted OR of 0.85 (95% CI, 0.67–1.08). No dose or duration effect was observed (Table 2). Sex, age (<70 or ≥70 years), and background cardiovascular risk did not modify the results associated with CaD (Table 3).
Figure 1.
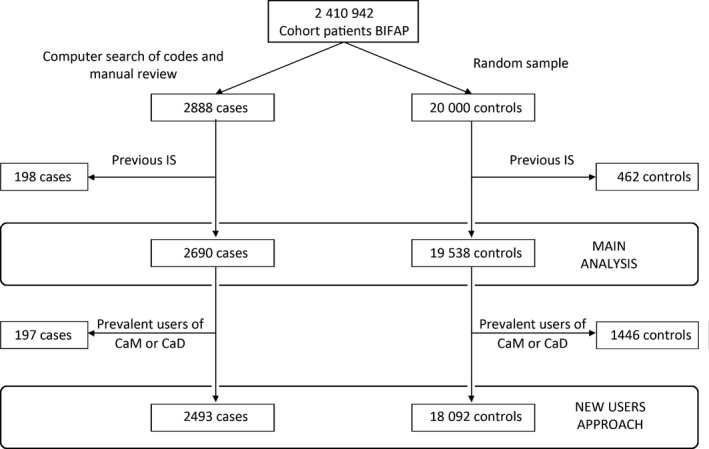
Flowchart of selection of cases and controls. BIFAP indicates Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria; CaD, calcium supplements with vitamin D; CaM, calcium supplements as monotherapy; IS, ischemic stroke.
Table 1.
Characteristics of Cases and Controls
| Cases (%) n=2690 | Controls (%) n=19 538 | Nonadjusted ORa (95% CI) | |
|---|---|---|---|
| Age mean, y (±SD) | 72.0 (10.8) | 72.0 (11.2) | ··· |
| Men | 1403 (52.16) | 10 246 (52.44) | ··· |
| Visits, n | |||
| <6 | 287 (10.67) | 3480 (17.81) | 1 (ref) |
| 6–15 | 909 (33.79) | 7918 (40.53) | 1.46 (1.27–1.68) |
| 16–24 | 782 (29.07) | 4790 (24.52) | 2.11 (1.82–2.44) |
| ≥25 | 712 (26.47) | 3350 (17.15) | 2.77 (2.38–3.21) |
| History of IHD (no AMI) | 174 (6.47) | 986 (5.05) | 1.34 (1.13–1.58) |
| History of AMI | 186 (6.91) | 1013 (5.18) | 1.37 (1.16–1.61) |
| History of TIA | 90 (3.35) | 395 (2.02) | 1.69 (1.33–2.13) |
| Heart failure | 153 (5.69) | 687 (3.52) | 1.67 (1.39–2.01) |
| Atrial fibrillation | 262 (9.74) | 974 (4.99) | 2.08 (1.80–2.41) |
| Hypertension | 1597 (59.37) | 9824 (50.28) | 1.48 (1.36–1.61) |
| Dyslipidemia | 978 (36.36) | 6511 (33.32) | 1.14 (1.04–1.24) |
| Diabetes mellitus | 907 (33.72) | 4028 (20.62) | 1.97 (1.80–2.15) |
| PAD | 108 (4.01) | 488 (2.50) | 1.64 (1.32–2.03) |
| COPD | 264 (9.81) | 1677 (8.58) | 1.18 (1.02–1.35) |
| Depression | 392 (14.57) | 2046 (10.47) | 1.46 (1.30–1.65) |
| Hyperuricemia | 120 (4.46) | 757 (3.87) | 1.18 (0.97–1.43) |
| Gout | 129 (4.80) | 685 (3.51) | 1.41 (1.16–1.71) |
| Renal failure | 127 (4.72) | 594 (3.04) | 1.58 (1.30–1.92) |
| Rheumatoid arthritis | 24 (0.89) | 189 (0.97) | 0.92 (0.60–1.40) |
| Osteoarthritis | 807 (30.0) | 5395 (27.61) | 1.13 (1.03–1.23) |
| Smoking | |||
| Never smoker | 920 (34.20) | 6818 (34.90) | 1 (ref) |
| Current smoker | 736 (27.36) | 4732 (24.22) | 1.15 (1.04–1.28) |
| Past smoker | 266 (9.89) | 1634 (8.36) | 1.20 (1.04–1.39) |
| No record | 786 (28.55) | 6354 (32.52) | 0.90 (0.81–0.99) |
| Alcohol abuse | 537 (19.96) | 3098 (15.86) | 1.36 (1.22–1.52) |
| BMI, kg/m2 | |||
| <25 | 332 (12.34) | 2212 (11.32) | 1 (ref) |
| 25–30 | 827 (30.74) | 5687 (29.11) | 0.97 (0.85–1.11) |
| >30 | 752 (27.96) | 4669 (23.90) | 1.07 (0.93–1.23) |
| No record | 779 (28.96) | 6970 (35.67) | 0.75 (0.65–0.86) |
| Current use of | |||
| Low‐dose aspirin | 507 (18.85) | 2369 (12.13) | 1.77 (1.59–1.98) |
| Nonaspirin antiplatelet | 155 (5.76) | 704 (3.60) | 1.66 (1.39–1.99) |
| α‐Blockers | 83 (3.09) | 556 (2.85) | 1.10 (0.87–1.39) |
| Calcium channel blockers | 411 (15.28) | 2392 (12.24) | 1.39 (1.24–1.56) |
| β‐Blockers | 293 (10.89) | 1490 (7.63) | 1.51 (1.33–1.73) |
| ACEIs | 608 (22.60) | 3529 (18.06) | 1.45 (1.31–1.60) |
| ARBs | 324 (12.04) | 1958 (10.02) | 1.25 (1.10–1.42) |
| Nitrates | 179 (6.65) | 896 (4.59) | 1.52 (1.29–1.80) |
| Oral anticoagulants | 150 (5.58) | 802 (4.10) | 1.39 (1.16–1.67) |
| Diuretics, high ceiling | 323 (12.01) | 1442 (7.38) | 1.81 (1.59–2.07) |
| Diuretics, low ceiling | 251 (9.33) | 2029 (10.38) | 0.91 (0.79–1.05) |
| Diuretics, K sparing | 50 (1.86) | 233 (1.19) | 1.58 (1.16–2.15) |
| Corticosteroids | 55 (2.04) | 407 (2.08) | 0.99 (0.75–1.32) |
| NSAIDs | 334 (12.42) | 2277 (11.65) | 1.10 (0.97–1.26) |
| Paracetamol | 481 (17.88) | 3354 (17.17) | 1.14 (1.01–1.29) |
| Metamizole | 114 (4.24) | 732 (3.75) | 1.12 (0.96–1.44) |
| Colchicine | 16 (0.59) | 75 (0.38) | 1.57 (0.91–2.70) |
| Allopurinol | 94 (3.49) | 578 (2.96) | 1.19 (0.95–1.49) |
| PPI | 606 (22.53) | 3803 (19.46) | 1.22 (1.10–1.35) |
| H2 blockers | 82 (3.05) | 616 (3.15) | 0.98 (0.77–1.24) |
ACEI indicates angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; H2, histamine receptor type 2; IHD, ischemic heart disease; NSAIDs, non‐steroidal anti‐inflammatory drugs; OR, odds ratio; PAD, peripheral artery disease; PPI, proton pump inhibitor; ref, reference; TIA, transient ischemic attack.
Adjusted only for matching factors (age, sex, and calendar year).
Table 2.
Risk of Nonfatal Ischemic Stroke Associated With the Use of Fixed‐Dose Combination of CaD and the Effect of Dose and Duration of Treatment
| Cases (%) n=2690 | Controls (%) n=19 538 | Nonadjusted ORa (95% CI) | Adjusted ORb (95% CI) | |
|---|---|---|---|---|
| CaD | ||||
| Nonuse | 2462 (91.52) | 17 825 (91.23) | 1 (ref) | 1 (ref) |
| Current | 87 (3.23) | 743 (3.80) | 0.83 (0.66–1.05) | 0.85 (0.67–1.08) |
| Recent | 80 (2.97) | 516 (2.64) | 1.11 (0.87–1.41) | 1.09 (0.85–1.40) |
| Past | 61 (2.27) | 454 (2.32) | 0.95 (0.72–1.24) | 0.92 (0.69–1.21) |
| Calcium dosec | ||||
| <1000 mg/d | 35 (1.30) | 342 (1.75) | 0.73 (0.51–1.03) | 0.76 (0.53–1.08) |
| ≥1000 mg/d | 37 (1.38) | 247 (1.26) | 1.07 (0.75–1.51) | 1.07 (0.74–1.53) |
| Unknown | 15 (0.56) | 154 (0.79) | 0.70 (0.41–1.19) | 0.73 (0.42–1.25) |
| Durationc | ||||
| ≤180 d | 41 (1.52) | 371 (1.90) | 0.79 (0.57–1.10) | 0.81 (0.58–1.13) |
| >180 d | 46 (1.71) | 372 (1.90) | 0.88 (0.64–1.20) | 0.90 (0.65–1.23) |
CaD indicates calcium supplements with vitamin D; CI, confidence interval; OR, odds ratio; ref, reference.
Adjusted only for matching factors (age, sex, and calendar year).
Among current users compared with nonusers.
Table 3.
Evaluation of the Potential Interaction of Fixed‐Dose Combination of CaD With Sex, Age, and Baseline Cardiovascular Risk on the Risk of Nonfatal Ischemic Stroke
| Current Use of CaD | Cases (%) n=2690 | Controls (%) n=19 538 | Nonadjusted ORa (95% CI) | Adjusted ORb (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 11 (0.78) | 92 (0.90) | 0.87 (0.47–1.64) | 0.86 (0.44–1.65) |
| Female | 76 (5.91) | 651 (7.01) | 0.83 (0.65–1.06) |
0.86 (0.67–1.12) Test for interaction, P=1.000 |
| Age | ||||
| <70 y | 16 (1.75) | 154 (2.30) | 0.75 (0.44–1.27) | 0.76 (0.43–1.32) |
| ≥70 y | 71 (4.00) | 589 (4.59) | 0.85 (0.66–1.10) |
0.87 (0.67–1.13) Test for interaction, P=0.668 |
| Cardiovascular risk | ||||
| Low‐intermediate | 58 (3.96) | 572 (4.10) | 0.96 (0.72–1.27) | 0.92 (0.69–1.23) |
| High | 29 (2.37) | 171 (3.06) | 0.76 (0.51–1.14) |
0.73 (0.48–1.10) Test for interaction, P=0.370 |
Fifty cases (1.86%) were current users of CaM compared with 293 controls (1.50%) (OR: 1.18; 95% CI, 0.86–1.61; Table 4). We observed a significantly increased risk at doses of ≥1000 mg/day (OR 2.09; 95% CI, 1.25–3.49; P=0.005) that was present in both women (OR: 1.77; 95% CI, 0.94–3.35; P=0.079) and men (OR: 3.00; 95% CI, 1.20–7.45; P=0.018; test for interaction, P=0.353). At lower daily doses, we did not observe an increased risk (OR: 0.76; 95% CI, 0.45–1.26). We did not observe a duration effect or an effect modification by sex, age, or background cardiovascular risk (Table 5).
Table 4.
Risk of Nonfatal Ischemic Stroke Associated With the Use of CaM and the Effect of Dose and Duration of Treatment
| Cases (%) n=2690 | Controls (%) n=19 538 | Nonadjusted ORa (95% CI) | Adjusted ORb (95% CI) | |
|---|---|---|---|---|
| CaM | ||||
| Nonuse | 2578 (95.84) | 18 769 (96.06) | 1 (ref) | 1 (ref) |
| Current | 50 (1.86) | 293 (1.50) | 1.25 (0.92–1.69) | 1.18 (0.86–1.61) |
| Recent | 32 (1.19) | 214 (1.10) | 1.09 (0.75–1.59) | 1.04 (0.71–1.53) |
| Past | 30 (1.12) | 262 (1.34) | 0.83 (0.56–1.21) | 0.79 (0.54–1.17) |
| Calcium dosec | ||||
| <1000 mg/d | 17 (0.63) | 155 (0.79) | 0.80 (0.48–1.32) | 0.76 (0.45–1.26) |
| ≥1000 mg/d | 21 (0.78) | 67 (0.34) | 2.29 (1.40–3.75) | 2.09 (1.25–3.49) |
| Unknown | 12 (0.45) | 71 (0.36) | 1.23 (0.67–2.27) | 1.22 (0.65–2.29) |
| Durationc | ||||
| ≤180 d | 24 (0.89) | 134 (0.69) | 1.31 (0.85–2.03) | 1.23 (0.79–1.93) |
| >180 d | 26 (0.97) | 159 (0.81) | 1.19 (0.78–1.81) | 1.13 (0.74–1.73) |
Table 5.
Evaluation of the Potential Interaction of CaM With Sex, Age, and Baseline Cardiovascular Risk on the Risk of Nonfatal Ischemic Stroke
| Current Use of CaM | Cases (%) n=2690 | Controls (%) n=19 538 | Nonadjusted ORa (95% CI) | Adjusted ORb (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 13 (0.93) | 45 (0.44) | 2.09 (1.12–3.89) | 1.77 (0.92–3.42) |
| Female | 37 (2.87) | 248 (2.67) | 1.08 (0.76–1.54) |
1.04 (0.73–1.50) Test for interaction, P=0.164 |
| Age | ||||
| <70 y | 11 (1.20) | 71 (1.06) | 1.13 (0.59–2.15) | 1.11 (0.56–2.21) |
| ≥70 y | 39 (2.20) | 222 (1.73) | 1.28 (0.91–1.81) |
1.19 (0.84–1.70) Test for interaction, P=0.860 |
| Cardiovascular risk | ||||
| Low‐intermediate | 28 (1.91) | 219 (1.57) | 1.23 (0.82–1.83) | 1.09 (0.73–1.64) |
| High | 22 (1.80) | 74 (1.32) | 1.34 (0.83–2.18) |
1.21 (0.73–2.00) Test for interaction, P=0.752 |
New‐User Approach
After excluding prevalent users of CaM or CaD, we had 2493 cases with a first episode of IS and 18 092 controls (Figure). Their characteristics are shown in Table S1. The ORs were similar to those of the main analysis (OR for CaD: 0.67; 95% CI, 0.44–1.00; OR for CaM: 1.20; 95% CI, 0.69–2.11; Tables S2 and S3). Consistent with the main analysis, we also detected a significantly increased risk at calcium daily doses of ≥1000 mg among current CaM users (OR: 3.99; 95% CI, 1.71–9.34; P=0.001; Table S3), both in women (OR: 4.57; 95% CI, 1.61–12.97; P=0.004) and in men (OR: 3.34; 95% CI, 0.74–15.07; P=0.116; test for interaction, P=0.741). No such dose effect was observed for CaD users (≥1000 mg/day: OR: 0.55; 95% CI, 0.26–1.16; <1000 mg/day: OR: 0.64; 95% CI, 0.34–1.20: Table S2). No duration effect was observed with either CaM or CaD. Sex, age, and background cardiovascular risk did not appear to be effect modification factors (Tables S4 and S5).
Discussion
The present study shows that CaM at high daily doses is associated with an increased risk of nonfatal IS, whereas no such a risk was observed when calcium supplements were used either at low‐doses or in association with vitamin D.
High serum calcium concentrations are linked to artery calcification,21, 22, 23 an important marker of atherosclerosis, and are considered a relevant predictor of hard ischemic events.24, 25, 26, 27 This mechanism has been postulated as the biological link between the use of calcium supplements and atherothombotic events.28 In a recent study, Anderson et al29 reported that calcium supplements were associated with an increased risk of atherosclerosis as measured by coronary artery calcification, whereas they found the opposite effect with dietary calcium. This apparent paradox could be explained by the abrupt increase of serum calcium that seems to occur after the intake of calcium supplements and not with dietary calcium.28, 29 Consequently, intermittent increases in serum calcium sustained over long periods would ultimately promote vascular calcification and the development of atherosclerosis. This could occur particularly when there is a positive calcium balance (eg, after calcium intake >1400 mg/day).29, 30
Our data support the hypothesis of a distinct effect of calcium when used in CaM or CaD. A number of studies seem to support this idea. In their meta‐analyses of randomized clinical trials, for instance, Lewis et al7 and Mao et al8 found no increased risk of AMI with CaD, whereas they detected quasisignificant ORs of 1.37 (95% CI, 0.98–1.92) and 1.28 (95% CI, 0.97–1.68), respectively, for CaM. For stroke, Mao et al8 also suggested a slightly different result for CaM (OR: 1.14) and for CaD (OR: 0.98), although CIs overlapped. Other studies, however, do not support this differential effect.3 An increasing body of evidence suggests that the deficiency of vitamin D is associated with higher cardiovascular morbidity and mortality31, 32 and that such deficiency is prevalent, even in countries with supposedly high sunlight exposure like Spain (some studies estimate figures as high as 56%).33 It is conceivable that the use of vitamin D supplements by patients, most of whom are vitamin D deficient, may offset potential cardiovascular damage induced by calcium supplements. Although it would have been interesting to assess the effect of vitamin D alone, the exposure was very low in our study and precluded any meaningful analysis.
According to our results, the amount of daily calcium intake (without vitamin D) may have a crucial role in the risk of IS. Similarly, in 2 studies performed in Denmark, Larsson et al34, 35 found an increased risk of stroke in both men34 and women35 who had dietary calcium intake >1000 mg/day with respect to those with a lower intake. In a recent meta‐analysis, Chung et al36 reviewed all observational studies that examined the association between total calcium intake (diet and supplements) and stroke risk, and the dose response found was highly inconsistent, but in most studies, there was no information on intake >1600 mg/day. In our study, we observed a risk only in patients with a daily dose of ≥1000 mg of calcium supplements in addition to dietary calcium intake (not recorded). Overall, it is likely that these patients had total daily intake of ≥1600 mg, precisely the part of the range at which most studies offer no data.
With an average incidence of IS of 460 cases per 100 000 person‐years in the US population aged ≥65 years,37 a relative risk of 2 for IS among users of high‐dose CaM would yield a number needed to treat of 217 patients per year to have 1 patient harmed, which is not a negligible population impact (see Table S6 for other scenarios).
The main strengths of our study are as follows. First, PCPs are the gatekeepers of the Spanish National Health System, and all patients, including those discharged from hospitals, should visit them to continue treatment; therefore, the recording of important diseases can be considered almost complete. Second, PCPs need to use a computer to fill in prescriptions, so the underrecording of prescription drugs can reasonably be excluded. Third, controls were randomly selected from the source population, which ensures their representativeness with respect to population exposure and reduces the possibility of a control selection bias. Fourth, researchers were blind to drug exposure when ascertaining cases, so avoiding a differential misclassification of cases influenced by exposure. Fifth, the results applying the new‐user approach were consistent with the main analysis, thus reinforcing their validity.
Our study has a number of limitations. First, we did not include fatal events because PCPs do not have a complete registry of deaths, and, in particular, there is no appropriate recording of cause of death. Consequently, our results cannot be extrapolated to fatal events. Second, although we made efforts to identify potential cases and validate the diagnosis of IS, the possibility of false positives and false negatives cannot be totally excluded; however, in our view, such potential misclassification would dilute any association between the exposure and the outcome and then could not explain the increased risk associated with high‐dose CaM. Third, we did not have information about the intake of calcium supplements with diet or with nonprescription medicinal products; if such intake were higher among nonusers of CaD or CaM (the reference category for all analyses), the measures of association would be distorted toward the null, assuming that calcium intake increases the risk of atherothrombotic events. Nevertheless, if this were true, the increased risk of IS observed with high‐dose CaM would be even greater, and the differential effect of CaM and CaD would still hold. Fourth, although the study has a large number of events, the exposure is limited (in particular, for CaM), and some estimates had wide CIs; however, the possibility that the association of high‐dose CaM with stroke is explained by chance is highly unlikely (P=0.005 in the main analysis and P=0.001 in the new‐user analysis), even in a scenario of multiple testing. Finally, despite our efforts to adjust for many potential confounding factors, the possibility of residual confounding still exists, as in any observational study, particularly for unmeasured factors.
In conclusion, the results from the present study do not support the hypothesis that CaD increases the risk of IS but add to the concerns relative to the safety of CaM, particularly when used at high doses.
Sources of Funding
BIFAP is funded and managed by the Spanish Agency for Medicines and Medical Devices. This study was supported by a research grant from Instituto de Salud Carlos III ‐ Ministerio de Economía, Industria y Competitividad (no. PI16/01353) (co‐funded by FEDER).
Disclosures
None.
Supporting information
Table S1. Characteristics of Cases and Controls: New‐User Approach
Table S2. Risk of Ischemic Stroke Associated With the Use of Fixed‐Dose Combination of Calcium Supplements With Vitamin D and the Effect of Dose and Duration of Treatment: New‐User Approach
Table S3. Risk of Ischemic Stroke Associated With the Use of Calcium Supplements in Monotherapy and the Effect o Dose and Duration of Treatment. New‐User Approach
Table S4. Evaluation of the Potential Interaction of Fixed‐Dose Combination of Calcium Supplements With Vitamin D With Sex, Age, and Baseline Cardiovascular Risk With Respect to the Risk of Ischemic Stroke
Table S5. Evaluation of the Potential Interaction of Calcium Supplements in Monotherapy With Sex, Age, and Baseline Cardiovascular Risk With Respect to the Risk of Ischemic Stroke
Table S6. Number Needed to Treat With High‐Dose Calcium Supplements to Have 1 Patient Harmed Per Year Under Different Scenarios
Acknowledgments
The authors would like to thank the contribution to the analytical part of the paper of Noelia Seco and Elisabet Sánchez de Pablo (pharmacy students at the University of Alcalá) and the excellent collaboration of primary care practitioners participating in BIFAP. The help of Andrew Maguire with the final paper edition is greatly appreciated.
(J Am Heart Assoc. 2017;6:e005795 DOI: 10.1161/JAHA.117.005795.)28522672
The results, discussion and conclusions are from the authors and do not represent the position of the Spanish Agency for Medicines and Medical Devices.
References
- 1. Cosman F, de Beur SJ, LeBoff MS, Leweicki EM, Tanner B, Randall S, Linday R. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2539–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ. 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta‐analysis. BMJ. 2011;342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattacharya RK. Does widespread calcium supplementation pose cardiovascular risk? Am Fam Physician. 2013;87:1–2. [PubMed] [Google Scholar]
- 5. Barice EJ, Hennekens C. Calcium and vitamin D supplementation: facts and myths. J Cardiovasc Pharmacol Ther. 2015;20:9–10. [DOI] [PubMed] [Google Scholar]
- 6. Prentice RL, Pettinger MB, Jackson RD, Wactawski‐Wende J, LaCroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZ, Datta M, LeBlanc ES, Cauley JA, Rossow JE. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis JR, Radavelli‐Bagatini S, Rejnmark L, Chen JS, Simpson JM, Lappe JM, Mosekilde L, Prentice RL, Prince RL. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta‐analysis of randomized clinical trials. J Bone Miner Res. 2015;30:165–175. [DOI] [PubMed] [Google Scholar]
- 8. Mao PJ, Zhang C, Tang L, Xian YQ, Li YS, Wang WD, Zhu XH, Qiu HL, He J, Zhou YH. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta‐analysis of randomized controlled trials. Int J Cardiol. 2013;169:106–111. [DOI] [PubMed] [Google Scholar]
- 9. Rautiainen S, Wang L, Manson J, Sesso HD. The role of calcium in the prevention of cardiovascular disease—a review of observational studies and randomized clinical trials. Curr Atheroscler Rep. 2013;15:362. [DOI] [PubMed] [Google Scholar]
- 10. García‐Poza P, de Abajo FJ, Gil MJ, Chacón A, Bryant V, García‐Rodríguez LA. Risk of ischemic stroke associated with non‐steroidal antiinflammatory drugs and paracetamol: a population‐based case‐control study. J Thromb Haemost. 2015;13:708–718. [DOI] [PubMed] [Google Scholar]
- 11. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 12. BIFAP . Base de datos para la investigación farmacoeopidemiológica en atención primaria. Available at: http://bifap.aemps.es/. Accessed October 30, 2016.
- 13. Requena G, Abbing‐Karahagopian V, Huerta C, De Bruin ML, Alvarez Y, Miret M, Hesse U, Gardarsdottir H, Souverein PC, Slattery J, Schneider C, Rottenkolber M, Schmiedl S, Gil M, De Groot MC, Bate A, Ruigómez A, García Rodríguez LA, Johansson S, de Vries F, Montero D, Schlienger R, Reynolds R, Klungel OH, de Abajo FJ. Incidence rates and trends of hip/femur fractures in five European countries: comparison using E‐healthcare records databases. Calcif Tissue Int. 2014;94:580–589. [DOI] [PubMed] [Google Scholar]
- 14. Requena G, Huerta C, Gardarsdottir H, Logie J, González R, Abbing‐Karahagopian V, Miret M, Schneider C, Souverein PC, Webb D, Afonso A, Boudiaf N, Martin E, Oliva B, Alvarez A, De Groot MC, Bate A, Johansson S, Schlienger R, Reynolds R, Klungel OH, de Abajo FJ. Hip/femur fractures associated with the use of benzodiazepines (anxiolytics, hypnotics and related drugs): a methodological approach to assess consistencies across databases from the PROTECT‐EU project. Pharmacoepidemiol Drug Saf. 2016;25(suppl 1):66–78. [DOI] [PubMed] [Google Scholar]
- 15. Brauer R, Douglas I, García‐Rodríguez LA, Downey G, Huerta C, de Abajo F, Bate A, Feudjo‐Tepie M, de Groot MC, Schlienger R, Reynolds R, Smeeth L, Klungel O, Ruigómez A. Risk of acute liver injury associated with use of antibiotics. Comparative cohort and nested case‐control studies using two primary care databases in Europe. Pharmacoepidemiol Drug Saf. 2016;25(suppl 1):29–38. [DOI] [PubMed] [Google Scholar]
- 16. WICC International Classification of Primary Care – 2nd Edition Wonca International Classification Committee. Available at: http://qicpd.racgp.org.au/media/57417/icpc-codes.pdf. Accessed December 12, 2016.
- 17. Walker A. Observation and Inference—An Introduction to the Methods of Epidemiology. Newton Lower Falls: Epidemiology Resources Inc.; 1991. [Google Scholar]
- 18. Toh S, García Rodríguez LA, Hernán MA. Analyzing partially missing confounder information in comparative effectiveness and safety research of therapeutics. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juutilainen A, Lehto S, Ronnemaa T, Pyoraia K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18‐year prospective population‐based study in Finnish subjects. Diabetes Care. 2005;28:2901–2907. [DOI] [PubMed] [Google Scholar]
- 20. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montalcini T, Gorgone G, Pujia A. Serum calcium level is related to both intima‐media thickness and carotid atherosclerosis: a neglect risk factor in obese/overweight subjects. J Transl Med. 2012;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubin MR, Rundek T, McMahon DJ, Lee HS, Sacco RL, Silverberg SJ. Carotid artery plaque thickness is associated with increased serum calcium levels: the Northern Manhattan study. Atherosclerosis. 2007;194:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bolland MJ, Wang TK, van Pelt NC, Horne AM, Mason BH, Ames RW, Grey AB, Ruygrok PN, Gamble GD, Reid IR. Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction. J Bone Miner Res. 2010;25:505–512. [DOI] [PubMed] [Google Scholar]
- 24. Lind L, Skarfors E, Berglund L, Lithell H, Ljunghall S. Serum calcium: a new, independent, prospective risk factor for myocardial infarction in middle‐aged men followed for 18 years. J Clin Epidemiol. 1997;50:967–973. [DOI] [PubMed] [Google Scholar]
- 25. Jorde R, Sundsfjord J, Fitzgerald P, Bønaa KH. Serum calcium and cardiovascular risk factors and diseases: the Tromsø study. Hypertension. 1999;34:484–490. [DOI] [PubMed] [Google Scholar]
- 26. Slinin Y, Blackwell T, Ishani A, Cummings SR, Enrud KE; MORE investigators . Serum calcium, phosphorus and cardiovascular events in postmenopausal women. Int J Cardiol. 2011;149:335–340. [DOI] [PubMed] [Google Scholar]
- 27. Wayths R, Zellinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–230. [DOI] [PubMed] [Google Scholar]
- 28. Bolland M, Grey A, Reid I. Calcium and cardiovascular risks. Aust Prescr. 2013;36:5–8. [Google Scholar]
- 29. Anderson JJB, Kruszka B, Delaney JAC, He K, Burke GL, Alonso A, Bild DE, Budoff M, Michos ED. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10‐year follow‐up of the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2016;5:e003815 DOI: 10.1161/JAHA.116.003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low‐ and high‐calcium diets. Kidney Int. 2012;81:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Creighton D, Ignaszewski A, Francis G. Vitamin D: new D‐fence against cardiovascular disease? B C Med J. 2012;54:136–140. [Google Scholar]
- 32. Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. [DOI] [PubMed] [Google Scholar]
- 33. Gradillas‐Garcia A, Alvarez J, Rubio JA, de Abajo FJ. Relationship between vitamin D deficiency and metabolic syndrome in adult population of the Community of Madrid. Endocrinol Nutr. 2015;62:180–187. [DOI] [PubMed] [Google Scholar]
- 34. Larsson SC, Virtanen MJ, Mars M, Männistö S, Pietinen P, Albanes D, Virtamo J. Magnessium, calcium, potassium and sodium intakes and risk of stroke in male smokers. Arch Intern Med. 2008;168:459–465. [DOI] [PubMed] [Google Scholar]
- 35. Larsson SC, Virtamo J, Volk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol. 2011;174:35–43. [DOI] [PubMed] [Google Scholar]
- 36. Chung M, Tang AM, Fu Z, Wang DD, Newberry SJ. Calcium intake and cardiovascular disease risk—an updated systematic review and meta‐analysis. Ann Intern Med. 2016;165:856–866. [DOI] [PubMed] [Google Scholar]
- 37. Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Cases and Controls: New‐User Approach
Table S2. Risk of Ischemic Stroke Associated With the Use of Fixed‐Dose Combination of Calcium Supplements With Vitamin D and the Effect of Dose and Duration of Treatment: New‐User Approach
Table S3. Risk of Ischemic Stroke Associated With the Use of Calcium Supplements in Monotherapy and the Effect o Dose and Duration of Treatment. New‐User Approach
Table S4. Evaluation of the Potential Interaction of Fixed‐Dose Combination of Calcium Supplements With Vitamin D With Sex, Age, and Baseline Cardiovascular Risk With Respect to the Risk of Ischemic Stroke
Table S5. Evaluation of the Potential Interaction of Calcium Supplements in Monotherapy With Sex, Age, and Baseline Cardiovascular Risk With Respect to the Risk of Ischemic Stroke
Table S6. Number Needed to Treat With High‐Dose Calcium Supplements to Have 1 Patient Harmed Per Year Under Different Scenarios


