Abstract
Background
Current guidelines suggest treating blood pressure above 180/105 mm Hg during the first 24 hours in patients with acute ischemic stroke undergoing any form of recanalization therapy. Currently, no studies exist to guide blood pressure management in patients with stroke treated specifically with mechanical thrombectomy. We aimed to determine the association between blood pressure parameters within the first 24 hours after mechanical thrombectomy and patient outcomes.
Methods and Results
We retrospectively studied a consecutive sample of adult patients who underwent mechanical thrombectomy for acute ischemic stroke of the anterior cerebral circulation at 3 institutions from March 2015 to October 2016. We collected the values of maximum, minimum, and average values of systolic blood pressure, diastolic blood pressure, and mean arterial pressures in the first 24 hours after mechanical thrombectomy. Primary and secondary outcomes were patients’ functional status at 90 days measured on the modified Rankin scale and the incidence and severity of intracranial hemorrhages within 48 hours. Associations were explored using an ordered multivariable logistic regression analyses. A total of 228 patients were included (mean age 65.8±14.3; 104 males, 45.6%). Maximum systolic blood pressure independently correlated with a worse 90‐day modified Rankin scale and hemorrhagic complications within 48 hours (adjusted odds ratio=1.02 [1.01–1.03], P=0.004; 1.02 [1.01–1.04], P=0.002; respectively) in multivariable analyses, after adjusting for several possible confounders.
Conclusions
Higher peak values of systolic blood pressure independently correlated with worse 90‐day modified Rankin scale and a higher rate of hemorrhagic complications. Further prospective studies are warranted to identify whether systolic blood pressure is a therapeutic target to improve outcomes.
Keywords: blood pressure, hemorrhage, outcome, stroke, thrombectomy
Subject Categories: Ischemic Stroke, High Blood Pressure, Revascularization, Quality and Outcomes, Complications
Clinical Perspective
What Is New?
Presently, in patients with acute ischemic stroke treated with mechanical thrombectomy, no data exist to guide blood pressure management after mechanical thrombectomy.
In this study, we reveal an association between higher peak systolic blood pressures within the first 24 hours after mechanical thrombectomy and worse patient outcomes, specifically greater hemorrhagic complications within 48 hours and worse functional status at 90 days after mechanical thrombectomy.
What Are the Clinical Implications?
Peak systolic blood pressure within 24 hours after mechanical thrombectomy may be a marker of poor outcome or may represent an intervenable, therapeutic target in patients with acute ischemic stroke.
Introduction
Several major clinical trials have demonstrated superiority of mechanical thrombectomy (MT) compared with standard medical treatment, including intravenous thrombolysis using tissue plasminogen activator (tPA) if eligible, for patients presenting with acute ischemic stroke (AIS) caused by a large anterior circulation vessel occlusion.1, 2, 3, 4, 5
An important difference between the 2 therapies is in their ability to achieve vessel recanalization. With intravenous tPA, a sustained, successful recanalization is achieved in less than 30% to 40% of the cases with proximal arterial occlusions.6, 7, 8, 9 Therefore, efforts to increase perfusion with permissive hypertension up to 180/105 mm Hg for the first 24 to 48 hours are commonly practiced in patients treated with intravenous tPA. However, unlike intravenous tPA, a successful recanalization can be achieved with MT in about 70% to 80% of cases, defined as Thrombolysis in Cerebral Infarction score ≥2b.1, 2, 3, 4, 5 Yet, the current guidelines suggest only treating blood pressure above 180/105 mm Hg for at least the first 24 hours after intraarterial intervention for AIS, similar to those receiving intravenous tPA.10
Presently, there is no evidence demonstrating an association of blood pressure after MT with clinical outcome. Therefore, in this study we aim to examine various blood pressure parameters within the first 24 hours following MT and identify an association with (1) functional outcome measured as the 90‐day modified Rankin scale (mRS) score, and (2) postthrombectomy hemorrhagic events.
Materials and Methods
Study Design
We conducted an observational, retrospective, multi‐institutional study to determine whether blood pressure within the first 24 hours after MT for AIS is associated with patient outcome. Patients treated at the following 3 US academic comprehensive stroke referral centers were studied: Houston Methodist Neurological Institute, Houston, TX; Vanderbilt University Medical Center, Nashville, TN; and University of Louisville, Louisville, KY. The study was approved by the research Review Board of each institution; and given its retrospective medical record review design without any patient intervention, consent was waived. The study protocol was designed a priori to data collection.
Patient Selection
We retrospectively reviewed the prospectively collected stroke databases at each institution. We included a consecutive sample of patients >18 years of age treated between March 2015 and October 2016 who underwent MT for AIS of the anterior cerebral circulation caused by occlusion of the intracranial internal carotid artery, proximal middle cerebral artery (M1 or M2), and proximal anterior cerebral artery (A1). We chose this time period to enrich inclusion of patients treated in all 3 institutions with standard, up‐to‐date endovascular treatment protocols based on the 5 major endovascular stroke trials.1, 2, 3, 4, 5 We excluded patients who had any 1 or more of the following: (1) a pre‐existing terminal condition, such as terminal cancer, (2) end‐stage heart or liver failure, (3) left ventricular assist device at the time of stroke, (4) perioperative stroke in the setting of major cardiovascular operation, and (5) a known prestroke mRS ≥4.
Outcome Measures and Covariates
Our primary study variables included blood pressure parameters. We recorded the maximum, minimum, and average values of systolic blood pressure (SBP), diastolic blood pressure, and mean arterial pressures in the first 24 hours after MT. These measurements were recorded at least once every hour during the first 24 hours after MT as part of standard of care. They were recorded using noninvasive methods only when an arterial line recording was unavailable. In addition, use of either a vasopressor or an antihypertensive agent to control blood pressure via continuous infusion was noted. Its initiation and choice of agent were decided by the treating physician.
Patients’ baseline characteristics and demographics were collected (Table 1). Other covariates related to the diagnosis and treatment of stroke recorded were National Institutes of Health Stroke Scale (NIHSS), laterality and location of vessel occlusion, prior treatment with intravenous tPA, estimated time from symptom onset to groin puncture, and postintervention recanalization assessment on the final digital subtraction angiography. Modern neurothrombectomy devices were used and choice of procedural anesthesia was decided by the interventionist. Extent of recanalization was determined with the modified Treatment in Cerebral Infarction (mTICI) score, categorized as 0 (no reperfusion), 1, 2a, 2b, and 3 (complete reperfusion).11
Table 1.
Summary of Patients’ (n=228) Demographics, Comorbiditiesa, Stroke Characteristics, Treatments, and Outcomes
| Characteristics | Value, n (%)b |
|---|---|
| Age, mean (SD), y | 65.8 (14.3) |
| Male sex | 104 (45.6) |
| Comorbidities | |
| Hypertension | 166 (72.8) |
| Hyperlipidemia | 104 (45.6) |
| Diabetes mellitus | 67 (29.4) |
| Recentc or active smoker | 50 (21.9) |
| Atrial fibrillation | 77 (33.8) |
| Antiplatelet drug use | 73 (32.0) |
| Anticoagulant use | 51 (22.4) |
| Stroke characteristics | |
| NIH stroke scale, mean (SD) | 16.3 (7.1) |
| ICA occlusion | 67 (29.4) |
| M1 occlusion | 131 (57.5) |
| M2 occlusion | 27 (11.8) |
| A1 occlusion | 3 (1.3) |
| Left circulation | 111 (48.7) |
| Stroke treatment | |
| IV thrombolysis | 119 (52.2) |
| Time to groin puncture, median (IQR), min | 260 (180, 375.8) |
| mTICI 0 | 9 (3.9) |
| mTICI 1 | 19 (8.3) |
| mTICI 2a | 17 (7.5) |
| mTICI 2b | 76 (33.3) |
| mTICI 3 | 107 (46.9) |
| Antihypertensive IV drip use | 56 (24.6) |
| Vasopressor IV drip use | 32 (14.0) |
| Outcomes | |
| No hemorrhage | 165 (72.4) |
| Asymptomatic hemorrhage | 50 (21.9) |
| Symptomatic hemorrhage | 13 (5.7) |
| mRS 0 | 27 (14.2) |
| mRS 1 | 21 (11.1) |
| mRS 2 | 23 (12.1) |
| mRS 3 | 24 (12.6) |
| mRS 4 | 29 (15.3) |
| mRS 5 | 12 (6.3) |
| mRS 6 | 54 (28.4) |
| Unknown | 38 (16.7) |
A1 indicates proximal segment of anterior cerebral artery; ICA, internal carotid artery; IQR, interquartile range; IV, intravenous; M1 and M2, proximal segments of middle cerebral artery; mRS, modified Rankin Scale; mTICI, modified Treatment in Cerebral Infarction score; NIH, National Institutes of Health.
Known at the time of stroke admission.
Values are reported as total number and percent, n (%), unless otherwise specified in the characteristic description.
Less than a month.
Our primary outcome of interest was patients’ functional status at 90 days measured on the mRS from 0 (no symptoms at all) to 6 (death). Our secondary outcome of interest was the incidence of intracranial hemorrhagic complications (specifically, intraparenchymal, subarachnoid, and/or intraventricular hemorrhages) within 48 hours after MT confirmed on brain imaging (computed tomography or magnetic resonance imaging). Hemorrhage was further classified as symptomatic if it was associated with a clinical worsening or an increase of ≥4 points on the NIHSS, as commonly defined.2, 4, 5 All patients received brain imaging after MT.
Statistical Analysis
Continuous variables are reported using standard descriptive statistical methods with measures of central tendency (mean or median) and variability (standard deviation, standard error of mean, or interquartile range) based on the normality of the distribution. All mean values of blood pressure parameters are plotted and reported with standard error of mean. Categorical variables are reported as proportions.
All categorical variables with more than 2 categories were treated as ordinal variables. The 90‐day mRS score was treated as a discrete 7‐category ordinal variable from 0 to 6. Similarly, hemorrhagic complication was also treated as an ordinal variable with the following categories of severities: none, asymptomatic, and symptomatic hemorrhages. Associations between the outcome variables and covariates were explored using both univariable, unadjusted ordered logistic regression analysis testing one predictor at a time as well as multivariable ordered logistic regression analysis to adjust for potential confounders.5, 12 An ordinal multivariable model was built by including all covariates (except blood pressure parameters) with unadjusted P≤0.1. Variables from this multivariable model were removed if their adjusted P value changed to >0.1. Prior variables excluded from the initial multivariable model were reintroduced one at a time; those with P≤0.1 in the multivariable model were kept in the model. Each blood pressure parameter was added to this final multivariable, covariate model to assess its independent correlation with outcome. Associations of blood pressure parameters with the outcome variables were re‐explored using a more general, backward stepwise regression to generate another multivariable, adjusted ordinal logistic model. The primary reason for this analysis was to further support and confirm our results. All covariates and blood pressure parameters with an unadjusted P≤0.1 were included in an initial multivariable model. Variables with a P>0.1 in the multivariable model were removed in a stepwise fashion. The proportional odds assumption was tested for all final models using the Brant test. The primary effect variable was the adjusted odds ratio reported with 95% CI and a corresponding P value.
Thirty‐eight (16.7%) patients were lost at 90‐day follow‐up; hence, their mRS scores were not available. While only complete data sets were analyzed and are presented here for mRS, the missing mRS values were imputed for a more inclusive analysis. No correlation was found between the study variables and loss of follow‐up. Hence, the mRS scores were considered to be missing completely at random and were imputed using multiple ordered logistic regression. A multivariable, adjusted model was created using the study variables with an adjusted P≤0.1, excluding the blood pressure parameters. Being a proxy or a strong predictor of 90‐day mRS, hospital discharge destination was included in the model (Table S1). Overall, a better fit was achieved with a model including discharge destination than without. Hospital discharge destination was treated as an ordinal variable with categories of home, inpatient rehabilitation, skilled‐nursing facility, and a grouped category for long‐term acute care facility and death before discharge. All variables with P≤0.05 in the multivariable, adjusted model were used to impute the missing mRS scores using multiple ordered logistic regression (Table S1). Given that 16.7% of the mRS scores were missing, 20 imputation data sets of mRS scores were created to ensure reproducibility.13, 14 Association of blood pressure parameters with mRS scores was re‐explored using these data sets.
All regression and imputation analyses were conducted using Stata Statistical Software for Windows, release 14 (StataCorp LP, College Station, TX). Graphical representations of data and Student t tests to compare means were done using GraphPad Prism version 7.00 for Windows (GraphPad Software, San Diego, CA). Statistical significance α was set at 0.05 for all statistical analyses. All P values are 2‐sided. For simultaneous, multiple hypothesis testing with blood pressure parameters in multivariable, adjusted regression models, statistical significance was adjusted using Bonferroni correction. All statistical analyses were reviewed in consultation with a professional biostatistician.
Results
Association of Covariates With Outcomes
A total of 335 consecutive patients were reviewed, of which 228 patients met the inclusion and exclusion criteria and were included for analysis. Summary statistics of their demographics, past medical history, stroke diagnosis, treatment, and outcomes are listed in Table 1. No sex‐based differences were present in our outcomes of interest.
In univariable, unadjusted analysis, the following variables were significantly associated with 90‐day mRS: age, history of hypertension, atrial fibrillation, NIHSS, location of vessel occlusion, treatment with intravenous tPA, time to groin puncture, mTICI score, use of antihypertensive intravenous drip, and hemorrhagic complication (Table S1). Higher age, NIHSS, severity of hemorrhagic complication, and a lower mTICI score continued to demonstrate a significant association with worse 90‐day mRS in multivariable, adjusted regression analysis.
In univariable, unadjusted analysis, the following variables were significantly associated with hemorrhagic complications within 48 hours after MT: history of diabetes mellitus, NIHSS, and need for antihypertensive intravenous drip (Table S2). History of diabetes mellitus, hyperlipidemia, and a higher NIHSS demonstrated a significant association with a greater severity of hemorrhagic complication in multivariable, adjusted regression analysis.
Association of Blood Pressure With Outcomes
In multivariable, adjusted analysis of complete cases (n=190), maximum SBP directly correlated with worse 90‐day mRS (odds ratio 1.02 [1.01–1.03], P=0.004; Table 2; Figure 1A). These odds ratios can be interpreted as the odds of higher categories of outcome versus lower categories (ie, mRS 0 versus 1–6, or mRS 0–1 versus 2–6) for a 1‐unit increase in the blood pressure parameter, given all other variables in the model are held constant. (Results after multiple imputations of the missing mRS scores are presented in Table S3). The maximum SBP also demonstrated significant correlation with hemorrhagic complications (1.02 [1.01–1.04], P=0.002; Table 2; Figure 2A). These results were substantiated with alternate backward, stepwise multivariable regression analyses (Table S4). Mean average and minimum values of SBP (Table 2; Figure 1B and 1C) and all parameters of diastolic blood pressure and mean arterial pressures did not correlate with outcomes in multivariable, adjusted analyses (Table 2; Figures S1 and S2).
Table 2.
Adjusted Odds Ratiosa From Multivariable Ordinal Regression Analyses of Study Outcomes With Systolic, Diastolic, and Mean Arterial Blood Pressure Parameters
| 90‐Day mRSb (n=190) | Hemorrhagic Complicationsc (n=228) | |||
|---|---|---|---|---|
| Odds Ratio | P Value | Odds Ratio | P Value | |
| SBP | ||||
| Maximum | 1.02 [1.01–1.03] | 0.004d | 1.02 [1.01–1.04] | 0.002d |
| Minimum | 1.00 [0.99–1.02] | >0.1 | 0.99 [0.97–1.01] | >0.1 |
| Average | 1.02 [1.00–1.04] | 0.05 | 1.01 [0.99–1.03] | >0.1 |
| DBP | ||||
| Maximum | 1.00 [0.99–1.02] | >0.1 | 1.00 [0.98–1.02] | >0.1 |
| Minimum | 0.98 [0.96–1.01] | >0.1 | 0.98 [0.95–1.01] | >0.1 |
| Average | 0.99 [0.97–1.02] | >0.1 | 1.00 [0.97–1.03] | >0.1 |
| MAP | ||||
| Maximum | 1.01 [0.99–1.03] | >0.1 | 1.01 [0.99–1.03] | >0.1 |
| Minimum | 1.00 [0.98–1.02] | >0.1 | 0.99 [0.97–1.01] | >0.1 |
| Average | 1.01 [0.99–1.04] | >0.1 | 1.01 [0.99–1.04] | >0.1 |
DBP indicates diastolic blood pressure; mRS, modified Rankin scale score; MAP, mean arterial blood pressure; SBP, diastolic blood pressure.
Odds ratios are reported with their corresponding 95% CIs.
mRS is treated as an ordinal variable from 0 (no symptoms at all) to 6 (death).
Hemorrhagic complication is treated as an ordinal variable with 3 categories: no hemorrhage, asymptomatic, and symptomatic hemorrhage.
Indicate P values meeting significance after Bonferroni correction of the significance level α.
Figure 1.
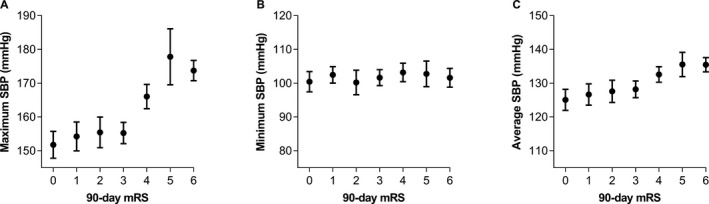
Mean values of maximum (A), minimum (B), and average (C) systolic blood pressure (SBP) plotted with 90‐day modified Rankin scale (mRS) scores. Error bars represent SEM.
Figure 2.
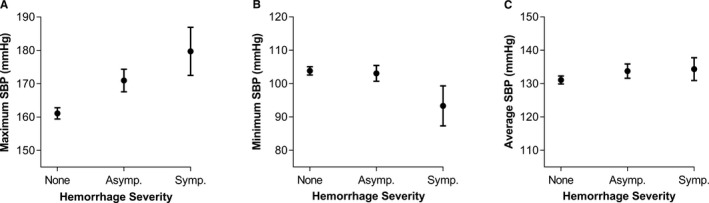
Mean values of maximum (A), minimum (B), and average (C) systolic blood pressure (SBP) plotted with hemorrhagic complications. Hemorrhagic complications are graded based on their severity: none, asymptomatic (Asymp.), and symptomatic (Symp.) hemorrhages. Error bars represent SEM.
Subanalyses in Recanalized and Nonrecanalized Patients
Subanalysis based on widely accepted definitions of successful (n=183) and unsuccessful (n=45) recanalization (mTICI ≤2a and ≥2b, respectively) was conducted. In patients with successful recanalization, maximum SBP also directly correlated with worse 90‐day mRS (adjusted odds ratio 1.02 [1.00–1.03], P=0.01; n=156) and severity of hemorrhagic complications (1.02 [1.00–1.03], P=0.05; n=183; Figure 3). While the minimum diastolic blood pressure inversely correlated with hemorrhagic complications as well (0.96 [0.93–1.00], P=0.04; Figure S3A), the observed differences in the means of these values were not large for practical purposes.
Figure 3.
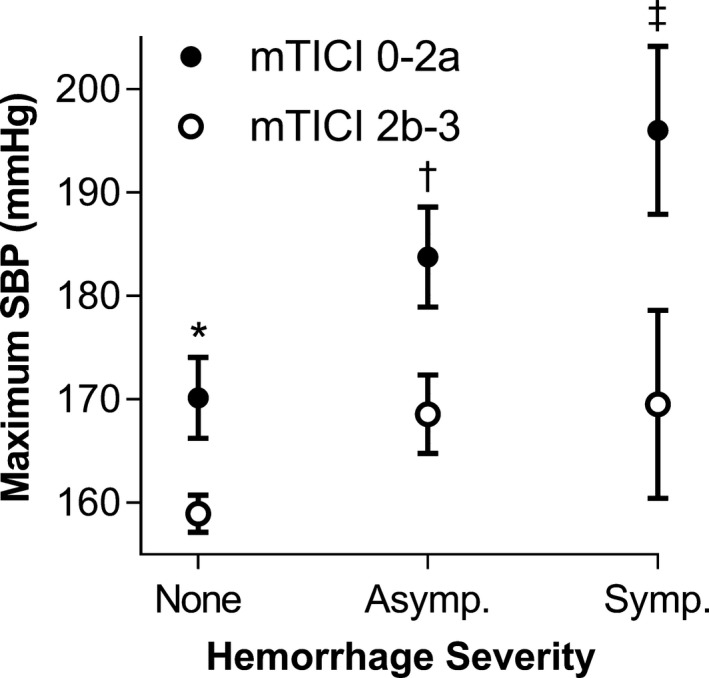
Mean maximum systolic blood pressure (SBP) plotted with hemorrhagic complications in nonrecanalized (mTICI 0‐2a, closed dots) and recanalized (mTICI 2b‐3, open dots) patients. Hemorrhagic complications are graded based on their severity: none, asymptomatic (Asymp.), and symptomatic (Symp.) hemorrhages. Error bars represent SEMs. *P<0.01, † P=0.03, and ‡ P=0.05 indicate P values of t tests comparing mean values of mTICI 0‐2a and 2b‐3 patients. (mTICI, modified Treatment in Cerebral Infarction score).
Of the patients with unsuccessful recanalization, only 34 had 90‐day mRS follow‐up. Therefore, the subanalysis was underpowered to reveal any significant correlation between the blood pressure parameters and 90‐day mRS. However, with respect to severity of hemorrhagic complications, a direct correlation was found with the maximum SBP (1.05 [1.01–1.10], P=0.01; Figure 3) as well as the maximum mean arterial pressure (1.06 [1.01–1.11], P=0.02; Figure S3B).
We compared the mean values of maximum SBP corresponding to the severity of hemorrhagic complications between the successfully and unsuccessfully recanalized patients. In successfully recanalized patients, hemorrhagic complications were observed at lower mean values of maximum SBP (Figure 3). Between recanalized and nonrecanalized patients, respectively, the mean values of maximum SBP (in mm Hg) were 159±1.8 [n=133] and 170±3.9 [n=32] (P=0.008) in patients who did not have hemorrhages; 169±3.8 [42] and 184±4.9 [8] (P=0.03) in patients who had asymptomatic hemorrhages; and 170±9.1 [8] and 196±8.1 [5] (P=0.05) in patients who had symptomatic hemorrhages.
Discussion
Our retrospective analysis of patients treated with MT for AIS at 3 institutions reveals that higher values of SBP in the first 24 hours after MT are independently associated with greater severity of hemorrhages within 48 hours and worse functional outcomes (mRS scores) at 90 days.
Nearly all prior studies have shown that higher blood pressure during the acute phase of stroke is associated with worse outcomes.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 A majority of these studies have demonstrated specifically that higher values of SBP immediately after treatment with intravenous tPA is associated with worse functional outcomes16, 28, 29 and hemorrhagic complications.16, 25, 28 However, a recent meta‐analysis found that blood pressure lowering within 3 days of stroke onset in patients does not improve outcome.30 Many of these studies included patients with heterogeneous stroke etiologies, such as hemorrhagic31, 32, 33, 34 and lacunar infarcts,31, 32, 33, 34, 35, 36, 37 and only 3 studies33, 34, 37 included a few, if any, patients who had received recanalization therapy only with intravenous tPA. Thus, high blood pressure has not yet been established as the cause of worse outcomes or a modifiable risk factor in patients with stroke, especially in patients with large‐vessel occlusion stroke treated with MT. We, therefore, first tested whether the previously observed associations of blood pressure parameters with outcomes were also present in patients with stroke who underwent MT, which achieves significantly higher rates of recanalization than intravenous tPA (<30–40% with tPA versus 70–80% with MT).1, 2, 3, 4, 5, 6, 7, 8, 9
It has been established that large‐vessel occlusion strokes have an ischemic penumbra that has impaired autoregulation and is more sensitive to changes in systemic blood pressure.38, 39, 40, 41, 42 Higher blood pressure in the setting of restoration of blood flow to ischemic core and penumbral areas may plausibly subject these regions to increased reperfusion injury.43 One such well‐established marker of reperfusion injury is hemorrhagic transformation.43 We hypothesize that elevated blood pressure in patients undergoing a therapy that is highly successful in restoring blood flow to these areas of impaired autoregulation with increased susceptibility to systemic pressures may result in greater reperfusion injury, demonstrable by hemorrhagic complications, and worse outcomes. Our results could suggest such a pathophysiological relationship.
We found increased incidence of hemorrhagic complications and poor functional outcomes in patients with higher peak SBP in the first 24 hours after MT. Further, hemorrhage, a known marker of reperfusion injury, was observed at lower mean values of peak SBP in patients who had successful recanalization compared with those who did not. Although a causal relationship between high SBP and worse outcomes cannot be inferred from our data, this suggests perhaps an increased susceptibility to reperfusion injury with recanalization. Therefore, while permissive hypertension up to SBP <180 mm Hg may be warranted in some cases of unsuccessful recanalization, in our study an overall independent, direct correlation was noted with worse outcomes and increasing peak SBP values up to 180 mm Hg in patients undergoing MT. Further studies are warranted to determine whether a lower threshold for SBP intervention is needed to potentially improve outcomes in these patients.
This study includes a large cohort of patients without a single institutional predominance, and our results are largely not limited by unique institutional practices. They are comparable to published trial data in the proportion of successful recanalization achieved with MT,1, 2, 3, 4, 5 incidence of symptomatic hemorrhages,2, 5 and mean time from symptom onset to groin puncture.2, 5 The latter 2 characteristics were slightly lower in other trials.1, 3, 4
The results herein must be interpreted with the limitations intrinsic to this study's retrospective observational design, such as inherent bias and heterogeneous practices of blood pressure management in individual patients. Few studies have demonstrated that total infarct volume is an important determinant of functional outcome.44, 45 Attempts to account for this variable were met with heterogeneity of intracranial imaging available, and therefore, its lack is a limitation that can be better addressed with a prospective study. This study was also underpowered to draw a meaningful association between SBP and outcome in patients with unsuccessful recanalization with MT. Hence, further prospective studies are warranted to support our results.
Conclusion
This study demonstrates that higher peak values of SBP in the first 24 hours after MT in patients with acute ischemic stroke due to large vessel anterior cerebral circulation occlusion are associated with worse functional outcomes at 90 days and greater severity of hemorrhagic complications within 48 hours after MT. Further prospective studies are warranted to identify whether SBP is a therapeutic target to improve outcomes or merely a marker of poor outcome in stroke patients treated with MT.
Disclosures
Volpi is part of the speakers’ bureau in Avanir Pharmaceuticals Inc, and Johnson & Johnson. He also serves as a consultant for Avanir Pharmaceuticals Inc. He holds stock in DiaMedica Therapeutics Inc. Froehler is a consultant for Medtronic Inc, and his research is funded by Stryker Neurovascular, Microvention Inc, Medtronic Inc, and Penumbra Inc. James holds stock in Remedy Pharmaceuticals, Inc.
Supporting information
Table S1. Odds Ratios* From Regression Analyses of Study Variables With 90‐Day mRS
Table S2. Odds Ratios* From Regression Analyses of Study Variables With Hemorrhagic Complications
Table S3. Adjusted Odds Ratios* From Multivariable Ordinal Regression Analyses† of 90‐Day mRS With Blood Pressure Parameters After Multiple Imputations of the 38 Missing mRS Values (Total n=228)
Table S4. Adjusted Odds Ratios* From Backward, Stepwise Multivariable Ordinal Regression Analyses of Study Outcomes With Systolic (SBP), Diastolic (DBP), and Mean Arterial (MAP) Blood Pressure Parameters
Figure S1. Mean values of maximum, minimum, and average diastolic (A, B, and C; DBP) and mean arterial (D, E, and F; MAP) blood pressure are plotted with 90‐day modified Rankin scale (mRS). Error bar represent SEMs.
Figure S2. Mean values of maximum, minimum, and average diastolic (A, B, and C; DBP) and mean arterial (D, E, and F; MAP) blood pressure are plotted with hemorrhagic complications. Hemorrhagic complications are graded based on their severity: none, asymptomatic (asymp.), and symptomatic (symp.) hemorrhages. Error bar represents SEMs.
Figure S3. In (A), mean minimum diastolic blood pressure (DBP) in recanalized (mTICI 2b‐3) and in (B), maximum mean arterial pressure (MAP) in nonrecanalized (mTICI 0‐2a) patients are plotted with hemorrhagic complications. Hemorrhagic complications are graded based on their severity: none, asymptomatic (asymp.), and symptomatic (symp.) hemorrhages. Error bar represents SEMs.
(J Am Heart Assoc. 2017;6:e006167 DOI: 10.1161/JAHA.117.006167.)28522673
Results of this study were partially presented as an abstract/poster presentation at the International Stroke Conference; February 22, 2017, in Houston, TX.
References
- 1. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; Investigators SP . Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 2. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M, Millan M, Urra X, Cardona P, Lopez‐Cancio E, Tomasello A, Castano C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez‐Perez M, Goyal M, Demchuk AM, von Kummer R, Gallofre M, Davalos A; Investigators RT . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; Investigators ET . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 4. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; Investigators E‐I . Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 5. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama a Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg‐Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; Investigators MC . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 6. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta‐analysis. Stroke. 2007;38:967–973. [DOI] [PubMed] [Google Scholar]
- 7. Lee KY, Han SW, Kim SH, Nam HS, Ahn SW, Kim DJ, Seo SH, Kim DI, Heo JH. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre‐ and post‐thrombolytic angiography in acute ischemic stroke patients. Stroke. 2007;38:192–193. [DOI] [PubMed] [Google Scholar]
- 8. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, Watson T, Goyal M, Demchuk AM. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real‐world experience and a call for action. Stroke. 2010;41:2254–2258. [DOI] [PubMed] [Google Scholar]
- 9. Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez‐Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW; Investigators C . Ultrasound‐enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. [DOI] [PubMed] [Google Scholar]
- 10. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H; American Heart Association Stroke C, Council on Cardiovascular N, Council on Peripheral Vascular D, Council on Clinical C . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 11. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, Mocco J, Wardlaw JM, Barnwell SL, Jovin TG, Linfante I, Siddiqui AH, Alexander MJ, Hirsch JA, Wintermark M, Albers G, Woo HH, Heck DV, Lev M, Aviv R, Hacke W, Warach S, Broderick J, Derdeyn CP, Furlan A, Nogueira RG, Yavagal DR, Goyal M, Demchuk AM, Bendszus M, Liebeskind DS; Cerebral Angiographic Revascularization Grading C, group SRw, Force STiCIT . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke. 2007;38:3055–3062. [DOI] [PubMed] [Google Scholar]
- 13. Bodner TE. What improves with increased missing data imputations? Struct Equ Modeling. 2008;15:651–675. [Google Scholar]
- 14. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Peng Y, Fan H, Chen M, Xu T, Zhang Y. Blood pressure and early clinical outcome among acute ischemic stroke patients. Can J Neurol Sci. 2011;38:225–229. [DOI] [PubMed] [Google Scholar]
- 16. Wu W, Huo X, Zhao X, Liao X, Wang C, Pan Y, Wang Y, Wang Y; Investigators T‐C . Relationship between blood pressure and outcomes in acute ischemic stroke patients administered lytic medication in the Tims‐China Study. PLoS One. 2016;11:e0144260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomii Y, Toyoda K, Suzuki R, Naganuma M, Fujinami J, Yokota C, Minematsu K. Effects of 24‐hour blood pressure and heart rate recorded with ambulatory blood pressure monitoring on recovery from acute ischemic stroke. Stroke. 2011;42:3511–3517. [DOI] [PubMed] [Google Scholar]
- 18. Tien YT, Chang MH, Lee YS, Liaw YF, Chen PL. Pulse blood pressure correlates with late outcome in acute ischemic stroke without significant culprit artery stenosis. J Stroke Cerebrovasc Dis. 2016;25:1229–1234. [DOI] [PubMed] [Google Scholar]
- 19. Serrano‐Ponz M, Rodrigo‐Gasque C, Siles E, Martinez‐Lara E, Ochoa‐Callejero L, Martinez A. Temporal profiles of blood pressure, circulating nitric oxide, and adrenomedullin as predictors of clinical outcome in acute ischemic stroke patients. Mol Med Rep. 2016;13:3724–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leonardi‐Bee J, Bath PM, Phillips SJ, Sandercock PA; Group ISTC . Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 21. Ishitsuka K, Kamouchi M, Hata J, Fukuda K, Matsuo R, Kuroda J, Ago T, Kuwashiro T, Sugimori H, Nakane H, Kitazono T. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension. 2014;63:54–60. [DOI] [PubMed] [Google Scholar]
- 22. Gill D, Cox T, Aravind A, Wilding P, Korompoki E, Veltkamp R, Kar A. A fall in systolic blood pressure 24 hours after thrombolysis for acute ischemic stroke is associated with early neurological recovery. J Stroke Cerebrovasc Dis. 2016;25:1539–1543. [DOI] [PubMed] [Google Scholar]
- 23. Geeganage C, Tracy M, England T, Sare G, Moulin T, Woimant F, Christensen H, De Deyn PP, Leys D, O'Neill D, Ringelstein EB, Bath PM. Relationship between baseline blood pressure parameters (including mean pressure, pulse pressure, and variability) and early outcome after stroke: data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST). Stroke. 2011;42:491–493. [DOI] [PubMed] [Google Scholar]
- 24. Castillo J, Leira R, Garcia MM, Serena J, Blanco M, Davalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–526. [DOI] [PubMed] [Google Scholar]
- 25. Butcher K, Christensen S, Parsons M, De Silva DA, Ebinger M, Levi C, Jeerakathil T, Campbell BC, Barber PA, Bladin C, Fink J, Tress B, Donnan GA, Davis SM; Investigators E . Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke. 2010;41:72–77. [DOI] [PubMed] [Google Scholar]
- 26. Berge E, Cohen G, Lindley RI, Sandercock P, Wardlaw JM, Sandset EC, Whiteley W. Effects of blood pressure and blood pressure‐lowering treatment during the first 24 hours among patients in the third international stroke trial of thrombolytic treatment for acute ischemic stroke. Stroke. 2015;46:3362–3369. [DOI] [PubMed] [Google Scholar]
- 27. Bentsen L, Ovesen C, Christensen AF, Christensen H. Does the admission blood pressure associate with short‐ and long term outcome in stroke patients treated with thrombolysis? A single centre study. Int J Hypertens. 2013;2013:610353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, Lees KR, Toni D; Investigators S . Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke‐International Stroke Thrombolysis Register (SITS‐ISTR). Stroke. 2009;40:2442–2449. [DOI] [PubMed] [Google Scholar]
- 29. Martins AI, Sargento‐Freitas J, Silva F, Jesus‐Ribeiro J, Correia I, Gomes JP, Aguiar‐Goncalves M, Cardoso L, Machado C, Rodrigues B, Santo GC, Cunha L. Recanalization modulates association between blood pressure and functional outcome in acute ischemic stroke. Stroke. 2016;47:1571–1576. [DOI] [PubMed] [Google Scholar]
- 30. Lee M, Ovbiagele B, Hong KS, Wu YL, Lee JE, Rao NM, Feng W, Saver JL. Effect of blood pressure lowering in early ischemic stroke: meta‐analysis. Stroke. 2015;46:1883–1889. [DOI] [PubMed] [Google Scholar]
- 31. Robinson TG, Potter JF, Ford GA, Bulpitt CJ, Chernova J, Jagger C, James MA, Knight J, Markus HS, Mistri AK, Poulter NR; Investigators C . Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post‐Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded‐endpoint trial. Lancet Neurol. 2010;9:767–775. [DOI] [PubMed] [Google Scholar]
- 32. Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, Jagger C. Controlling hypertension and hypotension immediately post‐stroke (CHHIPS): a randomised, placebo‐controlled, double‐blind pilot trial. Lancet Neurol. 2009;8:48–56. [DOI] [PubMed] [Google Scholar]
- 33. Bath PM, Woodhouse L, Scutt P, Krishnan K, Wardlaw JM, Bereczki D, Sprigg N, Berge E, Beridze M, Caso V, Chen C, Christensen H, Collins R, El Etribi A, Laska AC, Lees KR, Ozturk S, Phillips S, Pocock S, de Silva HA, Szatmari S, Utton S. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial‐factorial randomised controlled trial. Lancet. 2015;385:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ankolekar S, Fuller M, Cross I, Renton C, Cox P, Sprigg N, Siriwardena AN, Bath PM. Feasibility of an ambulance‐based stroke trial, and safety of glyceryl trinitrate in ultra‐acute stroke: the rapid intervention with glyceryl trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke. 2013;44:3120–3128. [DOI] [PubMed] [Google Scholar]
- 35. Sandset EC, Bath PM, Boysen G, Jatuzis D, Korv J, Luders S, Murray GD, Richter PS, Roine RO, Terent A, Thijs V, Berge E; Group SS . The angiotensin‐receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo‐controlled, double‐blind trial. Lancet. 2011;377:741–750. [DOI] [PubMed] [Google Scholar]
- 36. Oh MS, Yu KH, Hong KS, Kang DW, Park JM, Bae HJ, Koo J, Lee J, Lee BC; Valsartan Efficacy oN modesT blood pressUre REduction in acute ischemic stroke (VENTURE) study group . Modest blood pressure reduction with valsartan in acute ischemic stroke: a prospective, randomized, open‐label, blinded‐end‐point trial. Int J Stroke. 2015;10:745–751. [DOI] [PubMed] [Google Scholar]
- 37. He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, Tong W, Liu C, Xu T, Ju Z, Peng Y, Peng H, Li Q, Geng D, Zhang J, Li D, Zhang F, Guo L, Sun Y, Wang X, Cui Y, Li Y, Ma D, Yang G, Gao Y, Yuan X, Bazzano LA, Chen J. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311:479–489. [DOI] [PubMed] [Google Scholar]
- 38. Powers WJ. Acute hypertension after stroke: the scientific basis for treatment decisions. Neurology. 1993;43:461–467. [DOI] [PubMed] [Google Scholar]
- 39. Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. [DOI] [PubMed] [Google Scholar]
- 40. Olsen TS, Bruhn P, Oberg RG. Cortical hypoperfusion as a possible cause of ‘subcortical aphasia’. Brain. 1986;109(Pt 3):393–410. [DOI] [PubMed] [Google Scholar]
- 41. Jusufovic M, Sandset EC, Bath PM, Karlson BW, Berge E. Effects of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis. Int J Stroke. 2015;10:354–359. [DOI] [PubMed] [Google Scholar]
- 42. Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–57. [DOI] [PubMed] [Google Scholar]
- 43. Nour M, Scalzo F, Liebeskind DS. Ischemia‐reperfusion injury in stroke. Interv Neurol. 2013;1:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zaidi SF, Aghaebrahim A, Urra X, Jumaa MA, Jankowitz B, Hammer M, Nogueira R, Horowitz M, Reddy V, Jovin TG. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43:3238–3244. [DOI] [PubMed] [Google Scholar]
- 45. Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH, Hirsch JA, Gonzalez RG. Infarct volume is a pivotal biomarker after intra‐arterial stroke therapy. Stroke. 2012;43:1323–1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Odds Ratios* From Regression Analyses of Study Variables With 90‐Day mRS
Table S2. Odds Ratios* From Regression Analyses of Study Variables With Hemorrhagic Complications
Table S3. Adjusted Odds Ratios* From Multivariable Ordinal Regression Analyses† of 90‐Day mRS With Blood Pressure Parameters After Multiple Imputations of the 38 Missing mRS Values (Total n=228)
Table S4. Adjusted Odds Ratios* From Backward, Stepwise Multivariable Ordinal Regression Analyses of Study Outcomes With Systolic (SBP), Diastolic (DBP), and Mean Arterial (MAP) Blood Pressure Parameters
Figure S1. Mean values of maximum, minimum, and average diastolic (A, B, and C; DBP) and mean arterial (D, E, and F; MAP) blood pressure are plotted with 90‐day modified Rankin scale (mRS). Error bar represent SEMs.
Figure S2. Mean values of maximum, minimum, and average diastolic (A, B, and C; DBP) and mean arterial (D, E, and F; MAP) blood pressure are plotted with hemorrhagic complications. Hemorrhagic complications are graded based on their severity: none, asymptomatic (asymp.), and symptomatic (symp.) hemorrhages. Error bar represents SEMs.
Figure S3. In (A), mean minimum diastolic blood pressure (DBP) in recanalized (mTICI 2b‐3) and in (B), maximum mean arterial pressure (MAP) in nonrecanalized (mTICI 0‐2a) patients are plotted with hemorrhagic complications. Hemorrhagic complications are graded based on their severity: none, asymptomatic (asymp.), and symptomatic (symp.) hemorrhages. Error bar represents SEMs.


