Introduction
Hypertrophic cardiomyopathy (HCM) is a complex and heterogeneous disease with different anatomical variants, physiologic manifestations, and genetic underpinnings. Even asymptomatic patients with HCM are at potential risk for sudden cardiac death and require risk stratification and consideration of an implantable cardioverter‐defibrillator for primary or secondary prophylaxis. However, the progression to symptomatic heart failure depends most often on upper septal hypertrophy with systolic anterior motion of the anterior mitral valve leaflet causing left ventricular outflow tract (LVOT) obstruction, elevated gradients, mitral regurgitation, and often atrial fibrillation. Symptoms in patients with LVOT obstruction usually manifest as exertional dyspnea or chest pain, and β‐blockers or calcium channel blockers are the mainstay of medical therapy to reduce LV contractile forces and associated LVOT gradients. When symptoms are refractory to medical management, surgical myectomy or alcohol septal ablation (ASA) is considered, and both have been shown to be effective in carefully selected patients.1 Nonrandomized studies have determined that patients after surgical myectomy or ASA have an expected long‐term survival that is comparable to that of the general population, and superior to similar patients who do not undergo surgery.2, 3 Historically though, these procedures have been reserved for patients with severe symptoms and LVOT gradients ≥50 mm Hg, with the primary objective of symptom improvement, as reflected in major society guidelines.4, 5
In the current study by Veselka and colleagues in this issue of JAHA, the authors report the retrospective outcomes of 161 patients enrolled in the Euro‐ASA registry from 1996 to 2016 who underwent ASA despite being classified as only having New York Heart Association (NYHA) Class II symptoms.6 All patients had LVOT obstruction with gradients ≥50 mm Hg at rest or after provocation, and none reported angina or syncope. Importantly though, all patients reported symptoms that were refractory to medical therapy and a significantly reduced quality of life because of dyspnea. Reassuringly, the authors demonstrate that ASA in this population was safe with a 0.6% mortality at 30 days and a 9.4% rate of permanent pacemaker implantation, and show that long‐term mortality was comparable to a matched cohort in the general population. Patients in the study were followed for 895 patient‐years (median 4.8 years) of follow‐up. ASA was shown to be effective, with 88% of patients experiencing sustained resting gradient reductions ≤30 mm Hg at last follow‐up with only 9.3% of patients requiring repeat septal reduction (only 4 of whom went on to surgical myectomy). Importantly, the patients' symptoms seemingly improved, with 69% reporting NYHA Class I symptoms at last follow‐up, and only 2% of patients progressing to NYHA Class III symptoms. Therefore, the authors conclude that ASA was safe and effective in treating patients with symptomatic, medically refractory HCM with significant resting obstruction and NYHA Class II symptoms in this retrospective analysis.
There are several limitations of this retrospective study that should be mentioned. The first is that the authors do not have data on the use of provocative maneuvers to measure LVOT gradients postablation. The second is that we do not have data on the use of medications before or after ASA, which is a potential confounding variable, although we would have to assume that all patients were being treated with maximally tolerated doses. Finally, clinical improvement is only reported based on NYHA Class without other, objective measurements of quality of life or exercise capacity being available.
So how should the results of the current study be interpreted and applied? First, it must be noted that septal reduction procedures, either surgical or percutaneous, have not been shown in a randomized clinical trial to reduce long‐term mortality, and there are no data that support these invasive procedures in asymptomatic patients.4 There are, however, data to show that LVOT obstruction increases mortality risk in patients with hypertrophic obstructive cardiomyopathy and that after myectomy, mortality is similar to that of patients without LVOT obstruction, suggesting potential mortality benefit of myectomy. However, because of this type of indirect evidence, the primary objective of any intervention is to improve symptoms or quality of life. The current study is consistent with this recommendation, as all patients had both objectively severe gradients, and symptoms that significantly impacted their quality of life. In fact, the severity of obstruction in this cohort was quite high with a mean resting LVOT gradient of 63±32 mm Hg. Thus, despite being labeled as NYHA Class II, it is perhaps somewhat unfair to consider these patients mildly symptomatic. By definition, Class II symptoms are described as a slight limitation of physical activity, comfortable at rest, but ordinary physical activity results in symptoms of heart failure.7 Comparatively, Class III symptoms are defined as marked limitation of physical activity, comfortable at rest, but less than ordinary activity causes symptoms of heart failure.
There are obvious, potential limitations to using NYHA Class alone in evaluating patients with heart failure. Self‐reported symptoms are inherently subjective, and it is well known that some patients under‐report the severity of their limitations, especially when a decline is experienced gradually or over a long period of time. Thus, it is no surprise that society guidelines do not place a specific requirement for NYHA Class as a criterion for considering septal reduction techniques, and the severity of symptoms is left to the clinician to determine based on all available tools. As such, there has been much interest in studying more granular or more objective tools to help further stratify and follow patients with heart failure over time (Table). Cardiopulmonary exercise testing has been an important tool in the evaluation of patients with valve disease and hypertrophic obstructive cardiomyopathy when more mild symptoms are reported. In 1 such study, peak VO2 had a significant association with NYHA class, but the authors noted considerable overlap between NYHA classes I through III, indicating that many patients with significantly abnormal VO2 were classified as NYHA Class I or II only.8 Other parameters have been shown to be predictive of outcomes in patients with NYHA Class I and II symptoms undergoing exercise stress testing including achieved metabolic equivalents and heart rate recovery.9 The 6‐Minute Walk Test is an objective measure of exercise capacity that can be used, and is especially effective in following patients over time.10 Other tools have been developed to better quantify the subjective effects of hypertrophic cardiomyopathy on quality of life such as the Kansas City Cardiomyopathy Questionnaire, which may be useful in this population as poor quality of life was cited as a strong factor in patients undergoing ASA in the current study.11, 12 Finally, even a simple measurement of plasma natriuretic peptide (brain natriuretic peptide) has been shown to correlate with both NYHA Class and mortality in patients with HCM, and can be another useful tool in patients with questionable symptoms.13
Table 1.
Methods for Evaluating Intermediately Symptomatic Patients With Hypertrophic Cardiomyopathy and Obstruction
| Method | Description |
|---|---|
| New York Heart Association (NYHA) Class |
Class I: No limitation of physical activity. Class II: Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, or dyspnea. Class III: Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea. Class IV: Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases |
| Kansas City Cardiomyopathy Questionnaires (KCCQ) | 23‐item, self‐administered questionnaire. Clinical score includes physical limitations and total symptom frequency and burden. Overall Score includes the clinical score plus measures of the stability of symptoms, self‐efficacy or perceived ability to manage symptoms as an outpatient, quality of life, and social limitations. Also available as a short form |
| Cardiopulmonary Exercise Testing | Symptom‐limited treadmill exercise with respiratory gas analysis. Peak VO2 is measured over 30‐s intervals during the test and ventilatory threshold (the point where body demands exceed the capacity for aerobic metabolism) is calculated |
| 6‐Minute Walk Test | Measures distance walked in a 6‐minute time period |
| NT‐Pro‐BNP | Blood test that is elevated in a variety of heart failure conditions |
NT‐Pro‐BNP indicates N‐terminal pro‐brain natriuretic peptide.
Thus, in considering treatments for patients with HCM, we advocate for a comprehensive approach to symptom evaluation using both subjective and objective tools (Figure). Patients with seemingly intermediate symptoms should be evaluated by additional methods including cardiopulmonary exercise testing, 6‐Minute Walk Testing, measurement of natriuretic peptide, and/or completion of the Kansas City Cardiomyopathy Questionnaire. When these tests reveal limitations that are out of proportion to patient's self‐reported symptoms, consideration could be made to more aggressive medical management and possibly referral for septal reduction techniques. Careful tailoring of the procedural approach for such patients requires extensive expertise with these techniques, and a heart team approach involving the input of cardiac surgeons, interventional cardiologists, cardiac anesthesiologists, and cardiovascular imaging specialists is paramount to appropriate patient selection. Furthermore, the current American College of Cardiology/American Heart Association valve guidelines advocate for establishment of Heart Valve Centers of Excellence for treating complex patients, a recommendation that would certainly be relevant to the management of complex patients with HCM.14 In addition to demonstrating adequate procedural volume, low procedural complication rates, and high rates of procedural success, centers of excellence would have a mandate for active participation in data registry reporting and quality improvement processes, which are increasingly important as procedures become applied to lower‐risk populations. Just as utilization of a more comprehensive approach to preablative monitoring and determining candidacy for septal reduction therapies is warranted, it is important to follow patients after ASA or myectomy with similarly objective measures, including the use of standardized provocation techniques for measuring LVOT gradients, rather than relying on NYHA Class alone to demonstrate success.
Figure 1.
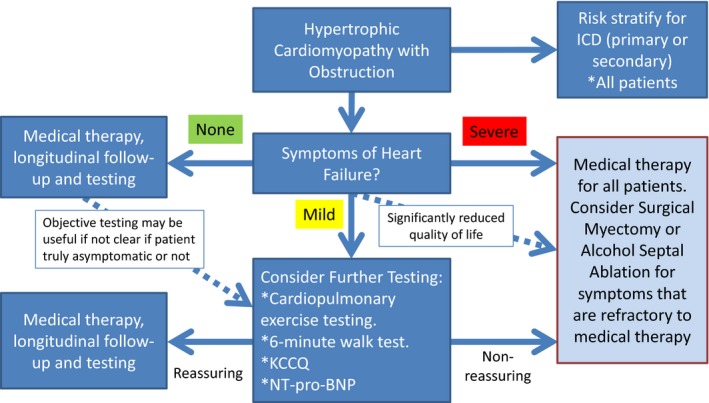
Algorithm for evaluating patients with hypertrophic cardiomyopathy with significant obstruction based on the severity of symptoms. ICD indicates implantable cardioverter defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
In conclusion, we agree with the authors that carefully selected patients with HCM who have significant obstructive pathology and symptoms that are refractory to medical therapy appear to benefit from alcohol septal ablation, and that such therapy is both safe and effective in reducing gradients and progression of symptoms. Importantly, this benefit seems to extend to the cohort of patients with seemingly more mild (NYHA Class II) symptoms, and as a result, we advocate for comprehensive evaluation of patients with hypertrophic obstructive cardiomyopathy using both subjective and objective measurements to determine the extent of symptoms and impact on quality of life, before making decisions about septal reduction therapies. Further study is needed to determine which of these tools are most effective in determining who might benefit from septal reduction therapies.
Disclosures
None.
J Am Heart Assoc. 2017;6:e006292 DOI: 10.1161/JAHA.117.006292.28512113
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Agarwal S, Tuzcu EM, Desai MY, Smedira N, Lever HM, Lytle BW, Kapadia SR. Updated meta‐analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;55:823–834. [DOI] [PubMed] [Google Scholar]
- 2. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. [DOI] [PubMed] [Google Scholar]
- 3. Sorajja P, Ommen SR, Holmes DR Jr, Dearani JA, Rihal CS, Gersh BJ, Lennon RJ, Nishimura RA. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2012;126:2374–2380. [DOI] [PubMed] [Google Scholar]
- 4. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 5. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. [DOI] [PubMed] [Google Scholar]
- 6. Veselka J, Faber L, Liebregts M, Cooper R, Januska J, Krejci J, Bartel T, Dabrowski M, Hansen P, Almaas V, Seggewiss H, Horstkotte D, Adlova R, Bundgaard H, Berg J, Stables R, Jensen M. Outcome of alcohol septal ablation in mildly symptomatic patients with hypertrophic obstructive cardiomyopathy: a long‐term follow‐up study based on the Euro‐ASA registry. J Am Heart Assoc. 2017;6:e005735 DOI: 10.1161/JAHA.117.005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 8. Sharma S, Elliott P, Whyte G, Jones S, Mahon N, Whipp B, McKenna WJ. Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am J Cardiol. 2000;86:162–168. [DOI] [PubMed] [Google Scholar]
- 9. Desai MY, Bhonsale A, Patel P, Naji P, Smedira NG, Thamilarasan M, Lytle BW, Lever HM. Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long‐term outcomes. JACC Cardiovasc Imaging. 2014;7:26–36. [DOI] [PubMed] [Google Scholar]
- 10. Pollentier B, Irons SL, Benedetto CM, Dibenedetto AM, Loton D, Seyler RD, Tych M, Newton RA. Examination of the six minute walk test to determine functional capacity in people with chronic heart failure: a systematic review. Cardiopulm Phys Ther J. 2010;21:13–21. [PMC free article] [PubMed] [Google Scholar]
- 11. Huff CM, Turer AT, Wang A. Correlations between physician‐perceived functional status, patient‐perceived health status, and cardiopulmonary exercise results in hypertrophic cardiomyopathy. Qual Life Res. 2013;22:647–652. [DOI] [PubMed] [Google Scholar]
- 12. Hawwa N, Vest AR, Kumar R, Lahoud R, Young JB, Wu Y, Gorodeski EZ, Cho L. Comparison between the Kansas City cardiomyopathy questionnaire and New York Heart Association in assessing functional capacity and clinical outcomes. J Card Fail. 2017;23:280–285. [DOI] [PubMed] [Google Scholar]
- 13. Geske JB, McKie PM, Ommen SR, Sorajja P. B‐type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61:2456–2460. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM III, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017. 2017. Available at: http://circ.ahajournals.org/content/early/2017/03/14/CIR.0000000000000503. Accessed May 11, 2017. [DOI] [PubMed] [Google Scholar]


