Abstract
Human papillomavirus (HPV) has been identified as the primary etiologic factor of cervical cancer, and subsets of anogenital and oropharyngeal cancers. HPV18 is the second most prevalent high-risk HPV type after HPV16. Furthermore, HPV18 is responsible for approximately 12% of cervical squamous cell carcinoma and 37% of cervical adenocarcinoma cases worldwide. In this study, we aimed to characterize the HPV18-E6-specific epitope and establish an HPV18 animal tumor model to evaluate the E6-specific immune response induced by our DNA vaccine. We vaccinated naïve C57BL/6 mice with a prototype DNA vaccine, pcDNA3-HPV18-E6, via intramuscular injection followed by electroporation, and analyzed the E6-specific CD8+ T cell responses by flow cytometry using a reported T cell epitope. We then characterized the MHC restriction element for the characterized HPV18-E6 epitope. Additionally, we generated an HPV18-E6-expressing tumor cell line to study the antitumor effect mediated by E6-specific immunity. We observed a robust HPV18-E6aa67-75 peptide-specific CD8+ T cell response after vaccination with pcDNA3-HPV18-E6. Further characterization demonstrated that this epitope was mainly restricted by H-2Kb, but was also weakly presented by HLA-A*0201, as previously reported. We observed that vaccination with pcDNA3-HPV18-E6 significantly inhibited the growth of HPV18-E6-expressing tumor cells, TC-1/HPV18-E6, in mice. An antibody depletion study demonstrated that both CD4+ and CD8+ T cells are necessary for the observed antitumor immunity. The characterization of HPV18-E6-specific T cell responses and the establishment of HPV18-E6-expressing tumor cell line provide infrastructures for further development of HPV18-E6 targeted immunotherapy.
Keywords: Human papillomavirus 18, E6 oncoprotein, DNA vaccine, immunotherapy, HPV18-E6 tumor model
Introduction
Human papillomavirus (HPV) is an etiological factor for several gynecological and head and neck malignancies, including cervical, penile, vaginal, anal, vulval and oropharyngeal cancer [1–4]. Unlike low-risk HPV types, high-risk HPV types, such as types 16 and 18, have high oncogenic potential and are often correlated with invasive cervical cancer [5, 6]. After HPV16, HPV18 is the second most carcinogenic HPV type. Together, HPV16 and 18 are responsible for causing nearly 70% of cervical cancer cases globally [7].
The most common histological type of cervical cancer is squamous cell carcinoma (SCC), accounting for around 80–90% of cases. Adenocarcinoma (ADC) is a less common cervical cancer histological type, contributing to around 10–20% of cases; however, ADC cases of the cervix have been on the rise over the past two decades [8]. Importantly, HPV18 is responsible for roughly 12% of SCC and 37% of ADC cases [9]. Thus, HPV18 and HPV18-associated cancers represent a significant global burden, and the development of treatment methods against HPV18-associated diseases is necessary.
While prophylactic vaccines have efficaciously provided protection against HPV infection in healthy patients, they are unable to clear pre-existing HPV infections or HPV-associated lesions [10]. Unlike prophylactic vaccines, therapeutic vaccines enhance the cell-mediated immune response to target and kill infected cells [11, 12]. Currently, however, no HPV18 tumor models are available to evaluate the efficacy of potential immunotherapies against HPV18-associated malignancies.
In this study, we developed and investigated the immunogenicity of a prototype therapeutic HPV18 DNA vaccine targeting HPV18-E6. We also characterized the HPV18-E6-specific CD8+ T cell responses elicited by our DNA vaccine in both wild type and HLA-A*0201 transgenic C57BL/6 mice. In addition, we established an HPV18-E6-expressing tumor cell line, TC-1/HPV18-E6, and validated the immunogenicity and antitumor effect of our vaccine against the TC-1/HPV18-E6 tumor model.
Materials and Methods
Mice
5- to 8-week-old female C57BL/6 mice were purchased from Charles River Laboratories (Frederick, MD). HLA-A*0201/Dd (AAD) transgenic mice under C57BL/6 background were kindly provided by Professor Victor Engelhard at the University of Virginia Health Sciences Center. These transgenic mice express chimeric HLA class I molecules comprising the α-1 and α-2 domains of HLA-A*0201 and the α-3 domain of H-2Dd. All mice were maintained at the Johns Hopkins University School of Medicine Oncology Animal Facility under specific pathogen-free conditions. All experimental procedures followed an approved animal protocol and were in accordance with recommendations for the proper use and care of laboratory animals.
Peptides, Antibodies
HPV18-E6aa67-75 peptide, KCIDFYSRI, was synthesized by GenScript (Piscataway, NJ). The peptide’s identity and purity were validated and confirmed via mass spectrometric analysis and high-performance liquid chromatography, respectively. FITC-conjugated rat anti-mouse IFN-γ (clone XMG1.2) and PE-conjugated anti-mouse CD8a (clone 53.6.7) antibodies were purchased from BD Pharmingen (San Diego, CA). Purified rat anti-mouse monoclonal antibodies against CD4 (clone GK1.5) and CD8 (clone 2.43) were purchased from BioXCell (West Lebanon, NH).
Cell Lines
HPV16-E6- and E7-expressing TC-1 [13] and MusPV-E6-expressing TC-1 tumor cells [14] have been previously described. To generate a tumor cell line that expresses HPV18-E6, TC-1 cells were transduced with lentivirus expressing HPV18-E6 and GFP. The cells were then sorted based on GFP expression (surrogate marker for HPV18-E6 expression) with a flow cytometry sorter. The cells were maintained in RPMI medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum. C1R is an Epstein-Barr virus-transformed B-cell cell line that has lost most of its HLA class I alleles, expressing only Cw0401 and trace amounts of B3503 [15]. The C1R murine MHC class I transfectants C1R/Db (which express H2-Db) and C1R/Kb (which express H2-Kb) were kindly provided by Professor Michael Edidin (Johns Hopkins University, Baltimore, MD). To generate a C1R cell line that expresses HLA-A*0201/Dd (C1R/AAD), C1R cells were transduced with retroviruses expressing AAD and sorted based on AAD expression with flow cytometry. These cell lines were cultured in RPMI-1640 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, 5×10−5 M β-mercaptoethanol, and 10% fetal bovine serum.
DNA Vaccine
To generate the DNA vaccine construct, the HPV18-E6 sequence was synthesized by GenScript and cloned into the pcDNA3 vector to generate the pcDNA3-HPV18-E6 vaccine. Plasmid construct was confirmed using DNA sequencing and the DNA was prepared using an endotoxin-free kit (QIAGEN, Valencia, CA).
DNA Vaccination
For intramuscular (IM) injection followed by electroporation, 50 μL of DNA was prepared and mice were immunized in the leg via IM (biceps femoris muscle) injection followed by electroporation with an ECM830 Square Wave Electroporation System (BTX Harvard Apparatus company, Holliston, MA, USA).
Western blot analysis for the detection of HPV18 E6 expression
To generate polyclonal antibodies specific to the HPV18-E6 protein, mice were vaccinated with pcDNA3-HPV18-E6 DNA vaccine. The mice received two more boosts at one-week intervals. One week after the last vaccination, serum from the vaccinated mice was collected and stored at −20°C until the assay. Cell lysates were prepared from either TC-1 or TC-1/HPV18-E6 cells, separated by SDS-PAGE and blotted with the polyclonal antibody against HPV18-E6. β-actin was used as protein loading control.
Intracellular Cytokine Staining
To detect HPV18-E6-specific CD8+ T cells, splenocytes (5×106 cells) from each experimental group were incubated for 20 hours with 1 μg/mL of indicated peptide in the presence of 1 μl/mL of GolgiPlug (BD Pharmingen, San Diego, CA). To determine the MHC restriction element for HPV18-E6aa67-75 peptide recognized by CD8+ T cells, C1R, C1R/Db, C1R/Kb, or C1R/AAD cells were loaded with HPV18-E6aa67-75 peptide and irradiated with a γ irradiator. After wash, these cells were co-cultured with splenocytes at an E:T ratio of 10 in the presence of GolgiPlug (1 μl/mL) for 20 hours at 37°C. To determine whether TC-1/HPV18-E6 cells are able to activate HPV18-E6-specific CD8+ T cells generated by pcDNA3-HPV18-E6 DNA vaccination, irradiated TC-1/HPV18-E6 cells were co-cultured with splenocytes at an E:T ratio of 10 in the presence of GolgiPlug (1 μl/mL) for 20 hours at 37°C. After incubation, the stimulated splenocytes were collected, washed with FACS wash buffer (PBS containing 0.5% BSA), and stained with PE-conjugated anti-mouse CD8α antibody. The cells were fixed using the Cytofix/Cytoperm kit (BD Pharmingen, San Diego, CA). Intracellular IFN-γ was stained with FITC-conjugated anti-mouse IFN-γ antibody. After wash, the cells were acquired with the FACSCalibur flow cytometer and analyzed with CELLQuest Pro software (BD Bioscience, Mountain View, CA).
In vivo tumor protection experiment
To test whether the immune responses induced by pcDNA3-HPV18-E6 DNA vaccination could protect mice from HPV18-E6-expressing tumor challenge, mice (5 mice/group) were immunized intramuscularly three times with 50 μg/mouse of pcDNA3-HPV18-E6 or pcDNA3 followed by electroporation with one-week intervals. The mice were challenged subcutaneously with 1×105/mouse of TC-1/HPV18-E6 cells one week after the last vaccination. Tumor growth was monitored by measurement with a digital caliper twice a week. Survival of the mice was monitored every other day.
In vivo tumor treatment experiment
To test whether the immune responses induced by pcDNA3-HPV18-E6 DNA vaccination could have therapeutic effects on established HPV18-E6-expressing tumors, mice (5 mice/group) were challenged subcutaneously with 7.5×104/mouse of TC-1/HPV-18-E6 cells. On day 3, the mice were immunized intramuscularly with 50 μg/mouse of pcDNA3-HPV18-E6 or pcDNA3 followed by electroporation three times with 4-day intervals. Tumor growth was monitored by measurement with a digital caliper twice a week. Survival of the mice was monitored every other day.
In vivo antibody depletion experiment
To determine the role of CD4+ or CD8+ T cell subsets in the protection against TC-1/HPV-18-E6 tumor cell challenge, mice were divided into 4 groups (10 mice/group). Three groups of mice were immunized intramuscularly with 50 μg/mouse of pcDNA3-HBV18-E6 followed by electroporation three times at one-week intervals. On the day of the third vaccination, one group of vaccinated mice was injected with anti-CD4 monoclonal antibody, and another group of immunized mice was injected with anti-CD8 monoclonal antibody through intraperitoneal injection (to deplete CD4+ or CD8+ T cell subsets, respectively). Both groups received injection for 3 days followed by injection once a week. 7 days after the last vaccination, all the mice were challenged subcutaneously with 1×105/mouse of TC-1/HPV18-E6 tumor cells. Tumor growth was monitored by measurement with a digital caliper twice a week. Survival of the mice was monitored every other day.
Statistical Analysis
Data expressed as mean ± standard deviation (SD) are representative of a minimum of two separate experiments. Comparisons between individual data points were made by two-tailed student’s t tests. Survival distributions for mice in different groups were compared through Kaplan-Meier curves and Log-rank tests. A p-value < 0.05 was considered statistically significant.
Results
Intramuscular administration of pcDNA3-HPV18-E6 DNA vaccine generates HPV18 E6-specific CD8+ T cell response in naïve mice
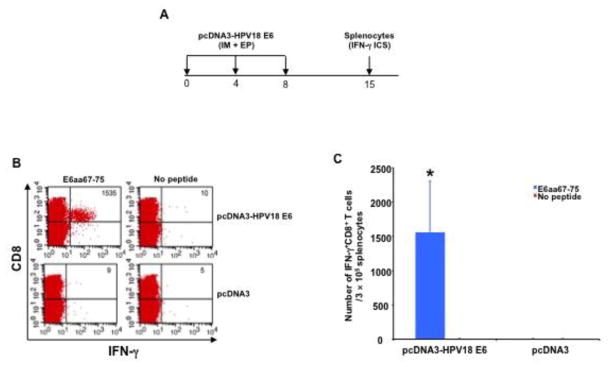
To evaluate the immunogenicity of the pcDNA3-HPV-18-E6 DNA vaccine, mice were vaccinated three times with IM pcDNA3-HPV18-E6 DNA or empty pcDNA3 (control) vector injection followed by electroporation. One week after the last vaccination, splenocytes were harvested, stimulated with a reported optimal HPV18-E6 epitope, HPV18-E6aa67-75 peptide [16], stained for surface CD8 and intracellular IFN-γ, and analyzed using flow cytometry (Figure 1A). Mice vaccinated with pcDNA3-HPV18-E6 generated a significant antigen-specific CD8+ T cell response while mice vaccinated with control vector did not generate any noticeable HPV18-E6 responses (Figure 1B–C).
Figure 1. Characterization of HPV18-E6 peptide-specific CD8+ T cell responses after DNA vaccination.
A. Schema of the experiment. Briefly, 5~8 weeks old female C57BL/6 mice (3 mice/group) were vaccinated with 50 μg/mouse of pcDNA3-HPV18-E6 or empty pcDNA3 vector DNA through IM injection followed by electroporation. The mice were boosted twice with the same regimen with 4-day intervals. 7 days after the last vaccination, splenocytes were harvested from the mice, stimulated with HPV18 E6aa67-75 peptide (1 μg/ml) at the presence of GolgiPlug (1 μl/ml) overnight at 37°C. The cells were then harvested, washed with PBS+0.5% BSA, surface stained with PE-conjugated anti-mouse CD8a antibody. After wash, the cells were then permeabilized, fixed and intracellularly stained with FITC-conjugated anti-mouse IFN-γ antibody. The cells were acquired with FACSCalibur flow cytometer and analyzed with CellQuest software. B. Representative flow cytometry data. C. Bar graph summary of the flow cytometry data. Data are represented as mean ± SD. p-values were calculated by 2-tailed Student’s t test. * = p<0.05.
Characterization of MHC class I restriction of HPV18 E6aa67-75-specific CD8+ T cell response in C57BL/6 mice
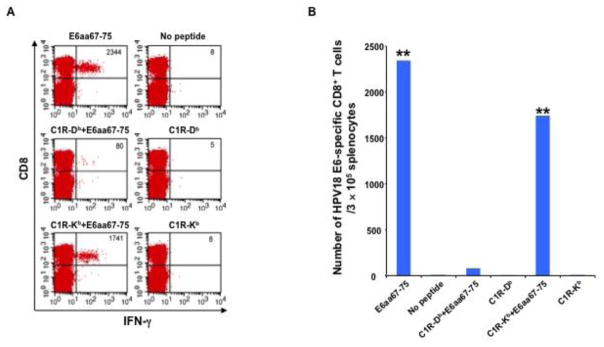
After confirming that HPV18-E6 DNA vaccination generates an E6aa67-75 epitope-specific CD8+ T cell response in naïve mice, we sought to determine the MHC class I restriction of this epitope. C57BL/6 mice received IM pcDNA3-HPV18-E6 vaccination followed by electroporation. One week after the last vaccination, splenocytes from mice were harvested, stimulated with 1) HPV18-E6aa67-75 peptides, or HPV18-E6aa67-75 peptide pulsed, irradiated 2) C1R/Db or 3) C1R/Kb cells, stained for surface CD8 and intracellular IFN-γ, and analyzed using flow cytometry. C1R/Kb cells could effectively present HPV18-E6aa67-75 peptide to activate E6-specific CD8+ T cells generated by pcDNA3-HPV18-E6 vaccine (Figure 2). In contrast, C1R/Db cells failed to present this peptide to activate vaccine-induced CD8+ T cells. These data demonstrated that the HPV18-E6aa67-75 epitope encoded in pcDNA3-HPV18-E6 vaccine sequence is presented through H2-Kb molecule in C57BL/6 mice.
Figure 2. Characterization of MHC class I restriction element for HPV18 E6aa67-75 peptide recognized by CD8+ T cells after DNA vaccination in C57BL/6 mouse.
5~8 weeks old female C57BL/6 mice were vaccinated with 30 μg/mouse of pcDNA3-HPV18-E6 DNA through IM injection followed by electroporation. The mice were boosted with the same regimen three times with 1-week interval. 7 days after the last vaccination, splenocytes were harvested from the mice, stimulated with either HPV18 E6aa67-75 peptide (1 μg/ml), or irradiated HPV18 E6aa67-75 peptide loaded C1R-Db, or C1R-Kb cells at the presence of GolgiPlug (1 μl/ml) overnight at 37°C. The cells were then collected, washed with PBS+0.5% BSA, surface stained with PE-conjugated anti-mouse CD8a antibody. After wash, the cells were then permeabilized, fixed and intracellularly stained with FITC-conjugated anti-mouse IFN-γ antibody. The cells were acquired with FACSCalibur flow cytometer and analyzed with CellQuest software. A. Representative flow cytometry data. B. Bar graph summary of the flow cytometry data. p-values were calculated by 2-tailed Student’s t test. ** = p<0.01.
Characterization of MHC class I restriction of HPV18 E6aa67-75-specific CD8+ T cell response in HLA-A*0201 transgenic C57BL/6 mice
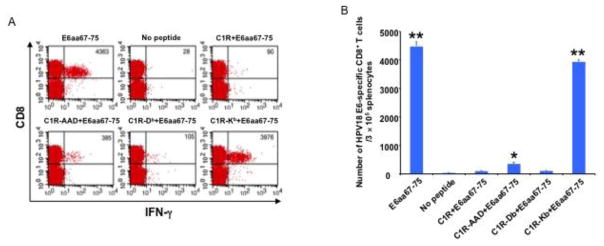
While the MHC class I restriction of HPV18-E6aa67-75 epitope in naïve C57BL/6 mice has not been previously explored, an earlier publication evaluated the MHC class I restriction of this epitope in HLA-A2/KB transgenic mice and determined that this peptide sequence binds mainly to HLA-A2 [17]. We demonstrated that pcDNA3-HPV-18-E6 vaccination generated E6aa67-75 epitope-specific CD8+ T cell response in naïve C57BL/6 mice (Figure 1). We also demonstrated that this epitope is presented through the H2-Kb molecule in naïve C57BL/6 mice (Figure 2). Thus, we sought to determine whether this epitope is also presented by HLA-A*0201 molecules. To do so, HLA-A*0201 transgenic C57BL/6 mice received IM pcDNA3-HPV18-E6 vaccination followed by electroporation. One week after the last vaccination, splenocytes from mice were harvested, stimulated with 1) HPV18-E6aa67-75 peptides, or HPV18-E6aa67-75 peptide pulsed, irradiated 2) C1R, 3) C1R/AAD, 4) C1R/Db, or 5) C1R/Kb cells, stained for surface CD8 and intracellular IFN-γ, and analyzed through flow cytometry. Splenocytes stimulated with HPV18-E6aa67-75 peptides or peptide-loaded C1R/Kb cells showed a strong E6aa67-75 specific CD8+ T cell response (Figure 3). Splenocytes stimulated with peptide-loaded C1R/AAD cells also showed a detectable peptide-specific CD8+ T cell response, while unstimulated splenocytes or splenocytes stimulated with peptide-loaded C1R or C1R/Db cells showed little to no detectable peptide-specific CD8+ T cell response.
Figure 3. Characterization of MHC class I restriction element for HPV18 E6aa67-75 peptide recognized by CD8+ T cells after DNA vaccination in HLA-A*0201 transgenic C57BL/6 mouse.
5~8 weeks old female HLA-A*0201 transgenic C57BL/6 mice were vaccinated with 50 μg/mouse of pcDNA3-HPV18-E6 DNA through IM injection followed by electroporation. The mice were boosted with the same regimen three times with 4-day interval. 7 days after the last vaccination, splenocytes were harvested from the mice, stimulated with either HPV18 E6aa67-75 peptide (1 μg/ml), or irradiated HPV18 E6aa67-75 peptide loaded C1R, or C1R-AAD, or C1R-Db, or C1R-Kb cells at the presence of GolgiPlug (1 μl/ml) overnight at 37°C. The cells were then collected, washed with PBS+0.5% BSA, surface stained with PE-conjugated anti-mouse CD8a antibody. After wash, the cells were then permeabilized, fixed and intracellularly stained with FITC-conjugated anti-mouse IFN-γ antibody. The cells were acquired with FACSCalibur flow cytometer and analyzed with CellQuest software. A. Representative flow cytometry data. B. Bar graph summary of the flow cytometry data. Data are represented as mean ± SD. p-values were calculated by 2-tailed Student’s t test. * = p<0.05; ** = p<0.01.
Establishment of HPV18 E6-expressing tumor model
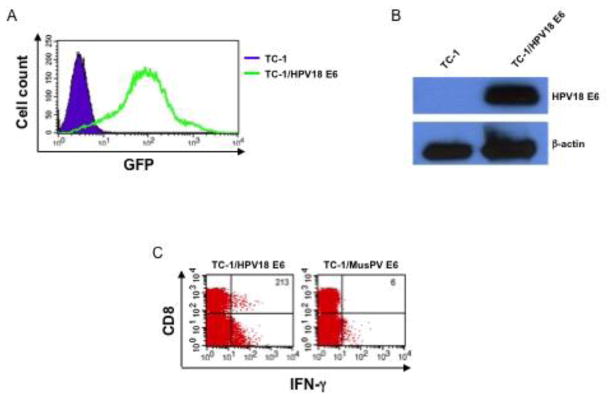
After demonstrating the ability of pcDNA3-HPV-18-E6 vaccine to generate an antigen-specific CD8+ T cell response in mice, we sought to determine whether this immune response could translate into antitumor effects. To evaluate the antitumor efficacy of the vaccine, we first generated a tumor model that expresses HPV18-E6 by transducing TC-1 cells with a plasmid encoding GFP and HPV18-E6. The expression of GFP by the transduced cell was evaluated using flow cytometry analysis as a surrogate marker for HPV18-E6 expression (Figure 4A). Western blots were then performed to confirm the expression of HPV18-E6 proteins by the transduced TC-1 cells (Figure 4B). In addition, we co-cultured the splenocytes from pcDNA3-HPV18-E6 vaccinated mice (Figure 1A) with HPV18-E6 or MusPV-E6 transduced TC-1 cells. Only the splenocytes co-cultured with HPV18-E6-transduced TC-1 cells generated a significant IFN-γ+ CD8+ T cell response (Figure 4C). Thus, we conclude that the HPV18-E6-transduced TC-1 tumor cell is a suitable model to evaluate the antitumor efficacy of pcDNA3-HPV-18-E6 vaccine.
Figure 4. Establishment of HPV18-E6-expressing tumor cell line.
HPV16-E6/E7-expressing murine tumor cell line, TC-1 was transduced with lentivirus expressing HPV18-E6 and GFP. The cells were then sorted based on GFP expression (surrogate marker for HPV18-E6 expression) with a flow cytometry sorter. A. Flow cytometry analysis of GFP expression by TC-1/HPV18-E6 tumor cells. B. Western blot analysis of HPV18-E6 protein expression by TC-1/HPV18-E6 tumor cells. Polyclonal antibody against HPV18-E6 was generated by vaccinated female C57BL/6 mice with pcDNA3-HPV18-E6 twice with 1-week interval. 7 days after the last vaccination, serum was collected from the immunized mice. Cell lysates were prepared from either TC-1 or TC-1/HPV18-E6 cells and separated by SDS-PAGE electrophoresis and blotted with the polyclonal antibody against HPV18-E6. β-actin was used as protein loading control. C. Characterization of HPV18-E6 CD8+ T cell-specific peptide presentation by TC-1/HPV18-E6 tumor cells. Splenocytes from the experiment described in Figure 1 were stimulated with irradiated TC-1/HPV18-E6, or TC-1/MusHPV-E6 at the presence of GolgiPlug (1 μl/ml) overnight at 37°C. The cells were then collected, washed with PBS+0.5% BSA, surface stained with PE-conjugated anti-mouse CD8a antibody followed by intracellular staining of IFN-γ. The cells were acquired with FACSCalibur flow cytometer and analyzed with CellQuest software.
HPV18-E6 DNA vaccination generates both protective and therapeutic antitumor effects against HPV18 E6-expressing tumor
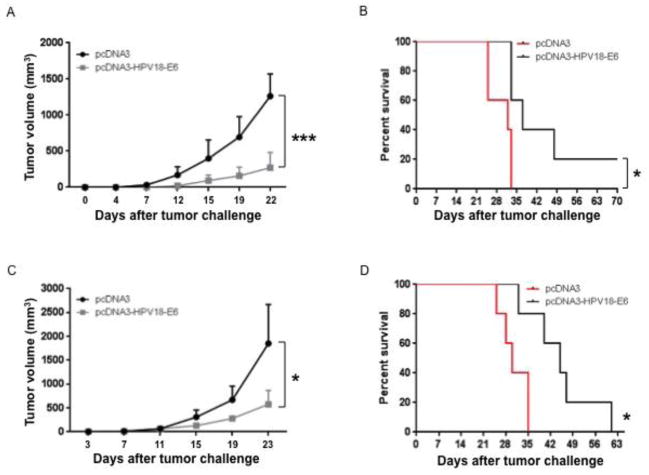
Next, we evaluated whether the HPV18-E6-specific CD8+ T cell responses generated by the pcDNA3-HPV18-E6 vaccine could prevent the growth of HPV-18-E6-expressing tumor. Mice were immunized with either pcDNA3-HPV18-E6 or control vector via IM injection followed by electroporation three times separated by one-week intervals. One week after the last vaccination, the mice were challenged subcutaneously with TC-1/HPV18-E6 tumor cells. We observed slower tumor growth rate and prolonged survival in mice vaccinated with pcDNA3-HPV18-E6 compared to those vaccinated with control (Figure 5A–B). To evaluate the therapeutic efficacy of the vaccine, we challenged mice with TC-1/HPV18-E6 tumor cells. Three days after tumor challenge, mice were immunized IM with either pcDNA3-HPV18-E6 or control followed by electroporation. Tumor-bearing mice vaccinated with pcDNA3-HPV18-E6 experienced a slower tumor growth rate and longer survival time compared to those vaccinated with control (Figure 5C-D). These results demonstrate the ability of the pcDNA3-HPV-18-E6 vaccine to generate both protective and therapeutic antitumor effects in HPV18 tumor-bearing mice.
Figure 5. Characterization of antitumor immunity generated by pcDNA3-HPV18-E6 DNA vaccine.
(A-B) To test whether the immune responses generated by pcDNA3-HPV18-E6 DNA vaccine could prevent TC-1/HPV18-E6 tumor growth, 5 ~ 8 weeks old female C57BL/6 mice (5 mice/group) were immunized with either 50 μg/mouse of pcDNA3-HBV18-E6 or empty pcDNA3 vector control through IM injection followed by electroporation three times with 1-week interval. The mice were challenged subcutaneously with 1 × 105/mouse of TC-1/HPV18-E6 cells one week after the last vaccination. Tumor growth was monitored by measurement with a digital caliper twice a week. Survival of the mice was monitored every other day. A. Line graph showing TC-1/HPV18-E6 tumor growth curve. B. Survival curve of TC-1/HPV18-E6 tumor-bearing mice. (C-D) To test whether the immune responses generated by pcDNA3-HPV18-E6 DNA vaccine could demonstrate therapeutic effect in TC-1/HPV18-E6 tumor-bearing mice, 5 ~ 8 weeks old female C57BL/6 mice (5 mice/group) were challenged subcutaneously with 7.5 × 104/mouse of TC-1/HPV18-E6 tumor cells. On day 3, the mice were immunized with either 50 μg/mouse of pcDNA3-HPV18-E6, or pcDNA3 through IM injection followed by electroporation three times with 4-day interval. Tumor growth was monitored by measurement with a digital caliper twice a week. Survival of the mice was monitored every other day. C. Line graph showing TC-1/HPV18-E6 tumor growth curve. D. Survival curve of TC-1/HPV18-E6 tumor-bearing mice. Data are represented as mean ± SD. p-values were calculated by 2-tailed Student’s t test or log rank test. * = p<0.05; *** = p<0.001.
Therapeutic antitumor effects generated by pcDNA3-HPV18-E6 vaccination are both CD4+ and CD8+ T cells dependent
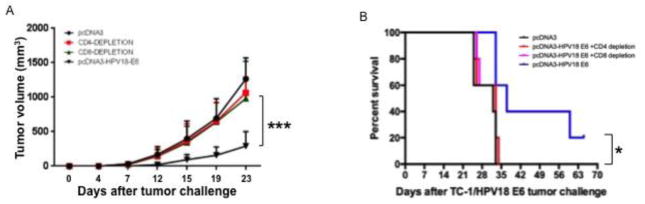
After demonstrating that pcDNA3-HPV18-E6 vaccination generates significant antigen-specific antitumor effects in mice, we investigated the role of CD4+ and CD8+ T cell subsets in post-vaccination antitumor immunity. To do so, mice were vaccinated IM with either pcDNA3-HPV18-E6 or control followed by electroporation, with or without CD4 or CD8 T cell depletion. One week after the final vaccination, mice were challenged subcutaneously with TC-1/HPV18-E6 tumor cells. As shown in Figure 6A, depletion of either CD4+ or CD8+ T cells abolishes the ability of pcDNA3-HPV18-E6 vaccination to control the growth of tumor in mice. The inability to control tumor growth in CD4+ or CD8+ T cell-depleted mice also translated into a worse survival rate, comparable to that of mice receiving empty vector control (Figure 6B). These data suggest that both CD8+ and CD4+ T cells contribute to the control of TC-1/HPV18-E6 tumors after pcDNA3-HPV18-E6 vaccination in mice.
Figure 6. Characterization of the role of T cell subset in the antitumor immunity generated by pcDNA3-HPV18-E6 DNA vaccine.
To determine the role of T cell subset, specifically the role of CD4+ or CD8+ T cells in the antitumor immunity generated by pcDNA3-HPV18-E6 DNA vaccine, forty 5~8 weeks old female C57BL/6 mice were divided into 4 groups (10 mice/group). Three groups of mice were immunized with 50 μg/mouse of pcDNA3-HPV18-E6 through IM injection followed by electroporation three times with 1-week interval. On the day of the third vaccination, one group of vaccinated mice was injected with anti-CD4 monoclonal antibody, and another group of immunized mice was injected with anti-CD8 monoclonal antibody through intraperitoneal injection for 3 days followed by once a week injection. 7 days after the last vaccination, all the mice were challenged subcutaneously with 1×105/mouse of TC-1/HPV18-E6 tumor cells. Tumor growth was monitored by measurement with a digital caliper twice a week. Survival of the mice was monitored every other day. A. TC-1/HPV18-E6 tumor growth curve. B. Survival curve of TC-1/HPV18-E6 tumor-bearing mice. Data are represented as mean ± SD. p-values were calculated by 2-tailed Student’s t test or log rank test. * = p<0.05; *** = p<0.001.
Discussion
In our study, we developed and investigated the immunogenicity of a prototype HPV18-E6 DNA vaccine construct in both naïve and HLA-A*0201 transgenic C57BL/6 mice. We showed that pcDNA3-HPV18-E6 vaccination elicited an antigen-specific CD8+ T cell response against HPV18-E6aa67-75 epitope. Using antigen-presenting cells that express only one type of MHC molecule, we showed that the HPV18-E6aa67-75 epitope is mainly presented through H-2Kb MHC class I molecules in both naïve and HLA-A*0201 transgenic C57BL/6 mice. We also demonstrated that pcDNA3-HPV18-E6 vaccination generated antitumor effects against HPV18-E6-expressing TC-1 tumor cells in mice, leading to tumor control and prolonged survival in a CD4- and CD8-dependent manner.
To date, most studies designing therapeutic HPV vaccine candidates have focused on HPV16 - the main contributor for HPV-associated cancer cases worldwide [18, 19]. HPV18 is the second most common HPV type found in cervical cancers [2, 18] and several studies have reported that HPV18 may have an equal or greater prevalence than HPV16 in HPV-associated oral cancer cases in countries including Brazil [20], the United Arab Emirates [21], Iran [22], and Taiwan [23]. We have previously generated two prototype therapeutic HPV DNA vaccines targeting HPV16-E6, the pcDNA3-HPV16-E6 (encoding wild type HPV16-E6 protein) and the pcDNA3-CRT/HPV16-E6 (encoding a fusion protein of HPV16-E6 with calreticulin) and evaluated the immunogenicity and antitumor effects of these vaccines in C57BL/6 mice [24]. In that study, using HPV16-E6 overlapping peptides, we showed that in vivo vaccination with pcDNA3-CRT/HPV16-E6 resulted in the generation of CD8+ T cell response specific to HPV16-E6aa50-57, while vaccination with pcDNA3-HPV16-E6 did not lead to the generation of noticeable antigen-specific immune responses. The results of our previous publication suggest that wild type HPV16-E6 protein is not very immunogenic in C57BL/6 mice, and thus, fusion to ER localizing protein CRT was necessary in order to generate HPV16-E6-specific antitumor immune effects in TC-1 tumor bearing C57BL/6 mice. In contrast, the prototype therapeutic HPV18 DNA vaccine developed in this study, pcDNA3-HPV18-E6 (encoding wild type HPV18-E6 protein), can generate significant level of HPV18 E6-specific CD8+ T cell response in healthy C57BL/6 mice as well as observable antitumor effects in TC-1/HPV18-E6 tumor-bearing mice (Figures 1, 5, and 6). Thus, we believe HPV18-E6 is more immunogenic than HPV16-E6 in C57BL/6 mice. However, because the immunogenicity of an antigen also varies greatly under different MHC class I setting, it is uncertain whether therapeutic HPV18-E6 vaccines will have better performance than therapeutic HPV16-E6 vaccines in clinical settings with diverse MHC class I backgrounds. Nonetheless, demonstrating that HPV18-E6 is immunogenic in C57BL/6 mice encourages further development and design of therapeutic vaccines targeting HPV18-E6 as potential treatment options against HPV18+ cancers that do not express HPV16 antigens.
Of note, the HPV18-E6 antigenic epitope characterized in this study, HPV18-E6aa67-75, has been evaluated by McCarthy et al. and was suggested to be presented via HLA-A2 MHC molecules [17]. In their study, McCarthy et al. evaluated the MHC binding specificity of HPV18-E6aa67-75 by incubating the peptide with cell lines that expresses HLA-A2 or murine MHC class I molecules and probed the cells for MHC class I upregulation using HLA-A2- or H-2Db-specific antibodies, suggesting greater expressions of HLA-A2 after incubation as compared to that of H-2Db. Here, we evaluated the MHC restriction of HPV18-E6aa67-75 using C1R cells, which lacks intrinsic MHC class I expression, transduced with AAD, H-2Db, or H-2Kb. We were able to observe a weak degree of HPV18-E6 specific CD8+ T cell activation after incubation with C1R/AAD cells, supporting McCarthy et al.’s findings (Figure 3B). However, we also observed a significantly stronger degree of HPV18-E6 specific CD8+ T cell activation after incubation with C1R/Kb cells, suggesting that HPV18-E6aa67-75 is also presented, perhaps more efficiently, by H-2Kb molecules (Figure 2B and 3B).
While the current study focused on characterizing the immune response against HPV18-E6aa67-75 since this epitope has been previously reported to be presented in mice [16, 17], other studies have reported the observation of HPV18-E6 specific immune response in human or in human MHC restricted fashion. For example, Chen et al. has previously identified that HPV18-E6 epitopes aa54-62 and aa84-92 can bind to HLA-A11 [25]. Nimako et al. reported the observation of HPV18-specific CTL response in CIN3 patients [26]. Smith et al. reported the observation of HPV18 E6-specific CTL response in healthy people and patients with lower genital tract neoplasia [27]. Lastly, Gallagher et al. reported the observation of HPV18-E6 epitope that is restricted to HLA-DRB1*15 in healthy young women [28]. Thus, our prototype therapeutic HPV18-E6 vaccine, which encodes the full length HPV18-E6 protein, may be able to present other HPV18-E6 epitopes under human MHC class I settings.
Importantly, we showed that the HPV18-E6-specific antitumor immune response elicited by pcDNA3-HPV18-E6 vaccination is both CD4+ and CD8+ T cell-dependent, as depletion of either T cell populations abolishes the observed antitumor effects (Figure 6). Previous studies have reported several immunization strategies that are capable of eliciting antigen-specific antitumor responses in CD4+ T cell-independent manner, such as supplementing CD40 signals [29] or introducing genes encoding IFN-β [30] or macrophage-derived chemokine [31] in the tumor location. Nonetheless, there is increasing evidence that suggests the need for CD4+ T cells in the generation of effective antitumor immunity, with potential roles such as maintaining the amount of antigen-specific CD8+ T cells, assisting cytotoxic T-lymphocyte (CTL) infiltration into tumor, and enhancing the function of tumor-specific CTLs [32–36]. In our previous study on therapeutic HPV16 DNA vaccine, we observed that IM injection of pNGVL4a-hCRTE6E7L2 DNA vaccine with electroporation can eliminate HPV16-E6/E7-expressing TC-1 tumors in a CD4-depleted setting; however, depletion of CD4 resulted in a lower number of antigen-specific CTLs after immunization [37]. Thus, CD4+ T cells appear to assist the antigen-specific CTL responses in various magnitudes depending on the target antigen, and the exact role of CD4+ T cells in the generation of HPV18-E6-specific immune responses requires further research.
It should also be mentioned that since the TC-1/HPV18-E6 tumor cell line used in the current study also expresses HPV16-E6 and HPV16-E7 oncoproteins, the expression of HPV18-E6 is not necessary for the tumor cells to maintain the cancerous characteristics. Thus, it is possible that the TC-1/HPV18-E6 tumor cells can develop resistance against pcDNA3-HPV18-E6 vaccination by suppressing the expression of HPV18-E6, thus explaining our finding in which the vaccination does not fully control and eliminate TC-1/HPV18-E6 tumor (Figure 5). Nonetheless, we have demonstrated using this model that pcDNA3-HPV18-E6 vaccination indeed leads to the generation of HPV18-E6-specific antitumor effects in C57BL/6 mice, which delays the growth of TC-1/HPV18-E6 tumors and prolongs the survival of tumor-bearing mice in comparison to vaccination with empty pcDNA3 vector (Figure 5). The generation of an improved HPV18 tumor model that relies on HPV18 E6 and/or E7 oncoproteins for the maintenance of its cancerous phenotypes can further strengthen the effort for future development and evaluation therapeutic vaccines targeting HPV18-E6.
In conclusion, our study demonstrates the capability of inducing immune responses that are specific for antigens encoded by HPV18, which is responsible for causing a significant amount of HPV-associated cancers in addition to HPV16. The characterization of HPV18-E6-specific CTL response and the establishment of HPV18-E6-expressing tumor cell line will serve as important infrastructures for further development of HPV18-E6 targeted immunotherapy.
Highlights.
HPV18 E6 DNA vaccination generates E6-specific CTL response against aa67-75 epitope
HPV18 E6aa67-75 epitope is presented mainly by H-2Kb and weakly by HLA-A*0201
Generation of HPV18-E6 expressing TC-1/HPV18-E6 preclinical tumor model
Antitumor immune response against TC-1/HPV18-E6 is CD4+ and CD8+ T cell dependent
Acknowledgments
This work was supported by the United States National Institutes of Health (NIH) Cervical Cancer Specialized Program of Research Excellence (SPORE) (P50 CA098252), R01 grant (CA114425), R21 grant (CA194896), and R21 grant (AI109259).
Abbreviations
- HPV
human papillomavirus
- SCC
squamous cell carcinoma
- ADC
adenocarcinoma
- CIN
cervical intraepithelial neoplasia
- E6
HPV early protein 6
- E7
HPV early protein 7
- HLA
Human leukocyte antigen
- MHC
major histocompatibility complex
- IFN-γ
interferon gamma
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- IM
intramuscular
Footnotes
Conflict of interest: The authors declare no conflict of interest exists.
Authors’ contributions
YM, SP, CFH, and TCW conceived and designed experiments and interpreted data. YM, AY, SP, and JQ performed and experiments. AY, SP, EF, CFH, and TCW wrote the manuscript. All authors have read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Current oncology reports. 2014;16:402. doi: 10.1007/s11912-014-0402-4. [DOI] [PubMed] [Google Scholar]
- 2.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell JH, Grandis JR, Ferris RL. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annual review of medicine. 2015 doi: 10.1146/annurev-med-051914-021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head & neck. 2013;35:747–55. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 5.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 6.Egawa N, Egawa K, Griffin H, Doorbar J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses. 2015;7:3863–90. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Dahlstrom LA, Ylitalo N, Sundstrom K, Palmgren J, Ploner A, Eloranta S, et al. Prospective study of human papillomavirus and risk of cervical adenocarcinoma. Int J Cancer. 2010;127:1923–30. doi: 10.1002/ijc.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen AA, Gheit T, Franceschi S, Tommasino M, Clifford GM, Group IHVS. Human Papillomavirus 18 Genetic Variation and Cervical Cancer Risk Worldwide. J Virol. 2015;89:10680–7. doi: 10.1128/JVI.01747-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26(Suppl 10):K53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23:75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang A, Farmer E, Lin J, Wu TC, Hung CF. The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus Res. 2017;231:148–65. doi: 10.1016/j.virusres.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer research. 1996;56:21–6. [PubMed] [Google Scholar]
- 14.Wang JW, Jiang R, Peng S, Chang YN, Hung CF, Roden RB. Immunologic Control of Mus musculus Papillomavirus Type 1. PLoS Pathog. 2015;11:e1005243. doi: 10.1371/journal.ppat.1005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zemmour J, Little AM, Schendel DJ, Parham P. The HLA-A, B “negative” mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J Immunol. 1992;148:1941–8. [PubMed] [Google Scholar]
- 16.Zhao L, Ren J, Feng J, Pang Z, Zhang ZX, Tan WJ, et al. Identification specific T lymphocyte epitopes on E6 protein of human papillomavirus type 18 in mice. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2010;24:406–8. [PubMed] [Google Scholar]
- 17.McCarthy C, Youde SJ, Man S. Definition of an HPV18/45 cross-reactive human T-cell epitope after DNA immunisation of HLA-A2/KB transgenic mice. Int J Cancer. 2006;118:2514–21. doi: 10.1002/ijc.21643. [DOI] [PubMed] [Google Scholar]
- 18.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 19.Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, et al. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 2016;15:989–1007. doi: 10.1586/14760584.2016.1157477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira MC, Soares RC, Pinto LP, Souza LB, Medeiros SR, de Costa AL. High-risk human papillomavirus (HPV) is not associated with p53 and bcl-2 expression in oral squamous cell carcinomas. Auris Nasus Larynx. 2009;36:450–6. doi: 10.1016/j.anl.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Mathew A, Mody RN, Patait MR, Razooki AA, Varghese NT, Saraf K. Prevalence and relationship of human papilloma virus type 16 and type 18 with oral squamous cell carcinoma and oral leukoplakia in fresh scrappings: a PCR study. Indian J Med Sci. 2011;65:212–21. [PubMed] [Google Scholar]
- 22.Seraj JM, Yazdani N, Ashtiani ZO, Seraj SM, Hasheminasab SM, Memar B, et al. TP53 gene expression in HPV-positive oral tongue SCC and its correlation with nodal metastasis. Pathol Res Pract. 2011;207:758–61. doi: 10.1016/j.prp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Lee LA, Huang CG, Liao CT, Lee LY, Hsueh C, Chen TC, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One. 2012;7:e40767. doi: 10.1371/journal.pone.0040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J, et al. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78:8468–76. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen HW, Leng CH, Liu HY, Cheng WF, Chang YW, Wu PY, et al. Identification of HLA-A11-restricted CTL epitopes derived from HPV type 18 using DNA immunization. Cancer Biol Ther. 2009;8:2025–32. doi: 10.4161/cbt.8.21.9732. [DOI] [PubMed] [Google Scholar]
- 26.Nimako M, Fiander AN, Wilkinson GW, Borysiewicz LK, Man S. Human papillomavirus-specific cytotoxic T lymphocytes in patients with cervical intraepithelial neoplasia grade III. Cancer research. 1997;57:4855–61. [PubMed] [Google Scholar]
- 27.Smith KL, Tristram A, Gallagher KM, Fiander AN, Man S. Epitope specificity and longevity of a vaccine-induced human T cell response against HPV18. Int Immunol. 2005;17:167–76. doi: 10.1093/intimm/dxh197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallagher KM, Man S. Identification of HLA-DR1- and HLA-DR15-restricted human papillomavirus type 16 (HPV16) and HPV18 E6 epitopes recognized by CD4+ T cells from healthy young women. J Gen Virol. 2007;88:1470–8. doi: 10.1099/vir.0.82558-0. [DOI] [PubMed] [Google Scholar]
- 29.Ribas A, Butterfield LH, Amarnani SN, Dissette VB, Kim D, Meng WS, et al. CD40 cross-linking bypasses the absolute requirement for CD4 T cells during immunization with melanoma antigen gene-modified dendritic cells. Cancer research. 2001;61:8787–93. [PubMed] [Google Scholar]
- 30.Brown JL, Barsoum J, Qin XQ. CD4+ T helper cell-independent antitumor response mediated by murine IFN-beta gene delivery in immunocompetent mice. J Interferon Cytokine Res. 2002;22:719–28. doi: 10.1089/10799900260100222. [DOI] [PubMed] [Google Scholar]
- 31.Lee JM, Merritt RE, Mahtabifard A, Yamada R, Kikuchi T, Crystal RG, et al. Intratumoral expression of macrophage-derived chemokine induces CD4+ T cell-independent antitumor immunity in mice. J Immunother. 2003;26:117–29. doi: 10.1097/00002371-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Liu DW, Tsao YP, Kung JT, Ding YA, Sytwu HK, Xiao X, et al. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000;74:2888–94. doi: 10.1128/jvi.74.6.2888-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–55. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 34.Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207:2469–77. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer research. 2010;70:8368–77. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Z, Cuss SM, Singh V, Gurusamy D, Shoe JL, Leighty R, et al. CD4+ T Cell Help Selectively Enhances High-Avidity Tumor Antigen-Specific CD8+ T Cells. J Immunol. 2015;195:3482–9. doi: 10.4049/jimmunol.1401571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng S, Song L, Knoff J, Wang JW, Chang YN, Hannaman D, et al. Control of HPV-associated tumors by innovative therapeutic HPV DNA vaccine in the absence of CD4+ T cells. Cell Biosci. 2014;4:11. doi: 10.1186/2045-3701-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]