Abstract
Development of spontaneous seizures is the hallmark of human epilepsy. There is a critical need for new epilepsy models in order to elucidate mechanisms responsible for leading to the development of spontaneous seizures and for testing new anti-epileptic compounds. Moreover, rodent models of epilepsy have clearly demonstrated that there are two independent seizure systems in the brain: 1) the forebrain seizure network required for the expression of clonic seizures mediated by forebrain neurocircuitry, and 2) the brainstem seizure network necessary for the expression of brainstem or tonic seizures mediated by brainstem neurocircuitry. In seizure naïve animals, these two systems are separate, but developing models that can explore the intersection of the forebrain and brainstem seizure systems or for elucidating mechanisms responsible for bringing these two seizure systems together may aid in our understanding of: 1) how seizures can become more complex over time, and 2) sudden unexpected death in epilepsy (SUDEP) since propagation of seizure discharge from the forebrain seizure system to the brainstem seizure system may have an important role in SUDEP because many cardiorespiratory systems are localized in the brainstem. The repeated flurothyl seizure model of epileptogenesis, as described here, may aid in providing insight into these important epilepsy issues in addition to understanding how spontaneous seizures develop.
Keywords: Epilepsy, Epileptogenesis, Flurothyl, Mouse, Spontaneous seizures, Seizure semiology changes
Background
Epilepsy is a complex and multifactorial disease defined by unprovoked spontaneous seizures. Approximately two-thirds of the epilepsy population are successfully treated with anticonvulsant drug regimens, but the remaining one third continue to experience seizures (Kwan and Brodie, 2000; Lindsten et al., 2001 ; Kwan et al., 2010 ; Loscher et al., 2013; Brodie, 2017). Given the complexities of genetic heterogeneity and inherent difficulties in studying the pathophysiology of epileptogenesis in humans, animal models of epilepsy have served important roles for understanding how spontaneous seizures develop. The mainstays of animal models of spontaneous seizures are either 1) chemically-induced status epilepticus models (SE: a condition characterized by continuous seizure activity) or electrically-induced SE models, or 2) traumatic brain injury (TBI) models. However, there are caveats with these models ( Pitkänen et al., 2006 ). For instance, at least 30 min of SE (but more typically 1-2 h of SE) are required for the appearance of spontaneous seizures with the presence of spontaneous seizures being highly dependent on the duration of SE (Lemos and Cavalheiro, 1995; Gorter et al., 2003 ; Curia et al., 2008 ; Loscher, 2013; Kandratavicius et al., 2014 ; Polli et al., 2014 ; Gorter et al., 2016 ). Following these prolonged bouts of SE, there can be a significant increase in mortality (Goodman, 1998; Curia et al., 2008 ; Scorza et al., 2009 ; Loscher, 2013; Reddy and Kuruba, 2013; Kandratavicius et al., 2014 ). Given that SE is a significant seizure event, substantial neuronal death occurs (Goodman, 1998; Curia et al., 2008 ; Scorza et al., 2009 ; Loscher, 2013; Reddy and Kuruba, 2013; Kandratavicius et al., 2014 ). Importantly, both SE and substantial neuronal death are not common findings in most human epilepsies. Lastly, TBI models in rodents also have caveats in that very large regions of the brain require damage to produce spontaneous seizures, and substantial brain damage is not a common observation found in human epilepsy ( Pitkänen et al., 2006 ). Therefore, new rodent models are needed limiting these caveats to continue to advance our understanding of epileptogenesis.
Experimental evidence suggests that there are two largely independent seizure systems that are responsible for the expression of generalized seizures ( Kreindler et al., 1958 ; Browning et al., 1981 ; Browning and Nelson, 1986; Magistris et al., 1988 ; Applegate et al., 1991 ). These two seizure systems are referred to as the forebrain seizure network and the brainstem seizure network. Whereas the forebrain seizure network is responsible for the expression of clonic seizures, the brainstem seizure network is responsible for the expression of brainstem (tonic) seizures ( Kreindler et al., 1958 ; Browning et al., 1981 ; Browning and Nelson, 1986; Magistris et al., 1988 ; Applegate et al., 1991 ). As such, forebrain neurocircuitry modulates the expression of clonic seizures, while brainstem neurocircuitry is both necessary and sufficient for the expression of a variety of tonic-brainstem seizure types. Notably, these seizure systems are mostly independent and the seizures elicited in one network do not readily spread to the other in seizure naïve rodents ( Kreindler et al., 1958 ; Browning et al., 1981 ; Browning and Nelson, 1986; Magistris et al., 1988 ; Applegate et al., 1991 ). Interestingly, BOLD fMRI and SPECT imaging has revealed the critical nature of brainstem structures in the expression of tonic seizures in humans and in animal models ( Blumenfeld et al., 2009 ; Varghese et al., 2009 ; DeSalvo et al., 2010 ). However, little is known regarding reorganizations that occur in the brainstem seizure network, or at the intersection of the forebrain seizure network and brainstem seizure network, which can both give rise to brainstem seizure expression.
Flurothyl is a volatile chemoconvulsant acting as a GABAA antagonist that was extensively used historically to induce seizures in severely depressed patients as an alternative to electroconvulsive shock therapy (Krasowski, 2000; Fink, 2014). There are three primary advantages of flurothyl as a chemoconvulsant. First, there are minimal stressors imparted on the rodents since flurothyl is highly volatile. It is infused into a chamber wherein the animal inhales the flurothyl thereby eliminating the need for injections. Second, flurothyl is rapidly eliminated unmetabolized through the lungs, thus eliminating potential confounds of residual convulsant remaining in the body ( Krantz et al., 1957 ; Dolenz, 1967). Finally, flurothyl-induced seizure durations are short (e.g., typically 15-60 sec depending on the seizure type expressed) due to the ease of controlling seizures by simply exposing the animals to room air.
The repeated flurothyl seizure model can be used to understand how seizures develop and become more complex over time, and to explore the mechanistic intersections of the forebrain seizure network and brainstem seizure network that may lead to more complex seizure types ( Applegate et al., 1997 ; Samoriski and Applegate, 1997; Samoriski et al., 1997; Ferland and Applegate, 1998a; 1998b and 1999). With the repeated flurothyl seizure model, C57BL/6J mice express clonic-forebrain seizures during eight flurothyl induction trials (Samoriski and Applegate, 1997; Papandrea et al., 2009 ). Following a one month incubation period and a rechallenge with flurothyl, C57BL/6J mice express a clonic-forebrain seizure that rapidly and uninterruptedly transitions into a tonic-brainstem seizure (Samoriski and Applegate, 1997; Ferland and Applegate, 1998b). We refer to these seizures as forebrain→brainstem seizures denoting the ictal progression from the forebrain seizure network to the brainstem seizure network ( Papandrea et al., 2009 ; Kadiyala et al., 2015 ). Lastly, C57BL6/J mice exposed to the repeated flurothyl seizure model rapidly develop spontaneous seizures that appear to remit without treatment following 1 month ( Kadiyala et al., 2016 ), in contrast to DBA2/J mice which also rapidly develop spontaneous seizures that do not remit (Kadiyala and Ferland, 2017). Here, we describe the methods for assaying mice in the repeated flurothyl seizure model, which was originally described 20 years ago ( Applegate et al., 1997 ; Samoriski and Applegate, 1997) and continues to be characterized.
Materials and Reagents
18 G needle
3 x 3 in. medium gauze pads (CVS, catalog number: 893120)
C57BL/6J male mice (6-7 weeks on arrival) (THE JACKSON LABORATORY, catalog number: 000664)
Aquarium sealant
-
Flurothyl (Bis(2,2,2-trifluoroethyl) ether or 2,2,2-trifluoroethyl ether) (Sigma-Aldrich, catalog number: 287571)
IMPORTANT: Perform all flurothyl exposures in a certified chemical fume hood with exhaust out of the laboratory, since the inhalation of flurothyl will result in seizures in humans.
95% ethanol (ethyl alcohol 190 proof) (PHARMCO-AAPER, catalog number: 111000190)
Petroleum jelly
10% flurothyl solution (see Recipes)
Equipment
All-clear vacuum Plexiglas desiccator chamber (Ted Pella, model: 2240-1)
20 ml glass syringe (Sigma-Aldrich, catalog number: Z101079)
Syringe pump (Kent Scientific, model: GENIE Plus)
Forceps (for removing the flurothyl saturated gauze pad from the chamber)
Wire mesh colander with at least ” square mesh
Chemical fume hood
Procedure
-
Construction of flurothyl chamber
The Plexiglas chamber needs to have a small hole drilled in the top of the chamber to allow for the fixing of an 18 G needle in this hole (the 18 G needle is cut at the luer lock end of the plastic edge of the needle and at the beveled edge of the needle resulting in a short 18 G blunted steel tube). The 18 G needle tube, upon fixing it to the top of the chamber, is connected to small diameter tubing on the outside of the chamber. At this point, seal the connection with the tubing and the top of the chamber with aquarium sealant to ensure that the chamber is air-tight. The no-tubing end of the 18 G tube should now be extended into the top of the chamber. Also, be sure to seal any other openings in the top half of the chamber with aquarium sealant (this is usually where the vacuum valve would be attached).
Next, tape a screen support to hold a gauze pad below where the 18 G needle tubing is hanging. The top and bottom of the chamber should be regularly greased with petroleum jelly to ensure an adequate seal between the top, bottom and O-ring of the chamber. The other end of the tubing is connected to a 20 ml glass syringe that is attached to a syringe pump (Figure 1).
-
Repeated flurothyl seizure model
Mice are allowed ad libitum access to food and water, and are maintained on a standard 12 h light-dark cycle with lights on at 7:00 AM. Mice are allowed to acclimate to the animal facility for ~1 week before seizure testing. Individual mice (7-8 weeks old) are exposed to 10% flurothyl (see Recipes section). Flurothyl is delivered to mice in a closed Plexiglas chamber. Flurothyl binds at the GABAA receptor where it acts as a noncompetitive antagonist (Krasowski, 2000). Since a receptor bound by a non-competitive antagonist will not be activated by binding of an agonist, and since ethanol is a positive allosteric modulator of the GABAA receptor, ethanol is unlikely to exert a major effect at the GABAA receptor in the presence of flurothyl.
Be sure to perform all flurothyl exposures in a certified chemical fume hood with exhaust out of the laboratory, since the inhalation of flurothyl will result in seizures in humans.
Ten percent flurothyl is infused via a syringe pump and glass syringe at a flow rate of 6 ml/h onto a gauze pad (folded in half) suspended from the top of the chamber by a screen (Figure 1). Since flurothyl is highly volatile, it rapidly vaporizes, leading to inhalation and subsequent seizure expression. The generalized seizure threshold (GST) is defined as the latency from the commencement of the infusion of flurothyl to the occurrence of an animal’s loss of postural control.
Once the animal losses its posture (i.e., expresses a generalized clonic seizure; grades 1-2 [see Table 1]), the chamber is opened to fresh air, resulting in the rapid elimination of flurothyl (and the flurothyl infusion pump is turned off). Mice with different genetic backgrounds will respond with different latencies to loss of postural control (i.e., GSTs). In different mouse strains, trial 1 GSTs can range from 200-500 sec ( Papandrea et al., 2009 ). However, the variability within strains is quite small given that this is a behavioral analysis. It is good practice to place a wire mesh colander over the bottom of the chamber, when the top is removed, to prevent the mouse from jumping out of the chamber. This is particularly important following the 28-day flurothyl incubation period and flurothyl rechallenge, since mice will often have brainstem seizures which can result in the mouse having wild running and bouncing seizures in which they can escape from the bottom half of the chamber. The wire mesh colander helps to contain the mouse in the bottom half of the chamber, while also exposing the animal to room air.
-
The latency to the first myoclonic jerk (behaviorally, myoclonic jerks are brief, but significant, contractions of the neck and body musculature, while maintaining postural control [ Applegate et al., 1997 ; Samoriski and Applegate, 1997]), the number of myoclonic jerks expressed before the onset of a generalized seizure, the latency to the loss of postural control (GST), the time to regain posture, the duration of the seizure (calculated as the time from the start of the generalized seizure to the time when the animal regains its posture and stops showing clonus of the limbs), and the type of seizure is recorded (Table 1) for each trial.
*In general, grades 1 and 2 are clonic-forebrain seizures comparable to a grade 5 seizure on the Racine scale for electrical kindling (Racine, 1972). Grades 3-7 are brainstem seizures. Importantly, mice recover their righting reflex before transitioning into grades 3-7 ( Kreindler et al., 1958 ; Browning and Nelson, 1986). We categorize mice having seizure grades 3-7 as forebrain→brainstem seizures to denote the progression of this type of seizure.
Upon seizure resolution, the animal is returned to its home cage until the next day. One mouse is tested at a time in the flurothyl chamber using a new gauze pad each time. Mice are given one 10% flurothyl-induced seizure per day for 8 days (the flurothyl induction phase) followed by a 4 week rest period (the flurothyl incubation period). We routinely try to keep the time intervals between induction trials as close to 24 h as possible (24 ± 4 h). Previous work has demonstrated the necessity of 8 induction trials and a 28-day incubation period for the effects observed (Samoriski and Applegate, 1997). The gauze pad, saturated in flurothyl, is removed from the top half of the chamber from the screening, using forceps, so as to not contaminate one’s gloves with flurothyl. The gauze pads are laid out under the fume hood to allow for flurothyl evaporation. Gauze pads can be reused the next day, since flurothyl will have evaporated completely from the gauze pad by this time.
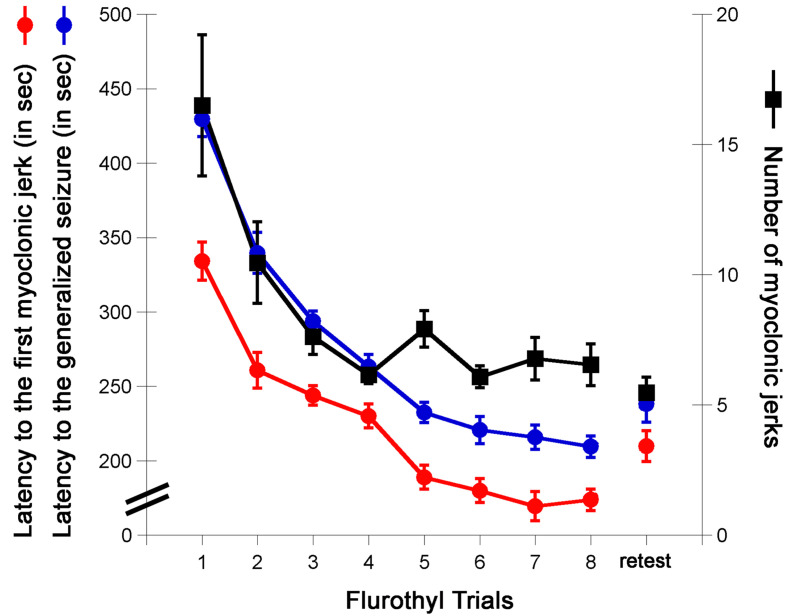
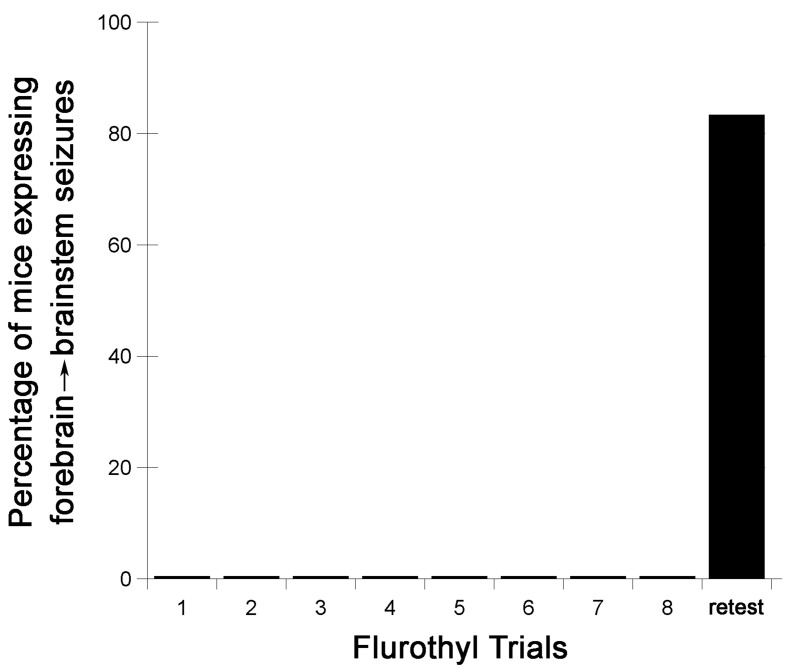
Following the 28-day incubation period, mice are again given a flurothyl exposure (retest/rechallenge) and the criteria described above are recorded. Representative examples from C57BL6/J mice of the observed changes in the latency to the first myoclonic jerk, the number of myoclonic jerks, and generalized seizure thresholds are shown (Figure 2). C57BL6/J mice typically do not express forebrain→brainstem seizures during the 8 flurothyl induction trials, but upon a 28-day incubation period and flurothyl retest, a significant percentage of mice now express this new seizure behavior (Figure 3).
Additionally, local field potential recordings from the cerebral cortex and hippocampus of C57BL6/J mice, during the 28-day incubation period, reveals that flurothyl-exposed mice develop spontaneous seizures following the 8th flurothyl induction trial with relatively high seizure occurrences ( Kadiyala et al., 2016 ). However, these seizures begin to remit over the 28-day incubation phase ( Kadiyala et al., 2016 ). Interestingly, DBA/2J mice similarly treated also develop spontaneous seizures with fewer seizure occurrences than C57BL6/J mice, but the spontaneous seizures observed in DBA/2J mice do not appear to remit (Kadiyala and Ferland, 2017).
Figure 1. The repeated flurothyl seizure model set-up.
Black arrows are pointing to the tubing with slack running from the syringe to the 18 G steel tube in the top of the chamber.
Table 1. Behavioral seizure classification grading scale.
| Seizure grade | Description of corresponding seizure behavior* |
| Grade 1 | Loss of posture associated with facial clonus, including chewing, and clonus of forelimbs and/or hindlimbs |
| Grade 2 | Grade 1 seizure followed by recovery of the righting reflex and low intensity bouncing |
| Grade 3 | Grade 1 and/or 2 features with recovery of the righting reflex followed by wild running and popcorning |
| Grade 4 | Grade 3 followed by forelimb and/or hindlimb treading |
| Grade 5 | Grades 3 and/or 4 followed by bilateral tonic extension of the forelimbs |
| Grade 6 | Grade 5 followed by bilateral tonic extension of the hindlimbs |
| Grade 7 | Grade 6 followed by immediate death |
Figure 2. Myoclonic jerk threshold, the number of myoclonic jerks, and the generalized seizure threshold (GST) in C57BL6/J mice.
The latency to the first myoclonic jerk (myoclonic jerk threshold in seconds), the number of myoclonic jerks expressed before the onset of a generalized seizure, and the latency to a generalized seizure (generalized seizure threshold) on each seizure trial in C57BL6/J mice. The retest trial is a rechallenge to flurothyl following a 28-day incubation period.
Figure 3. Flurothyl-induced seizure behaviors in C57BL6/J mice during 8 induction phase seizures, and a final flurothyl rechallenge (retest) after a 28-day incubation phase.
While none of the C57BL6/J mice expressed a complex forebrain→brainstem seizure during the flurothyl induction trials (these mice only expressed clonic-forebrain seizures), 75-100% of C57BL6/J mice will express forebrain→brainstem seizures following a rechallenge to flurothyl following a 28-day incubation period.
Data analysis
Repeated measures analyses of variance (ANOVA) are employed for comparisons between flurothyl-exposed and control groups across trials followed by post-hoc tests. Student’s t-tests are used to determine differences in seizure characteristics between trial 8 of the induction phase and the retest/rechallenge that followed the 28-day incubation period. Evaluation of differences (percentage) in the numbers of mice exhibiting clonic–forebrain seizures and the numbers of mice exhibiting forebrain→brainstem seizures, after flurothyl exposure, utilize Chi-square or Fisher Exact tests.
Notes
Be sure to perform ALL flurothyl exposures in a functional and annually certified chemical fume hood to ensure safety of laboratory personnel, given that flurothyl is an inhalant chemoconvulsant. Moreover, do not remove any materials that were exposed to flurothyl (i.e., gauze pads, etc.) in a liquid state, for at least 24 h, to ensure evaporation of flurothyl in the chemical fume hood.
As mice get older than 7-8 weeks of age, some mice do not have an obvious loss of posture with flurothyl exposure. They can present with very severe myoclonic jerks and even some bilateral forelimb clonus in the absence of loss of posture. Mice experiencing these behaviors often result in brainstem seizure expression, since the mice are continually exposed to flurothyl because the top of the chamber was not removed. Missing the clonic seizure (as defined by loss of posture) results in the chamber top not being removed and exposing the mice to room air. Continual flurothyl exposure will always result in a brainstem seizure, therefore, it is important to detect the generalized clonic seizure to terminate the trial, which may involve having to record EEGs to detect generalized seizures.
Recipes
-
Preparing the 10% flurothyl solution
10% flurothyl is made by diluting a 5 g ampule (~5 ml) of liquid flurothyl into 45 ml of 95% ethanol in a glass container
The flurothyl/ethanol mixture is then shaken to mix the flurothyl
Note: Flurothyl is always prepared and stored in a chemical fume hood (with the bottle and cap sealed with Parafilm).Be sure that the glass container is clearly labeled as a potential hazard as an inhalant chemoconvulsant.
Acknowledgments
This work was supported by an NIH/NINDS R01NS064283 grant to RJF. This work was adapted from previous publications (Samoriski and Applegate, 1997; Kadiyala et al., 2016 ).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Applegate C. D., Samoriski G. M. and Burchfiel J. L.(1991). Evidence for the interaction of brainstem systems mediating seizure expression in kindling and electroconvulsive shock seizure models. Epilepsy Res 10(2-3): 142-147. [DOI] [PubMed] [Google Scholar]
- 2.Applegate C. D., Samoriski G. M. and Ozduman K.(1997). Effects of valproate, phenytoin, and MK-801 in a novel model of epileptogenesis. Epilepsia 38(6): 631-636. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld H., Varghese G. I., Purcaro M. J., Motelow J. E., Enev M., McNally K. A., Levin A. R., Hirsch L. J., Tikofsky R., Zubal I. G., Paige A. L. and Spencer S. S.(2009). Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 4): 999-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodie M.J.(2017). Outcomes in newly diagnosed epilepsy in adolescents and adults: insights across a generation in Scotland. Seizure 44: 206-210. [DOI] [PubMed] [Google Scholar]
- 5.Browning R. A. and Nelson D. K.(1986). Modification of electroshock and pentylenetetrazol seizure patterns in rats after precollicular transections. Exp Neurol 93(3): 546-556. [DOI] [PubMed] [Google Scholar]
- 6.Browning R. A., Turner F. J., Simonton R. L. and Bundman M. C.(1981). Effect of midbrain and pontine tegmental lesions on the maximal electroshock seizure pattern in rats. Epilepsia 22(5): 583-594. [DOI] [PubMed] [Google Scholar]
- 7.Curia G., Longo D., Biagini G., Jones R.S.G., Avolia M.(2008). The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172(2-4):143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSalvo M. N., Schridde U., Mishra A. M., Motelow J. E., Purcaro M. J., Danielson N., Bai X., Hyder F. and Blumenfeld H.(2010). Focal BOLD fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. Neuroimage 50(3): 902-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolenz B. J.(1967). Flurothyl(Indoklon) side effects. Am J Psychiatry 123(11): 1453-1455. [DOI] [PubMed] [Google Scholar]
- 10.Ferland R. J. and Applegate C. D.(1998). The role of the ventromedial nucleus of the hypothalamus in epileptogenesis. Neuroreport 9(16): 3623-3629. [DOI] [PubMed] [Google Scholar]
- 11.Ferland R. J. and Applegate C. D.(1998). Decreased brainstem seizure thresholds and facilitated seizure propagation in mice exposed to repeated flurothyl-induced generalized forebrain seizures. Epilepsy Res 30(1): 49-62. [DOI] [PubMed] [Google Scholar]
- 12.Ferland R. J. and Applegate C. D.(1999). Bidirectional transfer between electrical and flurothyl kindling in mice: evidence for common processes in epileptogenesis. Epilepsia 40(2): 144-152. [DOI] [PubMed] [Google Scholar]
- 13.Fink M.(2014). The seizure, not electricity, is essential in convulsive therapy: the flurothyl experience. J ECT 30(2):91-93. [DOI] [PubMed] [Google Scholar]
- 14.Goodman J. H.(1998). Experimental models of status epilepticus. In: Peterson, S. L. and Albertson, T. E.(Eds.). Neuropharmacology Methods in Epilepsy Research. 95-125. Boca Raton: CRC Press. [Google Scholar]
- 15.Gorter J. A., Goncalves Pereira P. M., van Vliet E. A., Aronica E., da Silva Lopes, Lucassen F. H., P. J. (2003). Neuronal cell death in a rat model for mesial temporal lobe epilepsy is induced by the initial status epilepticus and not by later repeated spontaneous seizures. Epilepsia 44: 647-658. [DOI] [PubMed] [Google Scholar]
- 16.Gorter J. A., van Vliet E. A., da Silva Lopes, F. H. (2016). Which insights have we gained from the kindling and post-status epilepticus models? J Neurosci Methods 260:96-108. [DOI] [PubMed] [Google Scholar]
- 17.Kadiyala S. B. and Ferland R. J.(2017). Dissociation of spontaneous seizures and brainstem seizure thresholds in mice exposed to eight flurothyl-induced generalized seizures. Epilepsia Open 2(1): 48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadiyala S. B., Papandrea D., Tuz K., Anderson T. M., Jayakumar S., Herron B. J. and Ferland R. J.(2015). Spatiotemporal differences in the c-fos pathway between C57BL/6J and DBA/2J mice following flurothyl-induced seizures: A dissociation of hippocampal Fos from seizure activity. Epilepsy Res 109: 183-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadiyala S. B., Yannix J. Q., Nalwalk J. W., Papandrea D., Beyer B. S., Herron B. J. and Ferland R. J.(2016). Eight flurothyl-induced generalized seizures lead to the rapid evolution of spontaneous seizures in mice: A model of epileptogenesis with seizure remission. J Neurosci 36(28): 7485-7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandratavicius L., Balista P. A., Lopes-Aguiar C., Ruggiero R. N., Umeoka E. H., Garcia-Cairasco N., Bueno-Junior L. S., Leite J. P.(2014). Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat 10:1693-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krantz J. C. Jr. Truitt E. B. Jr. Ling A. S. and Speers L.(1957). Anesthesia. LV. The pharmacologic response to hexafluorodiethyl ether. J Pharmacol Exp Ther 121(3): 362-368. [PubMed] [Google Scholar]
- 22.Krasowski M. D.(2000). Differential modulatory actions of the volatile convulsant flurothyl and its anesthetic isomer at inhibitory ligand-gated ion channels. Neuropharmacology 39(7): 1168-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreindler A., Zuckermann E., Steriade M. and Chimion D.(1958). Electro-clinical features of convulsions induced by stimulation of brain stem. J Neurophysiol 21(5): 430-436. [DOI] [PubMed] [Google Scholar]
- 24.Kwan P., Arzimanoglou A., Berg A. T., Brodie M. J., Hauser W. A., Mathern G., Moshe S. L., Perucca E., Wiebe S., French J.(2010). Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 51: 1069-1077. [DOI] [PubMed] [Google Scholar]
- 25.Kwan P. and Brodie M. J.(2000). Early identification of refractory epilepsy. N Engl J Med 342(5): 314-319. [DOI] [PubMed] [Google Scholar]
- 26.Lemos T. and Cavalheiro E. A.(1995). Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp Brain Res 102(3): 423-428. [DOI] [PubMed] [Google Scholar]
- 27.Lindsten H., Stenlund H. and Forsgren L.(2001). Remission of seizures in a population-based adult cohort with a newly diagnosed unprovoked epileptic seizure. Epilepsia 42(8): 1025-1030. [DOI] [PubMed] [Google Scholar]
- 28.Loscher W.(2013). Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20(5): 359-368. [DOI] [PubMed] [Google Scholar]
- 29.Loscher W., Klitgaard H., Twyman R. E., and Schmidt D.(2013). New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 12: 757-776. [DOI] [PubMed] [Google Scholar]
- 30.Magistris M. R., Mouradian M. S. and Gloor P.(1988). Generalized convulsions induced by pentylenetetrazol in the cat: participation of forebrain, brainstem, and spinal cord. Epilepsia 29(4): 379-388. [DOI] [PubMed] [Google Scholar]
- 31.Papandrea D., Anderson T. M., Herron B. J. and Ferland R. J.(2009). Dissociation of seizure traits in inbred strains of mice using the flurothyl kindling model of epileptogenesis. Exp Neurol 215(1): 60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitkänen A., Schwartzkroin P. A., Moshé S. L.(2006). Models of seizures and epilepsy. Elsevier Academic Press. [Google Scholar]
- 33.Polli R. S., Malheiros J. M., Dos Santos R., Hamani C., Longo B. M., Tannus A. Mello, Covolan L. E., L. (2014). Changes in hippocampal volume are correlated with cell loss but not with seizure frequency in two chronic models of temporal lobe epilepsy. Front Neurol 5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racine R. J.(1972). Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3): 281-294. [DOI] [PubMed] [Google Scholar]
- 35.Reddy D. S. and Kuruba R.(2013). Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. International J Mol Sci 14:18284-18318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samoriski G. M. and Applegate C. D.(1997). Repeated generalized seizures induce time-dependent changes in the behavioral seizure response independent of continued seizure induction. J Neurosci 17(14): 5581-5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samoriski G. M., Piekut D. T. and Applegate C. D.(1997). Differential spatial patterns of Fos induction following generalized clonic and generalized tonic seizures. Exp Neurol 143(2): 255-268. [DOI] [PubMed] [Google Scholar]
- 38.Scorza F. A., Arida R. M., Naffah-Mazzacoratti Mda G., Scerni D. A., Calderazzo L., Cavalheiro E. A.(2009). The pilocarpine model of epilepsy: what have we learned? An Acad Bras Cienc 81(3): 345-65. [DOI] [PubMed] [Google Scholar]
- 39.Varghese G. I., Purcaro M. J., Motelow J. E., Enev M., McNally K. A., Levin A. R., Hirsch L. J., Tikofsky R., Paige A. L., Zubal I. G., Spencer S. S. and Blumenfeld H.(2009). Clinical use of ictal SPECT in secondarily generalized tonic-clonic seizures. Brain 8):2102-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]