Summary
The nicotinamide nucleotide transhydrogenase (TH) is an integral membrane enzyme that uses the proton motive force to drive hydride transfer from NADH to NADP+ in bacteria and eukaryotes. Here we solved a 2.2 Å crystal structure of the TH transmembrane domain (Thermus thermophilus) at pH 6.5. This structure exhibits conformational changes of helix positions from a previous structure solved at pH 8.5, and reveals internal water molecules interacting with residues implicated in proton translocation. Together with molecular-dynamic simulations, we show transient water flows across a narrow pore and a hydrophobic “dry” region in the middle of the membrane channel with key residues His42α2 (chain A) being protonated and Thr214β (chain B) displaying a conformational change, respectively, to gate the channel access to both cytoplasmic and periplasmic chambers. Mutation of Thr214β to Ala deactivated the enzyme. These data provide new insights into the gating mechanism of proton translocation in TH.
Keywords: membrane protein, molecular dynamics simulations, proton channel, transhydrogenase, X-ray crystallography
eTOC blurb
Padayatti et al. solved a high-resolution crystal structure of the transhydrogenase proton channel, and combined molecular dynamics simulations to elucidate the entire proton translocation pathway. Transient water flows were observed in the channel which are regulated by conformational change and protonation of key interacting residues.

Introduction
The nicotinamide nucleotide transhydrogenase (TH) is a proton translocating membrane protein which uses the proton motive force to drive hydride transfer from the reduced form of NAD+ (NADH) to nicotinamide adenine dinucleotide phosphate (NADP+), resulting in generation of NADPH, which is necessary for many anabolic reactions and, in many organisms, for the reduction of glutathione and cellular detoxification processes.
The holo-TH is a dimer with each protomer consisting of three distinct domains, each with a specific function (Figure 1A). Two hydrophilic nucleotide binding domains, dI and dIII bind NAD(H) and NADP(H), respectively, and hydride transfer occurs across the dI/dIII interface (Venning et al., 2001; Williams et al., 1994). The third domain, dII, is comprised of between 12 and 14 transmembrane helices (depending on species) and contains a proton-conducting pathway between the bulk aqueous phases on either side of the membrane (Leung et al., 2015). Hydride transfer from NADH to NADP+ is accompanied by the translocation of one proton from the “outside” (mitochondrial intermembrane space or bacterial periplasm) to the inside (mitochondrial matrix or bacterial cytoplasm) (Jackson et al., 2015). Under most physiological conditions the proton motive force down a concentration gradient drives protons across the membrane (out → in) which is coupled to the formation of NADPH.
Figure 1. Overall structural model of holo-TH and alignment of the dimeric dII structures at pH 6.5 and pH 8.5.
A. A cartoon representation of holo-TH architecture (based on PDB ID 409U). The holo-enzyme functions as a dimer with each protomer containing two cytosolic domains designated as dI (binds NAD(H)) and dIII (binds NADP(H)), and a transmembrane domain dII (proton channel).
B. Comparison of dimeric dII structures determined at pH 6.5 (this study, shown in color) and pH 8.5 (PDB ID 4O93, in grey) shows a slight change in the transmembrane (TM) positions. The TM helices in each protomer are slightly tilted about a vertical axis near the middle of the TM bundles (indicated by a red star). Structures shown is a periplasmic view of the dII dimer from T. thermophilus which contain two polypeptide chains α2 (three TM helices numbered from 2–4 shown in purple and green) and β (nine TM helices numbered from 6–14 in orange and cyan).
The 3-domain architecture of TH is conserved in all organisms but, depending on the species, these domains may comprise 1, 2 or 3 polypeptide chains. For example, the human mitochondrial TH has all 3 domains as parts of a single polypeptide subunit, whereas the enzyme from Thermus thermophilus has 3 different polypeptide subunits (α1, α2 and β) (Figure S1) (Leung et al., 2015). X-ray structures have been determined for the isolated nucleotide-binding domains of dI and dIII from several species, both with and without bound nucleotide (Bhakta et al., 2007; Johansson et al., 2005; Prasad et al., 1999; Prasad et al., 2002; Sundaresan et al., 2005; Sundaresan et al., 2003). Domain dI has always been a dimer in these structures and the soluble part of domain dIII crystallizes as a monomer (Prasad et al., 1999; Prasad et al., 2002). When these hydrophilic domains are combined in solution, they form a stable heterotrimer consisting of a dimer of domain dI and one copy of domain dIII (PDB ID 4J1T) (Bhakta et al., 2007). Nucleotide binding results in local conformational changes at the binding sites but there is no indication that the redox state of the nucleotides is associated with substantial conformational changes of the polypeptide away from the binding sites (Bragg and Hou, 2001; Sundaresan et al., 2003).
Recently, the structure of the isolated transmembrane domain (TMD) dII from T. thermophilus TH was determined along with a low resolution structure of the holo-enzyme (Leung et al., 2015). The membrane domain was solved at 2.8 Å resolution and was shown to be a dimer, with each protomer containing 12 transmembrane helices. The structure also revealed a putative proton channel within each protomer, flanked by transmembrane helices 3, 4, 9, 10, 13 and 14 (Throughout this manuscript the TM helix is numbered according to the topology of the human TH sequence which contains 14 TM helices; TM1 and TM5 are missing in the TH sequence from T. thermophilus (Figure S1)). The residues involved in the proton channel was previously identified by site-directed mutagenesis studies on the homologous E. coli TH as being important for function (Bragg and Hou, 2001; Olausson et al., 1995; Rydstrom et al., 1998; Yamaguchi et al., 2002). The structure of the holo-enzyme was solved to 6.9 Å and, as expected, showed the enzyme to be a dimer (Figure 1A) (Leung et al., 2015). Although the low resolution precluded any detailed analysis, the structure did show that the two copies of domain dIII in the dimer had very different orientations. In one protomer, domain dIII is oriented with its NADP(H) binding site adjacent to the domain dI NAD(H) binding site. In the second protomer, however, domain dIII is rotated so that the NADP(H) binding site is adjacent to the membrane domain. Crosslinking studies verified that both orientations were present in the preparation. These data are the basis of a proposed enzyme mechanism in which dIII alternates between a face-down orientation allowing NADP(H) binding to a face-up orientation allowing hydride transfer. In the face-down orientation, the redox state of bound NADP(H) determines whether a histidine residue in the middle of the membrane domain dII is protonically equilibrated to the cytoplasmic or periplasmic aqueous phase (Cotton et al., 1989; Jackson et al., 1999). In the face-up orientation, the NADP(H) bound to dIII comes into direct contact with the NAD(H) in dI to allow rapid, direct hydride transfer.
The pH is known to have a dramatic effect on the channel and enzymatic activity of TH (Bragg and Hou, 2001). A central histidine residue in the dII domain of TH has been postulated to interchange between protonated and non-protonated states to gate the channel access to either side of the membrane (Hutton et al., 1994). In the current work, the X-ray structure of the isolated membrane domain dII from the T. thermophilus TH has been determined in lipids at pH 6.5 to a resolution of 2.2 Å, revealing a conformational change in helix positions compared to the previous structure determined at pH 8.5 (Leung et al., 2015) along with internal water molecules found to interact with key residues within the putative proton channel. Molecular dynamics studies were conducted to examine the penetration of water molecules in a model of the TH protein embedded in a lipid bilayer. The results show water-filled chambers on both the periplasmic and cytoplasmic sides of the membrane as well as transient formation of water-wires across a narrow pore and a hydrophobic region in the middle of the membrane with key residues including Thr214β and His42α2 switched on to gate the channel access. Our data is consistent with biochemical evidence for the putative channel pathway in TH but provides new insights into the mechanism of the channel gating and proton translocation.
Results
X-ray crystallography
The construct of T. thermophilus domain dII used for crystallization contains two hydrophobic polypeptide chains: subunit α2 and a truncated version of subunit β from which the hydrophilic domain dIII has been removed from the C-terminus. Subunit α2 contains 3 transmembrane helices (TM2, TM3 and TM4; TM1 is present in the human sequence but missing in T. thermophilus) and the truncated β subunit contains 9 transmembrane helices (TM6 through TM14; TM 5 absent in T. thermophilus). The structure was solved to a resolution of 2.2 Å (Table 1) at pH 6.5 using lipidic cubic phase (LCP) crystallization with 1-(8Z-pentadecenoyl)-rac-glycerol (MAG 8.7, Avanti Polar Lipids, Alabaster, Alabama) as a host lipid. Overall, the new structure is similar to the previously reported one at pH 8.5 (PDB ID 4O93) (Leung et al., 2015). In the new crystal form (space group C2221), the asymmetric unit contains one molecule, and the biological dimer is represented by a crystallographic-symmetry related molecule. In the previously reported pH 8.5 structure, the asymmetric unit (space group C121) contains a biological dimer. Alignment of the pH 6.5 and pH 8.5 dimers (Figure 1B) (4,547 atoms) shows a RMSD of 1.24 Å for all atoms. The transmembrane helices (except the TM2) in the new structure are slightly tilted about a fulcrum near the middle of the TMD when compared to the pH 8.5 structure, thus showing a degree of conformational flexibility in the dII dimer (Figure 1B shows the periplasmic view).
Table 1.
Crystallographic data collection and refinement statistics
| α2:β(truncated) pH 6.5 | |
|---|---|
| Asymmetric unit | 1 monomer |
| Solvent content (%) | 54.3 |
| VM (Å3/Da) | 2.63 |
| Space group | C2 2 2 1 |
| Unit cell, a, b, c (Å) | 84.86,108.94,109.34 |
| α, β, γ (°) | 90, 90, 90 |
| Source | NECAT/ APS 24ID-C |
| Wavelength (Å) | 0.97910/1.03321 |
| Resolution range (Å) | 42.34 - 2.2 (2.27 - 2.20) |
| Total reflections | 64,201 (5487) |
| Unique reflections | 24,971 (2,189) |
| Completeness (%) | 96.7(93.5) |
| Redundancy | 2.6 (2.5) |
| I/σI | 10.1 (1.7) |
| Rmerge | 0.060 (0.664) |
| Rpim | 0.052 (0.525) |
| Reflections/test set | 24,951/1,310 |
| R-work/R-free | 0.188/0.229 |
| Rms Bonds (Å) | 0.019 |
| Rms Angles (°) | 1.78 |
| Phi, Psi geometry | 99.6% allowed |
| Average B factor | 47.0 |
| Wilson B (Å2) | 39.2 |
| Protein | 46.4 |
| Ligands | 71.6 |
| Water | 57.2 |
Numbers in parentheses are statistics of the highest resolution shell.
A total of 49 water molecules were observed bound to the protein in the current structure (Figure 2). Partial densities for two highly flexible lipid molecules (MAG 8.7) and several additional densities were modelled in the structure as bound additives from the crystallization solution, including polyethylene glycol and benzamidine-HCl (Figure 2A).
Figure 2. Cartoon representation of overall structure of dII domain at pH 6.5 and electrostatic potential displayed on solvent-accessible surface.
A. TM helices from the α2 subunit are shown in green. TM helices from the β (truncated) subunit are shown in cyan. The positions of all solvent molecules observed crystallographically are represented as balls. Other small molecules modelled into the density around the protein are represented as stick representation: MAG 8.7 (pink), Benzamidines (grey), and PEG 400 (yellow). I, II and III are views of dII domain from periplasm, side profile, and from cytosol, respectively.
B. The electrostatic potential displayed on the solvent accessible surface of membrane domain of TH. The scale shown as kT/e, at pH 7.0 and 25 °C. The potential gradient is shown with electropositive (blue) to electronegative (red) regions. The calculations are performed using APBS tool in Pymol. Putative channel entry points are indicated on both the periplasmic side and the cytosolic side.
Results from a channel-search algorithm (Voss and Gerstein, 2010) showed the presence of two solvent-accessible chambers, one on each side of the protein, which is consistent with findings in molecular dynamics (MD) (vide infra). The total volume of the chamber accessible to solvent on the cytosolic side is 543 Å3 with a surface area of 791 Å2, which are significantly larger than the total volume and surface area of the water-accessible chamber on the periplasmic side of the protein (volume 314 Å3 and area 376 Å2). The periplasmic chamber however reaches deeper into the proposed channel in comparison to the cytosolic chamber.
Electrostatic potential calculations for the solvent accessible surfaces (Figure 2B) showed cytosolic surface to be prominently electropositive (shown in blue) compared to periplasmic side. A comparison of crystallographically observed solvent positions with our calculations of solvent-accessible sites showed close agreement (Figure 2A&B).
We observed nine water molecules buried within the membrane-embedded part of TH in the pH 6.5 crystal structure (Figure 3). The placement of internal waters in the structure around conserved residues inside the channel were confirmed crystallographically by repeated analysis of several data sets resolved to similar resolutions. In the pH 6.5 structure, one water molecule (W1) observed within the cytoplasmic chamber is hydrogen bonded to His31α2, two water molecules (W2, W3) are part of the hydrogen bond network surrounding His42α2 in the middle of the membrane, and six water molecules are on the periplasmic side of domain dII (W4 – W9).
Figure 3. The pH 6.5 structure of dII showing the 9 bound internal water molecules.
W1 to W3 are shown as red balls. W4 to W6 are shown as purple balls. W7 to W9 are shown as orange balls. TM helices from the α2 subunit are shown in green. TM helices from the β (truncated) subunit are shown in cyan. I, II and III are views of dII domain from periplasm, side profile and cytosolic respectively. Putative channel entry positions are indicated by red arrows in the middle structure.
The interactions of these internal water molecules are shown in Figures 4 and 5. The locations of W1, W2 and W3 are shown in Figure 4A, and in greater detail for W2 and W3 in Figure 4B. The location of W1 marks the external end of the shallow cytoplasmic chamber (Figure 4A) and the surface of this chamber contains many polar residues. His31α2 that is hydrogen bonded to W1 is a highly conserved residue in all transhydrogenases. Residues participating in an extensive hydrogen bond network near the middle of the membrane (Ser38α2, Ser36α2, Asn39α2, His42α2, Asn75α2, Asn89β, Ser208β, Asn211β and Thr245β) are also highly conserved across different species. These residues and associated water molecules are likely positioned to provide a conduit for proton translocation.
Figure 4. The Cytosolic chamber of TH proton translocation machinery.
A. A view from the top of the dII domain from its cytosolic end. Three water molecules (W1, W2 and W3, purple balls) together with a cluster of hydrogen-bonded polar residues are shown to provide a backbone for the proton translocation. The Asp202β on the cytosolic end of TM13 is the last link from the transmembrane domain dII and its connection with Arg254β on a cytosolic loop (CL8) brings the communication link between dII and the cytosolic domain dIII (Leung et al., 2015).
B. The representation of the microenvironment of His42α2, and associated hydrogen bonded residues and water molecules (W2 and W3). Right and left panels are same views with a rotation of view point as indicated by the arrow. The yellow dashed lines represent the shortest distances. The two red balls represent waters W2 and W3 that are bound to Asn211β, and Asn39α2 respectively. The green mesh represent the |Fo-Fc| omit map contoured at 3.5 σ.
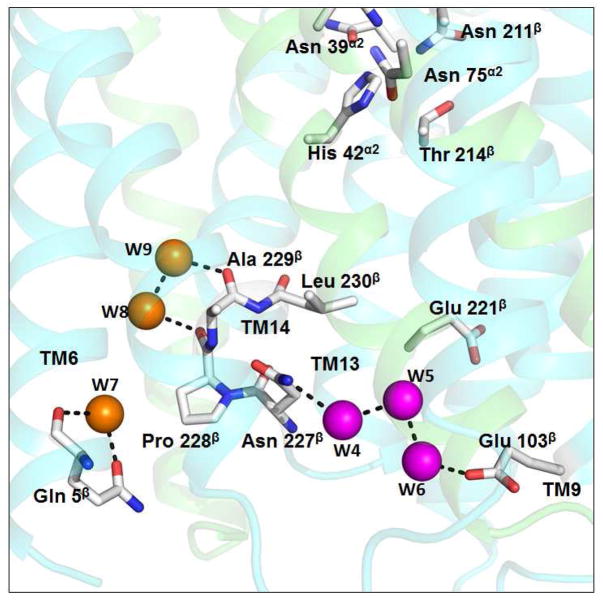
Figure 5. The proton transport machinery in periplasmic chamber.
Water molecules W4, W5 and W6 are clustered at the periplasmic entrance and hydrogen-bonded to Asn227β and Glu103β. The water molecules W8 and W9 are hydrogen bonded to W4 through main chain interactions involving residues Pro228β and Ala229β. The other major polar residue in the vicinity that may participate in the proton conduction from periplasmic chamber is Glu221β whose side chain in this structure is turned away from the channel.
Figure 5 shows the locations of the six water molecules on the periplasmic side of the pH 6.5 protein structure. Near the entrance of the deep periplasmic chamber (Figure 3), a cluster of three water molecules (W4, W5 and W6) forms a hydrogen bonded chain that connects Glu103β to Asn227β. Three additional water molecules (W7, W8 and W9) on the periplasmic side of the membrane form part of a chain that could represent a route for proton movement from the periplasmic aqueous phase to His42α2. Both W8 and W9 are part of a hydrogen bond network that includes Asn227β which is within the periplasmic chamber (Figure 5).
Molecular dynamics simulations
MD simulations were performed to determine the extent of access by water to both the periplasmic and cytoplasmic chambers (Figure 6). The blue-transparent surface shown in Figure 6A is a map highlighting regions within the protein that were highly occupied by water molecules during the simulation. This surface corresponds to the time-average probability of at least 20% for inserted water molecules. The simulation also showed that W3 remained bound throughout the 300-ns period, whereas W2 exchanged with the cytosol.
Figure 6. MD simulations of water flows in the dII domain.
A. Water positions determined in 300 ns MD simulations with His42α2 being fully protonated or cationic. The blue-transparent surface is a map highlighting regions within the protein that were highly occupied by water molecules and corresponds to the time-average probability of at least 20% for water insertion. A notable feature is the clear interruption of the proton channel by a “dry region” in the middle of the channel between Glu221β and Thr214β (shown within a vertical bracket), indicated by the discontinuity of the map, which became hydrated only transiently during the simulations as illustrated in Figure 7A. Blue arrow lines highlight the putative water entrance(s) from the periplasm. Thr214β is at −60 degree with respect to its N-Cα-Cβ-Cγ dihedral angle.
B. The time-average water content along each section of the proton channel. The solid blue represents the “water wire”, a region where connections form between polar residues within the cytosolic chamber at a certain point during the simulation. The grey line represents His42α2 and associated hydrogen bonded residues that forms the gating region separating the periplasmic chamber and the cytoplasmic chamber. The periplasmic chamber is connected to this gated barrier by the “dry region” (indicated by the orange line) comprised of hydrophobic aliphatic amino acids.
C. The average pore radii along the proton channel are calculated from the MD trajectory using the HOLE program (Smart, 1996).
Water occupancy profiles (< Nwat>) show water accessibility along the z axis of the channel (Figure 6B). A notable feature is a dry region that separates the periplasmic chamber from His42α2 by about 7.5 Å (Figures 6A and 6B). This region is surrounded by nonpolar aliphatic residues (Ala and Val), accounting for the minimal hydration. A pore radius profile along the z axis (Figure 6C) was determined to illustrate the degree of opening and accessibility of the proposed channel of the putative proton transfer pathway. The pore profile shows a narrow region (< 2 Å radius) that included both the dry region on the periplasmic side of His42α2 and the region from His42α2 to the cytoplasmic side.
The MD simulations were performed both with the assumption of His42α2 being in the cationic protonated state or in deprotonated neutral state. With His42α2 deprotonated, no water was observed to penetrate the dry region between His42α2 and the periplasmic chamber during the whole MD run of 300 ns. However, with His42α2 in the protonated state, a transient water wire was observed to form between Glu221β and His42α2 (between z = −2Å and z = 5Å, Figure 6A). In addition, a second transient water wire, which was present for most of the time during the simulation, was observed between His42α2 and Ser208β in the cytoplasmic chamber (between z = 5Å and z = 15Å) (Movie S1).
Figure 7A shows a snapshot of a water wire connecting His42α2 and Glu221β in the periplasmic chamber that was observed in a particular 300-ns simulation between 150 ns and 170 ns. The presence of the water wire coincided with a conformational change of Thr214β side chain located slightly below His42α2 (Figure 7B). Figure 7C shows the abrupt changes in the number of water molecules occupying this region of the protein corresponding to the formation and dissipation of the water wire. When the water wire had formed, the Thr214β side chain χ1 dihedral angle underwent a transition from −60° to +60° such that its Oγ atom rotated out of the proton pathway (Movie S1).
Figure 7. MD simulations revealed transient formation of water wires between Glu221β and Thr214β.
A. During the simulation, the “dry region” becomes transiently hydrated, resulting in the formation of a water wire establishing communication between the periplasmic chamber and His42α2. This is the result of a movement of the N-Cα-Cβ-Cγ dihedral angle of Thr214β from −60 to +60 degrees.
B. Conformational dynamics of Thr214β side chain during the simulation, quantified by its N-Cα-Cβ-Cγ dihedral angle.
C. Number of water molecules localized at the “dry region” of the proton channel during the simulation. The formation of water wires occurs between 150 and 170 ns when Thr214β is at +60 degree.
Enzymatic Activity of the Thr214βAla TH mutant
Thr214β was identified as a critical residue for proton transfer both in the crystal structure and in MD simulations. We tested its importance experimentally by generating and characterizing the Thr214βAla mutant TH. Compared to wild type, purified samples of the full-length mutant enzyme showed no significant loss of yield or any difference in its size-exclusion chromatography profile. We compared the enzymatic activities of the Thr 214βAla mutant to wild type TH using a well-established reverse TH activity assay (Rydstrom et al., 1998). The reverse TH activity measures NADPH reduction of an NAD+ analog (AcPyAD+) and this process is linked to proton pumping (Leung et al., 2015; Persson et al., 1987). Reverse TH activity of the Thr 214βAla mutant was diminished to only 3–5% of that measured for wild type at all three pHs analyzed (pH 6.5, 7.0 and 8.0) (Table 2).
Table 2. Reverse transhydrogenase activity of wild type and Thr214βAla mutant.
Values are represented as percentages of wild type enzyme activity measured at pH 8.0, which averaged 0.51 ± 0.07 μmole/mg/min (standard error from quadruple measurements).
| WT | Thr214βAla | |
|---|---|---|
| pH8.0 | 100 | 5 |
| pH7.0 | 82 | 5 |
| pH6.5 | 100 | 3 |
Discussion
The physiological purpose of the nicotinamide nucleotide transhydrogenase in most organisms is to utilize the proton motive force to drive hydride transfer from NADH to NADP+ to help maintain the pool of reduced NADPH (Nickel et al., 2015). One proton is translocated across the membrane (bacterial cytoplasmic membrane or mitochondrial inner membrane) for each hydride transfer (Hutton et al., 1994). The new high-resolution structure of the TMD, along with MD simulations and functional studies presented in the current work, support the previously proposed proton-conducting channel within TMD (dII) of the transhydrogenase (Leung et al., 2015).
Comparison of the dimeric dII structures solved at pH 6.5 vs 8.5 reveals only a small degree of conformational flexibility in the TMD region. It is possible that conformational changes in dII are related to the proton translocation or enzymatic activities affected by pH (Bragg and Hou, 2001), but further evidences are still needed to support this view. In this study both crystallographic as well as simulated water molecules are found within cytoplasmic and periplasmic invaginations on either side of His42α2, which is a functionally critical residue located in the middle of the membrane. His42α2 is postulated to be transiently protonated (Hutton et al., 1994) during proton translocation across the membrane and is shown here to be part of a hydrogen bond network of highly conserved polar residues together with two water molecules. The protein conformation captured in the pH 6.5 crystal structure allows water access between His42α2 and the cytoplasmic surface (Figures 6A and 7A), but a dry region of the protein separates His42α2 from the water-filled periplasmic cavity of the protein. Interestingly, our MD simulations indicate that transient water flows across this dry region only occur when His42α2 is in the protonated state, which is more likely at pH 6.5 or near a physiologic pH than at basic pHs. The current data are thus consistent with a model previously postulated for dII-mediated proton translocation (Jackson et al., 2015; Jackson et al., 1999; Leung et al., 2015) that occurs through a dynamic protonation and deprotonation of a central histidine residue. The proton motive force favors protonation of His42α2 from the periplasm and deprotonation to the cytoplasm and drives the chemical reaction in the forward direction, generating NADPH.
The current structure is of the TH isolated from T. thermophilus, whereas most of the biochemical characterization has been performed with the enzyme from E. coli (Bragg and Hou, 2001; Fjellstrom et al., 1999; Hu et al., 1995; Meuller and Rydstrom, 1999). In comparison, T. thermophilus TH has 924 amino acid residues divided among 3 polypeptide chains (α1, α2, β) containing 12 transmembrane helices, the E. coli TH has 972 residues divided between 2 polypeptide chains (α and β) with 13 transmembrane helices. Altogether, the sequences are 40% identical, with high conservation in portions of the TMD which are thought to be part of the proton channel. Site-directed mutagenesis on the TH from E. coli identified residues within the TMD that were concluded to be likely components of the proton channel: His91β, Ser139β, Gly252β and Asn222β. These residues correspond, respectively in the T. thermophilus TH, are Asn89β, Ser132β, Gly238β and Asn211β (Leung et al., 2015). Each of these enzymes has a single His residue in the channel that is near the middle of the membrane, but in the T. thermophilus TH the His is in TM3 (His42α2) and in the E. coli TH, the His is in TM9 (His91β). Nearly all homologous TH enzymes have sequence motifs similar to either the T. thermophilus TH or the E. coli TH. The representation of the hydrogen bond network in the T. thermophilus TH channel in Figure 4A can be converted to that in the E. coli TH by replacing Asn89β to a histidine and His42α2 to a serine.
The 2.2 Å structure of the transmembrane domain at pH 6.5 reveals nine water molecules (Figure 3). Two of these water molecules (W2 and W3) are hydrogen bonded to asparagines that are, in turn, hydrogen bonded to the central histidine (His42α2) and are part of the hydrogen bond network surrounding this residue. MD simulations further support the critical role of water molecules in forming hydrogen-bond “wires” to facilitate proton access to His42α2. The simulations in which His42α2 is protonated, water has a relatively high occupancy in a shallow invagination on the cytoplasmic side that reaches down as far as His42α2. Both W2 and W3 as well as W1 (Figure 4) are within the cytoplasmic chamber. In the simulations, W2 can be observed exchanging with bulk water on the cytoplasmic side of the membrane (Movie S1).
The X-ray structure also includes six additional water molecules (W4 – W9) on the periplasmic side of the membrane (Figure 5). MD simulations reveal a deep periplasmic cavity that allows water ready access to within about 7.5 Å from His42α2 (Figure 6A). Crystallographic water molecules W4, W5 and W6 are located in this periplasmic chamber. In MD simulations there is a dry region between His42α2 and the water-filled periplasmic chamber serving as a barrier to water access to His42α2. When His42α2 is protonated in the simulations, however, an occasional water wire is observed to penetrate through this barrier (Movie S1). The transient formation of this water wire requires rotation of the side chain of Thr214β, as shown in Figure 7. It is not clear whether this conformational change is relevant to the mechanism of the enzyme in vivo, but it does illustrate how small conformational changes can influence the pathways for proton diffusion through the protein.
Three additional water molecules, W7, W8 and W9 (Figure 5), are also observed crystallographically on the periplasmic side of the membrane but not part of the periplasmic aqueous chamber. These three water molecules suggest another possible route for water molecules to penetrate to His42α2 from the periplasmic bulk aqueous phase.
The current work is the first to implicate Thr214β as being important for proton translocation. This was first indicated by the MD computational work which correlated formation of a water wire within the proton channel to the conformation of the Thr214β side chain. Assays of Thr214βAla mutant TH activity (Table 2) demonstrated the loss of the enzymatic activity in the proton channel coupled assays.
We note that the current structure and MD simulations are for the isolated transmembrane domain of TH. The extramembranous nucleotide binding domains (dI and dIII) have been removed from this structure. Understanding the coupling mechanism of TH requires knowledge of how the binding of nucleotides to dI and dIII influences the proton channel in dII and vice versa. Assuming that protonation/deprotonation of the central histidine (e.g., His42α2 in the T. thermophilus TH) is part of the mechanism of proton translocation across the membrane, the proton channel may exist in other states that are stabilized by the binding of nucleotides in soluble domains. Certainly, high-resolution structures of the holo-TH in different states would be most important to clarify how these events are coupled.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by Qinghai Zhang, qinghai@scripps.edu. The MTA and other corporate partnerships at The Scripps Research Institute is handled directly through the Office of the Technology Development (http://www.scripps.edu/research/technology).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
The complete operon used for the expression of holo-TH was sub-cloned into bacterial expression vector pET21a. This vector is the primary source for all other sub-cloning are explained in detail under methods section. The bacterial strains used for sub-cloning and expression include DH5α and BL21DE3Δ Acr (knockout strain of bacterial endogenous efflux pump AcrAB).
METHOD DETAILS
Protein expression and purification
The protein construct used for current structural study included the transmembrane domain dII from Thermus thermophilus which constitutes α2 domain (chain-A, 1–94) and β domain (chain-B) truncated at residue 261. The expression and purification of dII were done as described previously (Leung et al., 2015). The final purified dII domain was obtained by size exclusion chromatography in a buffer containing 0.05 M Tris pH 8.0 with 0.1 M NaCl, 0.125 M Sucrose, 0.01% β-D-dodecyl maltoside (DDM, Anatrace, Maumee, OH) and protease inhibitor cocktail (Roche, Indianapolis, IN). The peak fractions were further concentrated to 60 mg/ml. The Thr214β-Ala mutant was generated using polymerase chain reaction-based site-directed mutagenesis protocol. Expression and purification of the wild type and mutant enzyme was performed as follows. The complete operon of TH from T. thermophilus (α2+β) with a C-terminal His-tag was co-expressed with polypeptide α1 in a pETDuet-1 bacterial expression vector (EMD Millipore, Billerica, MA). Proteins were co-purified from the overexpressed bacterial membranes by 6xHis affinity chromatography and Superose 6 gel filtration chromatography. The final purified protein was eluted into a buffer containing 0.05 M Tris pH 8.0 with 0.1 M NaCl and 0.01 % DDM. The presence of all the three polypeptides and association of polypeptide α1 with rest of the polypeptides (α2+β) was confirmed on SDS-PAGE after the final concentration step before used in the assays. Concentrations of purified proteins were determined using a SDS-PAGE based quantification using known concentrations of bovine serum albumin as standards on the gel.
LCP crystallization and data collection
LCP crystallization was performed by mixing protein samples (60 mg/ml) with MAG 8.7 at a protein/lipid ratio of 1:1 using a mechanical syringe mixer (Caffrey and Cherezov, 2009). The mixture was dispensed onto 96-well glass sandwich LCP plates (Marienfeld, Hampton Research, Aliso Viejo, CA) using a Gryphon LCP robot (Art Robbins, Sunnyvale, CA) as 25 nl boluses, around which 0.8 μl of precipitants were dispensed (Cherezov, 2011).
The dII domain crystals reported previously (PDB ID 4O93, transmembrane domain truncated at residue Leu261/B at 2.8 Å) were grown in LCP using precipitant solutions containing 0.1 M Tris-HCl pH 8.5, 0.3–0.4 M NH4-formate, 0.1 M Na-thiocyanate, and 18% (v/v) 1-4-butanediol (Leung et al., 2015). Repeated attempts failed to improve the resolution of these crystals. A search for new crystallization conditions resulted in identifying new crystal forms grown at pH 6.5. The pH 6.5 structures reported here were obtained from crystals grown in LCP made of MAG 8.7 and precipitant containing 30 % PEG 400 in 0.1 M MES pH 6.5 with 0.1–0.4 M magnesium nitrate and 1 to 2.5% benzamidine hydrochloride as additive. The data on LCP microcrystals were collected using synchrotron micro-crystallography and rasterring techniques implemented at GM/CA (23-ID-D) and NECAT (24-ID-C) beamlines of APS (Advanced Photon Source at Argonne National Labs) and processed to 2.2 Å resolution (Cherezov et al., 2009). The crystals used for data collection were 10 to 15 microns in the longest dimensions. A microbeam of 10 μm (wave length 1.03321) was used during the rasterring with 20-fold attenuation of the beam, 0.2° oscillation and 0.2 seconds exposure. After locating the crystals using automated rasterring tab on JBlueIce, the crystals were centered automatically by moving onto the corresponding grids that displayed the diffraction. Data sets of 20 to 30 degrees rotation per crystal were collected at a sample-to-detector (PILATUS) distance of 400 mm with 0.2° oscillation and 0.2 seconds exposure.
All diffraction data were processed and scaled using the programs XDS (Kabsch, 2010) and aimless/pointless (Evans, 2011). Initial phasing information was obtained by molecular replacement with Phaser (Mccoy et al., 2007) using a model of the dII domain monomer (PDB ID 4O93) as the search model. The solution was further refined through alternate cycles of model building in Coot (Emsley et al., 2010) and refinement in Refmac5 (Murshudov et al., 2011) until the data and model converged. Table 1 gives the data collection and refinement statistics. The refinement converged with a final Rwork = 18.8 % and Rfree = 22.9 %. The final resolution for the structure was truncated at 2.2 Å.
Channel Search protocols
A channel search was performed using a rolling probe method as described previously (Voss and Gerstein, 2010). The largest probe used had a radius in the range 5 to 6 Å, while a smaller probe radius ranged from 1.5 to 3 Å. Refined coordinates of the membrane domain (α2+β truncated), with and without the heteroatoms were used as the macromolecule for separate analysis. Among the many surface regions identified as possible channel entrances, one was found at the periplasmic side reaching deep into the protein transmembrane helices; and another on the cytosolic side shallower but with larger surface was selected. These were prioritized for further analysis.
Molecular Dynamics Simulations
Construction of in silico membrane-embedded transhydrogenase
MD simulations were performed for the dimeric dII domain of T. thermophilus TH. The system used for simulation was modeled with the dII dimer embedded in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) membrane, and was described by the CHARMM36 force field (Best et al., 2012; Klauda et al., 2010; MacKerell et al., 1998) Since the crystal structure provided the coordinates for only the heavy atoms, hydrogen atoms were introduced using PSFGEN of VMD (Humphrey et al., 1996). Twenty-three water molecules were modeled within the protein using DOWSER (Zhang and Hermans, 1996). Each His42α2 was treated as a “+1 charged residue” with both Nε and Nδ atoms of the imidazole ring protonated. To model the protein-embedded membrane complex, the first principal axis of the protein was aligned with the z axis using the OPM (Orientations of Protein in Membranes) database (Lomize et al., 2006; Lomize et al., 2012). The CHARMM-GUI webserver24 was used to model 390 lipid molecules around the aligned protein. The system was then solvated with 24,500 water molecules and NaCl at 200 mM, comprising of 90 Na+ and 99 Cl− ions, for a total of 133,000 atoms.
Simulation protocols
MD simulations comprised the following steps: (1) 0.5-ns simulation with restraints imposed on non-hydrogen atoms of the protein and 23 internal water molecules; (2) 0.5-ns simulations with backbone atoms of the protein and heavy atoms of the internal water molecules restrained; (3) 1-ns simulation with protein Cα atoms and internal water oxygen atoms restrained; (4) 1-ns simulation with only protein Cα atoms restrained; and (5) 300-ns unrestrained relaxation. The beginning of Steps 1, 2 and 3 were associated with 1000-step energy minimization. Harmonic potentials with the force constant k = 1 kcal/mol/Å2 were applied in the restrained simulations.
The simulations were performed using NAMD2 (Phillips et al., 2005) with a time step of 2 fs, the periodic boundary condition (PBC), a flexible cell which allows the system to change its dimension independently, and the isothermal-isobaric ensemble. All bonds involving hydrogen atoms were kept rigid using the SHAKE algorithm (Ryckaert et al., 1977). The particle mesh Ewald (PME) method (Darden et al., 1993) with a grid density of 1/Å3 was used to evaluate long-range electrostatic interactions in PBC without truncation. The cutoff for van der Waals interactions was set at 12 Å. The temperature was maintained at 310 K by Langevin dynamics with a damping coefficient γ of 1 ps−1. The Nose-Hoover Langevin piston method with a piston period of 200 fs was used to maintain the pressure at 1 atm.(Feller et al., 1995; Martyna, 1994)
Activity measurements of T. thermophilus holo-TH wild type and Thr214βAla mutant
Reverse TH activities for purified samples of both wild type and the mutant Thr214β-Ala were assayed as previously described (Yamaguchi et al., 2002). Reverse TH activity is measured as hydride transfer from NADPH to 3-acetylpyridine adenine dinucleotide (AcPyAD+, a NAD+ analogue), which is known to be coupled to proton transport (Yamaguchi and Stout, 2003). The details of this assay are described elsewhere (Jackson, 1991; Olausson et al., 1993; Sazanov and Jackson, 1995). The hydride transfer from NADPH to a NAD+ analogue, 3-acetylpyridine adenine dinucleotide (AcPyAD+) was assayed spectrophotometrically at 375 nm at room temperature in a reaction mixture volume (100 μl) containing 50 mM Tris HCl (adjusted to pH 6.5, 7.0 and 8.0, respectively), 1 mg/ml lysolecithin, 0.5 % Brij35, 0.3 mM β-NADPH, 0.3 mM AcPyAD+ and pure protein (~ 10 to100 μg). All data points used in the analysis were from at least four repeated measurements.
QUANTIFICATION AND STATISTICAL ANALYSIS
The statistical analysis of the assay data of standard deviations are obtained from the MS EXCEL built-in features.
DATA AND SOFTWARE AVAILABILITY
The co-ordinates for the structures reported here are deposited in PDB. The PDB IDs are 5UNI, 4O9U, and 4O9T.
Supplementary Material
The movie is from a simulation trajectory over the course of 300 ns, highlighting the putative proton channel in the dII domain of Thermus thermophilus TH. The proton loading site in the middle of the channel is separated from the cytoplasmic surface by a dry region. The formation of a water wire in this dry region is presumably critical for the enzymatic activity and is found during the simulation to involve a conformational change of Thr214. The movie is generated using VMD.
HIGHLIGHTS.
A 2.2 Å structure of transhydrogenase proton channel was determined in lipids.
The structures solved at two different pHs show conformational flexibility.
Water molecules and key interacting residues were revealed in the proton channel.
Molecular dynamics provide insights into the mechanism of channel gating.
Acknowledgments
We would like to dedicate this paper to the loving memory of Dr. C. David Stout (1947–2016) who enormously influenced all of us and was the driving force behind the understanding of transhydrogenase membrane protein structural studies. We thank synchrotron staff Sheila Trznadel, Craig Ogata and Steven Corcoran at APS (23ID-D and 24ID-C); David Neau of NECAT, Lisa Dunn, Doukov Tzanko and Mathews Irimpan of SSRL at experimental station 12-2 for generous beamtime help; Eric Johnson and Duncan McRee for comments on the manuscript. This work is supported by NIH grants 5R01GM103838 (to QZ) and R01HL16101 (to RBG). The atomic co-ordinates and structure factors have been deposited in the RCSB Protein Data Bank (PDB ID 5UNI). Molecular dynamics simulation resources were provided to RBG by the startup XSEDE grant TG-MCB150029. Use of Advanced Photon Source at GM/CA@APS has been funded in whole or in part with Federal funds from the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. The Eiger 16M detector was funded by an NIH–Office of Research Infrastructure Programs, High-End Instrumentation Grant (1S10OD012289-01A1). The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the NIGMS (including Grant P41GM103393).
Footnotes
Conflict of interests: Authors declare no conflicts of interest
Author Contributions:
P.S.P. performed the protein expression, purification, site directed mutagenesis, TH assays, crystallography, structure solution and refinement. J.H.L. made TMD and wild type holo-TH expression constructs. P.M. and E.T. performed MD simulations. A.I. and V.C. provided support for synchrotron data collection. V.C. provided suggestions in LCP crystallization and harvesting micro-crystals from sponge phase. S.M.S provided experimental support at the beamline. J.B.J. commented and edited the manuscript. C.D.S. involved in initial discussions and analysis of the data. P.S.P., P.M., R.B.G. and Q.Z. analyzed the data and wrote the paper. All authors participated in critical reading.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Best RB, Zhu X, Shim J, Lopes PE, Mittal J, Feig M, Mackerell AD., Jr Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J Chem Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta T, Whitehead SJ, Snaith JS, Dafforn TR, Wilkie J, Rajesh S, White SA, Jackson JB. Structures of the dI2dIII1 complex of proton-translocating transhydrogenase with bound, inactive analogues of NADH and NADPH reveal active site geometries. Biochemistry. 2007;46:3304–3318. doi: 10.1021/bi061843r. [DOI] [PubMed] [Google Scholar]
- Bragg PD, Hou C. Characterization of mutants of beta histidine91, beta aspartate213, and beta asparagine222, possible components of the energy transduction pathway of the proton-translocating pyridine nucleotide transhydrogenase of Escherichia coli. Arch Biochem Biophys. 2001;388:299–307. doi: 10.1006/abbi.2001.2298. [DOI] [PubMed] [Google Scholar]
- Caffrey M, Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nat Protoc. 2009;4:706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V. Lipidic cubic phase technologies for membrane protein structural studies. Curr Opin Struct Biol. 2011;21:559–566. doi: 10.1016/j.sbi.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V, Hanson MA, Griffith MT, Hilgart MC, Sanishvili R, Nagarajan V, Stepanov S, Fischetti RF, Kuhn P, Stevens RC. Rastering strategy for screening and centring of microcrystal samples of human membrane proteins with a sub-10 microm size X-ray synchrotron beam. J R Soc Interface. 2009;6(Suppl 5):S587–597. doi: 10.1098/rsif.2009.0142.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton NP, Lever TM, Nore BF, Jones MR, Jackson JB. The coupling between protonmotive force and the NAD(P)+ transhydrogenase in chromatophores from photosynthetic bacteria. Eur J Biochem. 1989;182:593–603. doi: 10.1111/j.1432-1033.1989.tb14868.x. [DOI] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L. Particle Mesh Ewald - an N.Log(N) Method for Ewald Sums in Large Systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PR. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr D. 2011;67:282–292. doi: 10.1107/S090744491003982X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller SE, Zhang YH, Pastor RW, Brooks BR. Constant-Pressure Molecular-Dynamics Simulation - the Langevin Piston Method. J Chem Phys. 1995;103:4613–4621. [Google Scholar]
- Fjellstrom O, Axelsson M, Bizouarn T, Hu X, Johansson C, Meuller J, Rydstrom J. Mapping of residues in the NADP(H)-binding site of proton-translocating nicotinamide nucleotide transhydrogenase from Escherichia coli. A study of structure and function. J Biol Chem. 1999;274:6350–6359. doi: 10.1074/jbc.274.10.6350. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang JW, Persson A, Rydstrom J. Characterization of the interaction of NADH with proton pumping E. coli transhydrogenase reconstituted in the absence and in the presence of bacteriorhodopsin. Biochim Biophys Acta. 1995;1229:64–72. doi: 10.1016/0005-2728(94)00187-a. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Hutton M, Day JM, Bizouarn T, Jackson JB. Kinetic resolution of the reaction catalysed by proton-translocating transhydrogenase from Escherichia coli as revealed by experiments with analogues of the nucleotide substrates. Eur J Biochem. 1994;219:1041–1051. doi: 10.1111/j.1432-1033.1994.tb18587.x. [DOI] [PubMed] [Google Scholar]
- Jackson JB. The Proton-Translocating Nicotinamide Adenine-Dinucleotide Transhydrogenase. J Bioenerg Biomembr. 1991;23:715–741. doi: 10.1007/BF00785998. [DOI] [PubMed] [Google Scholar]
- Jackson JB, Leung JH, Stout CD, Schurig-Briccio LA, Gennis RB. Review and Hypothesis. New insights into the reaction mechanism of transhydrogenase: Swivelling the dIII component may gate the proton channel. FEBS Lett. 2015;589:2027–2033. doi: 10.1016/j.febslet.2015.06.027. [DOI] [PubMed] [Google Scholar]
- Jackson JB, Peake SJ, White SA. Structure and mechanism of proton-translocating transhydrogenase. FEBS Lett. 1999;464:1–8. doi: 10.1016/s0014-5793(99)01644-0. [DOI] [PubMed] [Google Scholar]
- Johansson T, Oswald C, Pedersen A, Tornroth S, Okvist M, Karlsson BG, Rydstrom J, Krengel U. X-ray structure of domain I of the proton-pumping membrane protein transhydrogenase from Escherichia coli. J Mol Biol. 2005;352:299–312. doi: 10.1016/j.jmb.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Kabsch W. Xds. Acta Crystallogr D. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD, Jr, Pastor RW. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JH, Schurig-Briccio LA, Yamaguchi M, Moeller A, Speir JA, Gennis RB, Stout CD. Structural biology. Division of labor in transhydrogenase by alternating proton translocation and hydride transfer. Science. 2015;347:178–181. doi: 10.1126/science.1260451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomize MA, Lomize AL, Pogozheva ID, Mosberg HI. OPM: orientations of proteins in membranes database. Bioinformatics. 2006;22:623–625. doi: 10.1093/bioinformatics/btk023. [DOI] [PubMed] [Google Scholar]
- Lomize MA, Pogozheva ID, Joo H, Mosberg HI, Lomize AL. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 2012;40:D370–376. doi: 10.1093/nar/gkr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- Martyna GJ. Remarks on Constant-Temperature Molecular-Dynamics with Momentum Conservation. Phys Rev E. 1994;50:3234–3236. doi: 10.1103/physreve.50.3234. [DOI] [PubMed] [Google Scholar]
- Mccoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuller J, Rydstrom J. The membrane topology of proton-pumping Escherichia coli transhydrogenase determined by cysteine labeling. J Biol Chem. 1999;274:19072–19080. doi: 10.1074/jbc.274.27.19072. [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel AG, von Hardenberg A, Hohl M, Loffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015;22:472–484. doi: 10.1016/j.cmet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Olausson T, Fjellstrom O, Meuller J, Rydstrom J. Molecular biology of nicotinamide nucleotide transhydrogenase--a unique proton pump. Biochim Biophys Acta. 1995;1231:1–19. doi: 10.1016/0005-2728(95)00058-q. [DOI] [PubMed] [Google Scholar]
- Olausson T, Hultman T, Holmberg E, Rydstrom J, Ahmad S, Glavas NA, Bragg PD. Site-Directed Mutagenesis of Tyrosine Residues at Nicotinamide Nucleotide-Binding Sites of Escherichia-Coli Transhydrogenase. Biochemistry. 1993;32:13237–13244. doi: 10.1021/bi00211a036. [DOI] [PubMed] [Google Scholar]
- Persson B, Berden JA, Rydstrom J, van Dam K. ATP-driven transhydrogenase provides an example of delocalized chemiosmotic coupling in reconstituted vesicles and in submitochondrial particles. Biochim Biophys Acta. 1987;894:239–251. doi: 10.1016/0005-2728(87)90193-9. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad GS, Sridhar V, Yamaguchi M, Hatefi Y, Stout CD. Crystal structure of transhydrogenase domain III at 1.2 A resolution. Nat Struct Biol. 1999;6:1126–1131. doi: 10.1038/70067. [DOI] [PubMed] [Google Scholar]
- Prasad GS, Wahlberg M, Sridhar V, Sundaresan V, Yamaguchi M, Hatefi Y, Stout CD. Crystal structures of transhydrogenase domain I with and without bound NADH. Biochemistry. 2002;41:12745–12754. doi: 10.1021/bi020251f. [DOI] [PubMed] [Google Scholar]
- Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical-Integration of Cartesian Equations of Motion of a System with Constraints - Molecular-Dynamics of N-Alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]
- Rydstrom J, Hu X, Fjellstrom O, Meuller J, Zhang J, Johansson C, Bizouarn T. Domains, specific residues and conformational states involved in hydride ion transfer and proton pumping by nicotinamide nucleotide transhydrogenase from Escherichia coli. Biochim Biophys Acta. 1998;1365:10–16. doi: 10.1016/s0005-2728(98)00038-3. [DOI] [PubMed] [Google Scholar]
- Sazanov LA, Jackson JB. Cyclic-Reactions Catalyzed by Detergent-Dispersed and Reconstituted Transhydrogenase from Beef-Heart Mitochondria - Implications for the Mechanism of Proton Translocation. Bba-Bioenergetics. 1995;1231:304–312. doi: 10.1016/0005-2728(95)00096-2. [DOI] [PubMed] [Google Scholar]
- Smart OS. The simulation of substantial conformational transitions of proteins: progress in the application of path energy minimization in internal coordinate space. Biochem Soc Trans. 1996;24:125S. doi: 10.1042/bst024125s. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Chartron J, Yamaguchi M, Stout CD. Conformational diversity in NAD(H) and interacting transhydrogenase nicotinamide nucleotide binding domains. J Mol Biol. 2005;346:617–629. doi: 10.1016/j.jmb.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Yamaguchi M, Chartron J, Stout CD. Conformational change in the NADP(H) binding domain of transhydrogenase defines four states. Biochemistry. 2003;42:12143–12153. doi: 10.1021/bi035006q. [DOI] [PubMed] [Google Scholar]
- Venning JD, Rodrigues DJ, Weston CJ, Cotton NPJ, Quirk PG, Errington N, Finet S, White SA, Jackson JB. The heterotrimer of the membrane-peripheral components of transhydrogenase and the alternating-site mechanism of proton translocation. J Biol Chem. 2001;276:30678–30685. doi: 10.1074/jbc.M104429200. [DOI] [PubMed] [Google Scholar]
- Voss NR, Gerstein M. 3V: cavity, channel and cleft volume calculator and extractor. Nucleic Acids Res. 2010;38:W555–562. doi: 10.1093/nar/gkq395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Cotton NP, Thomas CM, Jackson JB. Cloning and sequencing of the genes for the proton-translocating nicotinamide nucleotide transhydrogenase from Rhodospirillum rubrum and the implications for the domain structure of the enzyme. Microbiology. 1994;140(Pt 7):1595–1604. doi: 10.1099/13500872-140-7-1595. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Stout CD. Essential glycine in the proton channel of Escherichia coli transhydrogenase. J Biol Chem. 2003;278:45333–45339. doi: 10.1074/jbc.M308236200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Stout CD, Hatefi Y. The proton channel of the energy-transducing nicotinamide nucleotide transhydrogenase of Escherichia coli. J Biol Chem. 2002;277:33670–33675. doi: 10.1074/jbc.M204170200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hermans J. Hydrophilicity of cavities in proteins. Proteins. 1996;24:433–438. doi: 10.1002/(SICI)1097-0134(199604)24:4<433::AID-PROT3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The movie is from a simulation trajectory over the course of 300 ns, highlighting the putative proton channel in the dII domain of Thermus thermophilus TH. The proton loading site in the middle of the channel is separated from the cytoplasmic surface by a dry region. The formation of a water wire in this dry region is presumably critical for the enzymatic activity and is found during the simulation to involve a conformational change of Thr214. The movie is generated using VMD.