Abstract
With the recent development of two effective treatments for patients with idiopathic pulmonary fibrosis, an accurate diagnosis is crucial. The traditional approach to diagnosis emphasises the importance of thorough clinical and laboratory evaluations to exclude secondary causes of disease. High-resolution CT is a critical initial diagnostic test and acts as a tool to identify patients who should undergo surgical lung biopsy to secure a definitive histological diagnosis of usual interstitial pneumonia pattern. This diagnostic approach faces several challenges. Many patients with suspected idiopathic pulmonary fibrosis present with atypical high-resolution CT characteristics but are unfit for surgical lung biopsy, therefore preventing a confident diagnosis. The state of the art suggests an iterative, multidisciplinary process that incorporates available clinical, laboratory, imaging, and histological features. Recent research has explored genomic techniques to molecularly phenotype patients with interstitial lung disease. In the future, clinicians will probably use blood-specific or lung-specific molecular markers in combination with other clinical, physiological, and imaging features to enhance diagnostic efforts, refine prognostic recommendations, and influence the initial or subsequent treatment options. There is an urgent and increasing need for well designed, large, prospective studies measuring the effect of different diagnostic approaches. Ultimately, this will help to inform the development of guidelines and tailor clinical practice for the benefit of patients.
Introduction
The availability of novel effective antifibrotic therapies,1 coupled with the limitations of traditional therapeutic combinations,2–4 have increased the urgency of making an accurate diagnosis of idiopathic pulmonary fibrosis.5 Traditional combined immunosuppression is associated with increased hospital admission and mortality,2 and the inconsistent efficacy of a simple acetylcysteine regimen3,4 strongly argues against the empirical use of these therapeutic combinations in patients with idiopathic pulmonary fibrosis. Whether early therapy with these interventions is warranted remains controversial.6 Over the past 15 years, substantial advances have facilitated the development of robust diagnostic pathways for patients with diffuse parenchymal lung diseases. These pathways incorporate evolving biological concepts, improved imaging techniques and interpretation, and a better understanding of the role of new lung-sampling methods and pathological features. These components of interstitial lung disease have been codified into the multidisciplinary team evaluation process that has become the standard of care. Increasingly, molecular markers from circulating (ie, blood) or lung-specific samples have been used to improve diagnostic and prognostic accuracy. In this Review, we describe the current state of the art in the diagnosis of idiopathic pulmonary fibrosis, with limited focus on the approach to prognostication and the current status of therapy, which is provided in other reviews.7,8
Medical history and clinical evaluation
A thorough clinical evaluation is central to the diagnosis of idiopathic pulmonary fibrosis.1 Clinical evaluation is, fundamentally, responsible for ruling out alternative causes of disease, such as chronic hypersensitivity pneumonitis (by excluding meaningful environmental exposures), connective tissue disease-related interstitial lung disease (by evaluating the patient and their serum for evidence of systemic autoimmunity), and drug toxicity. Although it is essential to identify exposures in the patient’s domestic and work environment in the evaluation of patients with suspected idiopathic pulmonary fibrosis, there is no validated questionnaire that can be used to ensure a comprehensive exposure evaluation. Thus, in practice, clinical evaluation has traditionally been more of an art than a science, with little objective knowledge regarding the effect of specific symptoms, signs, and presence of serum antibodies on the probability of disease.
Epidemiological evidence from multiple cohorts shows clear associations between clinical features and a diagnosis of idiopathic pulmonary fibrosis. Most prominent among these is age, with the incidence of disease increasing greatly in the seventh and eighth decades of life.9 These data have their origin in ageing-related pathogenic mechanisms that are prominently expressed in patients with idiopathic pulmonary fibrosis.10 As such, it has been hypothesised that patients above a certain age with fibrotic lung disease would need little further evaluation because age is a strong predictor of idiopathic pulmonary fibrosis. In a study of 135 patients with interstitial lung disease (97 of whom had idiopathic pulmonary fibrosis), Fell and colleagues11 reported a positive predictive value for idiopathic pulmonary fibrosis of 95% for patients aged 70 years or older. A second dataset, including multicentre data collection, substantiated that being older than 60 years increases the probability of a diagnosis of idiopathic pulmonary fibrosis.12 Although these findings need to be interpreted with caution given inherent biases in the study populations, they suggest that age could be a powerful predictive tool in the evaluation of suspected disease.
The importance of eliciting a detailed medical history, which is aimed at identifying all possible environmental exposures, extrapulmonary symptoms, and a salient family medical history, cannot be overstated. Without this history, a diagnosis of idiopathic pulmonary fibrosis might be made erroneously, since chronic hypersensitivity pneumonitis can present with a pattern of usual interstitial pneumonia,13–15 and this is a well recognised feature of connective tissue disease-related interstitial lung disease.16 Exposure to cigarette smoke is associated with distinct and overlapping clinical, radiological, and pathological features.17
Physical examination of the respiratory system has limited diagnostic specificity. Its primary use is identification of extrapulmonary signs, predominantly involving the skin and musculoskeletal systems, which might suggest that the usual interstitial pneumonia pattern is secondary to connective tissue disease.16 The potential contribution of comorbid autoimmune disorders to the diagnostic process is evolving.16 Scleroderma, rheumatoid arthritis, inflammatory myositis, and other connective tissue diseases can present with limited extrapulmonary involvement; thus, deliberate attention to the rheumatological examination is essential.16 In a study of diagnostic agreement between multidisciplinary teams evaluating 70 cases of diffuse parenchymal lung disease, five of seven teams constructed new diagnoses of connective tissue disease-related interstitial lung disease in about 10% of patients, emphasising the importance of formal rheumatology input within the multidisciplinary process.18 Common respiratory findings in idiopathic pulmonary fibrosis include dry, high-pitched inspiratory crackles and digital clubbing, although the clinical relevance and significance of these in prognosis and as potential biomarkers are unknown. Other examination findings could be particularly relevant for some interstitial pneumonias, including chronic hypersensitivity pneumonitis, in which an end-inspiratory wheeze has been described.19
There is little objective evidence to support a role for routine serological screening in patients with suspected idiopathic pulmonary fibrosis, but most experts believe that testing these patients for occult connective tissue disease is useful.20 Older patients (≥55 years) often have circulating evidence of autoimmunity (eg, positive rheumatoid factor or elevated antinuclear antibody titres).21–23 A study that compared 67 patients with idiopathic pulmonary fibrosis with 52 healthy, age-matched controls showed no difference in the basic serological profile, with 22% of patients with idiopathic pulmonary fibrosis and 21% of age-matched controls having at least one positive autoantibody24 Whether these serological abnormalities have biological relevance to idiopathic pulmonary fibrosis or suggest a latent connective tissue disease is unknown. The term interstitial pneumonia with autoimmune features has been suggested to describe patients with interstitial pneumonia who have non-specific serology or some autoimmune features, but who do not meet the required criteria for a specific connective tissue disease.16 However, interstitial pneumonia with autoimmune features may require a broader definition and certainly requires prospective validation.25
Although chronic hypersensitivity pneumonitis should always be considered in the differential diagnosis of patients with suspected idiopathic pulmonary fibrosis, the diagnostic pathway is not standardised. The role of specific IgG to known antigens,26 cultures from specimens obtained from the patient’s environment,27 bronchoalveolar lavage cellular analyses,28 and a bronchoprovocation test for a specific or suspected antigen, are all appropriate considerations but need validation in prospective studies.29 Testing for serum precipitins (ie, specific IgG against potential antigens) is subject to availability and has not been standardised.19,26 Additionally, the presence of specific IgG confirms that the patient was exposed to that antigen at some time in the past, but this does not necessarily equate to diagnosis of chronic hypersensitivity pneumonitis.26 Although bronchoalveolar lavage lymphocytosis raises the possibility of chronic hypersensitivity pneumonitis, it is nonspecific and insensitive.28 Whereas some clinicians advocate a specific inhalation challenge diagnostic test, this has not been fully standardised, is not widely available, and has a suboptimal negative predictive value.29 The diagnosis of chronic hypersensitivity pneumonitis is dependent on a high index of suspicion based on a thorough history and evolving data from imaging and pathological findings.
Imaging
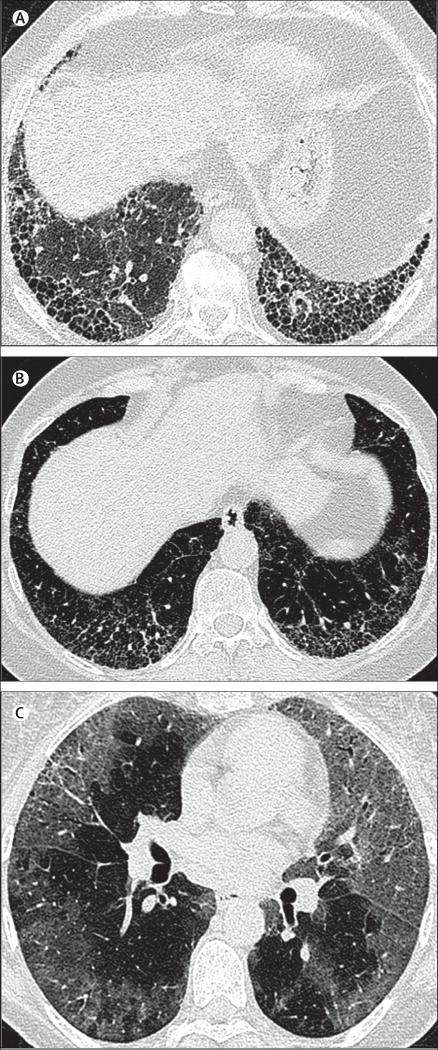
High-resolution CT of the chest has a central role in the initial evaluation of patients with suspected idiopathic pulmonary fibrosis and the results greatly influence subsequent management decisions. The most common high-resolution CT protocol used to evaluate diffuse lung disease is a volumetric acquisition of thin sections (usually ≤1·5 mm), combined with a high spatial frequency reconstruction algorithm. The 2011 international high-resolution CT criteria for a pattern of usual interstitial pneumonia define three diagnostic categories: usual interstitial pneumonia, possible usual interstitial pneumonia, and inconsistent with usual interstitial pneumonia (figure 1).20 High-resolution CT criteria for a diagnosis of usual interstitial pneumonia include the presence of honeycombing in a basal and subpleural distribution, without features considered incompatible with a diagnosis of idiopathic pulmonary fibrosis. In the correct clinical setting, a pattern of usual interstitial pneumonia on high-resolution CT obviates diagnostic surgical lung biopsy. If the pattern is one of possible usual interstitial pneumonia, or inconsistent with usual interstitial pneumonia, surgical lung biopsy is recommended to secure a definitive diagnosis of idiopathic pulmonary fibrosis.20
Figure 1. High-resolution CT imaging of interstitial pneumonia patterns: different diagnostic categories.
(A) Usual interstitial pneumonia pattern: axial chest high-resolution CT image taken at the level of the lower lobes, depicting multilayered subpleural honeycombing without evidence of features inconsistent with a usual interstitial pneumonia pattern. (B) Possible usual interstitial pneumonia: axial chest high-resolution CT image taken at the level of the lower lobes, depicting bilateral symmetrical reticular abnormalities containing areas of traction bronchiectasis, but no clear evidence of subpleural honeycombing. Although there are admixed areas of ground glass opacification, the predominant abnormality is of reticulation. (C) Inconsistent with usual interstitial pneumonia: axial chest high-resolution CT image taken below the level of the carina, depicting diffuse ground glass abnormalities in a predominantly peripheral distribution. Mild traction bronchiectasis is shown in the left upper lobe and right upper lobe, indicating that a proportion of the ground glass abnormality represents fibrosis.
Since the publication of the 2011 guidelines,20 thoracic radiologists have faced several challenges. First, a substantial proportion of patients with idiopathic pulmonary fibrosis do not present with typical high-resolution CT features of a usual interstitial pneumonia pattern. This point is highlighted in an idiopathic pulmonary fibrosis therapeutic trial,30 in which definite usual interstitial pneumonia (as described by the study protocol and corresponding to the current guideline high-resolution CT criteria20) was present in only about a third of enrolled patients. Furthermore, many patients are unable to undergo surgical lung biopsy because of advanced age or preclusive comorbidities, which ultimately denies them access to treatments that are only licensed when a diagnosis of idiopathic pulmonary fibrosis has been secured. In recognition of this limitation, several studies31–33 have attempted to expand the range of high-resolution CT features considered sufficiently accurate to diagnose idiopathic pulmonary fibrosis and to therefore preclude the need for biopsy. Two of these studies report that in patients with suspected idiopathic pulmonary fibrosis, a basal predominant coarse reticular abnormality without honeycombing might be predictive of histopathological usual interstitial pneumonia.31,33 A third study32 reported a high positive predictive value of possible usual interstitial pneumonia on high-resolution CT for histopathological usual interstitial pneumonia, although this study was limited by selection bias because it was performed in a cohort enriched for patients with idiopathic pulmonary fibrosis (ie, a clinical trial cohort from an idiopathic pulmonary fibrosis trial). Clarification of the prevalence of idiopathic pulmonary fibrosis among patients meeting high-resolution CT criteria for possible usual interstitial pneumonia requires further study. It is noteworthy that the inclusion criteria for a recent idiopathic pulmonary fibrosis drug trial permitted enrolment of patients with high-resolution CT appearances that met criteria for possible usual interstitial pneumonia.34
The second challenge is that diagnosis of idiopathic pulmonary fibrosis on the basis of high-resolution CT requires the presence of honeycombing; arguably implicit in this definition is that established fibrosis must be present.20 By contrast, an accurate diagnosis of idiopathic pulmonary fibrosis is crucial if improvement of clinical outcome is to be optimised.35 For example, a very limited subpleural reticular abnormality on high-resolution CT, without honeycombing, might not meet the criteria for a pattern of usual interstitial pneumonia; however, these findings in a man older than 75 years, without another identifiable cause, raise the probability of idiopathic pulmonary fibrosis and should prompt an aggressive approach to diagnosis.11,12 Moreover, a post-hoc subgroup analysis of a large randomised clinical trial assessing the safety and efficacy of nintedanib showed that patients with idiopathic pulmonary fibrosis with reticulation and traction bronchiectasis in the absence of honeycombing on high-resolution CT progress exactly like patients with the classic pattern of usual interstitial pneumonia, which is characterised by honeycombing.36 These findings lend support to a future change in the high-resolution CT criteria used to diagnose patients with idiopathic pulmonary fibrosis. The spectrum of high-resolution CT patterns seen in early idiopathic pulmonary fibrosis has not been investigated, but is particularly relevant given the current era of approved antifibrotic therapy. To characterise the earliest CT signs of fibrotic lung disease, researchers have investigated the prevalence and behaviour of interstitial lung abnormalities incidentally identified in patients undergoing CT for other reasons. Several studies have reported a prevalence of interstitial lung abnormalities of 7–10%,37,38 although these abnormalities appear to be more prevalent in smokers. Results of one study have shown that interstitial lung abnormalities with CT signs of fibrosis are progressive in some patients,37 whereas another study has linked progression to physiological worsening and increased mortality.39 Furthermore, a meta-analysis of data from the Framingham Heart study,40 the AGES-Reykjavik study41 the COPDGene study42 and the ECLIPSE study,43 supported the finding that the presence of interstitial lung abnormalities was associated with a greater risk of all-cause mortality; in one of these cohorts, the increased death rate was due to pulmonary disease, specifically pulmonary fibrosis.38 Given the potential confounders of age and smoking, the nature and therapeutic implications of interstitial lung abnormalities remain controversial.
Interobserver agreement for the identification of honeycombing and the high-resolution CT criteria for a pattern of usual interstitial pneumonia could be, at best, moderate.44,45 Although it is important to identify sources of disagreement when applying these criteria, there is also a growing perception that the initial high-resolution CT appearances in patients with suspected idiopathic pulmonary fibrosis are best seen as the starting point of a dynamic diagnostic process. In some cases, the impression given by the presenting high-resolution CT can ultimately be trumped by observed disease behaviour or initial response to therapy.46 Several ongoing studies are examining innovative imaging approaches (table).
Table.
Ongoing studies examining diagnostic approaches to idiopathic pulmonary fibrosis
| Study location | Primary goals | |
|---|---|---|
| General | ||
|
| ||
| NCT01915511 | Boehringer Ingelheim and Duke University | Define natural IPF history; define data on current practice patterns for IPF diagnosis; define the effect of IPF on quality of life; bank blood samples |
| NCT02772549 | University Hospital, Gentofte, Copenhagen | Define the diagnostic delay before an IPF diagnosis is made |
| NCT02479126 | Durham VA Medical Center and Boehringer Ingelheim | Define the prevalence of ILD in the VISN6 VA patient population; define the prevalence of IPF in theVISN6VA patient population; establish the accuracy of ICD-9 codes for diagnosis of ILD; compare the reported ICD-9 code diagnosis with the diagnosis made after chart review and re-interpretation of lung imaging and pathology; record the frequency of ICD-9 code diagnosis of ILD and clinic consultation with a pulmonary provider; define the prevalence of comorbid diabetes mellitus with ILD; compare the prevalence of diabetes with ILD as compared with the prevalence of diabetes in the general VA population |
| NCT00540475 | University of Pittsburgh, Temple University, University of Pennsylvania, Geisinger Clinic, and Milton S. Hershey Medical Center | Assess the extent of lung fibrosis in Pennsylvania; provide better access to standard of care and diagnosis of patients with pulmonary fibrosis in all regions of Pennsylvania; facilitate the translation of new therapeutic interventions from the bench to the bedside |
|
| ||
| Lung sampling techniques | ||
|
| ||
| NCT01714518 | Wissenschaftliches Institut Bethanien e.V | Assess the efficiency and safety of cryobiopsy compared with VATS in diagnosis of ILD |
| NCT02563730 | University Hospital Tuebingen | Clarify whether the addition of cryobiopsy can avoid surgical lung biopsy in a clinically relevant proportion of patients with suspected idiopathic interstitial pneumonia |
| NCT02235779 | Laval University | Evaluate the diagnostic yield, feasibility, and safety of transbronchial lung cryobiopsies done via bronchoscopy in the investigation of interstitial lung disease in comparison with VATS |
| NCT02075762;NCT01972685 | Johns Hopkins University and Duke University | Compare the sample size, architectural preservation, and diagnostic yield of bronchoscopic cryoprobe transbronchial lung biopsy, in comparison with bronchoscopic standard transbronchial lung biopsy and VATS lung biopsy for the diagnosis of ILD |
| NCT02579304 | Katholieke Universiteit Leuven | Evaluate the diagnostic value of transbronchial lung cryobiopsy and its procedural feasibility and safety in a prospective series of 20 patients with ILD who are referred for invasive histopathological diagnostic testing |
| NCT02763540 | University Hospital, Montpellier | Compare the pathological features of surgical open lung biopsies and cryobiopsies in non-1 PF ILD. Patients with non-1 PF ILD who are eligible for an open lung biopsy will undergo cryobiopsy at the same time. Interobserver agreement will be assessed for each pair of samples |
| ACTRN12615000718549 | Multiple centres | Validate transbronchial lung cryobiopsy against VATS biopsy in the diagnosis of ILD |
|
| ||
| Physiological techniques | ||
|
| ||
| NCT02827734 | Universitätsmedizin Mannheim | Define lung clearance and airway resistance in various ILDs |
|
| ||
| Imaging techniques | ||
|
| ||
| NCT01624753 | Singapore General Hospital | Explore the value of fibered confocal fluorescence microscopy in providing diagnostic information on fibrosis and inflammation of the distal air spaces associated with ILD without the need for lung biopsies; identify and catalogue distinct and discriminating features seen on images obtained from fibered confocal fluorescence microscopy, and correlate these findings with specific high-resolution CT features and pathological findings if available; create diagnostic criteria for fibered confocal fluorescence microscopy image interpretation of specific ILDs |
|
| ||
| Molecular markers | ||
|
| ||
| NCT00632307 | Lung Clinic Hemer | Define disease-specific clusters of volatile organic compounds in patients with different lung diseases, including ILD, using breath analysis with ion mobility spectrometry |
| NCT00258544 | University of Pittsburgh | Identify unique genetic markers in scarred lung that could ultimately lead to new approaches to the diagnosis and treatment of pulmonary fibrosis |
| N/A | Multiple centres | BRAVE-3 (Bronchial Sample Collection for a Novel Genomic Test)—to collect transbronchial biopsy specimens, clinical data, and pathology slides for external review to optimise a molecular profiling diagnostic test in patients with ILD |
IPF=idiopathic pulmonary fibrosis. ILD=interstitial lung disease. VA=Veterans Affairs. VATS=video-assisted thoracoscopic lung biopsy.
Pathology and biopsy techniques
Surgical lung biopsy remains an important diagnostic study in patients with suspected idiopathic pulmonary fibrosis whose high-resolution CT presentation is not typical of usual interstitial pneumonia. Its role is evolving given that complications, including acute exacerbations of the underlying fibrotic lung disease, can occur following surgical lung biopsy.47,48 The current recommendation (based on expert opinion and low quality evidence) is that tissue biopsy samples should be procured from more than one site within the lung, because of the high degree of variability in the distribution and morphology of the abnormalities.49 The potential effect of preoperative, radiologically directed site selection is not known. For most patients, video-assisted thoracic surgery is preferred, with a diagnostic yield equivalent to thoracotomy50
The histological features that reliably establish the usual interstitial pneumonia pattern are: a characteristic pattern of patchy (patchwork) collagen fibrosis; microscopic subepithelial foci of organising fibroblasts and myofibroblasts affiliated with evidence of epithelial injury and repair (fibroblast foci); architectural distortion in the form of tissue-destructive scarring and honeycomb change; a paucicellular, patchy infiltrate of mononuclear inflammatory cells; and absence of other findings (eg, cellular bronchiolitis with associated granulomatous inflammation characteristic of hypersensitivity pneumonia) to suggest an alternate diagnosis.51
A diagnosis made by surgical lung biopsy of usual interstitial pneumonia pattern is a strong predictor of outcome at the time of diagnosis in patients with idiopathic pulmonary fibrosis.52 The prognostic value of quantifying fibroblast foci in patients with clinically stable disease is controversial.53–56 The profusion of fibroblast foci had no prognostic importance when a quantitative method was applied in a standardised fashion to surgical lung biopsies from patients with idiopathic pulmonary fibrosis with no acute exacerbation.57 Patients with a surgical lung biopsy diagnosis of usual interstitial pneumonia and discordant findings on a chest high-resolution CT scan can have prolonged survival compared with patients in whom both high-resolution CT and surgical lung biopsy results show usual interstitial pneumonia.52
Separating patients with fibrotic variants of idiopathic non-specific interstitial pneumonia from patients with idiopathic pulmonary fibrosis goes beyond the multidisciplinary team at the time of initial diagnosis, and is an iterative process that requires continued follow-up. Changes that are indistinguishable from idiopathic non-specific interstitial pneumonia (ie, nonspecific interstitial pneumonia-like changes) can occur focally in an otherwise typical pattern of usual interstitial pneumonia.52,58 This occurrence is the most compelling argument for sampling more than one site at the time of surgical lung biopsy. Considering the potential for sampling errors, especially in patients who undergo surgical lung biopsy of a single site, and the relatively large subset of patients with idiopathic pulmonary fibrosis in whom high-resolution CT findings are not diagnostic, there are patients with idiopathic pulmonary fibrosis thought to have idiopathic non-specific interstitial pneumonia even after initial multidisciplinary evaluation.
Fibre-optic bronchoscopy with bronchoalveolar lavage and transbronchial biopsies have a potential role in addressing interstitial pneumonias other than idiopathic pulmonary fibrosis.59,60 Conversely, transbronchial biopsies are much less sensitive than surgical lung biopsy for identifying a pattern of usual interstitial pneumonia, although the potential value of transbronchial biopsies combined with multidisciplinary evaluation has not been properly evaluated. Only occasionally do transbronchial biopsies show a combination of characteristic histological features, limiting their value in diagnosing the pattern of usual interstitial pneumonia.61,62 The potential value of small closed biopsies could improve as molecular profiling techniques, with the potential to demonstrate diagnostic signatures, emerge.63
Transbronchial cryobiopsies by experienced operators yield larger samples than do transbronchial biopsies, although with higher complication rates.64 Preliminary, retrospectively collected data suggest that transbronchial cryobiopsies have a diagnostic yield that is greater than that reported with transbronchial biopsies in patients with idiopathic pulmonary fibrosis.65 However, the potential value of transbronchial cryobiopsies compared with surgical lung biopsy has not been prospectively tested in a robust, randomised trial.66 There are numerous ongoing studies examining the diagnostic value of transbronchial cryobiopsies (table).
Multidisciplinary evaluation of idiopathic pulmonary fibrosis
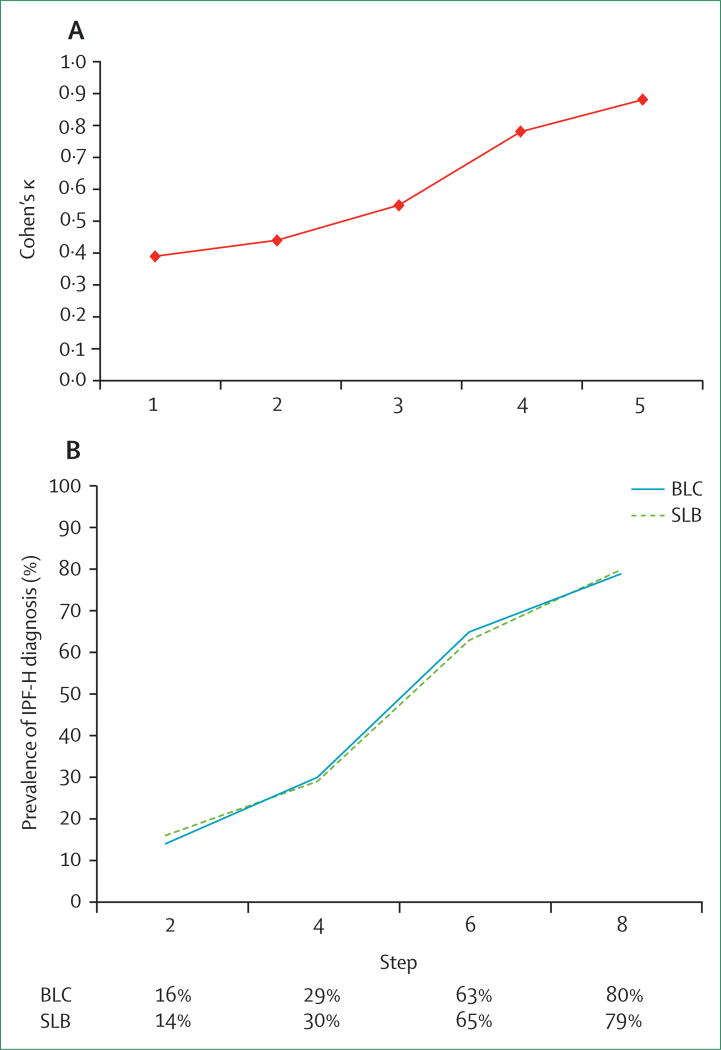
A 2002 statement by the American Thoracic Society and European Respiratory Society advocated a multidisciplinary approach to diagnosis of idiopathic interstitial pneumonias, involving a review of all the clinical, radiological, and pathological information (when biopsy material is available) from the patient.67 This approach was explored by a group of academic investigators who evaluated data from 59 patients with idiopathic interstitial pneumonias. Although a complete consensus was not reached in all cases, the investigators noted a significant improvement in agreement throughout the process (figure 2).68 A similar experiment looked at agreement within academic and community-based practices, as well as agreement between academic and community-based physicians, using a similar format.69 Improvement in agreement was again noted at each centre with multidisciplinary team input. However, there was overall poor agreement between academic and community practices. Community practices were more likely to assign a diagnosis of idiopathic pulmonary fibrosis compared with academic colleagues. The addition of transbronchial cryobiopsies has similar diagnostic value to surgical lung biopsy in the multidisciplinary diagnosis process (figure 2).70 Results from studies have also supported an improvement in diagnostic confidence among expert chest radiologists and pulmonologists with a multidisciplinary team approach.71 More recent studies also suggest diagnostic confidence is improved when a multidisciplinary approach to diagnosis is taken, involving expert chest radiologists, pathologists, and pulmonologists.71 As a result, the guidelines recommend use of a multidisciplinary discussion in the evaluation and diagnosis of idiopathic pulmonary fibrosis.20,72
Figure 2. Effect of multidisciplinary diagnostic approach on diagnosis in interstitial pneumonias.
(A) Interobserver agreement among clinicians and radiologists in the evaluation of patients with idiopathic interstitial pneumonias. Step 1: individual assessment of high-resolution CT data alone. Step 2: individual assessment of high-resolution CT plus clinical data. Step 3: group discussion of high-resolution CT plus clinical data. Step 4: group discussion of high-resolution CT, clinical, and surgical lung biopsy data. Step 5: consensus diagnosis among all participants. Adapted from Flaherty and colleagues.68 (B)The effect of bronchial cryobiopsy and surgical lung biopsy on diagnostic confidence in patients with idiopathic pulmonary fibrosis during multidisciplinary team evaluation. Step 2: addition of clinical and radiological data. Step 4: addition of bronchoalveolar lavage data. Step 6: addition of biopsy data. Step 8: addition of follow-up data. Reproduced with permission from Tomassetti and colleagues.69 BLC=bronchial cryobiopsy. IPF-H=idiopathic pulmonary fibrosis diagnosis made with high confidence. SLB=surgical lung biopsy.
Although diagnosis by a multidisciplinary team is associated with increased diagnostic agreement, this does not guarantee diagnostic accuracy. Since multidisciplinary team evaluation incorporates all available data at presentation, there is no baseline reference standard against which the veracity of this team diagnosis can be evaluated. One feasible surrogate marker of diagnostic accuracy in the setting of idiopathic pulmonary fibrosis is clinical outcome. Although the availability of antifibrotic therapy for patients with idiopathic pulmonary fibrosis has the potential to slow disease progression, at present, stabilisation of disease is not possible. Although a minority of patients with chronic hypersensitivity pneumonitis or fibrotic non-specific interstitial pneumonia might present in a similar way to those with idiopathic pulmonary fibrosis, in most cases, the inexorable progression of disease, despite therapy, is a distinguishing feature of idiopathic pulmonary fibrosis. Results from an international study involving seven expert multidisciplinary teams showed high levels of agreement for a diagnosis of idiopathic pulmonary fibrosis (Cohen’s κ=0.60) and for the diagnostic likelihood of idiopathic pulmonary fibrosis (κ=0.71).44 A diagnosis of idiopathic pulmonary fibrosis after multidisciplinary team evaluation has higher prognostic significance than a diagnosis rendered by either clinicians or radiologists in isolation.18 The diagnostic performance of clinicians during the first phase of the study, when they were asked to evaluate the cases without consultation with their radiologist or pathologist, was close (in terms of interobserver agreement and prognostic significance of an idiopathic pulmonary fibrosis diagnosis) to that of their respective multidisciplinary team meeting. Given that some physicians are tasked with evaluating patients with suspected idiopathic pulmonary fibrosis for consideration of antifibrotic therapy, but do not have access to appropriate radiological or pathological expertise, this observation is of particular importance and has a potential effect on clinical practice. Further research is needed to determine what other factors, such as biomarkers or quantitative imaging, might be needed to further refine the diagnostic process. Additionally, many questions remain about the composition and implementation of multidisciplinary team meetings (panel).
Biological markers
Capitalising on the evolving understanding of idiopathic pulmonary fibrosis biology to develop innovative biomarker approaches will facilitate an accurate diagnostic process. The rapidly evolving knowledge of genetics has identified a series of genetic determinants of pulmonary fibrosis.73 Numerous groups are exploring the role of genetic features as diagnostic markers,74 including a link between a MUC5B promoter polymorphism and the prevalence and progression of interstitial lung abnormalities.39
Although the pathogenesis of idiopathic pulmonary fibrosis remains unknown, abnormal extracellular matrix (ECM) remodelling is thought to contribute to the cellular alterations and relentless deposition of collagenous scar tissue in the lung.75,76 Several groups have focused on lung-specific sampling or circulating molecular markers.77 Previous studies have identified ECM-modifying enzymes—such as matrix metalloproteinase (MMP)-7 and MMP-1—as potential diagnostic biomarkers, particularly in combination, for idiopathic pulmonary fibrosis compared with chronic hypersensitivity pneumonitis.78 However, it is unclear how well these biomarkers could segregate patients with idiopathic pulmonary fibrosis from other clinical mimics, such as non-specific interstitial pneumonia and other idiopathic fibrotic disorders. MMP-7, along with surfactant protein (SP)-D and endothelin-1, is elevated in the plasma of at-risk, first-degree relatives of patients with familial idiopathic interstitial pneumonia.79 Similarly, it is possible to separate patients with idiopathic pulmonary fibrosis from healthy controls with MMP-degraded proteins,80 thus expanding previous work.81 What diagnostic value this provides is unclear until a comparison is made among the clinically relevant idiopathic interstitial pneumonias. Various additional circulating markers have shown variable diagnostic value, including YKL-40 and osteopontin.82 A combination of plasma SP-D, MMP-7, and osteopontin has proven particularly useful in distinguishing idiopathic pulmonary fibrosis from other interstitial pneumonias.83 Serum micro RNAs (miRNAs) have also been shown to separate patients with idiopathic pulmonary fibrosis from healthy controls,84 although the clinical relevance of this finding remains unclear. A similar diagnostic value has been suggested for peripheral blood transcriptomic signature.85
Inflammatory proteins could also have diagnostic potential in idiopathic pulmonary fibrosis. For example, S100A9 in bronchoalveolar lavage, but not in serum, modestly differentiated patients with idiopathic pulmonary fibrosis from other interstitial lung diseases and from healthy controls.86 Similarly, CCL18 is elevated in serum and bronchoalveolar lavage in various inflammatory and fibrotic interstitial pneumonias, but with a degree of overlap that limits diagnostic value.87 Lung gene expression has also been used to segregate patients with idiopathic pulmonary fibrosis from healthy controls,88,89 chronic hypersensitivity pneumonitis, and non-specific interstitial pneumonia.90 The most comprehensive approach tested the value of high-dimensional transcriptional data in differentiating the histological pattern of usual interstitial pneumonia from that of non-specific interstitial pneumonia, chronic hypersensitivity pneumonitis, and a range of other interstitial pneumonias; excellent sensitivity and specificity was shown.63 Similarly, it has been suggested that gene expression heterogeneity correlates with pathological heterogeneity.91 However, it is unclear how this approach will segregate various clinical diagnoses, how the heterogeneity in tissue abnormality will be accounted for, and how the unwieldy datasets will be simplified for use in clinical practice.
Many approaches are under investigation as potential robust diagnostic, molecular biomarkers. To ensure their fidelity and predictive value, these approaches should be compared among patients with a wide variety of interstitial pneumonias, identified prospectively across a variety of clinical settings.5 Additionally, sampling should be comprehensive to allow a rigorous examination of combinatorial models that include molecular markers plus other predictors of an idiopathic pulmonary fibrosis diagnosis (eg, increasing age11,12 or high-resolution CT scores32). There are ongoing prospective evaluations of molecular markers (table).
Summary and future directions
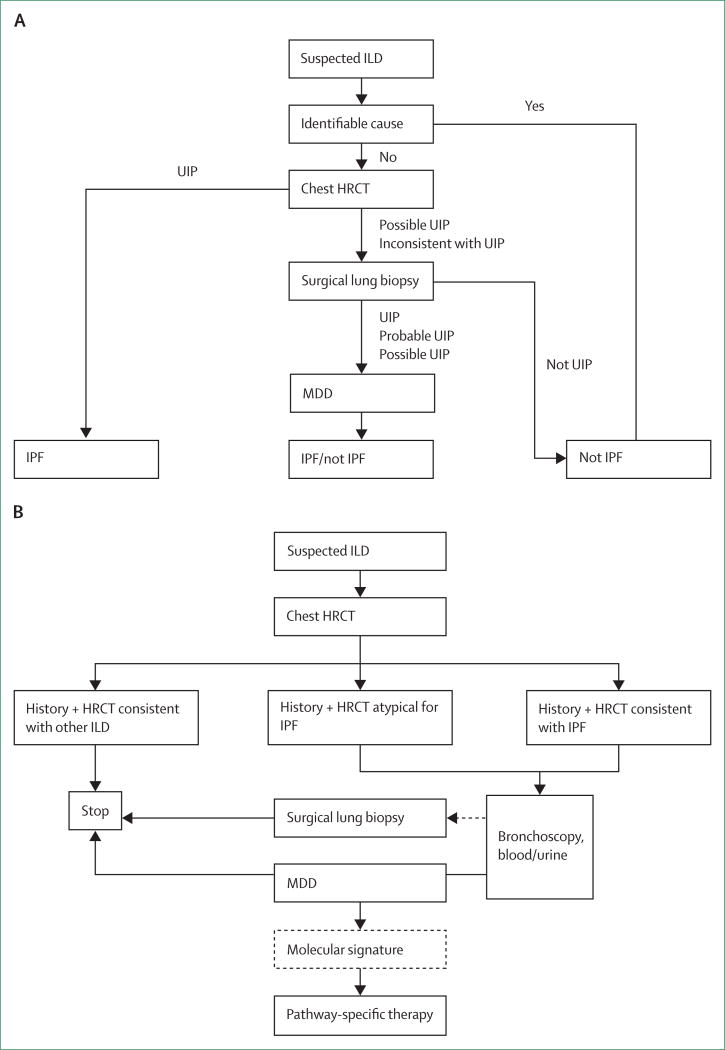
The rapid evolution of diagnostic and therapeutic options for interstitial pneumonias has altered the clinician’s approach to patients with these conditions. The traditional approach to diagnosis has emphasised the importance of thorough clinical and laboratory evaluations to exclude potential secondary causes of interstitial pneumonias (figure 3).20 High-resolution CT has proven to be a key initial diagnostic test.92 The imaging pattern has been used as a tool to identify patients who should undergo surgical lung biopsy to secure a histological diagnosis of usual interstitial pneumonia.20 The imaging features of usual interstitial pneumonia must be reconsidered and expanded, particularly within the setting of effective antifibrotic therapy.30,34,93 The current state of the art suggests the use of an iterative multidisciplinary team evaluation process, incorporating available clinical, laboratory, imaging, and histological features;49,68 however, the approval of effective pharmacotherapies places additional pressure on the multidisciplinary team to make a secure diagnosis of usual interstitial pneumonia. Emerging data suggest that many patients might not require a full team discussion. As such, future guideline developers might need to be mindful not to prevent physicians who do not have access to all the components of the multidisciplinary process from making a confident diagnosis of idiopathic pulmonary fibrosis. The evaluation process will probably include evolving genomic techniques that could usher in an new era of precision medicine in which molecular diagnostic tools will be used to enhance diagnostic, prognostic, and therapeutic decision making.7 This would allow clinicians to use blood-specific or lung-specific molecular markers alone, or in combination with other clinical, physiological, or imaging features; a surgical lung biopsy would then be required only if the molecular approach proved non-diagnostic (figure 3).
Figure 3. Traditional and future algorithms for the diagnosis of interstitial lung disease.
(A) Traditional approach to diagnosis of interstitial lung disease that includes high-resolution CT to identify the pattern of usual interstitial pneumonia. Surgical lung biopsy is recommended in patients with a high-resolution CT pattern of possible usual interstitial pneumoniaor inconsistent with usual interstitial pneumonia in an appropriate clinical setting. Multidisciplinary diagnosis is recommended as a key feature of the diagnostic pathway. Adapted from Raghu and colleagues.20 (B) Modified recommendation in which high-resolution CT retains a crucial initial diagnostic role. In cases in which high-resolution CT is diagnostic of an alternative process, a diagnosis is assured. As systemic or lung-specific biological markers evolve, these could become key diagnostic or prognostic markers that will supplement clinical and imaging evaluation, and potentially obviate the need for surgical lung biopsy. HRCT=high-resolution CT. ILD=interstitial lung disease. IPF=idiopathic pulmonary fibrosis. MDD=multidisciplinary diagnosis. UIP=usual interstitial pneumonia.
A major caveat to this approach is the potential efficacy of current, or future, antifibrotic agents in the majority of patients with fibrotic interstitial pneumonias. For example, pirfenidone has been investigated (NCT01933334) and nintedanib is being studied (NCT02597933) in scleroderma-associated interstitial lung disease. If either of these therapies proves to be efficacious, the clinician could attempt antifibrotic therapy before invasive diagnostic testing. There is an urgent and increasing need for well designed, large, prospective studies measuring the effects of different diagnostic approaches for patients with interstitial pneumonias, possibly integrating, in a cost-effective way, emerging technologies.
Key messages.
Given recent therapeutic developments, an accurate diagnosis of idiopathic pulmonary fibrosis is crucial
The traditional approach emphasises thorough clinical and laboratory evaluations to exclude secondary causes of pulmonary fibrosis
High-resolution CT is a key diagnostic test with evolving imaging criteria to diagnose the pattern of usual interstitial pneumonia
The current approach to diagnosis encourages an iterative, multidisciplinary process that incorporates all available data
Molecular techniques are being explored to enhance diagnostic efforts, refine prognostic recommendations, and influence the initial and subsequent therapeutic options
Panel: Key questions that remain in defining the role of the multidisciplinary team.
What patients should be included?
Should multidisciplinary team evaluation be mandated for all patients with interstitial lung disease?
Should all new interstitial lung disease cases be discussed?
Who should participate?
Who are the core participants that need to be present?
Is there added value from participation by non-pulmonary physicians, such as rheumatology, gastroenterology, or cardiology consultants?
Is there added value from participation from non-physician members such as nurses, research coordinators, social workers, and physicians in training?
What should the structure be for a multidisciplinary team evaluation?
Can multidisciplinary team evaluation be achieved virtually, using electronic means to present data remotely, rather than face-to-face?
Should multidisciplinary team evaluation occur only at regional centres?
Should community physicians be engaged in their own multidisciplinary teams?
How practical is multidisciplinary team evaluation for all cases of interstitial lung disease, both at community clinics and centres and regional centres of excellence for interstitial lung disease?
What data should be presented?
What are the essential elements that need to be presented for each case?
What is the value of presenting actual radiology or pathology images at the meeting, compared with presenting the interpretation of a radiologist or pathologist?
Search strategy and selection criteria.
We searched PubMed for articles published between Jan 1,1990, and Sept 8, 2016, with the term “diagnosis” or “high resolution computed tomography”, or “lung biopsy”, combined with the following individual search terms: “idiopathic pulmonary fibrosis”, “idiopathic interstitial pneumonia”, “pulmonary fibrosis”, “diffuse parenchymal lung disease”, “hypersensitivity pneumonia”, “interstitial lung disease”, “interstitial pneumonia”, and “connective tissue associated lung disease”. Articles from these searches and relevant references cited therein were reviewed. We also searched the ClinicalTrials.gov website for the term “diagnosis” combined with the terms “idiopathic pulmonary fibrosis”, “idiopathic interstitial pneumonia”, “pulmonary fibrosis”, “diffuse parenchymal lung disease”, “hypersensitivity pneumonia”, “interstitial lung disease”, “interstitial pneumonia”, and “connective tissue associated lung disease”. Only papers published in English were included.
Acknowledgments
FJM has been on steering committees for studies supported by Afferent, Bayer, Boehringer Ingelheim, Centocor, Gilead, and Veracyte. He has advised Adept, Axon, Clarion, Boehringer Ingelheim, Bellerophon, Genentech/Roche, Kadmon, Nycomed/Takeda, and Veracyte. He has presented continuing medical education for the American Thoracic Society, Academic CME, Falco, the National Association for Continuing Education, Miller, and Potomac. He has been a member of a Data Safety Monitoring Board for a study supported by Biogen. HRC has received personal fees from Medlmmune, Bayer, Biogen, Boehringer Ingelheim, Xfibra, Genoa, Gilead, GlaxoSmithKline, Mesoblast, Moerae Matrix, PharmAkea, Promedior, Prometic, Pulmatrix IUnity, Aeolys, aTyr pharmaceuticals, Pfizer, UCB Celltech, GBT, Veracyte, Patara, Samumed, Alkermes, Five Prime, and Takeda. He is a senior medical advisor for the Pulmonary Fibrosis Foundation. ESW has served as a co-investigator in studies supported by Boehringer Ingelheim, Kadmon, and EMD Serono. He is a medical advisory board member for the Pulmonary Fibrosis Foundation. KRF received personal compensation for work outside of the manuscript from Boehringer Ingelheim, Roche/Genentech, ImmuneWorks, Veracyte, Gilead, Biogen, Aeolus, Pharmakea, and grant support from Boehringer Ingelheim, Afferent. GR has consulted with Biogen, Boehringer Ingelheim, Celgene, Fibrogen, Gilead Sciences, Medlmmune, Promedior, Genentech/Roche, Sanfoi-Aventis, UCB, and Veracyte. He has served on a DSMB for Medlmmune. SLFW is a co-investigator in studies by Boehringer Ingelheim, and has spoken on behalf of Boehringer Ingelheim and Roche. LR has been on a Steering Committee for studies supported by Boehringer Ingelheim. He has advised InterMune, Medlmmune, Biogen, Sanofi-Aventis, Roche, Takeda, and Immuneworks. He has spoken on behalf of Shionogi.
Footnotes
Contributors
FJM, AC, HRC, KRF, JM, GR, SLFW, ESW, and LR made substantial contributions to the conception or design of the work reported. All authors reviewed and critically revised the manuscript for important content, and provided final approval of the submitted version.
Declaration of interests
AC and JM declare no competing interests.
References
- 1.Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ ALAT Clinical practice guideline: treatment of idiopathic pulmonary fibrosis, an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 2.Idiopathic Pulmonary Fibrosis Clinical Research Network. Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–77. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behr J, Bendstrup E, Crestani B, et al. Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2016;4:445–53. doi: 10.1016/S2213-2600(16)30044-3. [DOI] [PubMed] [Google Scholar]
- 5.Jones MG, Walsh SLF, Jones KD, Richeldi L. Idiopathic pulmonary fibrosis: securing a confident diagnosis for every patient. Eur Respir J. 2016;47:1057–59. doi: 10.1183/13993003.00265-2016. [DOI] [PubMed] [Google Scholar]
- 6.Brown KK. Counterpoint: should all patients with idiopathic pulmonary fibrosis, even those with more than moderate impairment, be treated with nintedanib or pirfenidone? No. Chest. 2016;150:276–78. doi: 10.1016/j.chest.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 7.Brownell R, Kaminski N, Woodruff PG, et al. Precision medicine: the new frontier in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:1213–18. doi: 10.1164/rccm.201601-0169CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sgalla G, Cocconcelli E, Tonelli R, Richeldi L. Novel drug targets for idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2016 doi: 10.1586/17476348.2016.1152186. published online Feb 26. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–16. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 10.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. An integral model. Am J Respir Crit Care Med. 2014;189:1161–72. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 11.Fell CD, Martinez FJ, Liu LX, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:832–37. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salisbury ML, Xia M, Murray S, et al. Predictors of idiopathic pulmonary fibrosis in absence of radiologic honeycombing: a cross sectional analysis of ILD patients undergoing lung tissue sampling. Respir Med. 2016;118:88–95. doi: 10.1016/j.rmed.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva CI, Muller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246:288–97. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 14.Churg A, Sin DD, Everett D, Brown K, Cool C. Pathologic patterns and survival in chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2009;33:1765–70. doi: 10.1097/PAS.0b013e3181bb2538. [DOI] [PubMed] [Google Scholar]
- 15.Morell F, Villar A, Montero MA, et al. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013;1:685–94. doi: 10.1016/S2213-2600(13)70191-7. [DOI] [PubMed] [Google Scholar]
- 16.Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–87. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty KR, Fell C, Aubry MC, et al. Smoking-related idiopathic interstitial pneumonia. Eur Respir J. 2014;44:594–602. doi: 10.1183/09031936.00166813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh SLF, Wells AU, Desai SR, et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case cohort study. Lancet Respir Med. 2016;4:557–65. doi: 10.1016/S2213-2600(16)30033-9. [DOI] [PubMed] [Google Scholar]
- 19.Morell F, Villar A, Ojanguren I, Munoz X, Cruz MJ. Hypersensitivity pneumonitis: challenges in diagnosis and management, avoiding surgical lung biopsy. Semin Respir Crit Care Med. 2016;37:395–405. doi: 10.1055/s-0036-1580692. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen SF, Bojesen SE, Schnohr P, Nordestgaard BG. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. BMJ. 2012;345:e5244. doi: 10.1136/bmj.e5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klippel JH, Stone JH, Crofford LJ, White PH, editors. Chapter 2. Primer on the rheumatic diseases. 13. New York: Springer New York; 2008. pp. 15–20. [Google Scholar]
- 23.Ruffatti A, Rossi L, Calligaro A, et al. Autoantibodies of systemic rheumatic diseases in the healthy elderly. Gerontology. 1990;36:104–11. doi: 10.1159/000213183. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Kim EJ, Lynch KL, et al. Prevalence and clinical significance of circulating autoantibodies in idiopathic pulmonary fibrosis. Respir Med. 2013;107:249–55. doi: 10.1016/j.rmed.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins B, Raghu G. Interstitial pneumonia with autoimmune features: the new consensus-based definition for this cohort of patients should be broadened. Eur Respir J. 2016;47:1293–95. doi: 10.1183/13993003.02084-2015. [DOI] [PubMed] [Google Scholar]
- 26.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 27.Millerick-May ML, Mulks MH, Gerlach J, et al. Hypersensitivity pneumonitis and antigen identification—an alternate approach. Respir Med. 2016;112:97–105. doi: 10.1016/j.rmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–14. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 29.Munoz X, Sanchez-Ortiz M, Torres F, Villar A, Morell F, Cruz MJ. Diagnostic yield of specific inhalation challenge in hypersensitivity pneumonitis. Eur Respir J. 2014;44:1658–65. doi: 10.1183/09031936.00060714. [DOI] [PubMed] [Google Scholar]
- 30.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–87. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 31.Gruden JF, Panse PM, Leslie KO, Tazelaar HD, Colby TV. UIP diagnosed at surgical lung biopsy, 2000–2009: HRCT patterns and proposed classification system. AJR Am J Roentgenol. 2013;200:W458–67. doi: 10.2214/AJR.12.9437. [DOI] [PubMed] [Google Scholar]
- 32.Raghu G, Lynch D, Godwin JD, et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med. 2014;2:277–84. doi: 10.1016/S2213-2600(14)70011-6. [DOI] [PubMed] [Google Scholar]
- 33.Chung JH, Chawla A, Peljto AL, et al. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest. 2015;147:450–59. doi: 10.1378/chest.14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safely of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 35.Cottin V, Richeldi L. Neglected evidence in idiopathic pulmonary fibrosis and the importance of early diagnosis and treatment. Eur Respir Rev. 2014;23:106–10. doi: 10.1183/09059180.00008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raghu G, Wells AU, Nicholson AG, et al. Effect of nintedanib in subgroups of idiopathic pulmonary fibrosis by diagnostic criteria. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201602-0402OC. published online June 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–71. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putman RK, Hatabu H, Araki T, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–81. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akari T, Putman RK, Hatabu H, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201512-2523OC. published online June 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vestbo J, Anderson W, Coxson HO, et al. on behalf of the ECLIPSE investigators. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE) Eur Respir J. 2008;31:869–73. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 44.Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM, Consort UIPO. Interobserver agreement for the ATS/ERS/JRS/ ALAT criteria for a UIP pattern on CT. Thorax. 2016;71:45–51. doi: 10.1136/thoraxjnl-2015-207252. [DOI] [PubMed] [Google Scholar]
- 45.Watadani T, Sakai F, Johkoh T, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–44. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 46.Wells AU. The revised ATS/ERS/JRS/ALAT diagnostic criteria for idiopathic pulmonary fibrosis (IPF)—practical implications. Respir Res. 2013;14(suppl 1):S2. doi: 10.1186/1465-9921-14-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193:1161–67. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]
- 48.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis, an international working group report. Am J Respir Crit Care Med. 2016;194:265–75. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 49.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller JD, Urschel JD, Cox G, et al. A randomized, controlled trial comparing thoracoscopy and limited thoracotomy for lung biopsy in interstitial lung disease. Ann Thorac Surg. 2000;70:1647–50. doi: 10.1016/s0003-4975(00)01913-5. [DOI] [PubMed] [Google Scholar]
- 51.Katzenstein AL, Mukhopadhyay S, Myers JL. Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum Pathol. 2008;39:1275–94. doi: 10.1016/j.humpath.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164:1722–27. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 53.Collard HR, Cool CD, Leslie KO, Curran-Everett D, Groshong S, Brown KK. Organizing pneumonia and lymphoplasmacytic inflammation predict treatment response in idiopathic pulmonary fibrosis. Histopathology. 2007;50:258–65. doi: 10.1111/j.1365-2559.2006.02554.x. [DOI] [PubMed] [Google Scholar]
- 54.Flaherty KR, Colby TV, Travis WD, et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167:1410–15. doi: 10.1164/rccm.200204-373OC. [DOI] [PubMed] [Google Scholar]
- 55.Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:173–77. doi: 10.1164/rccm.2109039. [DOI] [PubMed] [Google Scholar]
- 56.King TE, Jr, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–32. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 57.Hanak V, Ryu JH, de Carvalho E, et al. Profusion of fibroblast foci in patients with idiopathic pulmonary fibrosis does not predict outcome. Respir Med. 2008;102:852–56. doi: 10.1016/j.rmed.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Katzenstein AL, Zisman DA, Litzky LA, Nguyen BT, Kotloff RM. Usual interstitial pneumonia: histologic study of biopsy and explant specimens. Am J Surg Pathol. 2002;26:1567–77. doi: 10.1097/00000478-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Ohshimo S, Bonella F, Cui A, et al. Significance of bronchoalveolar lavage for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:1043–47. doi: 10.1164/rccm.200808-1313OC. [DOI] [PubMed] [Google Scholar]
- 60.Leslie KO, Gruden JF, Parish JM, Scholand MB. Trans bronchial biopsy interpretation in the patient with diffuse parenchymal lung disease. Arch Pathol Lab Med. 2007;131:407–23. doi: 10.5858/2007-131-407-TBIITP. [DOI] [PubMed] [Google Scholar]
- 61.Berbescu EA, Katzenstein AL, Snow JL, Zisman DA. Transbronchial biopsy in usual interstitial pneumonia. Chest. 2006;129:1126–31. doi: 10.1378/chest.129.5.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shim HS, Park MS, Park IK. Histopathologic findings of transbronchial biopsy in usual interstitial pneumonia. Pathol Int. 2010;60:373–77. doi: 10.1111/j.1440-1827.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- 63.Kim SY, Diggans J, Pankratz D, et al. Classification of usual interstitial pneumonia in patients with interstitial lung disease: assessment of a machine learning approach using high-dimensional transcriptional data. Lancet Respir Med. 2015;3:473–82. doi: 10.1016/S2213-2600(15)00140-X. [DOI] [PubMed] [Google Scholar]
- 64.Sharp C, McCabe M, Adamali H, Medford AR. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease—a systematic review and cost analysis. QJM. 2016 doi: 10.1093/qjmed/hcwl42. published online Aug 13. [DOI] [PubMed] [Google Scholar]
- 65.Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel NM, Borczuk AC, Lederer DJ. Cryobiopsy in the diagnosis of interstitial lung disease. A step forward or back? Am J Respir Crit Care Med. 2016;193:707–09. doi: 10.1164/rccm.201511-2313ED. [DOI] [PubMed] [Google Scholar]
- 67.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 68.Flaherty KR, King TE, Jr, Raghu G, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170:904–10. doi: 10.1164/rccm.200402-147OC. [DOI] [PubMed] [Google Scholar]
- 69.Flaherty KR, Andrei AC, King TE, Jr, et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med. 2007;175:1054–60. doi: 10.1164/rccm.200606-833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:745–52. doi: 10.1164/rccm.201504-0711OC. [DOI] [PubMed] [Google Scholar]
- 71.Tominaga J, Sakai F, Johkoh T, et al. Diagnostic certainty of idiopathic pulmonary fibrosis/usual interstitial pneumonia: the effect of the integrated clinico-radiological assessment. Eur J Radiol. 2015;84:2640–45. doi: 10.1016/j.ejrad.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 72.Cottin V, Crestani B, Valeyre D, et al. Diagnosis and management of idiopathic pulmonary fibrosis: French practical guidelines. Eur Respir Rev. 2014;23:193–214. doi: 10.1183/09059180.00001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spagnolo P, Grunewald J, du Bois RM. Genetic determinants of pulmonary fibrosis: evolving concepts. Lancet Respir Med. 2014;2:416–28. doi: 10.1016/S2213-2600(14)70047-5. [DOI] [PubMed] [Google Scholar]
- 74.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:770–78. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thannickal VJ, Henke CA, Horowitz JC, et al. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol. 2014;184:1643–51. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124:4673–77. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ley B, Brown KK, Collard HR. Molecular biomarkers in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2014;307:L681–91. doi: 10.1152/ajplung.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kropski JA, Pritchett JM, Zoz DF, et al. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191:417–26. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jenkins RG, Simpson JK, Saini G, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3:462–72. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 81.Leeming DJ, Sand JM, Nielsen MJ, et al. Serological investigation of the collagen degradation profile of patients with chronic obstructive pulmonary disease or idiopathic pulmonary fibrosis. Biomark Insights. 2012;7:119–26. doi: 10.4137/BMI.S9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res. 2012;159:218–27. doi: 10.1016/j.trsl.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White ES, Xia M, Murray S, et al. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201505-0862OC. published online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang G, Yang L, Wang W, Wang J, Wang J, Xu Z. Discovery and validation of extracellular/circulating micro RNAs during idiopathic pulmonary fibrosis disease progression. Gene. 2015;562:138–44. doi: 10.1016/j.gene.2015.02.065. [DOI] [PubMed] [Google Scholar]
- 85.Yang IV, Luna LG, Cotter J, et al. The peripheral blood transcriptome identifies the presence and extent of disease in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e37708. doi: 10.1371/journal.pone.0037708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hara A, Sakamoto N, Ishimatsu Y, et al. S100A9 in BALF is a candidate biomarker of idiopathic pulmonary fibrosis. Respir Med. 2012;106:571–80. doi: 10.1016/j.rmed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 87.Cai M, Bonella F, He X, et al. CCL18 in serum, BAL fluid and alveolar macrophage culture supernatant in interstitial lung diseases. Respir Med. 2013;107:1444–52. doi: 10.1016/j.rmed.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Bauer Y, Tedrow J, de Bernard S, et al. A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;52:217–31. doi: 10.1165/rcmb.2013-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meltzer EB, Barry WT, D’Amico TA, et al. Bayesian probit regression model for the diagnosis of pulmonary fibrosis: proof-of-principle. BMC Med Genomics. 2011;4:70. doi: 10.1186/1755-8794-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Selman M, Pardo A, Barrera L, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173:188–98. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DePianto DJ, Chandriani S, Abbas AR, et al. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax. 2015;70:48–56. doi: 10.1136/thoraxjnl-2013-204596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hodnett PA, Naidich DP. Fibrosing interstitial lung disease. A practical high-resolution computed tomography-based approach to diagnosis and management and a review of the literature. Am J Respir Crit Care Med. 2013;188:141–49. doi: 10.1164/rccm.201208-1544CI. [DOI] [PubMed] [Google Scholar]
- 93.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]