Summary
Introduction
Neuroprotective therapeutics are needed to treat glaucoma, an optic neuropathy that results in death of retinal ganglion cells (RGCs).
Areas covered
The BDNF/TrkB pathway is important for RGC survival. Temporal and spatial alterations in the BDNF/TrkB pathway occur in development and in response to acute optic nerve injury and to glaucoma. In animal models, BDNF supplementation is successful at slowing RGC death after acute optic nerve injury and in glaucoma, however, the BDNF/TrkB signaling is not the only pathway supporting long term RGC survival.
Expert Commentary
Much remains to be discovered about the interaction between retrograde, anterograde, and retinal BDNF/TrkB signaling pathways in both neurons and glia. An ideal therapeutic agent for glaucoma likely has several modes of action that target multiple mechanisms of neurodegeneration including the BDNF/TrkB pathway.
Keywords: Glaucoma, BDNF, TrkB, Retinal ganglion cells, neuroprotection
1.0 Glaucoma an optic neuropathy
Glaucoma is a progressive optic neuropathy that causes irreversible blindness and affects people throughout the world. Glaucoma is currently the second leading cause of blindness after cataracts, and the number of people afflicted with this disease is expected to rise to 79.6 million worldwide by 2020 [1,2]. Clinically, diagnosis of glaucomatous optic neuropathy is determined by the presence of both structural damage to the optic nerve and visual dysfunction [3,4]. These structural and functional changes are caused by the death of retinal ganglion cells (RGCs) and loss of their axons in the optic nerve [4]. RGCs are the final output neurons that collect visual input from the retina and transmit this information to the brain via action potentials along RGC axons [5]. RGC axons leave the retina and converge at the optic nerve head (ONH) where they pass out of the eye leaving a small depression as they form the optic nerve (ON). Loss of RGC axons combined with connective tissue alterations result in a widening and deepening of this depression that is characteristic of glaucoma [3,4].
Glaucoma is classified into two main types determined by the anatomy of the angle where the iris meets the cornea. Primary open angle glaucoma (POAG) is the most common occurring in 74% of the cases while primary closed angle glaucoma (PCAG) occurs less frequently [4]. Risk factors for glaucoma include elevated IOP, advancing age, non-Caucasian ethnicity, and a family history of glaucoma [6]. Although elevated IOP is the most significant risk factor for all types of glaucoma, elevated IOP does not always occur in glaucoma nor does lowering IOP always slow the progression of this disease [7–9]. Unfortunately, existing treatments for glaucoma are limited to eye drops, laser treatments, and surgical approaches designed to lower IOP [10]. Thus, a huge need exists for the development of neuroprotective therapeutics that will stop glaucomatous optic nerve degeneration, thereby preserving sight for millions of people.
A common characteristic of the molecular pathways implicated in glaucoma is that they lead to RGC death [11–14]. Many informative reviews have been published on mechanisms contributing to RGC apoptosis in glaucoma including mitochondrial dysfunction [15] and oxidative stress [16], endoplasmic reticulum (ER) stress and the unfolded protein response [17], neurotrophin deficits [11,18,19], excitotoxicity [20], ischemia [21], inflammation and glial activation [22,23]. Each of these pathways is a potential therapeutic target; however, our group has a keen interest in the role of brain derived neurotrophic factor (BDNF) and its cognate receptor tropomyosin-related kinase B (TrkB) in glaucoma.
In the present work, we review the relationship between the BDNF/TrkB pathway and RGC survival in the developing, healthy, and glaucomatous retina. A common theme emerges of temporal and spatial alterations in the BDNF/TrkB pathway throughout the visual system in response to RGC injury. Great strides have been made in identifying the critical role that the BDNF/TrkB signaling pathway plays in RGC survival in development and after optic nerve injury. Despite these advances, significant gaps of knowledge exist in our understanding of the molecular mechanisms that regulate the BDNF/TrkB pathway in the healthy versus glaucomatous retina. Understanding these mechanisms is difficult because the actions of the BDNF/TrkB signaling pathway extend across multiple compartments of the visual system. In addition, much remains to be learned about the differential role of the BDNF/TrkB pathway in neurons versus glia. Future studies to better understand the function of the BDNF/TrkB pathway in the healthy versus diseased visual system will yield new insights that are essential for the development of novel therapeutic strategies to treat glaucoma.
2.0 BDNF and its receptors
BDNF is a neurotrophin that functions both within and without the central nervous system where it regulates survival, development, function, and plasticity [24]. The neurotrophin family of proteins includes four mammalian neurotrophins: nerve growth factor (NGF), BDNF, neurotrophin-3 (NT-3), and neurotrophin 4/5 (NT-4/5) [25–29]. The discovery of the first neurotrophin, NGF, by Rita Levi-Montalcini in the early 1950’s was followed by identification of a second neurotrophin, BDNF, by Barde in 1982 [26,30]. BDNF structure and function follow a pattern similar to NGF. BDNF is synthesized as a precursor protein (~30–35 kD) that is proteolytically cleaved and processed to form mature BDNF (~14 kD) [31–33]. Proneurotrophins can either be cleaved intracellularly in the ER and Golgi by furin and proconvertases to form mature neurotrophin, or proneurotrophins can be secreted and cleaved extracellularly by plasmin and specific matrixmetaloproteases (MMPs) [34,35]. In their native state, both proBDNF and mature BDNF ligands exist as noncovalent homodimers [32]. Whether a cell secretes proBDNF, BDNF, or both varies with tissue, cell type, and culture conditions [36–38].
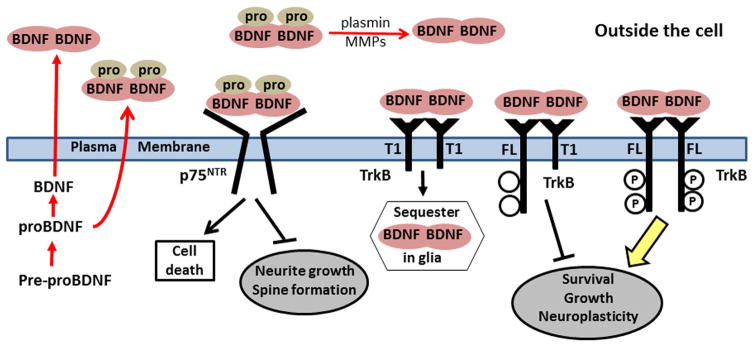
Neurotrophins interact with two main types of receptors: the Trk receptor tyrosine kinases and the p75 neurotrophin receptor (p75NTR) [25,39–41]. Each neurotrophin binds to specific Trk receptors: NGF with TrkA [42], BDNF and NT-4/5 with TrkB [43,44], and NT-3 mainly with TrkC [45]. In the CNS, neuronal activity increases the localization of TrkB to the cell surface where it can bind with BDNF [46,47]. Upon BDNF binding, TrkB dimerizes and autophosphorylates which activates tyrosine kinases that initiate signaling cascades. BDNF/TrkB signaling provides trophic support and modulation of dendrites and synapse formation through three main pathways; mitogen-activated protein kinases (MAPK), phosphatidylinositol 3-kinase (PI3K), and phospholipase C-ɣ (PLC-ɣ) [40,48]. The second BDNF receptor, p75NTR, has a much different function than TrkB. The p75NTR in association with Trk receptors enhances Trk receptor affinity for mature neurotrophin [49–51]. Alternatively, p75NTR can bind each of the neurotrophins directly, especially proneurotrophins [52,53]. The p75NTR does not have an intracellular kinase domain. Instead p75NTR, which can form dimers, signals through a combination of proteolytic events and association of effector molecules with its cytoplasmic tail [41,54,55]. Although the proNGF/p75NTR pathway is well-known for initiating apoptosis, proBDNF activation of p75NTR can also cause apoptosis as well as inhibit neurite growth and spine formation (Figure 1) [34,36,56].
Figure 1.
BDNF signaling is a balance between proBDNF/p75NTR and BDNF in glial cells or inhibit phosphorylation of FL-TrkB thereby preventing stimulation of survival, growth, and neuroplasticity.
The modulation of the BDNF/TrkB signaling pathway is complicated by the presence of TrkB splice variants expressed throughout the CNS. Three of the most common TrkB splice variants that are expressed within the CNS are T1, T2, and T-Shc [57–60]. TrkB splice variants have a normal extracellular domain that binds BDNF but lack the intracellular kinase domain [57–59]. Even though truncated TrkB is found abundantly in glia, it can also co-localize with full length TrkB in neurons [59,61–63]. Truncated TrkB can modulate full length TrkB signaling by inhibiting the movement of full length TrkB to the cell surface thus reducing availability of full length receptor to bind with BDNF [64]. Truncated TrkB can also act as a dominant negative receptor, forming a heterodimer with full length TrkB to prevent autophosphorylation and kinase activation of TrkB [65–68]. In addition, in non-neuronal cells, truncated TrkB can rapidly bind and internalize BDNF thereby preventing BDNF from diffusing away to adjacent cells or tissues [69]. Findings suggest that truncated TrkB may also have its own signaling functions independent of full length TrkB [70].
3.0 BDNF/TrkB in developing retina
Understanding the role of the BDNF/TrkB pathway in retinal development provides insights as to why the BDNF/TrkB pathway is important in the pathogenesis of glaucoma. RGCs are the only retinal neurons that extend their axons through the optic nerve to the brain (reviewed by Xiang, et. al., 1996 [5])Although RGC development and synapse formation is similar among vertebrates, much of our knowledge comes from studies in rodents, chicks, and tadpoles (Xenopus) [71]. One important difference between higher mammals such as primates and these experimental species is the distribution of RGC axon projections. In higher mammals the majority of RGC projections synapse in the lateral geniculate nucleus (LGN) with fewer axons extending to the superior colliculus (SC) [72,73]. In rodents, however, the majority of RGCs project to the SC [74], and in frogs and chicks RGCs project to the optic tectum, a brain region similar to superior colliculus in mammals [75,76]. During retinal development, RGCs are initially overproduced then undergo programmed cell death that coincides with successful formation of synapses [77–82].
In development, innervation of RGCs correlates with the spatial and temporal expression of BDNF in the visual system [83–85]. For example, in hamster, RGCs start populating the retina at embryological day 10 (E10), extend axons through the optic nerve, and arrive at the SC by E13, a time when BDNF protein levels are very low in both retina and SC [83,86,87]. BDNF levels rise in the SC (E14 to P4) as RGCs form side branches and BDNF remains high through P15 when arborization nears completion [83]. In the retina, BDNF levels do not rise until P12 to P18 when RGC axon arbors in the SC mature [83]. During the period of RGC death and synapse formation, BDNF expression is activity dependent [88]. At this time, both BDNF and TrkB mRNA and protein are expressed in retina and SC with strong BDNF and TrkB expression in RGC target areas. [83–85,89–94]. Whether BDNF/TrkB support of RGC survival during development is a result of retrograde, anterograde, or retinal sources of BDNF is a question that continues into adulthood.
Retrograde transport of target-derived BDNF in RGC survival and synapse formation is important during development. This paradigm is similar to the peripheral nervous system (PNS), where target derived BDNF is essential for the survival of select groups of neurons [95–97]. In the rodent visual system, BDNF injected into the SC decreases the rate of RGC developmental death in a manner that is consistent with retrograde transport of target-derived BDNF [98–100]. In contrast, BDNF and TrkB deficits increase the rate of RGC developmental death [101,102]. Despite BDNF/TrkB influence on the rate of RGC death, both BDNF null mice and TrkB null mice have normal numbers of RGCs in the mature retina [101–103]. BDNF/TrkB signaling also has a critical role in formation of RGC target connections. In postnatal rats, overexpression of BDNF in the SC results in more RGC projections to the ipsilateral colliculus [104]. In addition, BDNF supplementation to the optic tectum increases the complexity of RGC arbors in developing Xenopus and chick [105–108]. The ability of BDNF to undergo retrograde transport from SC to RGCs continues into adulthood and has significant implications during the pathogenesis of glaucoma [109–111].
BDNF also undergoes anterograde transport from RGCs to the brain during visual system development. In developing chick and postnatal rats, intraocular injection of BDNF results in anterograde transport of BDNF from RGCs in the retina to the optic tectum and superior colliculus, respectively [112,113]. In rats, anterograde transport of BDNF increases survival of post synaptic neurons in the SC and LGN and depletion of endogenous BDNF decreases survival of these post synaptic neurons [113,114]. In addition, depletion of retinal BDNF causes RGC axons to retract from the dorsal LGN but only during development [115]. Conflicting reports exist as to whether retinal Trk receptors are required to mediate this anterograde transport [114–116]. The ability of RGCs to deliver BDNF to the SC by anterograde transport also continues into adulthood [116,117].
Endogenous production of BDNF in the retina is also important for RGC survival during development [77,100]. In vitro studies show that RGC survival is enhanced by BDNF produced by RGCs suggesting a role for both autocrine and paracrine BDNF/TrkB signaling in the retina [89]. Supplementation of purified RGC cultures with BDNF increases RGC survival most robustly when RGC age corresponds to the developmental time point when target innervation occurs [118,119]. BDNF also increases survival of RGCs and neurite formation in embryonic and adult retinal explants [120–122]. The important relationship between BDNF and neuronal activity with RGC survival is demonstrated in cultures from older postnatal rats (P8) in which BDNF only enhances survival when accompanied by cAMP activation [123]. The cAMP elevation, which is associated with neuron depolarization, increases TrkB levels at the RGC surface [46]. Thus, cAMP induced sensitivity of RGCs to BDNF correlates with increased availability of TrkB to bind with BDNF at the cell surface [46]. Combining a TrkB agonist with forskolin, which elevates cAMP levels, enhances the increased survival of RGCs in culture [124]. The association between electrical activity and enhanced responsiveness of RGCs to BDNF has important implications for glaucoma, where injured RGCs may be less active hence less responsive to BDNF then their healthy counterparts.
Much remains to be discovered about the interplay between retrograde, anterograde, and retinal BDNF/TrkB signaling pathways. An elegant series of studies in Xenopus show that during RGC development, BDNF differentially modulates RGC architecture in the retina and optic tectum depending upon the location and source of BDNF. Intraocular injection of exogenous BDNF decreases the complexity of RGC dendritic arbors but has no effect on arborization of RGC axonal projections. In contrast, tectal-derived BDNF increases the arborization of both RGC axons in the optic tectum and RGC dendrites in the retina. Thus, BDNF can differentially modulate arbor formation of RGCs depending on the location and source of BDNF [105–107].
4.0 BDNF/TrkB in acute optic nerve injury
The importance of the BDNF/TrkB pathway in RGC development continues into adulthood, especially in response to optic nerve injury. In mature rodent retina, as in development, both BDNF and TrkB protein and mRNA are expressed in RGC and glia in the inner retina and in regions where RGC axons project in the brain [90,117,125–127]. Early studies of optic nerve injury utilized acute models such as axotomy and optic nerve crush After axotomy in rats and mice, 50–65% of RGCs die after one week, and 90% of RGCs die by 2 weeks post injury [128–130]. Similar to development, cell death after axotomy occurs by apoptosis as demonstrated by DNA fragmentation and caspase-3 expression in RGCs after axotomy [130,131]. Transient changes in BDNF/TrkB expression occur in response to optic nerve injury, however, differences in the severity of the insultmake comparisons between optic nerve crush and axatomy difficult. In rodent retinas, several studies show a BDNF gene and protein expression increase 2–5 days post injury followed by decline to control levels by 7 days post injury [132–134]. Although occasional studies do not report a decrease in BDNF expression after acute nerve injury, this may be attributable to differences in the severity of the insult [135]. In rodent retina, TrkB gene expression decreases starting at 3 days post axotomy [135,136]. TrkB deficits and ganglion cell loss are greater after optic nerve crush in mice lacking glial TrkB indicating that TrkB in glial cells plays a neuroprotective role after acute nerve injury [135]. Interestingly, in rats four weeks after axotomy, a high percentage of the few remaining RGCs are strongly TrkB positive [137]. In the SC of mice, transient increases in BDNF and TrkB have been reported in mice starting 6 hours after optic nerve crush which is earlier than in retina [133]. Overall, a general pattern emerges of an early increase in BDNF expression in response to injury followed by rapid RGC death accompanied by decreases in BDNF and TrkB expression. The pattern of BDNF and TrkB expression after acute optic nerve injury is similar to development in that the BDNF/TrkB response has spatio and temporal components closely associated with RGC survival. Alterations in the BDNF/TrkB signaling pathway appear to be an endogenous response of the retina to injury.
Numerous studies show that supplementation of the BDNF/TrkB pathway improves survival after acute optic nerve injury. In rats, BDNF exerts a strong protective effect compared to other neurotrophins [138–140]. In both rats and mice, even a single injection of BDNF significantly reduces RGC death from acute optic nerve injury [130,141,142]. In mice after axotomy, BDNF-mediated reduction in RGC death is accompanied by increased gene expression of the RGC markers Thy-1 and light neurofilament protein (NF-L), a major component of the RGC axons in the nerve fiber layer [143]. Interestingly, axotomy induced increases in GFAP, a marker of glial activation, were not reduced by BDNF treatment[143]. In cats and rats, prolonged delivery using multiple BDNF injections or virus mediated BDNF overexpression provide even greater neuroprotection, delaying RGC death for up to 6 weeks [141,144–147]. In cats, combined BDNF supplementation to both eye and visual cortex results in more RGC survival than supplementation to the eye alone [148]. In rats, BDNF supplementation in acute nerve injury is also accompanied by increased axonal sprouting and branch length, however, BDNF does not stimulate regeneration of RGC axons into peripheral nerve grafts or the optic nerve [141,147]. In rats, TrkB gene transfer before axotomy also improves RGC survival, an effect that is amplified by a single intravitreal injection of BDNF [136]. In rats, intravitreal injections of TrkB agonists also increase RGC survival after axotomy [124,149]. The synergistic action of BDNF with TrkB overexpression underscores the need for both BDNF and TrkB to support RGC survival. Although supplementation of BDNF and TrkB after acute optic nerve injury significantly slows the rate of RGC death, BDNF and TrkB supplementation alone does not sustain long term survival. The finding that BDNF and TrkB are not the only requirements for prolonged RGC survival is not surprising. Optic nerve crush and axotomy are extreme injuries and RGCs require a complex set of nutritional requirements when maintained in purified cultures, in vitro [46,123].
One interesting observation from studies of acute optic nerve injury is that BDNF supplementation interacts with other factors such as the activation of microglial cells. Microglia play a central role in the CNS response to injury [150]. Whether microglia can secrete BDNF in response to optic nerve injury is unknown, however, activated microglia secrete BDNF in vitro and murine microglial BDNF is needed in the brain for TrkB phosphorylation, which is a mediator of synaptic plasticity [151,152]. In response to axotomy, dual effects of microglia activation in conjunction with BDNF supplementation have been reported. On one hand, in rats BDNF supplementation delays microglial activation after axotomy [153] and inhibition of microglial cells by treatment with a microglia suppressing factor enhances RGC viability and axon regeneration [154]. Whether this delay in microglial activation is a cause or effect of delayed RGC death is an area of ongoing study [155]. On the other hand, in rats, supplementation with BDNF after axotomy increases activity of nitric oxide synthase (NOS) and activates microglial cells [156,157]. Combined treatment of BDNF with a free radical scavenger or NOS inhibitor, increases BDNF mediated RGC survival after axotomy [156]. Another interesting finding is that in rats, lens injury in conjunction with axotomy protects RGCs and enhances outgrowth of RGC axons into the distal nerve [141,158–160]. This response is macrophage dependent and BDNF independent [159,160]. When BDNF is administered with lens injury and axotomy, an additive increase in RGC survival is observed, however, axon regeneration is absent [158]. The interplay between BDNF and microglia or macrophages is important in the pathogenesis of glaucoma where the final therapeutic goal is not only to prevent RGC death but to regenerate axons and restore visual function.
5.0 BDNF/TrkB in glaucoma
Although much is learned from acute models of optic nerve injury, development of more realistic glaucoma models has provided better systems with which to investigate the BDNF/TrkB pathway. A variety of rodent ocular hypertension models are available for modeling glaucoma (for reviews see [161,162]). Methods to raise intraocular pressure include restricting aqueous outflow by damaging venous drainage from the anterior chamber or by blocking the access of aqueous humor to the trabecular meshwork with microbeads. A variety of genetic mouse models are also available including the DBA2J mouse which develops ocular hypertension and glaucoma with age [163–165]. Compared to acute injury models, glaucoma models have a slower rate of RGC apoptosis that is accompanied by damage to the optic nerve head [131]. The optic nerve head in both humans and animal models is susceptible to stress caused by ocular hypertension. As nerve fibers exit the eye at the ONH, they pass through the mesh-like lamina cribrosa that is stretched or displaced in response to changes in intraocular pressure. One cause of axonal damage in glaucoma is thought to be due to pinching of nerve fibers and blood vessels as they pass through the pores of the lamina cribrosa (reviewed in [166]).
Glaucoma induced changes in BDNF and TrkB function and expression are especially evident at the ONH. Immunohistochemical (IHC) analyses show that in healthy primate and rat eyes, BDNF and TrkB expression is relatively uniform throughout nerve bundles and connective tissue of the ONH [110,167]. In the ONH of glaucomatous primates, however, many nerve bundles have degenerated leaving accumulations of BDNF and TrkB in remaining bundles as well as in astrocytic fibers [110]. In rats, IHC analysis of BDNF expression at the ONH shows a sharp decrease in BDNF signal 7 days post onset of ocular hypertension but a week later astrocytic fibers display robust BDNF signal. Whether total BDNF/TrkB protein levels are altered or just redistributed in this region is hard to accurately access by IHC. Western blot analysis of human post mortem ONH tissue from glaucomatous eyes shows a decrease in BDNF and phosphorylated TrkB expression at the ONH that is also seen in the ONH of mice, 8 weeks post onset of ocular hypertension in a microbead model of glaucoma [168]. These observations are consistent with in vitro studies in which TrkB and phophorylated TrkB are reduced in lamina cribrosa and ONH astrocyte cultures after oxygen glucose deprivation [169]. Although BDNF expression in these cultures varies depending on BDNF isoform and cell type, secretion of BDNF is reduced [169]. These results highlight some of the difficulties in teasing apart the complex role of BDNF/TrkB signaling in neuronal, glial, and cribrosal elements of the ONH.
One important factor disrupting normal physiology and expression of BDNF and TrkB in the visual system is glaucoma-induced axon dysfunction. Acute increases and decreases in IOP disrupt anterograde and retrograde transport through axons of the optic nerve [170–172]. Ocular hypertension, mechanically compresses axon bundles anterior to the lamina cribrosa and causes axons posterior to the lamina cribrosa to dilate and fill with vesicles [110,170–172]. In acute ocular hypertension, BDNF transport from SC to retina is reduced and both BDNF and TrkB accumulate posterior to the ONH [110,111]. More recent studies utilizing the DBA/2J mice show that axon dysfunction and degeneration occurs before RGC loss [173–175]. How these axonal changes specifically impact RGCs and BDNF/TrkB signaling pathways throughout the visual pathway is an area of ongoing study. Another factor influencing axonal transport in the region of the lamina cribrosa is low cerebral spinal fluid (CSF) pressure. An analysis of patient data shows that low CSF pressure is correlated with normal tension glaucoma [176,177]. Low CSF pressure with normal IOP creates a trans lamina cribrosa pressure gradient similar to the pressure gradiant caused by ocular hypertension. A short term reduction of CSF pressure in rats causes a reduction in both anterograde and retrograde transport but whether lowered CSF hinders transport of BDNF from the superior colliculus to the retina has not been determined [178].
In retina, similar to the ONH, the duration and severity of glaucomatous insult influence the temporal and spatial expression of BDNF and TrkB. BDNF is expressed in inner layers of the healthy retina, especially the ganglion cell layer where it co-localizes with TrkB in RGCs [167,179]. TrkB expression in the inner retina extends throughout the NFL, GCL, and IPL with robust in signal in RGC axons [60,110,179]. In glaucomatous monkey retinas and hypertensive rats, IHC analyses show an overall reduction in BDNF signal with focal accumulations of BDNF and TrkB in the IPL. TrkB is also concentrated in the GCL [110,167]. The temporal nature of the BDNF/TrkB response to ocular hypertension combined with variation in glaucoma models make comparisons between studies difficult. In one set of studies, BDNF gene and protein expression is increased in retinas 4 weeks after episcleral vein cauterization in Wistar rats [180]. In contrast, Guo and colleagues show a general decline in BDNF gene expression that tends to correspond with increasing optic nerve damage when assayed at 5 weeks after induction of ocular hypertension in Brown Norway rats [181]. Although this trend was not statistically significant, other investigators have reported a significant decrease in BDNF gene and protein expression in mouse retina 8 weeks after induction of ocular hypertension using microbeads and laser photocoagulation [168,182]. Interestingly, in Guo’s work, proBDNF protein significantly decreases with the grade of optic nerve injury. Less data is available on variation of TrkB expression with ocular hypertension. Although TrkB gene expression is reduced in retinas with more severe optic nerve injury, TrkB and pTrkB protein levels are unchanged 4–5 weeks after induction of ocular hypertension [180,181].
The importance of BDNF and TrkB deficits in the pathology of optic neuropathies is demonstrated in mice lacking one or both BDNF or TrkB allels. Although BDNF (−/−) null mice have normal numbers of RGCs in the mature retina, their axons are hypomyelinated and mice are not viable beyond three weeks of age [102]. Young heterozygous BDNF (+/−) mice have normal RGC numbers and axon myelination, yet they are more sensitive to ocular hypertension with increased visual dysfunction and loss of cells in the GCLs then wild type counterparts. By 1 year of age BDNF (+/−) mice show signs of age-related optic neuropathy. Similar to BDNF deficient mice, TrkB (−/−) null mice have normal RGC numbers with reduced myelination but are not viable after P16 [103]. TrkB (+/−) mice express 25% of normal TrkB and lose 20% of their RGCs by 3 months of age [103]. Thus, although BDNF and TrkB signaling does not determine the final number of RGCs populating the retina at the end of development, these proteins are essential to prevent RGC degeneration in adult animals.
6.0 BDNF/TrkB : Therapeutic implications
The majority of studies testing BDNF therapies in animal models of glaucoma have shown that BDNF supplementation is successful at slowing the progression of RGC degeneration. In rats, multiple intraocular injections of BDNF at weekly intervals significantly increase survival of RGCs by roughly 10% after 33 days of ocular hypertension [183]. In a more acute lasar photocoagulation model of ocular hypertension in rats, a single BDNF injection improves survival but not axonal transport for the majority of RGCs [184]. Interestingly, although BDNF treatment protects the Brn3a positive RGCs, it fails to protect the intrinsically photosensitive ganglion cells, which account for a small fraction (2.5%) of the RGC population [184,185]. In lieu of multiple intraocular injections, which are not well tolerated in the small rodent eye, topical administration of BDNF in eye drops or BDNF gene therapy are less invasive approaches. Topical application of BDNF in the form of eye drops rescued visual function and increased numbers of Brn3a positive ganglion cells in 7 month old DBA/2J mice [186]. Although several studies have demonstrated that neurotrophins such as NGF and BDNF can be delivered to the retina and ON via eye drops, the effectiveness of this mode of neurotrophin delivery is still being validated [187]. Another strategy of BDNF delivery is to use gene therapy with adeno-associated virus (AAV) mediated BDNF overexpression systems to stimulate long term production of BDNF within the retina. BDNF overexpression rescues ganglion cells and improves visual function for at least 9 weeks after an brief and acute elevation of IOP [188]. Interestingly, BDNF synthesized by RGCs appears to have paracrine actions rescuing neighboring neurons that do not express BDNF [188]. BDNF induced protection of RGCs extends to rat glaucoma models, where BDNF overexpression leads to significant RGC survival after 4 weeks of ocular hypertension [189].
A recent study by Feng and colleagues provides hope that long-term protection of RGCs by BDNF is possible [182]. This group used a tamoxifen-induced Cre recombinase system to upregulate BDNF and to protect mouse retinas from sustained IOP elevation. Conditional BDNF overexpressing mice have reduced axon and ganglion cell loss, improved visual acuity and function, and preservation of RGC dendritic fields. Beneficial effects are maintained for up to 6 months [182]. The conditional BDNF overexpressing mice have a neuroprotective advantage over BDNF injections, topical BDNF application, and retinal AAV systems because these mice overexpress BDNF throughout their visual system. Thus, BDNF supplementation effectively occurs in retina, ONH, SC, and visual cortex simultaneously. This paper does not address the effects of BDNF overexpression in the vasculature in response to ocular hypertension, however, this factor may have therapeutic benefits as well. Although delivery of BDNF to the entire visual system is presently not possible in the human patient, the results from Feng’s study highlight the importance of understanding the role of BDNF/TrkB signaling pathway in all parts of the visual system.
Work to understand how glaucoma alters the BDNF/TrkB pathway in SC is just beginning. Two recent studies highlight the complexity of the BDNF/TrkB pathway in this region. In one study, vector-induced BDNF overexpression in the SC did not increase RGC survival or alter BDNF levels in the retina during ocular hypertension [133]. Perhaps raising BDNF levels in the SC does not guarantee transfer of BDNF to RGCs. In one of the few studies on the effect of glaucoma on the SC, Crish and colleagues use the DBA/2J model to show that BDNF accumulates in astrocytes of the glaucomatous SC, even though BDNF mRNA levels are reduced [190]. The authors propose that in response to declines in RGC axonal function, astrocytes of the SC sequester BDNF in an effort to shield target neurons from toxic insults [190]. Given the important role of BDNF/TrkB signaling in the formation of RGC arbors and synapses with SC neurons during development, a better understanding of how the structure and physiology of these connections are altered in glaucoma is critical for identifying novel therapeutic approaches. In DBA/2J mice, synapses between RGCs and neurons of the SC are maintained for a period of time after anterograde transport deficits occur, suggesting that a therapeutic window exists for restoring RGC axonal function before synapses at the SC degenerate [190,191].
7.0 What about proBDNF?
Much remains to be learned about the function of the BDNF/TrkB pathway in both neurons and glia of the healthy and injured visual system including the role of the BDNF precursor, proBDNF. Although mature BDNF has been studied in relation to RGC survival, much less is known about proBDNF/p75NTR signaling pathways. Even though expression of p75NTR in RGCs is low, p75NTR expression is robust in Müller cells, the main glial cell in the retina [192,193]. As much as 80% of the total BDNF in the retina is estimated to be proBDNF [181]. Although the role of proBDNF and p75NTR in the glaucomatous retina is not well studied, increased p75NTR gene expression is accompanied by decreases in proBDNF protein in rat retinas after 5 weeks of ocular hypertension [181]. Interestingly, cultured Müller cells secrete both proBDNF and BDNF in response to treatment with glutamate, the main excitatory neurotransmitter in the retina [194]. ProBDNF’s cousin, proNGF, has already been implicated in RGC death via proNGF/p75NTR mediated secretion of the cytokine TNF-α [193]. In addition, inhibition of p75NTR increases RGC survival after axotomy and this survival is enhanced by co-administration of NGF or TrkA agonist [192]. Collectively, these findings suggest that the role of proBDNF in the pathogenesis of glaucoma is an area deserving future study.
8.0 TrkB regulation and splice variants
The role of TrkB in modulating BDNF trophic support in the glaucomatous retina is not well understood and is another area deserving additional study. Although TrkB is expressed by glia and neurons of the inner retina, details regarding the cell specific localization of TrkB receptor isoforms and their role in regulation of the BDNF/TrkB signaling pathway are still emerging [195]. TrkB signaling is further complicated by the influence of modulatory proteins that associate with the TrkB cytoplasmic tail. For example, in a mouse model of glaucoma, the SH2 domain-containing phosphatase-2 (Shp-2) protein binds to the TrkB receptor in RGCs to inhibit TrkB phosphorylation [196]. This finding may explain, in part, why BDNF supplementation alone is not sufficient to rescue ganglion cells in glaucoma [196]. In glial cells, TrkB signaling plays an important neuroprotective role in response to optic nerve injury [135], however, questions regarding the role of full length versus truncated TrkB signaling remain. Future studies using glaucoma models in combination with tissue specific, conditional BDNF and TrkB knockout mice will aid in dissecting the specific roles and mechanisms of neuronal versus glial BDNF/TrkB signaling pathways in the glaucomatous retina [135,197].
9.0 Expert commentary
The importance of BDNF in human health and disease is becoming increasingly evident especially in light of findings from analyses of the human genome. Studies show that reduced levels of BDNF are associated with heart failure, cognitive disorders, and skeletal muscle energy metabolism [198–201]. Although no large studies have identified BDNF as a biomarker for glaucoma, two small exploratory studies report BDNF deficits in tears and serum of patients with glaucoma [202,203]. The discovery that a mutation in the BDNF pro domain is present in approximately 25% of the population has significant health implications [204]. This single nucleotide polymorphism (SNP) rs6265 affects intracellular trafficking and activity-dependent secretion of BDNF and is associated with impaired memory and hippocampal function [204,205]. This same SNP, rs6265, is associated with the progression of primary open angle glaucoma in a Polish population [206]. This finding is significant in light of the current gene profiling work being done across the globe. Whether additional BDNF related genes are implicated in the progression of glaucoma remains to be seen.
Proper function of the BDNF/TrkB pathway is critical to RGC survival and visual system function in retinal development, in the adult visual system, and in response to optic nerve injury. The importance of the BDNF/TrkB pathway throughout the visual system is evident by the spatio and temporal changes in BDNF/TrkB expression during development and after optic nerve injury. These BDNF/TrkB responses are cell type and region specific extending from the retina, through the ONH, to RGC synapses with neurons of the SC. One emerging concept is that BDNF is not just important in neurons. Much remains to be learned about the differential role of the BDNF/TrkB pathway in glia. Astrocytes, Müller cells, microglia, and lamina cribrosa cells can all secrete BDNF making these cells likely players in the maintenance of healthy BDNF/TrkB signaling throughout the visual system.
Whether the BDNF/TrkB pathway is a worthy therapeutic target in glaucoma is an ongoing debate. A good therapeutic target for glaucoma should accomplish 2 goals. First, survival of RGCs needs to be preserved and second, neuronal signals between RGC axons and their targets need to be restored. Although the BDNF/TrkB pathway is not the sole source of RGC trophic support in the retina, supplementation of BDNF and TrkB in models of acute nerve injury and ocular hypertension helps to preserve RGC survival by delaying RGC death. Prevention of RGC death is the first step in neuroprotection. This neuroprotection is enhanced by combining BDNF supplementation with additional trophic factors or compounds that reduce oxidative stress.
The finding that BDNF and TrkB are not the only requirements for prolonged RGC survival should not deter scientists from trying to understand the complexities of BDNF signaling pathways that are essential for proper RGC function. Currently, our knowledge about the molecular mechanisms regulating the BDNF/TrkB pathway in glaucoma is limited. Not only does the outcome of BDNF/TrkB signaling affect interactions between the retina, ONH, and SC, but BDNF/TrkB signaling also affects the interactions between glia and neurons within each of these regions. Other areas that are highly relevant to glaucoma include modulation of full length TrkB signaling by truncated TrkB and the role of proBDNF/p75NTR signaling pathways. Future studies in these areas will provide new insights that are required for the development of novel neuroprotective treatments for glaucoma.
10.0 Five year view
In the next five years, future studies into the differential roles of the BDNF/TrkB pathway in glia versus neurons will provide new insights that will aid in the development of novel therapeutic approaches for glaucoma. An ideal therapeutic agent for glaucoma is likely to be one that has several modes of action that target multiple mechanisms of neurodegeneration including restoration of healthy BDNF/TrkB function. The ONH has high energy requirements, abundant mitochondria, and is considered the initial site of glaucomatous injury [207]. These characteristics make the ONH an ideal beneficiary of therapeutics that reduce oxidative and ER stress. A therapeutic strategy aimed at preserving function of the ONH may have the secondary effect of restoring healthy BDNF/TrkB signaling throughout the visual system. Our group is interested in sigma-1 receptor, a potential therapeutic target that meets these criteria. Sigma-1 receptor is an inter-organelle signaling modulator known to mediate oxidative and ER stress as well as BDNF processing and secretion [208–210]. A better understanding of the cellular response to stress and tissue repair will lead to new approaches to support the integrity and function of the ONH thereby hopefully preserving healthy BDNF/TrkB signaling pathways. Whether the future therapeutic agent is a sigma-1 receptor agonist or another deserving candidate, development of neuroprotective therapies for glaucoma is greatly needed to preserve vision for the many people afflicted by this sight-threatening disease.
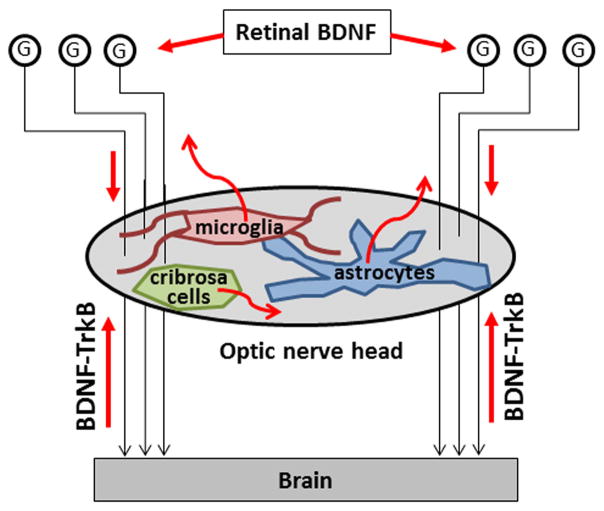
Figure 2.
BDNF is an essential neurotrophin for survival of retinal ganglion cells (G). Retrograde, anterograde and endogenous sources of BDNF are all thought to play a role in the visual system response to optic nerve injury and glaucoma.
Key issues.
Neuroprotective therapeutics are needed to treat glaucoma.
The BDNF/TrkB pathway is critical to retinal ganglion cell survival.
Temporal and spatial alterations in the BDNF/TrkB pathway are a common theme.
Duration and severity of optic nerve injury alter expression of BDNF and TrkB.
BDNF supplementation slows RGC death after nerve injury and in glaucoma.
The BDNF/TrkB pathway is not the only requirement for long term RGC survival.
The interaction between retrograde, anterograde, and retinal BDNF/TrkB signaling pathways in both neurons and glia is an important area of future study.
An ideal therapeutic agent for glaucoma will need to modulate multiple neurodegenerative pathways including the BDNF/TrkB pathway.
Acknowledgments
This work was supported in part by a National Institute of Health 5K08EY021758 grant and an American Glaucoma Society young clinician scientist award (KEB).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Contributor Information
BA Mysona, Augusta University Department of Cellular Biology and Anatomy, James and Jean Culver Vision Discovery Institute. Address: Augusta University Department of Cellular Biology and Anatomy, Health Sciences Campus, 1120 15th Street, Augusta, GA 30912, USA, Phone: (706) 721-3891, Fax: (706) 721-6120
J Zhao, Medical College of Georgia, Department of Ophthalmology at Augusta University, James and Jean Culver Vision Discovery Institute. Address: Medical College of Georgia, Department of Ophthalmology at Augusta University, 1120 15th Street, Augusta, GA 30912, USA, Phone: (706) 721-3891, Fax: (706) 721-1158
KE Bollinger, Medical College of Georgia, Department of Ophthalmology at Augusta University, Augusta University Department of Cellular Biology and Anatomy, James and Jean Culver Vision Discovery Institute. Address: Medical College of Georgia, Department of Ophthalmology at Augusta University, 1120 15th Street, Augusta, GA 30912, USA, Phone: (706) 721-6175, Fax: (706) 721-1158
References
- 1.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82(11):887–888. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 5.Xiang M, Zhou H, Nathans J. Molecular biology of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996;93(2):596–601. doi: 10.1073/pnas.93.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worley A, Grimmer-Somers K. Risk factors for glaucoma: what do they really mean? Aust J Prim Health. 2011;17(3):233–239. doi: 10.1071/PY10042. [DOI] [PubMed] [Google Scholar]
- 7.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109(1):77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 8.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004;138(3 Suppl):S19–31. doi: 10.1016/j.ajo.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Crawley L, Zamir SM, Cordeiro MF, Guo L. Clinical options for the reduction of elevated intraocular pressure. Ophthalmol Eye Dis. 2012;4:43–64. doi: 10.4137/OED.S4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Baltmr A, Duggan J, Nizari S, Salt TE, Cordeiro MF. Neuroprotection in glaucoma - Is there a future role? Experimental eye research. 2010;91(5):554–566. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Nickells RW. The cell and molecular biology of glaucoma: mechanisms of retinal ganglion cell death. Investigative ophthalmology & visual science. 2012;53(5):2476–2481. doi: 10.1167/iovs.12-9483h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18(1):39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 15.Lopez Sanchez MI, Crowston JG, Mackey DA, Trounce IA. Emerging Mitochondrial Therapeutic Targets in Optic Neuropathies. Pharmacol Ther. 2016 doi: 10.1016/j.pharmthera.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Nita M, Grzybowski A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid Med Cell Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anholt RR, Carbone MA. A molecular mechanism for glaucoma: endoplasmic reticulum stress and the unfolded protein response. Trends Mol Med. 2013;19(10):586–593. doi: 10.1016/j.molmed.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Wang R, Thrimawithana T, et al. The nerve growth factor signaling and its potential as therapeutic target for glaucoma. BioMed research international. 2014;2014:759473. doi: 10.1155/2014/759473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afarid M, Torabi-Nami M, Zare B. Neuroprotective and restorative effects of the brain-derived neurotrophic factor in retinal diseases. J Neurol Sci. 2016;363:43–50. doi: 10.1016/j.jns.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Casson RJ. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin Experiment Ophthalmol. 2006;34(1):54–63. doi: 10.1111/j.1442-9071.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- 21.Choi J, Kook MS. Systemic and Ocular Hemodynamic Risk Factors in Glaucoma. BioMed research international. 2015;2015:141905. doi: 10.1155/2015/141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mac Nair CE, Nickells RW. Neuroinflammation in Glaucoma and Optic Nerve Damage. Prog Mol Biol Transl Sci. 2015;134:343–363. doi: 10.1016/bs.pmbts.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Russo R, Varano GP, Adornetto A, et al. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur J Pharmacol. 2016 doi: 10.1016/j.ejphar.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 25.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi-Montalcini R, Cohen S. In Vitro and in Vivo Effects of a Nerve Growth-Stimulating Agent Isolated from Snake Venom. Proc Natl Acad Sci U S A. 1956;42(9):695–699. doi: 10.1073/pnas.42.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernfors P, Ibanez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991;7(5):857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- 30.Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116(2):321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- 31.Teng KK, Felice S, Kim T, Hempstead BL. Understanding proneurotrophin actions: Recent advances and challenges. Dev Neurobiol. 2010;70(5):350–359. doi: 10.1002/dneu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolbeck R, Jungbluth S, Barde YA. Characterisation of neurotrophin dimers and monomers. Eur J Biochem. 1994;225(3):995–1003. doi: 10.1111/j.1432-1033.1994.0995b.x. [DOI] [PubMed] [Google Scholar]
- 33.Suter U, Heymach JV, Jr, Shooter EM. Two conserved domains in the NGF propeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. EMBO J. 1991;10(9):2395–2400. doi: 10.1002/j.1460-2075.1991.tb07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 35.Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 36.Koshimizu H, Kiyosue K, Hara T, et al. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto T, Rauskolb S, Polack M, et al. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11(2):131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Siao CJ, Nagappan G, et al. Neuronal release of proBDNF. Nat Neurosci. 2009;12(2):113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 41.Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5(11):1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein R, Lamballe F, Bryant S, Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992;8(5):947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- 44.Ip NY, Stitt TN, Tapley P, et al. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10(2):137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- 45.Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- 46.Meyer-Franke A, Wilkinson GA, Kruttgen A, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21(4):681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du J, Feng L, Yang F, Lu B. Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol. 2000;150(6):1423–1434. doi: 10.1083/jcb.150.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11(3):272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 49.Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999;18(3):616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18(7):321–326. [PubMed] [Google Scholar]
- 51.Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2(8):699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Tebar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4(4):487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Tebar A, Dechant G, Gotz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO J. 1992;11(3):917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skeldal S, Matusica D, Nykjaer A, Coulson EJ. Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling?: Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR) Bioessays. 2011;33(8):614–625. doi: 10.1002/bies.201100036. [DOI] [PubMed] [Google Scholar]
- 55.Vilar M, Charalampopoulos I, Kenchappa RS, et al. Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron. 2009;62(1):72–83. doi: 10.1016/j.neuron.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teng HK, Teng KK, Lee R, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(22):5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11(1):143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shelton DL, Sutherland J, Gripp J, et al. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15(1 Pt 2):477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- 60.Jelsma TN, Friedman HH, Berkelaar M, Bray GM, Aguayo AJ. Different forms of the neurotrophin receptor trkB mRNA predominate in rat retina and optic nerve. J Neurobiol. 1993;24(9):1207–1214. doi: 10.1002/neu.480240907. [DOI] [PubMed] [Google Scholar]
- 61.Armanini MP, McMahon SB, Sutherland J, Shelton DL, Phillips HS. Truncated and catalytic isoforms of trkB are co-expressed in neurons of rat and mouse CNS. Eur J Neurosci. 1995;7(6):1403–1409. doi: 10.1111/j.1460-9568.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 62.Frisen J, Verge VM, Fried K, et al. Characterization of glial trkB receptors: differential response to injury in the central and peripheral nervous systems. Proc Natl Acad Sci U S A. 1993;90(11):4971–4975. doi: 10.1073/pnas.90.11.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck KD, Lamballe F, Klein R, et al. Induction of noncatalytic TrkB neurotrophin receptors during axonal sprouting in the adult hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(9):4001–4014. doi: 10.1523/JNEUROSCI.13-09-04001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haapasalo A, Sipola I, Larsson K, et al. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J Biol Chem. 2002;277(45):43160–43167. doi: 10.1074/jbc.M205202200. [DOI] [PubMed] [Google Scholar]
- 65.Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(10):3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haapasalo A, Koponen E, Hoppe E, Wong G, Castren E. Truncated trkB.T1 is dominant negative inhibitor of trkB.TK+-mediated cell survival. Biochem Biophys Res Commun. 2001;280(5):1352–1358. doi: 10.1006/bbrc.2001.4296. [DOI] [PubMed] [Google Scholar]
- 67.Ninkina N, Adu J, Fischer A, Pinon LG, Buchman VL, Davies AM. Expression and function of TrkB variants in developing sensory neurons. EMBO J. 1996;15(23):6385–6393. [PMC free article] [PubMed] [Google Scholar]
- 68.Li YX, Xu Y, Ju D, Lester HA, Davidson N, Schuman EM. Expression of a dominant negative TrkB receptor, T1, reveals a requirement for presynaptic signaling in BDNF-induced synaptic potentiation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95(18):10884–10889. doi: 10.1073/pnas.95.18.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biffo S, Offenhauser N, Carter BD, Barde YA. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121(8):2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- 70.Baxter GT, Radeke MJ, Kuo RC, et al. Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17(8):2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isenmann S, Kretz A, Cellerino A. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res. 2003;22(4):483–543. doi: 10.1016/s1350-9462(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 72.Perry VH, Cowey A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience. 1984;12(4):1125–1137. doi: 10.1016/0306-4522(84)90007-1. [DOI] [PubMed] [Google Scholar]
- 73.Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- 74.Hofbauer A, Drager UC. Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. The Journal of comparative neurology. 1985;234(4):465–474. doi: 10.1002/cne.902340405. [DOI] [PubMed] [Google Scholar]
- 75.Mey J, Thanos S. Development of the visual system of the chick. I. Cell differentiation and histogenesis. Brain Res Brain Res Rev. 2000;32(2–3):343–379. doi: 10.1016/s0165-0173(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 76.Holt CE, Harris WA. Order in the initial retinotectal map in Xenopus: a new technique for labelling growing nerve fibres. Nature. 1983;301(5896):150–152. doi: 10.1038/301150a0. [DOI] [PubMed] [Google Scholar]
- 77.Frade JM, Bovolenta P, Martinez-Morales JR, Arribas A, Barbas JA, Rodriguez-Tebar A. Control of early cell death by BDNF in the chick retina. Development. 1997;124(17):3313–3320. doi: 10.1242/dev.124.17.3313. [DOI] [PubMed] [Google Scholar]
- 78.Perry VH, Henderson Z, Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. The Journal of comparative neurology. 1983;219(3):356–368. doi: 10.1002/cne.902190309. [DOI] [PubMed] [Google Scholar]
- 79.Galli-Resta L, Ensini M. An intrinsic time limit between genesis and death of individual neurons in the developing retinal ganglion cell layer. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(7):2318–2324. doi: 10.1523/JNEUROSCI.16-07-02318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guerin MB, McKernan DP, O’Brien CJ, Cotter TG. Retinal ganglion cells: dying to survive. Int J Dev Biol. 2006;50(8):665–674. doi: 10.1387/ijdb.062159mg. [DOI] [PubMed] [Google Scholar]
- 81.Sengelaub DR, Finlay BL. Cell death in the mammalian visual system during normal development : I. Retinal ganglion cells. The Journal of comparative neurology. 1982;204(4):311–317. doi: 10.1002/cne.902040402. [DOI] [PubMed] [Google Scholar]
- 82.Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 83.Frost DO, Ma YT, Hsieh T, Forbes ME, Johnson JE. Developmental changes in BDNF protein levels in the hamster retina and superior colliculus. J Neurobiol. 2001;49(3):173–187. doi: 10.1002/neu.1073. [DOI] [PubMed] [Google Scholar]
- 84.Herzog KH, Bailey K, Barde YA. Expression of the BDNF gene in the developing visual system of the chick. Development. 1994;120(6):1643–1649. doi: 10.1242/dev.120.6.1643. [DOI] [PubMed] [Google Scholar]
- 85.Cohen-Cory S, Fraser SE. BDNF in the development of the visual system of Xenopus. Neuron. 1994;12(4):747–761. doi: 10.1016/0896-6273(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 86.Sengelaub DR, Dolan RP, Finlay BL. Cell generation, death, and retinal growth in the development of the hamster retinal ganglion cell layer. The Journal of comparative neurology. 1986;246(4):527–543. doi: 10.1002/cne.902460409. [DOI] [PubMed] [Google Scholar]
- 87.Jhaveri S, Edwards MA, Schneider GE. Initial stages of retinofugal axon development in the hamster: evidence for two distinct modes of growth. Exp Brain Res. 1991;87(2):371–382. doi: 10.1007/BF00231854. [DOI] [PubMed] [Google Scholar]
- 88.Karlsson M, Hallbook F. Kainic acid, tetrodotoxin and light modulate expression of brain-derived neurotrophic factor in developing avian retinal ganglion cells and their tectal target. Neuroscience. 1998;83(1):137–150. doi: 10.1016/s0306-4522(97)00340-0. [DOI] [PubMed] [Google Scholar]
- 89.Cohen-Cory S, Escandon E, Fraser SE. The cellular patterns of BDNF and trkB expression suggest multiple roles for BDNF during Xenopus visual system development. Dev Biol. 1996;179(1):102–115. doi: 10.1006/dbio.1996.0244. [DOI] [PubMed] [Google Scholar]
- 90.Perez MT, Caminos E. Expression of brain-derived neurotrophic factor and of its functional receptor in neonatal and adult rat retina. Neurosci Lett. 1995;183(1–2):96–99. doi: 10.1016/0304-3940(94)11123-z. [DOI] [PubMed] [Google Scholar]
- 91.Rickman DW, Brecha NC. Expression of the proto-oncogene, trk, receptors in the developing rat retina. Vis Neurosci. 1995;12(2):215–222. doi: 10.1017/s0952523800007896. [DOI] [PubMed] [Google Scholar]
- 92.Garner AS, Menegay HJ, Boeshore KL, et al. Expression of TrkB receptor isoforms in the developing avian visual system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(5):1740–1752. doi: 10.1523/JNEUROSCI.16-05-01740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hallbook F, Backstrom A, Kullander K, Ebendal T, Carri NG. Expression of neurotrophins and trk receptors in the avian retina. The Journal of comparative neurology. 1996;364(4):664–676. doi: 10.1002/(SICI)1096-9861(19960122)364:4<664::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 94.Escandon E, Soppet D, Rosenthal A, et al. Regulation of neurotrophin receptor expression during embryonic and postnatal development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(4):2054–2068. doi: 10.1523/JNEUROSCI.14-04-02054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klein R, Smeyne RJ, Wurst W, et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75(1):113–122. [PubMed] [Google Scholar]
- 96.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368(6467):147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 97.Altar CA, Cai N, Bliven T, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389(6653):856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 98.Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(6):2097–2107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spalding KL, Cui Q, Harvey AR. The effects of central administration of neurotrophins or transplants of fetal tectal tissue on retinal ganglion cell survival following removal of the superior colliculus in neonatal rats. Brain Res Dev Brain Res. 1998;107(1):133–142. doi: 10.1016/s0165-3806(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 100.Spalding KL, Rush RA, Harvey AR. Target-derived and locally derived neurotrophins support retinal ganglion cell survival in the neonatal rat retina. J Neurobiol. 2004;60(3):319–327. doi: 10.1002/neu.20028. [DOI] [PubMed] [Google Scholar]
- 101.Pollock GS, Robichon R, Boyd KA, et al. TrkB receptor signaling regulates developmental death dynamics, but not final number, of retinal ganglion cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(31):10137–10145. doi: 10.1523/JNEUROSCI.23-31-10137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci. 1997;9(5–6):397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- 103.Rohrer B, LaVail MM, Jones KR, Reichardt LF. Neurotrophin receptor TrkB activation is not required for the postnatal survival of retinal ganglion cells in vivo. Exp Neurol. 2001;172(1):81–91. doi: 10.1006/exnr.2001.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Isenmann S, Cellerino A, Gravel C, Bahr M. Excess target-derived brain-derived neurotrophic factor preserves the transient uncrossed retinal projection to the superior colliculus. Mol Cell Neurosci. 1999;14(1):52–65. doi: 10.1006/mcne.1999.0763. [DOI] [PubMed] [Google Scholar]
- 105.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378(6553):192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 106.Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(22):9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(17):7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276(5317):1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- 109.Fournier AE, Beer J, Arregui CO, Essagian C, Aguayo AJ, McKerracher L. Brain-derived neurotrophic factor modulates GAP-43 but not T alpha1 expression in injured retinal ganglion cells of adult rats. J Neurosci Res. 1997;47(6):561–572. [PubMed] [Google Scholar]
- 110.Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Investigative ophthalmology & visual science. 2000;41(3):764–774. [PubMed] [Google Scholar]
- 111.Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41(11):3460–3466. [PubMed] [Google Scholar]
- 112.von Bartheld CS, Byers MR, Williams R, Bothwell M. Anterograde transport of neurotrophins and axodendritic transfer in the developing visual system. Nature. 1996;379(6568):830–833. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- 113.Caleo M, Menna E, Chierzi S, Cenni MC, Maffei L. Brain-derived neurotrophic factor is an anterograde survival factor in the rat visual system. Curr Biol. 2000;10(19):1155–1161. doi: 10.1016/s0960-9822(00)00713-2. [DOI] [PubMed] [Google Scholar]
- 114.Spalding KL, Tan MM, Hendry IA, Harvey AR. Anterograde transport and trophic actions of BDNF and NT-4/5 in the developing rat visual system. Mol Cell Neurosci. 2002;19(4):485–500. doi: 10.1006/mcne.2001.1097. [DOI] [PubMed] [Google Scholar]
- 115.Menna E, Cenni MC, Naska S, Maffei L. The anterogradely transported BDNF promotes retinal axon remodeling during eye specific segregation within the LGN. Mol Cell Neurosci. 2003;24(4):972–983. doi: 10.1016/s1044-7431(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 116.Butowt R, von Bartheld CS. Anterograde axonal transport of BDNF and NT-3 by retinal ganglion cells: roles of neurotrophin receptors. Mol Cell Neurosci. 2005;29(1):11–25. doi: 10.1016/j.mcn.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Avwenagha O, Bird MM, Lieberman AR, Yan Q, Campbell G. Patterns of expression of brain-derived neurotrophic factor and tyrosine kinase B mRNAs and distribution and ultrastructural localization of their proteins in the visual pathway of the adult rat. Neuroscience. 2006;140(3):913–928. doi: 10.1016/j.neuroscience.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 118.Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rodriguez-Tebar A, Jeffrey PL, Thoenen H, Barde YA. The survival of chick retinal ganglion cells in response to brain-derived neurotrophic factor depends on their embryonic age. Dev Biol. 1989;136(2):296–303. doi: 10.1016/0012-1606(89)90256-x. [DOI] [PubMed] [Google Scholar]
- 120.Thanos S, Bahr M, Barde YA, Vanselow J. Survival and Axonal Elongation of Adult Rat Retinal Ganglion Cells. Eur J Neurosci. 1989;1(1):19–26. doi: 10.1111/j.1460-9568.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 121.Castillo B, Jr, del Cerro M, Breakefield XO, et al. Retinal ganglion cell survival is promoted by genetically modified astrocytes designed to secrete brain-derived neurotrophic factor (BDNF) Brain Res. 1994;647(1):30–36. doi: 10.1016/0006-8993(94)91395-1. [DOI] [PubMed] [Google Scholar]
- 122.Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol. 1994;25(8):953–959. doi: 10.1002/neu.480250805. [DOI] [PubMed] [Google Scholar]
- 123.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15(4):805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 124.Hu Y, Cho S, Goldberg JL. Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Investigative ophthalmology & visual science. 2010;51(3):1747–1754. doi: 10.1167/iovs.09-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9(8):2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kawamoto Y, Nakamura S, Nakano S, Oka N, Akiguchi I, Kimura J. Immunohistochemical localization of brain-derived neurotrophic factor in adult rat brain. Neuroscience. 1996;74(4):1209–1226. doi: 10.1016/0306-4522(96)00245-x. [DOI] [PubMed] [Google Scholar]
- 127.Vecino E, Caminos E, Ugarte M, Martin-Zanca D, Osborne NN. Immunohistochemical distribution of neurotrophins and their receptors in the rat retina and the effects of ischemia and reperfusion. Gen Pharmacol. 1998;30(3):305–314. doi: 10.1016/s0306-3623(97)00361-3. [DOI] [PubMed] [Google Scholar]
- 128.Rabacchi SA, Bonfanti L, Liu XH, Maffei L. Apoptotic cell death induced by optic nerve lesion in the neonatal rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(9):5292–5301. doi: 10.1523/JNEUROSCI.14-09-05292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(7):4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sanchez-Migallon MC, Valiente-Soriano FJ, Nadal-Nicolas FM, Vidal-Sanz M, Agudo-Barriuso M. Apoptotic Retinal Ganglion Cell Death After Optic Nerve Transection or Crush in Mice: Delayed RGC Loss With BDNF or a Caspase 3 Inhibitor. Investigative ophthalmology & visual science. 2016;57(1):81–93. doi: 10.1167/iovs.15-17841. [DOI] [PubMed] [Google Scholar]
- 131.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Investigative ophthalmology & visual science. 1995;36(5):774–786. [PubMed] [Google Scholar]
- 132.Gao H, Qiao X, Hefti F, Hollyfield JG, Knusel B. Elevated mRNA expression of brain-derived neurotrophic factor in retinal ganglion cell layer after optic nerve injury. Investigative ophthalmology & visual science. 1997;38(9):1840–1847. [PubMed] [Google Scholar]
- 133.Dekeyster E, Geeraerts E, Buyens T, et al. Tackling Glaucoma from within the Brain: An Unfortunate Interplay of BDNF and TrkB. PLoS One. 2015;10(11):e0142067. doi: 10.1371/journal.pone.0142067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang AD, Makowiecki K, Bartlett C, Rodger J. Low intensity repetitive transcranial magnetic stimulation does not induce cell survival or regeneration in a mouse optic nerve crush model. PLoS One. 2015;10(5):e0126949. doi: 10.1371/journal.pone.0126949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harada C, Azuchi Y, Noro T, et al. TrkB Signaling in Retinal Glia Stimulates Neuroprotection after Optic Nerve Injury. Am J Pathol. 2015;185(12):3238–3247. doi: 10.1016/j.ajpath.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(10):3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cui Q, Tang LS, Hu B, So KF, Yip HK. Expression of trkA, trkB, and trkC in injured and regenerating retinal ganglion cells of adult rats. Investigative ophthalmology & visual science. 2002;43(6):1954–1964. [PubMed] [Google Scholar]
- 138.Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Investigative ophthalmology & visual science. 1996;37(4):489–500. [PubMed] [Google Scholar]
- 139.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602(2):304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- 140.Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Perez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Experimental eye research. 2009;89(1):32–41. doi: 10.1016/j.exer.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 141.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91(5):1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanchez-Migallon MC, Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M. Brain derived neurotrophic factor maintains Brn3a expression in axotomized rat retinal ganglion cells. Experimental eye research. 2011;92(4):260–267. doi: 10.1016/j.exer.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 143.Chidlow G, Casson R, Sobrado-Calvo P, Vidal-Sanz M, Osborne NN. Measurement of retinal injury in the rat after optic nerve transection: an RT-PCR study. Molecular vision. 2005;11:387–396. [PubMed] [Google Scholar]
- 144.Isenmann S, Klocker N, Gravel C, Bahr M. Short communication: protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur J Neurosci. 1998;10(8):2751–2756. doi: 10.1046/j.1460-9568.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 145.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95(7):3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Investigative ophthalmology & visual science. 2001;42(5):966–974. [PubMed] [Google Scholar]
- 147.Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(12):3887–3894. doi: 10.1523/JNEUROSCI.16-12-03887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weber AJ, Viswanathan S, Ramanathan C, Harman CD. Combined application of BDNF to the eye and brain enhances ganglion cell survival and function in the cat after optic nerve injury. Investigative ophthalmology & visual science. 2010;51(1):327–334. doi: 10.1167/iovs.09-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bai Y, Xu J, Brahimi F, Zhuo Y, Sarunic MV, Saragovi HU. An agonistic TrkB mAb causes sustained TrkB activation, delays RGC death, and protects the retinal structure in optic nerve axotomy and in glaucoma. Investigative ophthalmology & visual science. 2010;51(9):4722–4731. doi: 10.1167/iovs.09-5032. [DOI] [PubMed] [Google Scholar]
- 150.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gomes C, Ferreira R, George J, et al. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. Journal of neuroinflammation. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sobrado-Calvo P, Vidal-Sanz M, Villegas-Perez MP. Rat retinal microglial cells under normal conditions, after optic nerve section, and after optic nerve section and intravitreal injection of trophic factors or macrophage inhibitory factor. The Journal of comparative neurology. 2007;501(6):866–878. doi: 10.1002/cne.21279. [DOI] [PubMed] [Google Scholar]
- 154.Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(2):455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Galindo-Romero C, Valiente-Soriano FJ, Jimenez-Lopez M, et al. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Investigative ophthalmology & visual science. 2013;54(2):974–985. doi: 10.1167/iovs.12-11207. [DOI] [PubMed] [Google Scholar]
- 156.Klocker N, Cellerino A, Bahr M. Free radical scavenging and inhibition of nitric oxide synthase potentiates the neurotrophic effects of brain-derived neurotrophic factor on axotomized retinal ganglion cells In vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(3):1038–1046. doi: 10.1523/JNEUROSCI.18-03-01038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Klocker N, Kermer P, Gleichmann M, Weller M, Bahr M. Both the neuronal and inducible isoforms contribute to upregulation of retinal nitric oxide synthase activity by brain-derived neurotrophic factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(19):8517–8527. doi: 10.1523/JNEUROSCI.19-19-08517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pernet V, Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129(Pt 4):1014–1026. doi: 10.1093/brain/awl015. [DOI] [PubMed] [Google Scholar]
- 159.Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Investigative ophthalmology & visual science. 2000;41(12):3943–3954. [PubMed] [Google Scholar]
- 160.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(12):4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Morrison JC, Cepurna WO, Johnson EC. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Experimental eye research. 2015;141:23–32. doi: 10.1016/j.exer.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morgan JE, Tribble JR. Microbead models in glaucoma. Experimental eye research. 2015;141:9–14. doi: 10.1016/j.exer.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 163.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investigative ophthalmology & visual science. 1998;39(6):951–962. [PubMed] [Google Scholar]
- 164.Chang B, Smith RS, Hawes NL, et al. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nature genetics. 1999;21(4):405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- 165.Fernandes KA, Harder JM, Williams PA, et al. Using genetic mouse models to gain insight into glaucoma: Past results and future possibilities. Experimental eye research. 2015;141:42–56. doi: 10.1016/j.exer.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wallace DM, O’Brien CJ. The role of lamina cribrosa cells in optic nerve head fibrosis in glaucoma. Experimental eye research. 2016;142:102–109. doi: 10.1016/j.exer.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 167.Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Investigative ophthalmology & visual science. 2000;41(2):431–442. [PubMed] [Google Scholar]
- 168.Gupta V, You Y, Li J, et al. BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma. Biochimica et biophysica acta. 2014;1842(9):1567–1578. doi: 10.1016/j.bbadis.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 169.Lambert WS, Clark AF, Wordinger RJ. Neurotrophin and Trk expression by cells of the human lamina cribrosa following oxygen-glucose deprivation. BMC neuroscience. 2004;5:51. doi: 10.1186/1471-2202-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Minckler DS, Bunt AH. Axoplasmic transport in ocular hypotony and papilledema in the monkey. Arch Ophthalmol. 1977;95(8):1430–1436. doi: 10.1001/archopht.1977.04450080140018. [DOI] [PubMed] [Google Scholar]
- 171.Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Investigative ophthalmology & visual science. 1977;16(5):426–441. [PubMed] [Google Scholar]
- 172.Minckler DS, Bunt AH, Klock IB. Radioautographic and cytochemical ultrastructural studies of axoplasmic transport in the monkey optic nerve head. Investigative ophthalmology & visual science. 1978;17(1):33–50. [PubMed] [Google Scholar]
- 173.Buckingham BP, Inman DM, Lambert W, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(11):2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Howell GR, Libby RT, Jakobs TC, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179(7):1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Investigative ophthalmology & visual science. 2008;49(12):5412–5418. doi: 10.1167/iovs.08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ren R, Jonas JB, Tian G, et al. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology. 2010;117(2):259–266. doi: 10.1016/j.ophtha.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Z, Liu D, Jonas JB, et al. Axonal Transport in the Rat Optic Nerve Following Short-Term Reduction in Cerebrospinal Fluid Pressure or Elevation in Intraocular Pressure. Investigative ophthalmology & visual science. 2015;56(8):4257–4266. doi: 10.1167/iovs.14-16045. [DOI] [PubMed] [Google Scholar]
- 179.Vecino E, Garcia-Crespo D, Garcia M, Martinez-Millan L, Sharma SC, Carrascal E. Rat retinal ganglion cells co-express brain derived neurotrophic factor (BDNF) and its receptor TrkB. Vision Res. 2002;42(2):151–157. doi: 10.1016/s0042-6989(01)00251-6. [DOI] [PubMed] [Google Scholar]
- 180.Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neurobiol. 2004;58(3):341–354. doi: 10.1002/neu.10293. [DOI] [PubMed] [Google Scholar]
- 181.Guo Y, Johnson E, Cepurna W, Jia L, Dyck J, Morrison JC. Does elevated intraocular pressure reduce retinal TRKB-mediated survival signaling in experimental glaucoma? Experimental eye research. 2009;89(6):921–933. doi: 10.1016/j.exer.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Feng L, Chen H, Yi J, Troy JB, Zhang HF, Liu X. Long-Term Protection of Retinal Ganglion Cells and Visual Function by Brain-Derived Neurotrophic Factor in Mice With Ocular Hypertension. Investigative ophthalmology & visual science. 2016;57(8):3793–3802. doi: 10.1167/iovs.16-19825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ko ML, Hu DN, Ritch R, Sharma SC, Chen CF. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett. 2001;305(2):139–142. doi: 10.1016/s0304-3940(01)01830-4. [DOI] [PubMed] [Google Scholar]
- 184.Valiente-Soriano FJ, Nadal-Nicolas FM, Salinas-Navarro M, et al. BDNF Rescues RGCs But Not Intrinsically Photosensitive RGCs in Ocular Hypertensive Albino Rat Retinas. Investigative ophthalmology & visual science. 2015;56(3):1924–1936. doi: 10.1167/iovs.15-16454. [DOI] [PubMed] [Google Scholar]
- 185.Galindo-Romero C, Jimenez-Lopez M, Garcia-Ayuso D, et al. Number and spatial distribution of intrinsically photosensitive retinal ganglion cells in the adult albino rat. Experimental eye research. 2013;108:84–93. doi: 10.1016/j.exer.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 186.Domenici L, Origlia N, Falsini B, et al. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS One. 2014;9(12):e115579. doi: 10.1371/journal.pone.0115579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lambiase A, Mantelli F, Bonini S. Nerve growth factor eye drops to treat glaucoma. Drug News Perspect. 2010;23(6):361–367. doi: 10.1358/dnp.2010.23.6.1472299. [DOI] [PubMed] [Google Scholar]
- 188.Ren R, Li Y, Liu Z, Liu K, He S. Long-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressure. Investigative ophthalmology & visual science. 2012;53(2):1003–1011. doi: 10.1167/iovs.11-8484. [DOI] [PubMed] [Google Scholar]
- 189.Martin KR, Quigley HA, Zack DJ, et al. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Investigative ophthalmology & visual science. 2003;44(10):4357–4365. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- 190.Crish SD, Dapper JD, MacNamee SE, et al. Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience. 2013;229:55–70. doi: 10.1016/j.neuroscience.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010;107(11):5196–5201. doi: 10.1073/pnas.0913141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lebrun-Julien F, Morquette B, Douillette A, Saragovi HU, Di Polo A. Inhibition of p75(NTR) in glia potentiates TrkA-mediated survival of injured retinal ganglion cells. Mol Cell Neurosci. 2009;40(4):410–420. doi: 10.1016/j.mcn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 193.Lebrun-Julien F, Bertrand MJ, De Backer O, et al. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proc Natl Acad Sci U S A. 2010;107(8):3817–3822. doi: 10.1073/pnas.0909276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Taylor S, Srinivasan B, Wordinger RJ, Roque RS. Glutamate stimulates neurotrophin expression in cultured Muller cells. Brain research. Molecular brain research. 2003;111(1–2):189–197. doi: 10.1016/s0169-328x(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 195.Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(20):8919–8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gupta VK, You Y, Klistorner A, Graham SL. Shp-2 regulates the TrkB receptor activity in the retinal ganglion cells under glaucomatous stress. Biochimica et biophysica acta. 2012;1822(11):1643–1649. doi: 10.1016/j.bbadis.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 197.Rios M, Fan G, Fekete C, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15(10):1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 198.Takashio S, Sugiyama S, Yamamuro M, et al. Significance of low plasma levels of brain-derived neurotrophic factor in patients with heart failure. Am J Cardiol. 2015;116(2):243–249. doi: 10.1016/j.amjcard.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 199.Weinstein G, Beiser AS, Choi SH, et al. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014;71(1):55–61. doi: 10.1001/jamaneurol.2013.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Matthews VB, Astrom MB, Chan MH, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52(7):1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 202.Ghaffariyeh A, Honarpisheh N, Shakiba Y, et al. Brain-derived neurotrophic factor in patients with normal-tension glaucoma. Optometry. 2009;80(11):635–638. doi: 10.1016/j.optm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 203.Ghaffariyeh A, Honarpisheh N, Heidari MH, Puyan S, Abasov F. Brain-derived neurotrophic factor as a biomarker in primary open-angle glaucoma. Optom Vis Sci. 2011;88(1):80–85. doi: 10.1097/OPX.0b013e3181fc329f. [DOI] [PubMed] [Google Scholar]
- 204.Anastasia A, Deinhardt K, Chao MV, et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun. 2013;4:2490. doi: 10.1038/ncomms3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 206.Nowak A, Szaflik JP, Gacek M, et al. BDNF and HSP gene polymorphisms and their influence on the progression of primary open-angle glaucoma in a Polish population. Arch Med Sci. 2014;10(6):1206–1213. doi: 10.5114/aoms.2014.45089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23(1):53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 208.Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31(12):557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 210.Fujimoto M, Hayashi T, Urfer R, Mita S, Su TP. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse. 2012;66(7):630–639. doi: 10.1002/syn.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]