Highlights
-
•
Secondary uterine sarcomas occur in < 1% of patients treated with CCRT.
-
•
Secondary uterine sarcomas can occur > 5 years from CCRT.
-
•
The median survival period from the secondary tumor occurrence was only 4 months.
-
•
All our patients had heterologous carcinosarcoma of the uterine corpus.
Keywords: Secondary uterine carcinosarcoma, Concurrent chemoradiotherapy, Cervical cancer
1. Introduction
Cervical cancer is the fourth most common cancer in women, with estimated 528,000 new cases and 266,000 deaths worldwide in 2012 (Cancer IAfRo, 2012). Advances in screening and treatments have improved prognoses in recent years. Cancer survivors, however, often live with long-term consequences of the disease and its treatment, including a higher risk of developing new primary cancers. This risk has been quantified to be 14% higher in cancer survivors in the U.S. as compared with the general population; particularly for cervical cancer survivors the risk was 32% (Curtis et al., 2006).
More than half of cervical cancer patients received initial radiotherapy (RT), either alone or in combination with a surgery. Therefore, several studies have reported a correlation between RT for cervical cancer and subsequent cancer risk. Significant lower risk was seen in patients with cancers of the breast and corpus uteri and melanoma of the skin (Curtis et al., 2006). During a follow-up study of 104,760 one-year survivors of cervical cancer, cases of the uterine corpus cancer did not show a statistically significant increase (Chaturvedi et al., 2007). A Japanese cohort study that enrolled 2167 cervical cancer patients who had underwent RT, showed no significant risk of secondary primary uterine malignancies (Ohno et al., 2007). Furthermore, a report from Japan in 5725 patients treated with radiation therapy showed that significant deficits of second cancer were observed in the uterus, colon, liver, and gallbladder (Arai et al., 1991).
On the other hand, Teng et al. reported that a significantly higher risk was observed for secondary cancers of the uterus (Teng et al., 2015). In a report from Japan, a site-based analysis revealed an excess of second cancers in the rectum and uterine body and occurrences of acute leukemia (Ota et al., 2007).
We found three cases of uterine carcinosarcoma as a secondly primary malignancy after concurrent chemoradiotherapy (CCRT) for cervical cancer, and report on these cases with a literature review.
2. Patients and methods
We retrospectively analyzed 313 patients with stage IB to IVA (18 patients in stage IB1, 39 IB2, 16 IIA, 132 IIB, 100 III, and 8 IVA) cervical cancer who were treated with CCRT between 1997 and 2013 at our hospital. None of the patients had received prior treatment. All patients provided written informed consent. Patient charts were reviewed for clinicopathological data and this was a contemporary cohort of patients. This retrospective study was approved by the Institutional Review Board of our university (#1043).
All patients were treated with anterior–posterior and posterior–anterior parallel–opposed ports, or with the four-field technique of whole-pelvic external beam radiotherapy (WP-EBRT). A 50-Gy dose of WP-EBRT was delivered in 25 fractions. A center shield (4-cm wide at the midline) was used in some patients after delivery of the 40-Gy dose. High-dose-rate intracavitary brachytherapy (HDR-ICBT) was delivered once per week at a fractional dose of 6 Gy that was given 1–3 times at point A for a total dose of 6–18 Gy. Boost EBRT doses of 6–20 Gy in 1–4 fractions were applied to the pelvic walls and/or nodal metastases (≥ 10 mm in a short-axis diameter) for patients with nodular parametrial involvement. The CCRT regimen consisted of cisplatin at 20 mg/m2 for 5 days every 3 weeks or 40 mg/m2 weekly, administered concomitantly with RT. Follow-up examinations were conducted every month for the first year, every 2 months for the second year, and every 3–6 months thereafter.
3. Results and case reports
Among these 313 patients, we found three cases (0.96%) with a second uterine corpus malignancy after CCRT (Table 1).
Table 1.
Summary of three patients who developed second primary uterine malignancy.
| Age (years) | Latency (months) | Histology | Treatment | Survival | |
|---|---|---|---|---|---|
| 1 | 56 | 76 | CS (endometrial stromal sarcoma, rhabdomyosarcoma, clear cell carcinoma, serous carcinoma) | TAH + BSO + LN sampling, Paclitaxel + carboplatin | 20 months, DOD |
| 2 | 80 | 118 | CS (undifferentiated sarcoma, undifferentiated carcinoma) | Best supportive care | 2 months, DOD |
| 3 | 70 | 68 | CS (osteosarcoma, clear cell carcinoma) | Exploratory laparotomy | 4 months, DOD |
CS; carcinosarcoma, TAH; total abdominal hysterectomy, BSO; bilateral salpingo-oophorectomy, LN; lymph node, DOD; dead of disease.
In Case 1, a 50-year-old woman was diagnosed with squamous cell carcinoma of the cervix stage IIB and treated by CCRT (2 cycles of cisplatin, 20 mg/m2 for 5 days), achieving a complete response. After 6 years and 4 months' post-treatment, she complained of vaginal discharge, and was diagnosed with a uterine corpus tumor by ultrasound and magnetic resonance imaging (MRI) (Fig. 1, left). The patient underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node sampling. Histological examination revealed a uterine corpus carcinosarcoma that comprised a high grade endometrial stromal sarcoma, rhabdomyosarcoma, clear cell carcinoma, and serous carcinoma (Fig. 1, right). She received six cycles of paclitaxel and carboplatin postoperatively, but developed a recurrence 4 months later, and died of the disease 20 months after that.
Fig. 1.
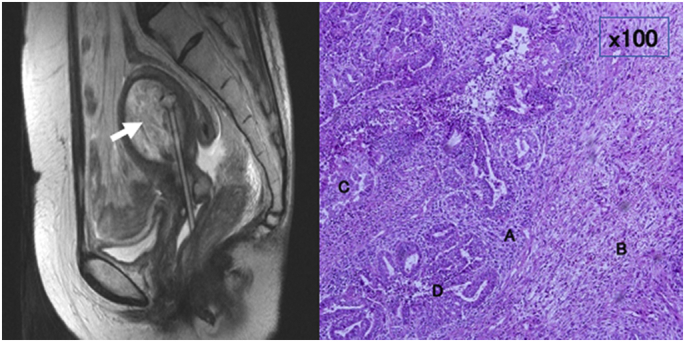
Magnetic resonance imaging T2-weighted sagittal image shows uterine cavity is occupied with high-intensity tumor (arrow) (left panel). Tumor cells comprise of high-grade endometrial stromal sarcoma (A), rhabdomyosarcoma (B), clear cell carcinoma (C), and serous carcinoma (D) (Hematoxylin-Eosin staining × 100) (right panel).
In Case 2, a 70-year-old woman was diagnosed with squamous cell carcinoma of the cervix stage IIIB and treated by CCRT (2 cycles of cisplatin, 20 mg/m2 for 5 days), achieving a complete response. Nine years and 10 months after completion of the treatment, she complained of abdominal bulging, and was diagnosed with a uterine corpus tumor by ultrasound and computed tomography. The endometrial biopsy specimen showed a uterine corpus carcinosarcoma comprising an undifferentiated sarcoma and undifferentiated carcinoma. The patient had a poor performance status due to the tumor and a past cerebral infarction; therefore, she was treated with the best supportive care. She died of the disease in 2 months.
In Case 3, a 63-year-old woman was diagnosed with squamous cell carcinoma of the cervix stage IIA, and treated by CCRT (2 cycles of cisplatin, 20 mg/m2 for 5 days) achieving a complete response. An artificial anus was constructed due to radiation colitis after 3 years. Five years and 8 months after completion of the treatment, she was diagnosed with a uterine corpus tumor using MRI (Fig. 2, left). Surgery was performed, but it involved an exploratory laparotomy and endometrial biopsy due to severe peritoneal carcinomatosis. Histological examination of the endometrial biopsy revealed carcinosarcoma that comprised of osteosarcoma and clear cell carcinoma (Fig. 2, right). She died of the disease after 4 months.
Fig. 2.
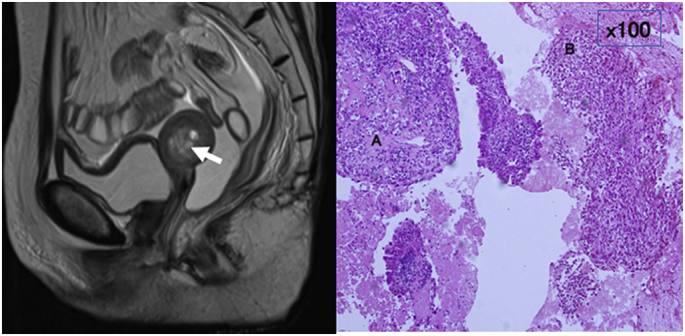
Magnetic resonance imaging T2-weighted sagittal image shows uterine cavity is occupied with high-intensity tumor and hemorrhage (arrow) (left panel). Carcinosarcoma comprising of osteosarcoma (A) and clear cell carcinoma (B) (Hematoxylin-Eosin staining × 100) (right panel).
4. Discussion
Among 313 patients with a prior history of CCRT for cervical cancer in our institute, 3 patients (0.96%) developed a primary second malignancy of the uterine corpus. The RT doses ranged from 58 to 64 Gy. The latency time from the CCRT to the development of the carcinosarcoma ranged from 68 to 118 months, with a median of 76 months. The median survival period from the secondary tumor occurrence was 4 months (range, 2–20 months). All the patients had heterologous carcinosarcoma as the second uterine corpus malignancy. Therefore, post-RT sarcoma should be discussed alongside cervical cancers. To consider a RT-associated sarcoma, some criteria are proposed as follows: within a previously irradiated field; a significant amount of radiation; a latency period of several years (at least 3–5 years); and histologically proven and different from the primary neoplasm (Cahan and Woodhard, 1948, Arlen et al., 1971, Murray et al., 1999). Our cases satisfied the above-mentioned criteria, although it cannot be completely ruled out that the occurrence of uterine carcinosarcoma in these patients might be de novo.
Exposure to radiation has been long associated with increased risk of sarcoma and there has been considerable interest in identifying a genetic expression profile that can differentiate between radiation-related and sporadic tumors. Although we did not complete chromosomal and immunohistochemical examinations in our patients, the several inspections for pathogenesis of RT-induced sarcoma have been reported as follows: the loss of material from 3p21-3pter detected by comparative genomic hybridization (Mertens et al., 2000), a role of p53 gene mutations (Nakanishi et al., 1998), and the KIT protein overexpression without mutation (Komdeur et al., 2003).
The uterine sarcoma mostly reported in patients treated with RT for cervical cancer, and for benign conditions such as menorrhagia in early reports. Duran et al. (1980) reported 9 patients with carcinosarcoma of the uterus associated with a pelvic RT as compared with 8 non RT-associated patients. Patients with post-RT tumors presented at a younger age and with symptoms indicative of extensive intraabdominal disease and two-thirds of the patient's neoplasms were classified as heterologous carcinosarcoma. The latent interval ranged from 9 to 30 years, with a median of 18 years. Mark et al. (1996) reported on 13 patients diagnosed with uterine sarcomas having a prior history of RT. The conditions for which these patients had received RT included one choriocarcinoma, four menorrhagias, six cervical cancers, and two ovarian cancers. The RT doses ranged from 40 to 80 Gy and the latency time from the RT to the development of sarcoma ranged from 3 to 30 years, with a median of 17 years. The five-year disease-specific survival was 17%. The authors estimated that the absolute risk ranged from 0.03 to 0.8%. Arai et al. (1991) reported on 13 cases of second uterine cancer (0.023%) in patients after the RT, consisting of eight malignant mesotheliomas, three adenocarcinomas of the body, a leiomyosarcoma, and a rhabdomyosarcoma. The post-RT sarcoma of the gynecologic tract is a relatively late event associated with a poor prognosis. These findings were consistent with our cases.
Our patients were treated with anterior–posterior and posterior–anterior parallel–opposed ports, or with the four-field technique of WP-EBRT. Hall et al. summarized the new technologies, such as three-dimensional conformal radiation therapy (3D-CRT) and intensity-modulated radiation therapy (IMRT), on radiation-induced second cancers (Hall and Wuu, 2003). 3D-CRT reduces the volume of normal tissues receiving a high dose, with an increase in dose to the target volume. A decrease in the number of second malignancy might be expected. IMRT involves more fields and the increased total body exposure, due to leakage radiation, leading to increase the risk of second cancers. However, risk for subsequent uterine body malignancy remains unknown.
Secondary uterine corpus malignancies could develop > 5 years after the completion of CCRT, although it is a very rare event. Long-term follow-up data revealed that our method of CCRT for locally advanced carcinoma of the uterine cervix is effective, with a low incidence of second malignancies of the uterine corpus.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- Arai T., Nakano T., Fukuhisa K., Kasamatsu T., Tsunematsu R., Masubuchi K., Yamauchi K., Hamada T., Fukuda T., Noguchi H., Murata M. Second cancer after radiation therapy for cancer of the uterine cervix. Cancer. 1991;67:398–405. doi: 10.1002/1097-0142(19910115)67:2<398::aid-cncr2820670214>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Arlen M., Higinbotham N.L., Huvos A.G., Marcove R.C., Miller T., Shah I.C. Radiation induced sarcoma of bone. Cancer. 1971;28:1087–1099. doi: 10.1002/1097-0142(1971)28:5<1087::aid-cncr2820280502>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cahan W.G., Woodhard H.Q. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1:3–29. doi: 10.1002/1097-0142(194805)1:1<3::aid-cncr2820010103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cancer IAfRo Cervical Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. 2012. http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp (Accessed April 30, 2017)
- Chaturvedi A.K., Engels E.A., Gilbert E.S., Chen B.E., Storm H., Lynch C.F., Hall P., Langmark F., Pukkala E., Kaijser M., Andersson M., Fosså S.D., Joensuu H., Boice J.D., Kleinerman R.A., Travis L.B. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J. Natl. Cancer Inst. 2007;99:1634–1643. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- Curtis R.E., Freedman D.M., Ron E., Ries L.A.G., Hacker D.G., Edwards B.K., Tucker M.A., Fraumeni J.F. National Cancer Institute; Bethesda (MD): 2006. New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000. [Google Scholar]
- Duran J.V., Nochomovitz L.E., Prem K.A., Dehner L.P. Postirradiation mixed Mullerian tumors of the uterus. Cancer. 1980;45:1625–1631. doi: 10.1002/1097-0142(19800401)45:7<1625::aid-cncr2820450718>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Hall E.J., Wuu C.S. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- Komdeur R., Hoekstra H.J., Molenaar W.M., Van Den Berg E., Zwart N., Pras E., Plaza-Menacho I., Hofstra R.M., Van Der Graaf W.T. Clinicopathologic assessment of postradiation sarcomas: KIT as a potential treatment target. Clin. Cancer Res. 2003;9:2926–2932. [PubMed] [Google Scholar]
- Mark R.J., Poen J., Tran L.M., Fu Y.S., Heaps J., Parker R.G. Postirradiation sarcoma of the gynecologic tract. A report of 13 cases and a discussion of the risk of radiation-induced gynecologic malignancies. Am. J. Clin. Oncol. 1996;19:59–64. doi: 10.1097/00000421-199602000-00013. [DOI] [PubMed] [Google Scholar]
- Mertens F., Larramendy M., Gustavsson A., Gisselsson D., Rydholm A., Brosjö O., Mitelman F., Knuutila S., Mandahl N. Radiation-associated sarcomas are characterized by complex karyotypes with frequent rearrangements of chromosome arm 3p. Cancer Genet. Cytogenet. 2000;116:89–96. doi: 10.1016/s0165-4608(99)00105-3. [DOI] [PubMed] [Google Scholar]
- Murray E.M., Werner D., Greeff E.A., Taylor D.A. Postradiation sarcomas: 20 cases and literature review. Int. J. Radiat. Oncol. Biol. Phys. 1999;45:951–961. doi: 10.1016/s0360-3016(99)00279-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Tomita Y., Myoui A., Yoshikawa H., Sakai K., Kato Y., Ochi T., Aozasa K. Mutations of the p53 gene in post radiation sarcoma. Lab. Investig. 1998;78:727–733. [PubMed] [Google Scholar]
- Ohno T., Kato S., Sato S., Fukuhisa K., Nakano T., Tsujii H., Arai T. Long-term survival and risk of second cancers after radiotherapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:740–745. doi: 10.1016/j.ijrobp.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Ota T., Takeshima N., Tabata T., Hasumi K., Takizawa K. Treatment of squamous cell carcinoma of the uterine cervix with radiation therapy alone: long-term survival, late complications, and incidence of second cancers. Br. J. Cancer. 2007;97:1058–1062. doi: 10.1038/sj.bjc.6604005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C.J., Huon L.K., Hu Y.W., Yeh C.M., Chao Y., Yang M.H., Chen T.J., Hung Y.P., Liu C.J. Secondary primary malignancy risk in patients with cervical cancer in Taiwan: a Nationwide population-based study. Med. (Baltimore) 2015;94:e1803. doi: 10.1097/MD.0000000000001803. [DOI] [PMC free article] [PubMed] [Google Scholar]


