Abstract
During 2014 and 2015 an outbreak of Ebola deemed a Public Health Emergency of International Concern affected a number of West African countries. The outbreak underscored the need for a vaccine against Ebola. An unprecedented and to great extent collaborative effort built on the availability of a number of candidate vaccines that could enter into clinical phase evaluation. A series of international consultations and activities were led by WHO as a contribution to the unprecedented global efforts to develop and assess an Ebola vaccine. WHO consulted widely, and immediately fostered interactions with the international scientific, ethics, regulatory, vaccine development, public health partners, industry and funders’ communities and participated in consortia to facilitate Ebola vaccine assessments. WHO also fostered key activities to ensure the optimal policy and deployment of Ebola vaccines, if licensed. WHO has convened a broad global coalition of experts to develop a Blueprint and a platform for accelerated R&D, in order to avert full-blown epidemics.
Introduction
On August 8, 2014, the Director-General of WHO declared that the Ebola virus disease (EVD) outbreak in West Africa represented a Public Health Emergency of International Concern under the 2005 International Health Regulations.1 What followed was a chain of events leading to the acceleration of timelines for vaccine development. The unprecedented outbreak underlined the need for a vaccine to combat the disease. For some years before this outbreak, scientists and, governments had created promising data on several Ebola vaccine candidates. Still, development was delayed in part because public sector investment mechanisms were not entirely in place to support pre-emptive clinical assessment of these candidates, and there was a perception that regulatory pathways were limited for Ebola vaccines.
Several independent reviews have highlighted the fact that the world and WHO were not prepared to respond to this epidemic [1,2]. However, clinical trials for Ebola vaccines were initiated in record time and WHO provided valuable technical leadership [2,3•]. This article summarizes some of the main actions taken by WHO since August 2014 and highlight some lessons we learned. It also illustrates how these experiences are contributing to shape a WHO Blueprint for research and development for emerging/re-emerging diseases likely to cause outbreaks in the near future and for which countermeasures are not available or are insufficient.
What actions were implemented?
Fostering fora for global collaboration on matters critical to Ebola vaccines research and development pathways
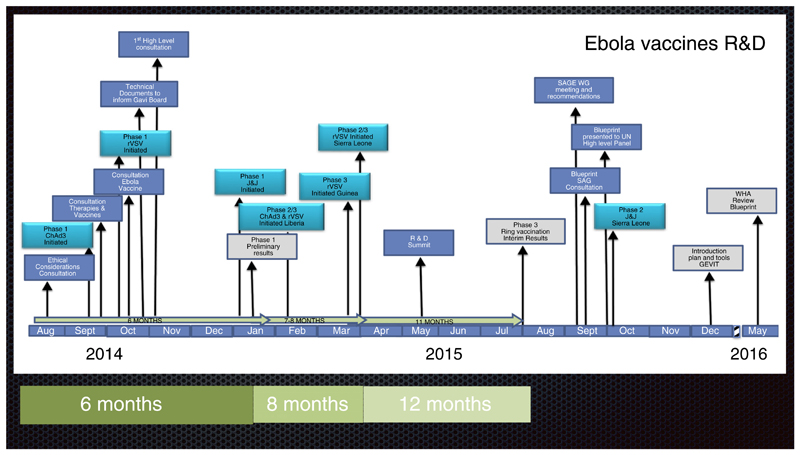
After the declaration of the Public Health Emergency, what followed was a collaborative effort, to an important extent guided by WHO, among global public health organizations, pharmaceutical companies, regulatory agencies, and nongovernmental organizations to accelerate the development of Ebola vaccines from preclinical research to clinical trials. WHO contributions were framed by WHO’s core functions which include: providing leadership on matters critical to health and engaging in partnerships where joint action is needed; shaping the research agenda and stimulating the generation, translation and dissemination of valuable knowledge; setting norms and standards and promoting and monitoring their implementation; articulating ethical and evidence-based policy options; providing technical support, catalyzing change, and building sustainable institutional capacity; and monitoring the health situation and assessing health trends. WHO consulted widely, and immediately fostered interactions with the international scientific, ethics, regulatory, vaccine development, public health partners, industry and funders’ communities on key activities to implement on an emergency basis (Figure 1).
Fig. 1.
Collaborative efforts, adaptation of the traditional R&D model, compressed timeframes and, unprecedented partnerships formed.
Identifying the need to establish R&D priorities, a WHO convened international meeting reviewed available Ebola therapies and vaccine options. It focused on addressing three questions: firstly, do these products work and are they safe?; secondly, can they be developed more rapidly in order that they might be moved from the laboratory to the field? and; thirdly, can they be scaled up to serve the necessary demand? Emphasis was put on the need for leadership, prioritization, and coordination. There was agreement that oversight of the research agenda for evaluation and its implementation would be best performed under the coordination of WHO. The need to build research capacity skills in the countries with EVD outbreaks was stressed. WHO was also encouraged to continue the work on a mechanism for evaluating preclinical data to determine the priority of interventions to be further assessed. Informed by available data and international experts, WHO regularly updated a document that summarizes the data on categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola [4••,5].
WHO led face-to-face and virtual interactions among high-level government representatives, development partners and representatives from Ebola-affected countries, scientists, vaccine manufacturers, regulatory authorities, international organizations, funding agencies and civil society representatives. The aim was to discuss and agree on how to fast track the testing and the deployment of promising vaccines. Three important consensus commitments came from these interactions:
First, phase 1 clinical trials of the two most advanced vaccines should be completed as soon as possible. Without waiting for the results of the phase 1, measures should be taken by all stakeholders to initiate efficacy trials in the three most affected countries before the end of 2014. All candidate vaccines must be tested until they prove to be unsafe and/or inefficacious. Second, the pharmaceutical companies developing vaccines should commit to scaling up their production capacity in 2015. Third, as community engagement was seen as essential, there was a need to generate the necessary partnerships between local communities, national governments, and other stakeholders to ensure success. All parties concluded that even in the emergency setting, clinical trial data would be required on vaccine candidates to determine how best they could complement the public health response and called upon WHO to enhance coordinating of efforts and to ensure effective communication between the various actors [6,7].
Furthermore, in October 2015 the first of a series of consultations on the Science of Ebola were convened to obtain the overview necessary to achieve integration of relevant scientific work from different disciplines [8–10]. These consultations concluded that investing in research is vital for ensuring effective prevention and response to future outbreaks. They underlined the need to ensure that investments in addressing the current outbreak would not be without legacy, but would be leveraged to continue implementation of essential research, and that there was an urgent need to mainstream research into public health sector development throughout future work in affected countries.
A series of consultations on regulatory approaches for expediting development and availability of Ebola vaccines took place [11,12]. The regulatory experts debated around the following objectives: firstly, identify the critical regulatory pathways for the lead candidate Ebola vaccines and the main regulatory challenges; secondly, agree on anticipated timelines for availability of these vaccines for both clinical trials and potential future large-scale deployment and; thirdly, mapping out possible avenues to address the regulatory challenges while keeping safety as a main concern.
Regulatory support was provided through African Vaccine Regulatory Forum (AVAREF) meetings, whereby regulators from high-income authorities, attended consultations in support of African regulators (North-South collaboration) [13]. In addition AVAREF enabled African authorities with experience of pre-licensure vaccine trials to support those in the most affected countries with less experience of clinical trials (South-South collaboration). WHO assembled representatives from GlaxoSmithKline (GSK), makers of Chimpanzee Adenovirus Serotype-3 vaccine ChAd3, along with members of national ethics and medicines regulatory authorities from Cameroon, Ghana, Guinea, Liberia, Mali, Nigeria, Senegal, and Sierra Leone for a joint review of the clinical trial application for the Phase II trial of the GSK vaccine [14]. WHO developed a mechanism for the Emergency Use Authorization Listing (EUAL) of vaccines intended to assist interested UN procurement agencies and Member States on the acceptability for use of a specific vaccine in the context of a public health emergency. EUAL is a special procedure for vaccines during public health emergencies when the community may be more willing to tolerate less certainty about the precise level of efficacy of products, given the morbidity and/or mortality of the disease and the shortfall of treatment and/or prevention options [15••].
WHO will continue to support international and national efforts to identify suitable alternatives for fast-tracked regulatory evaluation of Ebola vaccines and offer expert oversight and guidance to countries.
Promoting scientific debate on trial designs and enabling parallel implementation of Phase 1, 2 and 3 clinical trials of Ebola vaccines
A WHO expert consultation assessed the status of Ebola candidate vaccines, with the overarching objective to take stock of the many efforts under way to rapidly evaluate candidate Ebola vaccines for safety and efficacy. All agreed that the ultimate goal was to have a fully tested and licensed product that can be scaled up for use in mass vaccination campaigns. WHO used a set of criteria and experts reviews of the data to objectively assess which vaccine candidates would be proactively fast-tracked for clinical evaluation. The criteria included availability of Good Manufacturing Practice grade vials after lot release for clinical trials, and that 100% efficacy had been documented in non-human primates with acceptable preclinical safety. Both ChAd3-ZEBOV and rVSV-ZEBOV were the two candidate vaccines that met those criteria as of August 2014 [16]. The Ad26/MVA and Novavax (recombinant protein) candidate vaccines met the criteria later in the epidemic.
A consultation including ethicists and trial methodologists pondered the question of what clinical trial designs were ethically acceptable and mapped out the issues that were relevant for utilizing various study designs in the outbreak context. Participants concluded that in principle, as long as standard requirements for human research ethics are met, all scientifically recognized methodologies and study designs should be considered to be ethically acceptable. Nonetheless, they underlined that a number of issues regarding intervention characteristics, design and site and participant-related issues must be accounted for. Real-time data collection and sharing was deemed a scientific and moral imperative to ensure that potential vaccines could be quickly evaluated and developed [17]. Regular teleconferences served as a forum to critically discuss protocols proposed for Ebola vaccines trials and to discuss progress and challenges [18–22].
Continuous assessment of vaccine attributes to inform long-term use of Ebola vaccines to control or prevent future outbreaks
It was agreed during the high level consultations that WHO would develop Ebola Vaccine Target Product Profiles for Ebola Vaccines. The target product profiles (TPPs) for Ebola vaccines provides guidance to vaccine developers and highlight the use of Ebola vaccines in two likely scenarios: firstly, reactive/emergency use, to interrupt an existing outbreak and to break chains of transmission, and secondly, prophylactic use, to protect frontline healthcare and other workers at high risk in future outbreaks [23]. The TPP proposes that a single vaccine may not possess a complete set of characteristics needed in the short and long term and that different types of vaccines may be needed to cover the range of uses.
Engaging in partnerships where joint action was deemed necessary
The African and European VSV-Ebola consortium (VEBCON)
The Public Health Agency of Canada donated 800 vials of the vesicular stomatitis virus (VSV) — Zaire Ebola virus vaccine candidate (rVSV-ZEBOV) to WHO. By September 2014, WHO have created an African and European VSV-Ebola consortium (VEBCON) to initiate dose-escalation phase 1 clinical trials with rVSV-ZEBOV vaccine candidate (rVSV-ZEBOV) to allow optimized rapid decisions on dose and safety [24]. These phase 1-trials were conducted in Hamburg, Germany (NCT02283099), Kilifi, Kenya (NCT02296983), Lambarene, Gabon (PACTR2014000089322), and Geneva, Switzerland (NCT02287480) and were supported financially through WHO from a grant from the Wellcome Trust Foundation and the German Center for Infection Research.
Based in part on the data from these studies [25••,26••] researchers selected the vaccine dose for a phase 3 efficacy study initiated in Guinea (PACTR201503001057193) and for a phase 3 trial conducted in Sierra Leone (PACTR201502001037220).
The Guinea vaccine consortium
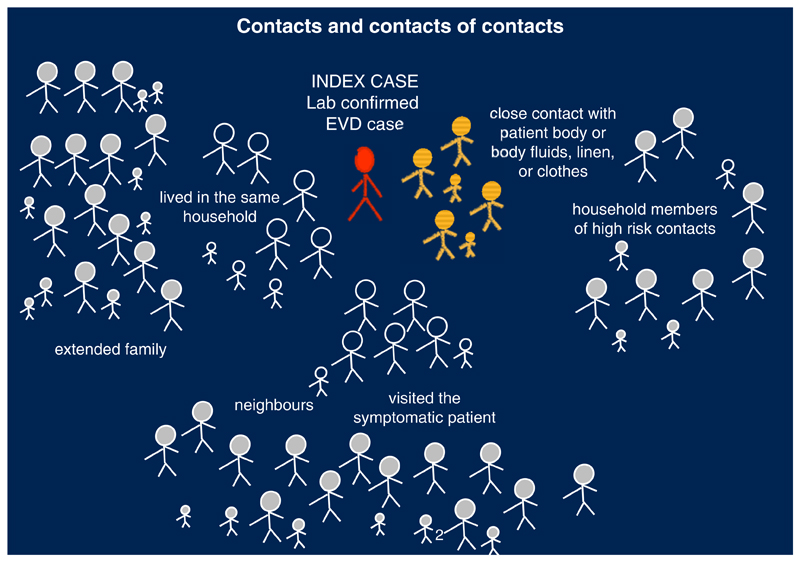
It was established in the periphery and at the end of WHO’s first high-level Ebola vaccine meeting in October 2014 during an informal side meeting. Plans had been drawn up to assess Ebola candidate vaccines in Sierra Leone and Liberia. Guinea, where the outbreak had begun, was perceived as an exceptionally challenging setting for assessing a vaccine. Given the request of Guinea to be part of the Ebola vaccine development effort, some participants met to discuss options to design a trial for Guinea. The Guinea vaccine Consortium held its first meeting in November 2015 in Geneva where options of protocol designs were considered. Funding was secured in January 2015. The Consortium designed a novel cluster trial design called a ‘ring vaccination trial,’ in which a ‘ring’ of people (contacts and contacts of contacts) around a newly discovered Ebola patient is vaccinated. Entire rings were randomized to receive vaccination either immediately or after 21 days (Figure 2). If significantly more people contract Ebola in the delayed rings, the vaccine is deemed effective [27••].
Fig. 2.
What is a vaccination ring?
The Guinea Ebola vaccine trial is the coordinated effort of many international agencies. WHO was the regulatory sponsor of the study, which is implemented by the Ministry of Health of Guinea, WHO, Médecins sans Frontières (MSF), EPICENTRE and the Norwegian Institute of Public Health. The trial is funded by WHO, with support from the Wellcome Trust, the United Kingdom Department for International Development, the Norwegian Ministry of Foreign Affairs to the Norwegian Institute of Public Health through the Research Council of Norway, the Canadian Government through the Public Health Agency of Canada, Canadian Institutes of Health Research, International Development Research Centre and Department of Foreign Affairs, Trade and Development and MSF. The trial team includes experts from The University of Bern, the University of Florida, the London School of Hygiene and Tropical Medicine, Public Health England, the European Mobile Laboratories among others. Only four months after the first discussions started about a potential efficacy trial in Guinea, WHO and partners began an Ebola ring vaccination trial to evaluate the efficacy of the rVSV-ZEBOV candidate vaccine (Figure 3). Preliminary results published in August 2015 suggest that the vaccine is safe and efficacious [28••]. The trial was also expanded into Sierra Leone in September 2015 [29].
Fig. 3.
A timeline ….
Anticipating the need for policy guidance and the potential vaccine deployment challenges to provide technical support to affected countries
To rapidly implement campaigns in the affected countries once vaccine becomes available, public health officials need to plan the most optimal vaccination strategies as soon as feasible. Starting in 2014, WHO Strategic Advisory Group of Experts on immunization (SAGE) reviewed a framework for making recommendations regarding use of Ebola vaccines. The SAGE recommended vaccination strategies should target of those at highest risk of exposure. Considerations included: specific scenario relating to the epidemiology and the type of authorization for vaccine use; objectives for vaccination (primary — stopping transmission, secondary — individual protection); prioritization of target populations; and additional considerations [30,31]. A Global Ebola Vaccine Implementation Team (GEVIT) was created under WHO leadership including countries most affected by the EVD outbreak and key partners, who would be closely involved in procuring and introducing an Ebola vaccine [32]. The objective was to develop a framework to enable timely roll out of a licensed vaccine in public health Ebola emergencies. It also aimed to engage stakeholders in the affected countries in the decision-making and in identifying priority groups for vaccination. Operational issues and community engagement are also considered to anticipate any barriers to vaccine acceptance. During the 2016 World Economic Forum in Davos, Gavi announced support ($5 million USD) to Merck for production of 300 000 doses in the next few months in exchange for a commitment to seek licensure in 2017. This commitment is intended to permit a stockpile of rVSV-ZEBOV vaccine (and potentially other vaccines as more evidence emerges) for readiness for the inevitable next outbreak [40].
What did we learn?
The Ebola epidemic taught us that the global community can muster the capacity and resources in the face of adversity to curb the spread of disease. The accelerated start and implementation of Ebola vaccine clinical trials in the challenging conditions of an outbreak were only possible thanks to the cooperative efforts of national authorities in the affected countries, scientists, research funders, regulatory agencies in the USA, Europe, and West Africa, pharmaceutical companies, nongovernmental organizations, and affected communities [41]. In all in 2014–2015, 13 vaccines were in clinical phase of development in 41 clinical trials of which 39% are located in Africa [33]. Regulatory and ethics timelines were faster than ever before. Surging human resources into the assessment process contributed to this; the rigour of the assessment process was maintained with the emphasis on product quality and participant safety. Protocol development occurred within a few weeks thanks to the involvement of many of the chief researchers and methodologists. Safety and immunogenicity data was available as soon as February 2015 from some of the trials [33]. Phase 2 and 3 trials were planned and initiated in record time in each of the three worst affected countries (Liberia and Sierra Leone and Guinea). The first trial initiated was in Liberia (PREVAIL) in February, 2015 only 6 months after the global public health emergency was declared (in collaboration with US-NIH [34]) followed by the STRIVE started only in April trial in Sierra Leone (in collaboration with US-CDC; [35]), and the ‘Ring Vaccination trial’ in Guinea which started in March 2015 [27••,28••]. These trials are an example of international partnerships with researchers and authorities from the Ebola affected countries. WHO facilitated complex interactions between numerous stakeholders and enabled the parallel testing of different candidate Ebola vaccines. Despite these unprecedented achievements, the overall Ebola vaccine research and development effort could have moved even faster if there had been investments earlier to assess candidate vaccines through phase 1 or 2 trials and a system to prioritize the most promising candidate vaccines. WHO’s mandate and capacity to combine, coordinate, and drive a varied combination of assets towards a common goal calls for an expanded role in R&D. At the request of its 194 Member States, WHO has convened a broad global coalition of experts to develop a Blueprint and a platform for accelerated R&D, in order to avert full-blown epidemics [36•,37•]. The R&D Blueprint is a global strategy and preparedness plan to ensure that targeted R&D can strengthen the emergency response and aims to reduce the time between the declaration of an international public health emergency and the availability of effective tests, vaccines and treatments [38]. While conventional containment measures will remain cornerstones of a health emergency response a repertoire of effective health technologies could be the key to pre-empting full-blown epidemics, and limiting their human, social and economic losses. It proposes that this can be achieved by: firstly, acting together to identify the top global disease threats on which to focus initial R&D efforts; secondly, creating a R&D roadmap for those threats and by identifying the basic and applied research, and health technologies needed to contain this emerging threat; thirdly, developing organizational frameworks for the coordination of national and international actors and inclusion of scientists in low-income and middle-income countries as equal partners and by building the capability to conduct clinical trials for vaccines and therapeutics against emerging disease threats in low-income and middle-income countries; fourthly, developing a plan to evaluate the impact of the R&D Blueprint and continuously improve the Blueprint and create a high-level checklist to monitor preparedness and impact; and fifthly, exploring funding models for R&D preparedness and response [39••]. WHO has convened a broad global coalition of experts to develop the blueprint and a platform for accelerated research and development. The Blueprint has be presented to WHO Member States at the next World Health Assembly in May 2016.
Footnotes
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.World Health Organization. Report of the Ebola Interim Assessment Panel. 2015 Jul; http://www.who.int/entity/csr/resources/publications/ebola/ebola-panel-report/en/index.html.
- 2.Moon S, Sridhar D, Pate MA, Jha AK, et al. Will Ebola change the game? Ten essential reforms before the next pandemic. The report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. Lancet. 2015;28:2204–2221. doi: 10.1016/S0140-6736(15)00946-0. [Epub 2015 Nov 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellcome Trust and the Center for Infectious Disease Research and Policy (CIDRAP) University of Minnesota. Recommendations for Accelerating the Development of Ebola Vaccines: Report & analysis. 2015 Feb; http://www.cidrap.umn.edu/sites/default/files/public/downloads/ebola_virus_team_b_report-final-021615.pdf.
- 4.World Health Organization. Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola. 2015 Jul; http://www.who.int/medicines/ebola-treatment/2015_0703TablesofEbolaDrugs.pdf?ua=1.
- 5.World Health Organization. WHO Consultation on potential Ebola therapies and vaccines held by the World Health Organization. 2014 Sep; https://www.google.ch/url?sa=t&rct=jȦq=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwiigbrj_NjKAhUFnA4KHUlSB7MQFggcMAA&url=http%3A%2F%2Fwww.who.int%2Fcsr%2Fresources%2Fpublications%2Febola%2Febola-therapies%2Fen%2F&usg=AFQjCNGLavMgAmQD8TYIaKHz5Xx_tvT1iw&sig2=LfPl5JNOlYpakUhy1ZAo_A.
- 6.World Health Organization. WHO high-level meeting on Ebola vaccines access and financing: Full Summary Report. 2014 Oct; http://apps.who.int/iris/bitstream/10665/137184/1/WHO_EVD_Meet_EMP_14.2_eng.pdf?ua=1.
- 7.World Health Organization. Second WHO High-level Meeting on Ebola Vaccines Access and Financing. 2015 Jan; http://www.who.int/entity/mediacentre/events/2015/ebola-vaccine-access/en/index.html.
- 8.World Health Organization. How Can Science Inform Our Response to Ebola Virus Disease? Note of a Consultation Held on 2014. 2014 Oct; http://www.who.int/medicines/ebola-treatment/meetings/20141007_Ebola_Science_Committee-First_TC-MeetingNote.pdf?ua=1.
- 9.World Health Organization. Meeting of the WHO Ebola Science Committee. 2014 Nov; http://www.who.int/entity/medicines/ebola-treatment/meetings/ebola_science_committee/en/index.html.
- 10.World Health Organization. Meeting of the WHO Ebola Science Committee. 2015 Mar; http://www.who.int/entity/medicines/ebola-treatment/meetings/science-committee-march/en/index.html.
- 11.World Health Organization. First Teleconference on Regulatory Approaches for Expediting Development and Availability of Ebola Vaccines. 2014 Oct; http://www.who.int/entity/medicines/ebola-treatment/meetings/2014-1030_1stT_RegEbola_vaccines_summary.pdf?ua=1.
- 12.World Health Organization. First Teleconference on Regulatory Approaches for Expediting Development and Availability of Ebola Vaccines. 2015 Jan; http://www.who.int/entity/medicines/ebola-treatment/meetings/2015-0127_2ndTC_RegEbola.pdf?ua=1.
- 13.Ninth Annual Meeting of the African Vaccine Regulatory Forum (AVAREF); Pretoria South Africa. 2014. Nov, http://www.who.int/entity/medicines/ebolatreatment/meetings/phaseII_clinical_trial_meeting/en/index.html. [Google Scholar]
- 14.World Health Organization. Report of the Joint Review Meeting Facilitated by the World Health Organization for the GSK ChAd3 Ebola Vaccine Clinical Trials Application. 2014 http://www.who.int/entity/immunization_standards/vaccine_regulation/2014-1217_BriefingAfricanNRA_EC.pdf?ua=1.
- 15.World Health Organization. Emergency Use Assessment and Listing Procedure (EUAL) for Candidate Vaccines for Use in the Context of a Public Health Emergency. 2015 Jul; http://www.who.int/medicines/news/EUAL-vaccines_7July2015_MS.pdf.
- 16.World Health Organization. Experimental Ebola Vaccines WHO Consultation on Ebola Vaccines: Ebola Situation Assessment. 2014 Oct; http://www.who.int/mediacentre/news/ebola/01-october-2014/en/
- 17.World Health Organization. Ethical Issues Related to Study Design for Trials on Therapeutics for Ebola Virus Disease. WHO Ethics Working Group meeting. 2014 Oct; http://www.who.int/csr/resources/publications/ebola/ethical-evd-therapeutics/en/
- 18.World Health Organization. First Teleconference on Vaccine Clinical Trials Design in Guinea Liberia and Sierra Leone. 2014 Oct; http://www.who.int/entity/medicines/ebola-treatment/2014-1028_Minutes-1stTC_on_vaccine_clinical_trials.pdf?ua=1.
- 19.World Health Organization. Second Teleconference on Vaccine Clinical Trials Design in Guinea Liberia and Sierra Leone. 2014 Nov; http://www.who.int/entity/medicines/ebola-treatment/2014-1125_Minutes-2ndTC_on_vaccine_clinical_trials.pdf?ua=1.
- 20.World Health Organization. Third Teleconference on Vaccine Clinical Trials Design in Guinea Liberia and Sierra Leone. 2014 Dec; http://www.who.int/entity/medicines/ebola-treatment/3rd-teleconference-vaccines.pdf?ua=1.
- 21.World Health Organization. Fourth Teleconference on Vaccine Clinical Trials Design in Guinea Liberia and Sierra Leone. 2015 Mar; http://www.who.int/entity/medicines/ebola-treatment/4th_teleconference_vaccine_clinical_trials.pdf?ua=1.
- 22.World Health Organization. Fifth Teleconference on Vaccine Clinical Trials Design in Guinea Liberia and Sierra Leone. 2015 Jul; http://www.who.int/entity/medicines/ebola-treatment/meetings/fifth_tel-conf_clinictrials/en/index.html.
- 23.World Health Organization. WHO Ebola Vaccine Target Product Profile. 2015 Jan; http://www.who.int/immunization/research/target-product-profile/ebolavaccine/en/
- 24.World Health Organization. VSV Consortium Overview and key questions. 2014 https://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwiSzbzpkdnKAhVBfRoKHUAPDN0QFggcMAA&url=http%3A%2F%2Fwww.who.int%2Fimmunization%2Fdiseases%2Febola%2F3-rVSV_Phase_1_Michael_29_Sep_14_v2.pdf&usg=AFQjCNGUY1yV0nJkE5cuJhF2ppLN6F6YQw&sig2=_J6Ss7LLJ0Wvcuu2Dn6FSw&bvm=bv.113034660,d.d2s.
- 25.Agnandji ST, Huttner A, Zinser ME, Njuguna P, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe — Preliminary Report. N Engl J Med. 2015 Apr; doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regules JA, Beigel JH, Paolino KM, Voell J, et al. A recombinant vesicular stomatitis virus Ebola vaccine — preliminary report. N Engl J Med. 2015 Apr; doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebola ça suffit ring vaccination trial consortium. The ring vaccination trial: a novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. Br Med J. 2015;351:h3740. doi: 10.1136/bmj.h3740. http://www.bmj.com/content/351/bmj.h3740 (Published 27.07.15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:p857–p866. doi: 10.1016/S0140-6736(15)61117-5. http://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(15)61117-5.pdf. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Ebola ça suffit! — Phase III Vaccine Trial in Guinea: Questions and Answers. 2016 Jan; http://www.who.int/medicines/ebola-treatment/q-a_ebola-ca-suffit/en/
- 30.World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization October 2015 — conclusions and recommendations. Wkly Epidemiol Rec. 2015;90:681–700. http://www.who.int/wer/2015/wer9050.pdf?ua=1. 90th YEAR/11. No. 50. [PubMed] [Google Scholar]
- 31.World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2015 — conclusions and recommendations. Wkly Epidemiol Rec. 2015 May 29;90:261–280. http://www.who.int/wer/2015/wer9022.pdf?ua=1. 90th YEAR/11. No. 22. [PubMed] [Google Scholar]
- 32.World Health Organization. First workshop of the partners group on Ebola vaccines deployment. Summary report. 2015 Feb; http://www.who.int/healthsystems/publications/vaccines-deployment-workshop/en/
- 33.World Health Organization. Ebola R&D Landscape of clinical candidates and trials. Public report. 2015 Oct; http://www.who.int/medicines/ebola-treatment/EbolaR_D_public-report_updt2015.pdf.
- 34.Kennedy SB, Neaton JD, Lane HC, Kieh MWS. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: design, procedures, and challenges. Clin Trials. 2016 Jan; doi: 10.1177/1740774515621037. http://ctj.sagepub.com/content/early/2016/01/08/1740774515621037.long. [DOI] [PubMed] [Google Scholar]
- 35.United States Centers for Disease Control and Prevention (CDC) Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) Questions and Answers. 2015 Dec; http://www.cdc.gov/vhf/ebola/strive/qa.html.
- 36.World Health Organization. Ebola Virus Disease Outbreak and Issues Raised: Follow-up to the Special Session of the Executive Board on the Ebola Emergency (Resolution EBSS3.R1) and the Sixty-eighth World Health Assembly (Decision WHA68(10))Executive Board EB138/27 138th Session 22 January 2016 Provisional Agenda Item 9.1. 2014 http://apps.who.int/gb/ebwha/pdf_files/EB138/B138_27-en.pdf.
- 37.World Health Organization. Special Session of the Executive Board on Ebola EBSS3.R1 Agenda Item 3 25 January 2015. Ebola: Ending the Current Outbreak, Strengthening Global Preparedness and Ensuring WHO's Capacity to Prepare for and Respond to Future Large-scale Outbreaks and Emergencies with Health Consequences. 2015 http://apps.who.int/gb/ebwha/pdf_files/EBSS3/EBSS3_R1-en.pdf.
- 38.World Health Organization. WHO Ebola Research and Development Summit. Geneva: 2015. May, http://www.who.int/medicines/ebola-treatment/Executive-summary-WHO-Ebola-RandD-summit.pdf?ua=1&ua=1. [Google Scholar]
- 39.World Health Organization. A Research and Development Blueprint for Action to Prevent Epidemics. 2015 http://www.who.int/csr/research-and-development/en/
- 40.http://www.gavi.org/Library/News/Press-releases/2016/Ebola-vaccine-purchasing-commitment-from-Gavi-to-prepare-for-future-outbreaks/.
- 41.Kanapathipillai R, Henao Restrepo AM, Fast P, Wood PhD, Dye D, Kieny MP, Moorthy CV. Ebola vaccine — an urgent international priority. N Engl J Med. 2014;371:2249–2251. doi: 10.1056/NEJMp1412166. [DOI] [PubMed] [Google Scholar]