Abstract
Young-onset autoimmune diabetes associated with additional autoimmunity usually reflects a polygenic predisposition but rare cases result from monogenic autoimmunity. Diagnosing monogenic autoimmunity is crucial for patients’ prognosis and clinical management. We sought to identify novel genetic causes of autoimmunity presenting with neonatal diabetes (NDM; diagnosis <6 months).
We performed exome sequencing in a patient with NDM and autoimmune lymphoproliferative syndrome and his unrelated, unaffected parents and identified compound heterozygous null mutations in LRBA. Biallelic LRBA mutations cause Common Variable Immunodeficiency-8, however NDM has not been confirmed in this disorder.We sequenced LRBA in 169 additional patients with diabetes diagnosed <1 year without mutations in the 24 known NDM genes. We identified recessive null mutations in 8 additional probands, of which 3 had NDM (<6 months). Diabetes was the presenting feature in 6 of 9 probands. Six of 17 (35%) patients both born to consanguineous parents and with additional early-onset autoimmunity had recessive LRBA mutations.
LRBA testing should be considered in patients with diabetes diagnosed <12 months, particularly if they have additional autoimmunity or are born to consanguineous parents. A genetic diagnosis is important as it can enable personalized therapy with abatacept, a CTLA4 mimetic, and inform genetic counselling.
Clustering of diabetes with early-onset autoimmunity in very early childhood is usually due to a combination of extreme polygenic risk and environmental exposure. Rarely, a mutation in a single gene is the aetiological cause and the identification of the underlying monogenic defect can give important insights into mechanisms of beta-cell autoimmunity and pathways of immune tolerance(1–14). Due to significant clinical overlap, discriminating patients with causative mutations in a single gene from those with a polygenic aetiology remains a challenge.
A prompt diagnosis of monogenic autoimmunity is crucial as it informs clinical management and targeted therapies may be possible. FOXP3 mutations in males cause Immunodysregulation, Polyendocrinopathy, Enteropathy, X-Linked (IPEX) syndrome(14) which can be treated with a haematopoietic stem cell transplant (HSCT). If performed early HSCT can cure the life threatening enteropathy as well as prevent the onset of autoimmune-mediated diabetes(15). In an individual with polyarthritis, scleroderma and autoimmune haemolytic anaemia resulting from an activating STAT3 mutation, treatment with tocilizumab, a monoclonal antibody against IL-6, resulted in marked improvement in their symptoms(16). Patients with Common Variable Immunodeficiency-8 (CVID-8), caused by recessively inherited mutations in Lipopolysaccharide-responsive Beige-like Anchor protein (LRBA), can be successfully treated with Abatacept, a mimetic for CTLA-4. CTLA-4 is a potent suppressive receptor that acts as an immune checkpoint and is post-translationally regulated by LRBA. (1).
Monogenic autoimmune disease often presents extremely early; for example, mutations in the STAT3, FOXP3 or IL2RA genes commonly present with neonatal diabetes(13, 14, 17). Mutations in LRBA typically presents with severe autoimmune disease early in childhood and diabetes is a feature in 22% of patients, however neonatal diabetes has not been confirmed (2).
We identified biallelic mutations in LRBA in an individual with neonatal diabetes diagnosed at 7 weeks and additional early-onset autoimmunity of unknown cause. Wego on to show that this is a relatively common aetiology of neonatal or infancy-onset diabetes when patients have additional early-onset autoimmune disease and are born to consanguineous parents.
Research Design and Methods
Gene discovery using exome sequencing
The initial case presented in diabetic keto-acidosis (blood glucose concentration: 53 mmol/L) at the age of seven weeks and developed thrombocytopenia and autoimmune lymphoproliferative disease aged three years. To define the genetic aetiology, having excluded all 24 known causes of neonatal diabetes, we used exome sequencing and trio analysis of the proband and his unaffected, unrelated parents to search for de novo heterozygous mutations and/or compound heterozygous mutations. Exome sequencing was performed using Agilent’s SureSelect Human All Exon kit (v5) with paired end 100bp read length sequencing undertaken on an Illumina HiSeq 2500. For single nucleotide variant identification, the resulting reads were aligned to the hg19 reference genome according to GATK(18, 19) best practice guidelines. An in house script was used to remove synonymous variants, those outside the coding region or conserved splice site, and variants present in dbSNP131 or the ExAC database with a MaF greater than 0.1%, as previously reported(13). We used the R software package ExomeDepth(20) to detect copy number variation. Coverage and read depth data for the trio is provided in supplementary table S1.
Follow up testing in selected neonatal/infancy onset diabetes
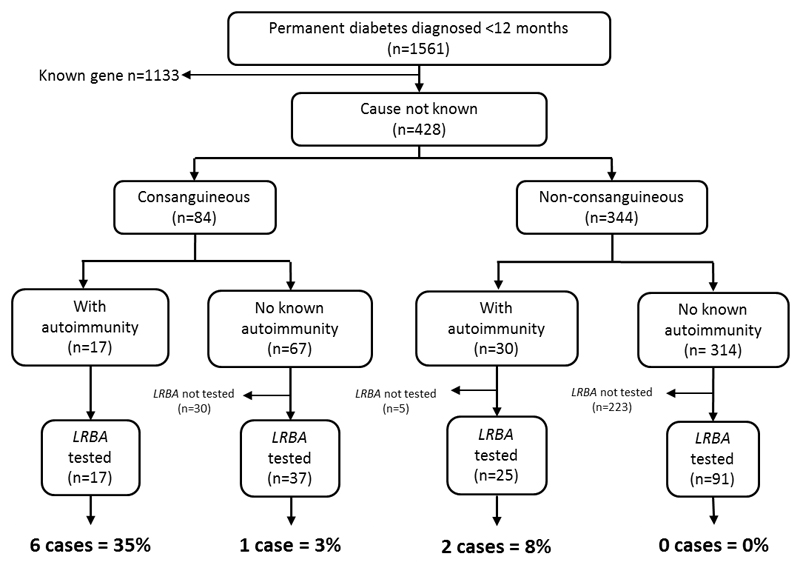
In our cohort of 1561 patients diagnosed with diabetes before the age of 12 months, 169 did not have a mutation in a known gene and were screened for mutations in LRBA (figure 2). Of these, 54 patients were consanguineous and within this group 17 individuals had autoimmune disease. Autoimmune disease was also present in 25 of 116 non-consanguineous patients . Consanguineous unions were defined as previously described (21), either known related parents (n=25) or patients who were from regions with high levels of consanguineous unions (n=29)(21). The additonal autoimmune disease was diagnosed before 5 years and included hypothyroidism (15/42), Coeliac disease/autoimmune enteropathy (16/42) and inflammatory arthritis (3/42) (further details are provided in supplementary table S2).
Figure 2. Flow diagram showing the testing strategy for LRBA screening in individuals diagnosed with diabetes diagnosed before 12 months of age.
The pick up rates of LRBA mutations, when individuals are subgrouped according to consanguinity and additional autoimmune disease, are provided.
The 24 known causes of neonatal diabetes had been previously excluded by next generation-sequencing (13, 22), and methylation specific MLPA (MRC Holland) in all 169 patients . Targeted next generation sequencing of the 58 exons and flanking intronic regions of LRBA (NM_006726.4) was performed as previously described(23) in the 169 patients with diabetes diagnosed before 1 year. Putative mutations were confirmed by Sanger sequencing or by droplet digital PCR (details available on request). When available samples from affected siblings and unaffected parents underwent mutation testing.. Clinical information was collected from the patient’s medical records by the referring clinician. All subjects and/or their parents gave informed consent for genetic testing. The study was approved by the Genetic Beta Cell Research Bank, Exeter, U.K. with ethical approval from the North Wales Research Ethics Committee, U.K.
Results
Molecular Genetics
We initially searched for de novo mutations in the proband and unrelated, unaffected parent trio. This identified four coding variants in the proband, all of which were present in the Exome Aggregation Consortium (ExAC) database(24) of >60,000 patients not diagnosed with any severe paediatric disease (see supplementary table S3). We considered these unlikely to be causative and switched our analysis to look for recessive causes.
We identified compound heterozygous mutations in LRBA and PKHD1L1. The two novel null mutations in LRBA (p.D1053fs*2; c.3156del and p.S2659*; c.7976C>G) were considered likely to be pathogenic as bi-allelic mutations in this gene are known to cause Common Variable Immunodeficiency-8 (CVID-8)(7). Variants in PKHD1L1 have not been associated with Mendelian disease and there are 549 individuals in the ExAC database (controls without severe paediatric disease) with homozygous loss of function mutations (24), suggesting that loss of PKHD1L1 does not cause childhood-onset disease.
Whilst diabetes has been reported as a feature in 11/57 patients(1–12) with LRBA mutations, only two patients were diagnosed before the age of one year; one at 4 months and one at 7 months. The median age of diabetes diagnosis in the other patients was two years (range 1-9 years). LRBA encodes the Lipopolysaccharide-responsive beige-like anchor protein - an essential post-translational regulator of the CTLA-4 receptor involved in the suppression of regulatory T cells(1).
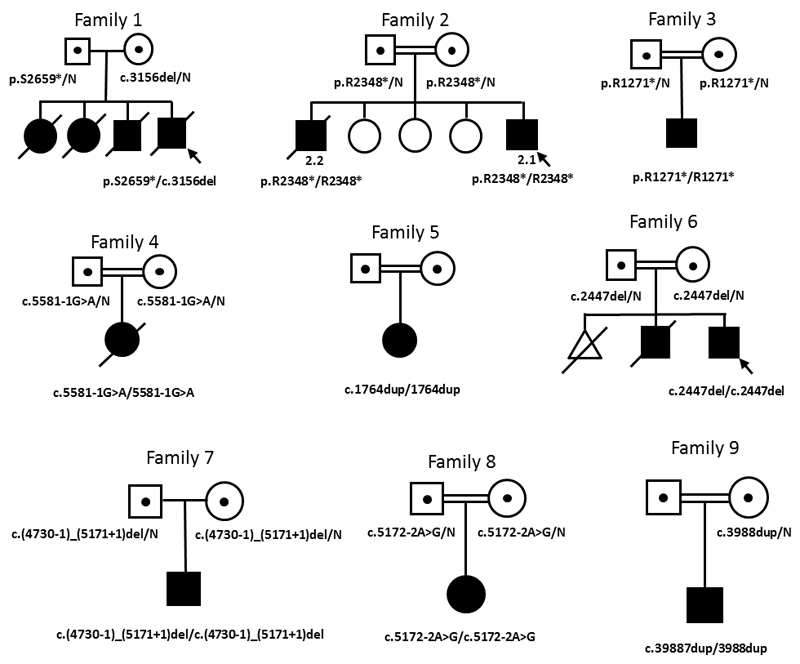
Sequence analysis of LRBA in 169 patients diagnosed with diabetes before 12 months identified homozygous null mutations in eight additional probands and one affected sibling (see table , figure 1, supplementary figure S2). All mutations introduce premature termination codons (3 nonsense, 4 frameshift, 2 mutations affecting splicing and one whole exon deletion) and are predicted to result in complete loss of the LRBA protein. Carrier status was confirmed in parents when samples were available (see figure 1).
Figure 1. Family pedigrees of patients with LRBA mutations.
Filled symbols represent affected individuals and dots within symbols represent heterozygous unaffected carriers. Double lines signify parents are related. Genotypes are provided below affected individuals and carriers. When no genotype is given, samples were unavailable for testing.
Table 2 shows the distribution of individuals with LRBA mutations by age of diabetes diagnosis and parental consanguinity. Interestingly the highest proportion of those with a mutation were diagnosed with diabetes between 6 and 12 months. We identified LRBA mutations in 9 of 1561 patients diagnosed with diabetes before 12 months, giving a minimum prevalence of 0.6% in our cohort (Table 2).
Table 2. Minimum prevalence of LRBA mutations in our cohort of patients diagnosed with diabetes before 12 months. *Born to consanguineous parents. †Born to unrelated parents.
| <6 months | 6-12 months | <12 months | ||||
|---|---|---|---|---|---|---|
| Consang* | Non-consang† | Consang | Non-consang | Consang | Non-consang | |
| Total number of patients | 338 | 892 | 63 | 268 | 401 | 1160 |
| Number with other known genetic cause | 299 | 761 | 17 | 56 | 316 | 817 |
| Number LRBA tested in | 31 | 74 | 23 | 41 | 54 | 116 |
| Number of LRBA cases identified | 3 | 1 | 4 | 1 | 7 | 2 |
| Minimum prevalence (%) | 0.9% | 0.1% | 6.3% | 0.4% | 1.7% | 0.2% |
Seven of 54(13%) consanguineous patients had LRBA mutations whilst a mutation was identified in only 2 of 25 (8%) non-consanguineous patients with autoimmunity. Strikingly, when the criteria of consanguinity and autoimmunity were combined, 6 of 17 (35%) patients harboured mutations in LRBA (figure 2). Using these two criteria therefore greatly increased the likelihood of identifying an LRBA mutation.
Clinical characteristics
The proband presented in severe diabetic ketoacidosis at the age of seven weeks (blood glucose 53 mmol/L). He was treated with a full replacement dose of insulin, was negative for anti-GAD antibodies and had a HbA1c prior to his death of 7.0% (53 mmol/mol). Thrombocytopenia and autoimmune lymphoproliferative disease were reported at the age of three years with additional features including right hemiparesis and neuromotor retardation also noted at this time. The patient died shortly before his fourth birthday as a result of an intracranial haemorrhage caused by thrombocytopenia.
Detailed follow up after genetic analysis revealed the proband’s three elder siblings had also died in childhood at another hospital; two older sisters died at ages 12 years and 3.5 years due to complications relating to immunodeficiency and his older brother died at the age of 6.5 years as a result of immunodeficiency and severe enteropathy. Diabetes was not reported in these individuals and DNA was not available for testing. This family history suggests the siblings were also compound heterozygous for the LRBA mutations , fitting with the inheritance pattern of LRBA. The parents were unaffected in keeping with previous reports that haploinsufficiency of LRBA does not cause CVID-8 (5, 6)
All 10 patients with bi-allelic LRBA mutations were diagnosed with diabetes in the first 15 months of life (median: 7.5 months, range: 6 weeks – 15 months) and four of these patients met the criteria for neonatal diabetes, having been diagnosed with diabetes before the age of 6 months. All had insulin doses suggesting full replacement was required (table 1). Positivity for anti-GAD antibodies (90 U/mL; normal range <25 U/mL) was detected in just 1 of the 6 patients in whom pancreatic antibody screening with GAD/IA2/ZnTransporter was possible (table 1). This low prevalence of autoantibodies is in keeping with these patients having autoimmunity with a distinct mechanism to that seen in type 1 diabetes.
Table 1. Clinical features of patients with LRBA mutations.
*All mutations are homozygous unless otherwise indicated and are described according to HGVS guidelines based on the longest isoform, NM_006726.4. Disorders reported are based on the clinical diagnosis made by the patients’ physician and were not always confirmed by diagnostic investigations such as biopsies. †Most recent HbA1c recorded. ‡Upper respiratory tract infections. ND – no data. TPO Ab – thyroid peroxidase antibody.
| Patient | 1 | 2.1 | 2.2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype* | p.D1053fs/p.S2659* (c.3156del/c.7976C>A) |
p.R2348* (c.7042C>T) |
p.R2348* (c.7042C>T) |
p.R1271* (c.3811C>T) |
p.? (c.5581-1G>A) |
p.M589fs (c.1764dup) |
p.P816fs (c.2447del) |
p.? (c.(4729+1_4730-1)_(5171+1_5172-1)del) | p.? (c.5172-2A>G) | p.I1330fs (c.3988dup) |
| Birth weight [g] (gestation [weeks]) | 2600 (35) | 3200 (39) | 3200 (unknown) | 2700 (40) | 3200 (38) | 2750 (39) | 3200 (40) | 2965 (40) | 2970 (40) | 3000 (40) |
| Sex | Male | Male | Male | Male | Female | Female | Male | Male | Female | Male |
| Current Age (years) | Deceased | 1 | Deceased | 8 | Deceased | 2 | 6 | 26 | 1 | 4 |
| Known consanguinuity | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Ethnicity | Turkish | Moroccan | Moroccan | Omani | Omani | Iranian | Egyptian | Chinese | Turkish | Pakistani |
| Diabetic features | ||||||||||
| Age at onset | 7 weeks | 6 weeks | 15 months | 4 months | 5 months | 9 months | 9 months | 10 months | 8 months | 7 months |
| Treatment (dose) | Insulin (1U/kg/day) | Insulin (1U/kg/day) | Insulin (1.2U/kg/day) | Insulin (1U/kg/day) | Insulin (0.6U/kg/day) | Insulin (0.7U/kg/day) | Insulin (2U/kg/day) | Insulin (0.6U/kg/day) | Insulin (0.9U/kg/day) | Insulin (1.7U/Kg/day) |
| HbA1c† (mmol/mol) | 7.0% (53) | 7.1% (54) | 6.6% (49) | (7.1% (54) | 8.3% (67) | ND | ND | 8.7% (72) | 7.2% (55) | ND |
| Antibody Status | GAD Negative | GAD/IA2/ZnT8 Negative | ND | GAD/IA2 Negative | GAD positive | ND | ND | GAD negative | GAD/IA2 Negative | ND |
| Immunodysregulatory features | ||||||||||
| Haemato-logical disorders | Thrombocytopenia; Autoimmune lymphoproliferative disease |
_ | Thrombocytopenia; Autoimmune lymphoproliferative disease |
Agammaglobulinaemia; Autoimmune lymphoproliferative disease |
_ | _ | Thrombocytopenia; Autoimmune haemolytic anaemia |
Pernicious anaemia | _ | _ |
| Gastro-intestinal disorders | _ | Autoimmune enteropathy | Autoimmune enteropathy; Hepatosplenomegaly |
Hepatosplenomegaly | Autoimmune enteropathy | _ | Episodes of diarrhoea | Coeliac disease | _ | Chronic diarrhoea |
| Endocrine disorders | _ | _ | _ | _ | _ | _ | Autoimmune hypothyroidism | TPO Ab positive (sub clinical hypothyroidism) |
_ | _ |
| Recurrent infections | _ | _ | Died of septic shock following unknown infection | Recurrent chest infections (Aspergillus spp.) | _ | _ | Pneumonia | _ | Pneumonia; Otitis media |
URTI‡, septicaemia |
| Other features | ||||||||||
| Cleft lip; Developmental delay; Hemiparesis. Died from intracranial bleed |
_ | _ | Lymphocytic interstitial pneumonia | Died from Nephroblastoma | _ | History of convulsions; Multiple cerebral infarctions |
Parenchymal calcification of kidneys | _ | _ | |
In 6 of the 9 probands diabetes was the presenting feature. Autoimmune disorders were present in 8 of the 9 probands (table 1) and included haematological manifestations (5/8), autoimmune enteropathy (3/8) and hypothyroidism (1/8). The remaining proband is the result of a consanguineous union and has presented with diabetes at 9 months, which is still the only clinical feature at 2 years. In three patients recurrent respiratory infections were reported. The prognosis was poor as three of the patients are deceased; the original proband died from a cerebral haemorrhage likely caused by thrombocytopenia, a second patient developed complications associated with nephroblastoma and a third child died of sepsis (table1).
Conclusions
We have identified 10 different loss of function LRBA mutations in nine probands and one sibling (figure 1 and table 1) with 4 cases having neonatal diabetes. This study increases the total number of genetic causes of neonatal diabetes to 25, and the genetic causes of severe early-onset autoimmunity that includes neonatal diabetes to 4; the others being FOXP3 (14), IL2RA (17), and STAT3 (13).
In our cohort of patients with diabetes diagnosed before 12 months, 0.6% have recessively inherited LRBA mutations. In contrast to other monogenic autoimmune diabetes subtypes the highest pick-up rate for LRBA mutations was in those individuals diagnosed with diabetes between 6 and 12 months. The combined criteria of autoimmune disease and consanguinity identified a high proportion of patients with LRBA mutations. Six of the 9 probands with LRBA mutations were suspected or proven to be consanguineous and have additional autoimmune disease. Using these criteria in our patients with infancy-onset diabetes we identified a causative mutation in 35% (6/17) of patients; (see figure 2). We therefore recommend testing for LRBA mutations is considered in all patients diagnosed with diabetes before the age of 12 months, particularly those who have additional autoimmunity and are the result of consanguineous union .
Identifying LRBA mutations early is crucial as it may allow for the introduction of optimal treatment strategies before the disease progresses. Genetic testing of all causes of neonatal diabetes is now predominantly by targeted panels (e.g. (23, 25, 26)) occurring immediately after the diagnosis of diabetes with in the first 6 months of life(22). Targeted sequencing should include LRBA as in all 4 probands with neonatal diabetes this was the first feature of their multisystem autoimmune disorder and for one proband is currently the only feature at the age of two years.
Recessive mutations in LRBA are a known cause of Common Variable Immunodeficiency 8 (CVID-8, MIM #614700)(7) which often includes early-onset autoimmunity, immune dysregulation, recurrent infections and hypogammaglobinaemia with variable penetrance (1–12, 27). Neonatal diabetes had not been confirmed as a feature of this disorder. The extra-pancreatic features observed in our cohort are at a similar prevalence to those reported in patients with biallelic LRBA mutations (supplementary Figure S1), consistent with a diagnosis of CVID-8.
Five of the 6 patients in whom testing was possible were negative for pancreatic antibodies. This suggests that the mechanism of autoimmunity may be distinct to that observed in early-onset type 1 diabetes. It may be that the autoantigens which are the target of the immune response are as yet uncharacterised, that they are not islet specific, or that the autoimmunity is cell-based rather than antibody driven. Further work to elucidate the true mechanism underlying the development of diabetes in CVID-8 is warranted and may give new insights into the pathophysiology of type 1 diabetes.
All patients we describe have functionally similar bialleleic null mutations but despite this there is considerable variation in their phenotype. For example patient 3 presented with neonatal diabetes (diagnosed at 4 months) and has immunodeficiency, autoimmune lymphoproliferative disease, hepatosplenomegaly, lymphocytic interstitial pneumonia and recurrent chest infections diagnosed before the age of 8 years, whereas patient 7 was diagnosed with diabetes at the age of 10 months and has coeliac disease, pernicious anaemia and subclinical hypothyroidism at the age of 26 years (see table 1). The variable phenotype of patients with homozygous missense mutations seen in previous studies was not statistically different from those with protein truncating mutations; the age of onset of the first symptom is similar in both groups (median age of onset; missense mutations: 1.75 years versus nonsense mutations: 2 years, p = 0.79) (1, 5, 7, 8, 11). It therefore seems likely that additional genetic and/or environmental factors influence the severity of the disease and the specific organs affected in these patients.
The autoimmunity observed in patients with LRBA mutations is considered to result from the loss of an essential immune regulatory pathway and a reduction in the suppressive action of regulatory T cells, therefore a disruption of immune tolerance(1). It was recently shown that LRBA prevents the lysosomal degradation of the CTLA-4 receptor, facilitating its trafficking to the surface of T cells during T cell receptor (TCR) stimulation(1). CTLA-4 is a potent suppressor, blocking co-stimulation of the TCR and therefore negatively regulating immune responses (28). A loss of LRBA therefore results in increased CTLA-4 degradation diminishing this inhibitory pathway on T-cell activation and resulting in unchecked activation of immunologic responses.
Identifying the underlying genetic aetiology is clinically important for these patients as understanding the disease mechanism may allow the use of personalized therapy. Abatacept, a CTLA-4 mimetic that replaces the action of the lost suppressive receptor, has been used to treat 12 patients with LRBA mutations so far and all showed improvement in their autoimmune features(1, 5). Therapy with abatacept had not been attempted in our patients at the time of reporting. HSCT is also an option for these patients, with successful outcome reported in 3 of 4 patients with LRBA mutations in whom it has been attempted(5, 10, 12).
In conclusion, we have identified LRBA mutations in 9 probands with early-onset diabetes (< 1 year) of whom 8 had additional autoimmune features. In 4 of these patients diabetes was diagnosed before 6 months confirming the role of this gene in the aetiology of neonatal diabetes. As diabetes was the presenting feature in 6/9 individuals we recommend that testing for LRBA mutations is considered in all patients with newly diagnosed neonatal diabetes, and in those with infancy onset (< 12 months) diabetes, especially when a recessive inheritance is suspected or additional autoimmune features are present. . A genetic diagnosis is critical not only for counseling on recurrence risk but it can also allow for immunomodulatory agents such as abatacept to be considered as part of the treatment regimen.
Supplementary Material
Acknowledgements
The authors thank Benjamin Bunce of The Royal Devon and Exeter NHS Foundation Trust and Richard Caswell, Matthew Wakeling and Thomas Laver of the University of Exeter Medical School for their assistance. This work was supported by a Wellcome Trust Senior Investigator Award to SE and ATH (grant number: 098395/Z/12/Z). ATH is an NIHR senior investigator. EDF is a Naomi Berrie Fellow in Diabetes Research. SEF has a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant Number: 105636/Z/14/Z). Additional support came from the University of Exeter and the NIHR Exeter Clinical Research Facility.
Footnotes
Author Contributions
S.E, A.T.H and S.E.F designed the study. M.B.J and E.D-F performed the genetic analysis and interpreted the data. H.L-A performed bioinformatics analysis, A.A-S, N.E, Z.S, M.B, Z.I, A.H, I.U, S.A and D.G recruited patients, provided clinical information and contributed to discussion, M.B.J, E.D-F, A.T.H and S.E.F. wrote the manuscript which was reviewed/edited by all authors.
Guarantor statement
A.T.H is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
References
- 1.Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015 Jul 24;349(6246):436–40. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 2.Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015 Jan;135(1):217–27. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alangari A, Alsultan A, Adly N, Massaad MJ, Kiani IS, Aljebreen A, et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012 Aug;130(2):481–8 e2. doi: 10.1016/j.jaci.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns SO, Zenner HL, Plagnol V, Curtis J, Mok K, Eisenhut M, et al. LRBA gene deletion in a patient presenting with autoimmunity without hypogammaglobulinemia. J Allergy Clin Immunol. 2012 Dec;130(6):1428–32. doi: 10.1016/j.jaci.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gamez-Diaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016 Jan;137(1):223–30. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Levy E, Stolzenberg MC, Bruneau J, Breton S, Neven B, Sauvion S, et al. LRBA deficiency with autoimmunity and early onset chronic erosive polyarthritis. Clin Immunol. 2016 Jul;168:88–93. doi: 10.1016/j.clim.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, Herholz P, Trujillo-Vargas CM, Phadwal K, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012 Jun 8;90(6):986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revel-Vilk S, Fischer U, Keller B, Nabhani S, Gamez-Diaz L, Rensing-Ehl A, et al. Autoimmune lymphoproliferative syndrome-like disease in patients with LRBA mutation. Clin Immunol. 2015 Jul;159(1):84–92. doi: 10.1016/j.clim.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Schreiner F, Plamper M, Dueker G, Schoenberger S, Gamez-Diaz L, Grimbacher B, et al. Infancy-Onset T1DM, Short Stature, and Severe Immunodysregulation in Two Siblings With a Homozygous LRBA Mutation. J Clin Endocrinol Metab. 2016 Mar;101(3):898–904. doi: 10.1210/jc.2015-3382. [DOI] [PubMed] [Google Scholar]
- 10.Seidel MG, Hirschmugl T, Gamez-Diaz L, Schwinger W, Serwas N, Deutschmann A, et al. Long-term remission after allogeneic hematopoietic stem cell transplantation in LPS-responsive beige-like anchor (LRBA) deficiency. J Allergy Clin Immunol. 2015 May;135(5):1384–90 e1-8. doi: 10.1016/j.jaci.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serwas NK, Kansu A, Santos-Valente E, Kuloglu Z, Demir A, Yaman A, et al. Atypical manifestation of LRBA deficiency with predominant IBD-like phenotype. Inflamm Bowel Dis. 2015 Jan;21(1):40–7. doi: 10.1097/MIB.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 12.Tesi B, Priftakis P, Lindgren F, Chiang SC, Kartalis N, Lofstedt A, et al. Successful Hematopoietic Stem Cell Transplantation in a Patient with LPS-Responsive Beige-Like Anchor (LRBA) Gene Mutation. J Clin Immunol. 2016 Jul;36(5):480–9. doi: 10.1007/s10875-016-0289-y. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Lango Allen H, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014 Aug;46(8):812–4. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d'Hennezel E, Bin Dhuban K, Torgerson T, Piccirillo CA. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2012 May;49(5):291–302. doi: 10.1136/jmedgenet-2012-100759. [DOI] [PubMed] [Google Scholar]
- 15.Nademi Z, Slatter M, Gambineri E, Mannurita SC, Barge D, Hodges S, et al. Single centre experience of haematopoietic SCT for patients with immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Bone Marrow Transplant. 2014 Feb;49(2):310–2. doi: 10.1038/bmt.2013.181. [DOI] [PubMed] [Google Scholar]
- 16.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015 Jan 22;125(4):591–9. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3168–71. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11 0 1–33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011:43. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plagnol V, Curtis J, Epstein M, Mok KY, Stebbings E, Grigoriadou S, et al. A robust model for read count data in exome sequencing experiments and implications for copy number variant calling. Bioinformatics. 2012 Nov 1;28(21):2747–54. doi: 10.1093/bioinformatics/bts526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bittles AH. A community genetics perspective on consanguineous marriage. Community Genet. 2008;11(6):324–30. doi: 10.1159/000133304. [DOI] [PubMed] [Google Scholar]
- 22.De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015 Sep 5;386(9997):957–63. doi: 10.1016/S0140-6736(15)60098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellard S, Lango Allen H, De Franco E, Flanagan SE, Hysenaj G, Colclough K, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013 Sep;56(9):1958–63. doi: 10.1007/s00125-013-2962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016 Aug 17;536(7616):285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnefond A, Philippe J, Durand E, Muller J, Saeed S, Arslan M, et al. Highly sensitive diagnosis of 43 monogenic forms of diabetes or obesity through one-step PCR-based enrichment in combination with next-generation sequencing. Diabetes Care. 2014 Feb;37(2):460–7. doi: 10.2337/dc13-0698. [DOI] [PubMed] [Google Scholar]
- 26.Gao R, Liu Y, Gjesing AP, Hollensted M, Wan X, He S, et al. Evaluation of a target region capture sequencing platform using monogenic diabetes as a study-model. BMC Genet. 2014 Jan 29;15:13. doi: 10.1186/1471-2156-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkhairy OK, Abolhassani H, Rezaei N, Fang M, Andersen KK, Chavoshzadeh Z, et al. Spectrum of Phenotypes Associated with Mutations in LRBA. J Clin Immunol. 2016 Jan;36(1):33–45. doi: 10.1007/s10875-015-0224-7. [DOI] [PubMed] [Google Scholar]
- 28.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998 Dec 18;282(5397):2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.