Summary
Objective
Global data on cervical lesion incidence and progression in HIV-positive is essential for understanding the natural history of cervical neoplasia and informing screening policy.
Methods
A systematic review was performed summarizing the incidence and progression of cervical lesions in HIV-positive women.
Results
Of 5,882 HIV-positive women from 15 studies, incidence ranged from 4.9 to 21.1 cases per 100 woman-years for any cervical lesion and 0.4 to 8.8 cases per 100 woman-years for high grade cervical lesions. HIV-positive women showed a median 3-fold higher incidence of cervical lesions compared to HIV-negative women. Of 1,099 HIV-positive women from 11 studies, progression from low to high grade lesions ranged from 1.2 to 26.2 cases per 100 woman-years. Both incidence and progression rates increased with lower CD4 counts. The effect of antiretroviral therapy on the natural history of cervical neoplasia remains unclear.
Conclusions
HIV-positive women have higher incidence and progression of cervical neoplasia. Cervical cancer screening should be integrated into HIV treatment programs.
Keywords: cervical cancer, cervical lesions, HIV, incidence, progression
Introduction
In 2008, an estimated 33 million people were HIV infected worldwide, with 2.7 million newly acquired HIV infections, most of which occurred in less developed countries1. HIV prevalence continues to rise due to both the high rate of new infections and the beneficial increase in life expectancy from antiretroviral therapy (ART). HIV is increasingly becoming a disease of women, with almost 50% of prevalent cases occurring in females1. This trend is particularly noticeable in sub-Saharan Africa where females have approximately three times the HIV prevalence of males1.
Many resource limited nations that have high HIV prevalence also have high rates of cervical cancer because of limitations of screening and treatment. Cervical cancer is caused by infection with carcinogenic HPV genotypes2 and HIV infection is strongly associated with a higher prevalence, incidence, and persistence of human papillomavirus (HPV) infection3–6. Women with HIV have a higher prevalence of cervical squamous epithelial lesions (SIL) and invasive cervical cancer7–13. Recognizing this connection, the CDC declared invasive cervical cancer as an AIDS-defining illness in 199314. Additionally, reducing cervical cancer in HIV-positive women is a primary focus of a joint public-private international initiative launched in 2011, the Pink Ribbon Red Ribbon initiative15.
The large number of women living with HIV underscores the need to better understand the natural history of cervical precancer and cancer development in this high-risk population. While reports have linked HIV-related immunosuppression with an increased incidence of cervical precancer lesions and invasive cervical cancer3,16, there is no global review compiling data on rates of incidence and progression of cervical neoplasia in HIV-positive women. Thus, we systematically reviewed the current literature on cervical precancer and cancer among HIV-positive women worldwide and examine important factors related to immunosuppression, including CD4+ T-cell count and ART use.
Methods
In order to identify studies related to cervical lesion incidence, a systematic literature search was conducted in MEDLINE for all studies indexed through January 31, 2012 with no specified start date using the following search terms: (uterine cervical neoplasms (MeSH term) OR pap abnormality OR squamous intraepithelial lesions) AND cohort studies (MeSH term) limited to AIDS (keyword for HIV-related studies). Articles written in languages other than English were translated and reviewed for inclusion criteria. References of identified publications were also used to identify additional published articles for review. For cervical lesion incidence, studies were included if: i) the study sample included 20 or more HIV-positive women; ii) a cohort design was used to assess incidence of SIL or greater and/or cervical intraepithelial neoplasia (CIN)1 or greater; and iii) study participants had normal cervical screen at baseline. Where possible, incidence rates were split for high grade versus low grade SIL and CIN (HSIL/CIN2–3 and LSIL/CIN1, respectively).
Articles reporting on cervical lesion progression were selected from the chosen incidence articles as well as through a separate MEDLINE search. The MEDLINE search was conducted for all studies indexed by January 12, 2012 using the following search terms: uterine cervical neoplasms (MeSH term) AND progression limited to AIDS (which limits the search to HIV-specific publications). Studies were included in the progression analysis if: i) the study sample included 20 or more HIV-positive women; ii) a cohort design was used to estimate progression to SIL or greater and/or CIN1 or greater; and iii) study participants had atypical cells of unknown significance (ASCUS), low-grade SIL (LSIL), or low-grade CIN (CIN1) diagnosis at baseline.
Incidence and progression rates were reported as stated in the articles or estimated from reported number of cases, subjects and median or mean follow-up time. Additionally, study cumulative risks of incidence and of progression were reported. Risk ratios were reported as stated in the articles or estimated using the ratio of the study cumulative risks. In this review, the term ART is broadly used to mean any ART use (monotherapy or greater). However, in the tables and results, ART and highly active antiretroviral therapy (HAART) were labeled as written in the individual articles with ART typically referring to monotherapy or dual therapy and HAART referring to triple therapy or greater. When the study included both women using ART and HAART, the term “mixed ART” is applied. When more than one article based on the same study population met the selection criteria for inclusion, the article with the largest number of women was used, unless multiple articles could contribute to separate analyses.
Results
Incidence of cervical lesions
From 451 screened abstracts, our search identified 15 cohort studies presenting data on the incidence of cervical neoplasia with observational data on 5882 HIV-positive women in total (Table 1). Most studies were conducted in the European Union (N=7; 47%)7,17–22, with 1 including women from France and French Guiana10, and 1 including women from Europe and South Africa23. Three studies were conducted in the United States24–26, 2 were conducted solely in Africa27,28, and 1 took place in Thailand29. No studies came from Australia, Central America, or Eastern Europe. Study populations were primarily from health clinics, sexually transmitted disease clinics, and drug treatment centers. Less than half of the studies (N=6, 40%) had both HIV-positive and HIV-negative populations. Median age of study participants ranged from 29 to 43, with most studies having a median age in the low 30s. The number of participants with follow-up data in the included studies ranged from 27 to 1,931 HIV-positive women; most had 100 or more HIV-positive study participants. Study lengths ranged from less than a year to 8 years, with most studies reporting follow-up visits every six months. In 2 of the studies, all study participants were receiving HAART21,30. Eleven of the studies had a proportion of the participants receiving ART (3 ART, 3 HAART, and 5 mixed HAART/ART)7,10,18,19,22–26,28,29, 1 study had no participants receiving ART27, and 1 study did not specify ART use20.
Table 1.
Incidence rate estimates of cervical neoplasia from HIV-positive populations
| Author/year Country Study Population |
Median (mean) age, years |
ART use |
Women with follow-up data (N) |
Median (mean) follow- up time, months |
Assessment method (intervals) |
Baseline | Outcome | Cases (N) | Cases / 100 woman-years |
Study cumulative risk |
|---|---|---|---|---|---|---|---|---|---|---|
| Chalermchockcharoenkit et al. 201129 Thailand STD clinic 2004–2009 |
HIV+ (30) | mixed* | HIV+ 444 | HIV+ 10 | cytology† (6 months) | normal | LSIL | HIV+ 60 | HIV+16.2‡ | HIV+ 14% |
| normal | HSIL | HIV+ 18 | HIV+ 4.9‡ | HIV+ 4% | ||||||
| normal | SIL | HIV+ 78 | HIV+ 21.1‡ | HIV+ 18% | ||||||
|
| ||||||||||
| Omar et al. 201128 South Africa HIV wellness clinic 2003–2009 |
HIV+ 32 | mixed* | HIV+ 832 | HIV+ 30 | cytology(6–12 months) | normal | LSIL | HIV+ 183 | HIV+ 9.6 | HIV+ 22% |
| normal | HSIL | HIV+ 61 | HIV+ 3.3 | HIV+ 7% | ||||||
| normal | SIL | HIV+ 244 | HIV+ 12.9 | HIV+ 29% | ||||||
|
| ||||||||||
| Massad et al. 200825 USA Women’s Interagency HIV Study (WIHS) university, public, and private medical centers and clinics 1994–2006 |
HIV+/− 43§|| | mixed¶ | HIV+ 1931|| HIV− 533|| |
HIV+/− 101§|| | cytology (6 months) | normal | HSIL | NS | HIV+ 0.4 HIV− 0.1 |
HIV+ 4% HIV− 1% |
| normal | SIL | NS | HIV+ 6.0 HIV− 1.1 |
HIV+ 43% HIV− 11% |
||||||
|
| ||||||||||
| Kitchener et al. 200723 5 European nations and South Africa Hospital and community health Centers 2000–2004 |
HIV+ 33|| | mixed* | HIV+ 552 | HIV+ 30|| | cytology (6 months) | normal | SIL | HIV+ 117 | HIV+ 8.5 | HIV+ 21% |
|
| ||||||||||
| Sirera et al. 200721 Germany University HIV clinic1997–2005 |
HIV+ (35) | all* | HIV+ 133 | HIV+ 57** | cytology† (12 months) | normal | SIL | HIV+ 47 | HIV+ 7.4‡ | HIV+ 35% |
|
| ||||||||||
| Soncini et al. 200722 Italy University Hospital 1993–2003 |
NS | mixed†† | HIV+ 101 | HIV+ (42) | cytology†, colposcopy(12 months) | normal | LSIL | HIV+ 7 | HIV+ 2.0‡ | HIV+ 7% |
| normal | HSIL | HIV+ 31 | HIV+ 8.8‡ | HIV+ 30% | ||||||
| normal | SIL | HIV+ 38 | HIV+10.7‡ | HIV+ 38% | ||||||
|
| ||||||||||
| Lehtovirta et al. 200619 Finland University gynecological clinic 1989–2003 |
HIV+ (31) || | mixed†† | HIV+ 55 | HIV+ (53) || | cytology (6 months) | normal | LSIL | HIV+ 18 | HIV+ 7.4‡ | HIV+ 33% |
| normal | HSIL | HIV+ 6 | HIV+ 2.5‡ | HIV+ 11% | ||||||
| normal | SIL | HIV+ 24 | HIV+ 9.9‡ | HIV+ 44% | ||||||
|
| ||||||||||
| Hawes et al. 200627 Senegal STD clinics, sex workers, oversampling HR-HPV/HIV− 1994–1998 |
HIV+ (32) HIV− (31) |
none | HIV+ 246 HIV− 381 |
HIV+/− (26) § | cytology (4 months) | normal | HSIL | HIV+ 36** HIV− 35** |
HIV+ 6.4** HIV− 4.2** |
HIV+ 15% HIV− 9% |
|
| ||||||||||
| Schuman et al. 200326 USA HIV Epidemiology Research Study (HERS) 1993–1999 |
HIV+ 35 HIV− 34 |
mixed†† | HIV+ 774 HIV− 391 |
HIV+ 48 HIV− 48 |
cytology† (6 months) | normal | HSIL | HIV+ 47 HIV− 4 |
HIV+ 1.6 HIV− 0.3 |
HIV+ 7% HIV− 1% |
| normal | SIL | HIV+ 224 HIV− 34 |
HIV+ 11.5 HIV− 2.6 |
HIV+ 35% HIV− 9% |
||||||
|
| ||||||||||
| Branca et al. 200330 Italy DIANAIDS project, clinics 1997–1999 |
HIV+ (34) || HIV− (33) || |
all* | HIV+ 73 HIV− 44 |
HIV+ (11) || HIV− (20) || |
cytology (NS) | normal | LSIL | HIV+ 5 HIV− 0 |
HIV+ 7.8‡ HIV− 0‡ |
HIV+ 7% HIV− 0% |
| normal | HSIL | HIV+ 4 HIV− 1 |
HIV+ 6.2‡ HIV− 1.4‡ |
HIV+ 5% HIV− 2% |
||||||
| normal | SIL | HIV+ 9 HIV− 1 |
HIV+ 14.0‡ HIV− 1.4‡ |
HIV+ 12% HIV− 2% |
||||||
|
| ||||||||||
| Ellerbrock et al. 200024 USA New York Cervical Disease Study; STD clinics and drug treatment centers 1991–1996 |
HIV+ ≥ 35 HIV− ≥ 34 |
mixed‡‡ | HIV+ 328 HIV− 325 |
HIV+ (30) HIV− (33) |
cytology, colposcopy, histology confirmation (6 months) | normal | LSIL | HIV+ 61 HIV− 12 |
HIV+ 7.6 HIV− 1.3 |
HIV+ 19% HIV− 4% |
| normal | HSIL | HIV+ 6 HIV− 4 |
HIV+ 0.7 HIV− 0.4 |
HIV+ 2% HIV− 1% |
||||||
| normal | SIL | HIV+ 67 HIV− 16 |
HIV+ 8.3 HIV− 1.8 |
HIV+ 20% HIV− 5% |
||||||
|
| ||||||||||
| Delmas et al. 20007 12 European Countries European Study Group on Natural History of HIV Infection in Women; gynecology, STD, and drug treatment centers 1993–1998 |
HIV+ 31|| | mixed†† | HIV+ 229 | HIV+ 24 | cytology† (6 months) | normal | LSIL | HIV+ 61 | HIV+ 13.3‡ | HIV+ 27% |
| normal | HSIL | HIV+ 6 | HIV+ 1.3‡ | HIV+ 3% | ||||||
| normal | SIL | HIV+ 67 | HIV+ 14.6‡ | HIV+ 29% | ||||||
|
| ||||||||||
| Petry et al. 199920 Germany university immunology center 1990–1998 |
HIV+ (33) | NS | HIV+ 88 | HIV+ (21)† | cytology, colposcopy, histology confirmation (NS) | normal | CIN II/III | HIV+ 7 | HIV+ 4.6‡ | HIV+ 8% |
|
| ||||||||||
| Six et al. 199810 France and French Guiana medical centers and STD clinics 1993–1995 |
HIV+ 3|| HIV− 29|| |
mixed‡‡ | HIV+ 160 HIV− 118 |
HIV+/− 13§|| | cytology† (6 months) | normal | ASCUS and SIL | HIV+ 33 HIV− 6 |
HIV+ 19.0‡ HIV− 4.7‡ |
HIV+ 21% HIV− 5% |
|
| ||||||||||
| Heard et al. 199518 France HIV gyn. clinic 1991–1993 |
NS | mixed‡‡ | HIV+ 27 | HIV+ 18 | cytology and colposcopy (6 months) | normal | SIL | HIV+ 2 | HIV+ 4.9‡ | HIV+ 7% |
ART=antiretroviral therapy, HAART=highly active antiretroviral therapy, HIV=human inmmunodeficiency virus, SIL=squamous intraepithelial lesion, LSIL = low grade SIL, HSIL = high grade SIL, ASCUS=atypical cells of unknown significance, CIN=cervical intraepithelial neoplasia, STD=sexually transmitted disease, NS=not specified;
HAART;
Some cases confirmed by histology;
Estimated from [[incident cases / (N normal at baseline * mean or median follow-up time)] * 100;
HIV+ / − combined;
For larger cohort;
Mixed ART/HAART, additional reference used32;
From personal communication with author;
Mixed ART/HAART;
ART.
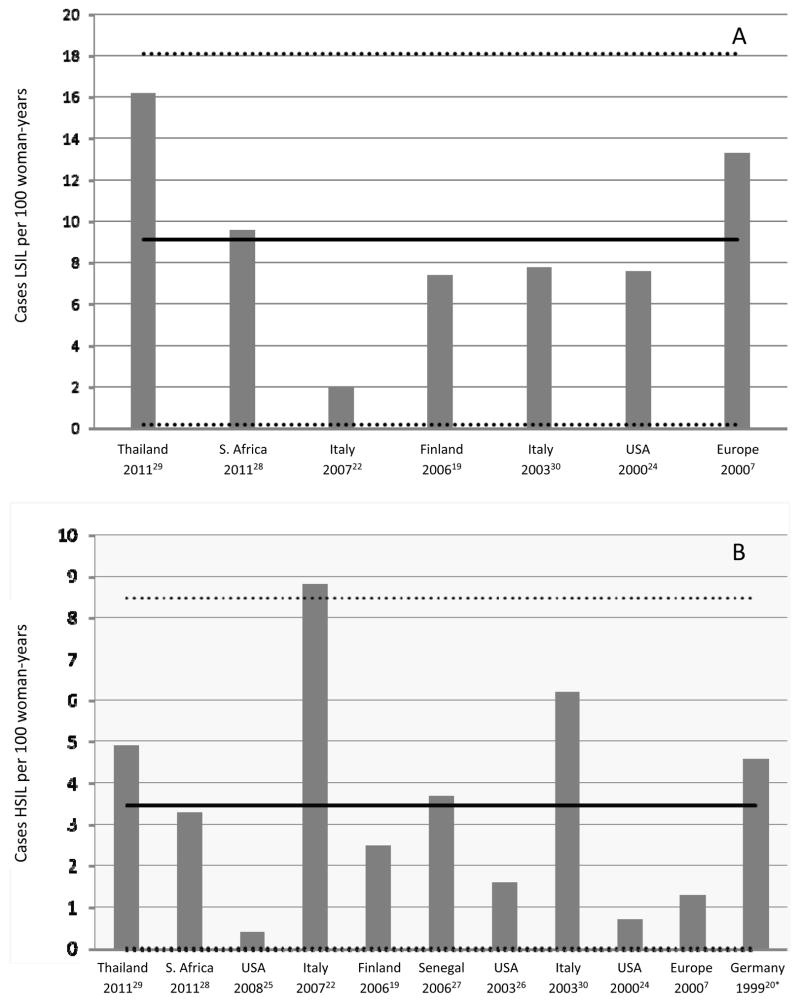
Cervical lesions were identified through cytology in all of the studies, with 8 confirming some or all diagnoses with histology. Incidence rates of any SIL for HIV-positive women who had normal cytology at study entry ranged from 4.9 to 21.1 cases per 100 women-years (Table 1). In all studies that included HIV-negative populations (N=6, 40%), the incidence rate of any SIL was greater in HIV-positive than HIV-negative participants, with a median 3-fold increase in incidence (range 1.5 – 10.0). Incidence rates of LSIL ranged from 2.0 to 16.2 cases per 100 women years (Table 1 and Figure 1). Rates of HSIL/CIN2–3 for HIV-positive women varied from 0.4 to 8.8 cases per 100 women-years. Studies with both HIV-positive and HIV–negative populations showed higher rates of HSIL/CIN2–3 for HIV–positive participants. As an additional measure, study cumulative incidence of LSIL and of HSIL, though not comparable between studies as study length was variable, also consistently showed higher risk for HIV-positive participants than for HIV-negative participants within the same study.
Figure 1.
Incidence rates reported or calculated for LSIL (A) or HSIL (B) in HIV-positive women. Rates are reported as cases per 100 woman-years. Solid lines show unweighted mean rate. Dotted lines show 95% confidence intervals around the unweighted mean. *This study reports CIN2–3.
Incidence stratified by CD4 counts and antiretroviral use
Seven studies included analysis of lesion incidence by CD4 count categories (Table 2) 7,10,18,19,21,26,27. As the studies varied in length of follow-up, and varied in the categorization of CD4 counts, comparisons of cumulative incidence between studies were not meaningful. Within study comparisons, however, show that in 6 studies, lower CD4 count was associated with an increased incidence of abnormal cervical cytology7,10,19,21,26,27, although most estimates were not statistically significant. Two studies assessed nadir CD4 counts and reported no statistically significant relationship with incidence29,31.
Table 2.
Cervical neoplasia incidence estimates stratified by CD4 count in HIV-positive populations
| Author/year Country |
Median (mean) follow-up time, months* | Assessment method | Baseline | Outcome | ART use | CD4 count | Women with follow-up data (N) | Study cumulative risk | Risk Ratio(95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Sirera et al. 200721 Germany |
57† | cytology‡ | normal | SIL | all§ | > 200 | 110 | 33% | 0.4 (0.1, 1.0)|| |
| ≥ 200 | 20 | 55% | REF | ||||||
|
| |||||||||
| Lehtovirta et al. 200619 Finland |
(31) | cytology | normal | SIL | mixed¶ | ≥ 400 | NS | NS | REF |
| <400 | NS | NS | 1.6 (0.7, 3.7)** | ||||||
|
| |||||||||
| Hawes et al. 200627 Senegal |
(26) | cytology | normal | HSIL | none | > 500 | 102 | 6% | REF |
| 200–500 | 95 | 20% | 1.6 (0.5, 4.9)†† | ||||||
| < 200 | 48 | 39% | 1.7 (0.5, 5.6)†† | ||||||
|
| |||||||||
| Schuman et al. 200326 USA |
35 | cytology‡ | normal | SIL | mixed¶ | > 500 | NS | NS | REF |
| 200–500 | NS | NS | 1.8 (1.1, 2.8) | ||||||
| < 200 | NS | NS | 2.1 (1.3, 3.6) | ||||||
|
| |||||||||
| Delmas et al. 20007 12 European Countries |
24 | cytology‡ | normal | SIL | none | ≥ 500 | NS | NS | REF |
| 200–499 | NS | NS | 1.9 (1.0, 3.6)‡‡ | ||||||
| < 200 | NS | NS | 2.9 (1.1, 8.0)‡‡ | ||||||
| all¶ | ≥ 500 | NS | NS | REF | |||||
| 200–499 | NS | NS | 1.3 (0.6, 2.8)‡‡ | ||||||
| < 200 | NS | NS | 1.7 (0.8, 3.5)‡‡ | ||||||
| mixed¶ | ≥ 500 | 87 | 22% | REF | |||||
| 200–499 | 99 | 31% | 1.6 (0.9, 2.8)‡‡ | ||||||
| < 200 | 43 | 44% | 1.9 (1.0, 3.7)‡‡ | ||||||
|
| |||||||||
| Six et al. 199810 France and French Guiana |
13 | cytology‡ | normal | ASCUS and SIL | none | > 500 | 46 | 17% | REF |
| < 500 | 51 | 27% | 1.6§§ | ||||||
|
| |||||||||
| Heard et al. 199518 France |
18 | cytology and colposcopy | normal | SIL | mixed|||| | ≥ 200 | 22 | 9% | NS |
| < 200 | 5 | 0% | NS | ||||||
ART=antiretroviral therapy, HAART=highly active antiretroviral therapy, SIL=squamous intraepithelial lesion, HSIL = high grade SIL, ASCUS=atypical cells of unknown significance, CI = confidence interval, NS=not stated
For larger cohort;
From personal communication with author;
Some cases confirmed by histology;
HAART;
Odds ratio;
Mixed ART/HAART;
Adjusted for age;
Adjusted for age, employment as commercial sex worker, parity, birth in Senegal, and cytology reader;
Adjusted for HPV detection;
Estimated from the ratio of the study cumulative risks;
ART.
Five studies presented risk ratios for SIL incidence by ART use (Table 3)19,22,26,31,32. Of the 4 studies that reported on HAART use specifically22,26,31,32, 2 studies found a reduced incidence and 2 studies found an increased incidence of SIL for users of HAART. However, only 1 of these estimates, finding reduced incidence, was statistically significant22. Two studies reported on ART use, with one reporting a decrease and 1 reporting an increase in incidence of SIL19,26. Again, only one study, finding increased incidence, found this association to be statistically significant26.
Table 3.
Cervical neoplasia incidence estimates stratified by anti-retroviral use in HIV-positive populations
| Author/year Country |
Median (mean) follow-up time, months* | Assessment method | Baseline | Outcome | ART use | Women with follow-up data (N) | Risk Ratio(95% CI) |
|---|---|---|---|---|---|---|---|
| Minkoff et al. 201032 USA |
NS | cytology | normal | SIL | none | NS | REF |
| HAART† | NS | 0.7 (0.3,1.9) | |||||
|
| |||||||
| Sirera et al. 200831 Germany |
(35) | cytology‡ | normal | SIL | none | 37 | REF |
| HAART | 90 | 1.8 (0.7, 4.7) § | |||||
|
| |||||||
| Soncini et al., 200722 Italy |
NS | cytology‡ colposcopy | normal | SIL | none | 31 | REF |
| HAART | 43 | 0.3 (0.1, 0.7) || | |||||
|
| |||||||
| Lehtovirta et al. 200619 Finland |
(31) | cytology | normal | SIL | None | NS | REF |
| ART¶ | NS | 0.8 (0.4, 1.8)** | |||||
|
| |||||||
| Schuman et al. 200326 USA |
35 | cytology‡ | normal | SIL | None | NS | REF |
| Sub-HAART†† | NS | 1.6 (1.1, 2.1) | |||||
| HAART | NS | 1.2 (0.5, 2.9) | |||||
SIL=squamous intraepithelial lesion, ART=antiretroviral therapy, HAART=highly active antiretroviral therapy,
CI = confidence interval, NS=not stated
For larger cohort;
Includes only women adherent to HAART;
Some cases confirmed by histology;
Odds ratio;
Hazard ratio;
Mixed ART/HAART;
Adjusted for age;
non-HAART combination therapy or monotherapy.
Progression of low grade lesions
From 219 screened abstracts, our search for cervical lesion progression studies in HIV-positive women identified 11 cohort studies, including data from 1099 HIV-positive women in total (Table 4). Four of the studies were conducted in the USA33–36, 4 in the European Union with one including women from France and French Guiana 7,10,37,38, 2 in Africa28,39, and 1 in Brazil. No studies came from Asia, Australia, Central America, or Eastern Europe. Half of the studies (N=5, 45%) had both HIV-positive and HIV-negative populations. Median age of study participants ranged from 27 to 38, with the majority of studies having a median age in the low 30s. Eight of the studies reported a proportion of the participants receiving ART (2 ART, 1 HAART, and 5 mixed ART/HAART) 7,10,28,33–36,38. In 3 studies ART use was not specified37,39,40. In none of the study populations were all participants receiving ART. The number of participants ranged from 28 to 242 HIV-positive women, with most having fewer than 100 participants. Study lengths ranged from one year to 7 years, with the majority reporting follow-up visits every six months. To assess the grade of cervical lesions, most studies used cytology, with 6 studies (56%) confirming some or all diagnoses with histology.
Table 4.
Progression rate estimates of cervical neoplasia from HIV-positive populations
| Author/year Country Study Population |
Median (mean) age, years | ART use | Women with progression follow-up data (N) | Median (mean) follow-up time for progression, months | Assessment method (intervals) | Baseline | Outcome | Progressed (N) | Progression cases / 100 woman-years | Study cumulative risk |
|---|---|---|---|---|---|---|---|---|---|---|
| Omar et al. 201128 South Africa HIV wellness clinic 2003–2009 |
HIV+ 32* | mixed† | HIV+ 242 | HIV+ 31* | cytology (6–12 months) | LSIL | HSIL | HIV+52 | HIV+ 9.8 | HIV+ 22% |
|
| ||||||||||
| Duerr et al. 200633 USA HIV Epidemiology Research Study (HERS) 1993–1999 |
HIV+ 35‡ HIV− 34‡ |
mixed§ | HIV+ 148 HIV− 48 |
HIV+ 53* HIV− 53* |
cytology (6 months) | ASCUS | SIL | HIV+ 89 HIV− 12 |
HIV+13.7|| HIV− 5.7|| |
HIV+ 60% HIV− 25% |
|
| ||||||||||
| Nappi et al. 200537 Italy University clinic1990–1997 |
HIV+ (27)* HIV− (28)* |
NS | HIV+ 28 HIV− 30 |
NS | cytology confirmed by histology (3–6 months) | LSIL | HSIL | HIV+ 15 HIV− 7 |
NS | HIV+ 54% HIV− 23% |
|
| ||||||||||
| Del Mistro et al. 200438 Italy Infectious disease units of city hospitals 1994–2002 |
HIV+ 33* | mixed§ | HIV+ 88 | NS | cytology¶ (6–12 months) | LSIL | HSIL | HIV+ 6 | NS | HIV+ 7% |
|
| ||||||||||
| Kirby et al. 200434 USA University clinic 1996-NS |
HIV+ 37* | mixed** | HIV+ 64 | HIV+ 29 | cytology (6 months) | ASCUS | HSIL | HIV+ 5 | HIV+ 3.2|| | HIV+ 8% |
|
| ||||||||||
| Massad et al. 200435 USA Women’s Interagency HIV Study (WIHS) university, public, and private medical centers and clinics 1994–2002 |
HIV+ (38) HIV− (31) |
mixed§ | HIV+ 202 HIV− 21 |
HIV+/−(40) †† | cytology¶ (6 months) | CIN1 | CIN 2–3 or HSIL | HIV+ 8 HIV− 0 |
HIV+ 1.2 HIV− 0 |
HIV+ 4% HIV− 0% |
|
| ||||||||||
| Robinson et al. 200236 USA 26 AIDS Clinical Trials Group sites, isotretinoin treatment study‡‡1996–1999 |
NS | mixed§ | HIV+ 52 | HIV+ 11 | cytology confirmed by histology (3–6 months) | CIN1 | CIN2/3 | HIV+ 13 | HIV+26.2|| | HIV+ 25% |
|
| ||||||||||
| Calore et al. 200140 Brazil Infectology Institute1996–1999 |
HIV+ (35) | NS | HIV+ 31 | NS | cytology (24–48 months) | LSIL | HSIL | HIV+ 2 | NS | HIV+ 7% |
|
| ||||||||||
| Delmas et al. 20007 12 European Countries European Study Group on Natural History of HIV Infection in Women; gynecology, STD, and drug treatment centers 1993 – 1998 |
HIV+ 31* | mixed§ | HIV+ 115 | HIV+ 18 | cytology¶ (6 months) | LSIL | HSIL | HIV+ 11 | HIV+ 6.4|| | HIV+ 10% |
|
| ||||||||||
| La Ruche et al. 199939 Côte d’Ivoire Gynecology clinics 1995–1996 |
NS | NS | HIV+ 38 HIV− 56 |
NS | cytology (5 months) | LSIL | HSIL | NS | NS | HIV+ 18% HIV− 0% |
|
| ||||||||||
| Six et al. 199810 France and French Guiana medical centers and STD clinics 1993–1995 |
HIV+ 31* HIV− 29* |
mixed** | HIV+ 47 HIV− 7 |
HIV+/− 13*†† | cytology¶ (6 months) | LSIL | HSIL | HIV+ 12 HIV− 0 |
HIV+ 23.6|| HIV− 0|| |
HIV+ 26% HIV− 0% |
ART=antiretroviral therapy, HAART=highly active antiretroviral therapy, HIV=human immunodeficiency virus, ASCUS=atypical cells of unknown significance, SIL=squamous intraepithelial lesion, LSIL = low grade SIL, HSIL = high grade SIL, CIN = cervical intraepithelial neoplasia, NS=not specified
For larger cohort;
HAART;
Additional reference used26;
Mixed ART/HAART;
Estimated from [[number progressed / (N at baseline * mean follow-up time)] * 100;
Some cases confirmed by histology;
ART;
HIV+ / − combined;
Control arm.
Cervical lesion progression rates from LSIL/CIN1 to greater than LSIL/CIN1(most commonly HSIL/CIN2–3) for HIV-positive women ranged from 1.2 to 26.2 cases per 100 women-years. Two studies followed women with ASCUS at baseline33,34, finding a rate of 13.7 cases of SIL per 100 women-years and 3.2 cases of HSIL per 100 women-years. Study cumulative risks, though not comparable between studies, were higher for HIV-positive participants than for HIV-negative participants in all five studies that included both populations10,33,35,37,39.
Progression of lesions stratified by CD4 counts
Three studies reported progression of cervical lesions separated by CD4 count categories (Table 5)10,33,37. The cumulative risks of progression in the studies presenting these data appeared to show a trend of increased risk of progression with decreasing CD4 count10,37. Risk ratios for progression were higher with lower CD4 count33,37, however only 1 of the studies reported statistical significance for this association. Nadir CD4 count was not evaluated. None of the studies clearly delineated progression risk for ART users versus non-users.
Table 5.
Progression estimates for cervical lesions stratified by CD4 count in HIV-positive populations
| Author/year Country |
ART use | median (mean) follow-up time for progression, months* | Assessment method | Baseline | Outcome | CD4 count | Number of women with follow-up data | Study cumulative risk | Risk Ratio(95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Duerr et al. 200633 USA |
mixed† | 53 | cytology | ASCUS | SIL | >500 | NS | NS | REF |
| 200–500 | NS | NS | 1.4 (0.7, 2.6) | ||||||
| <200 | NS | NS | 1.7 (0.8, 3.6) | ||||||
| Nappi et al. 200537 Italy |
NS | NS | cytology confirmed by histology | LSIL | HSIL | >200 | 15 | 33% | REF |
| <200 | 13 | 77% | 6.67‡ (1.2, 35.7) | ||||||
| Six et al. 199810 France and French Guiana |
mixed§ | 13 | cytology|| | LSIL | HSIL | >500 | 9 | 0% | NS |
| 200–500 | 18 | 31% | NS | ||||||
| <200 | 20 | 41% | NS |
ART=antiretroviral therapy, CI= confidence interval, HAART=highly active antiretroviral therapy, HIV=human immunodeficiency virus, LSIL = low grade SIL, HSIL = high grade SIL, NS=not specified
For larger cohort;
Mixed ART/HAART;
Odds ratio, not risk ratio;
ART;
Some cases confirmed by histology.
Discussion
Our comprehensive search identified 15 studies reporting incident cervical lesions and 11 studies reporting progression of cervical lesions for HIV-positive women. These studies showed a wide range of incidence and progression rates for cervical lesions in HIV-positive populations. In studies with both HIV-positive and HIV-negative populations, the incidence rate of cervical lesions was a median three-fold higher in HIV-positive women as compared to HIV-negative study participants. HIV-positive women were also at least twice as likely to have cervical lesions that progressed in severity as compared to HIV-negative women. The majority of studies showed that lower CD4 counts were associated with increased incidence and progression of cervical lesions in HIV-positive women, however, this relationship was usually not statistically significant, often due to small sample size per strata of CD4 counts.
The evidence on ART use and cervical lesion incidence was more inconsistent. Though one study showed a reduction in incidence with HAART use versus non-HAART use22, most studies found no significant difference between HAART/ART use and non-use19,26,31,32. Associations may be confounded by the practice of initiating ART use only in patients with low CD4 counts or lack comparability due to differences in CD4 count recovery, HIV viremia, and follow-up time after ART initiation. Additionally, ART treatment has evolved since its introduction so it is important to note that many of the study populations that received ART were receiving single or dual drugs as compared to current standard of triple drug combinations (HAART) currently in practice. Life expectancy of HIV-positive individuals on HAART is also increasing and thus a greater number of HIV-positive women may be susceptible to cervical cancer and associated diseases in the future. In addition, an important gap in the literature is the effect of HAART use and progression of cervical lesions. Minkoff et al. (2010) found a significant increase in clearance of SILs among adherent women on HAART (adjusted odds ratio 2.4; 95% confidence interval 1.1–5.2) as compared to pre-HAART32. Two more recent studies, published after the cut off for this analysis, also found a reduction in progression or increase in regression for consistent users of HAART41,42. The relationship of HAART use and progression may become more defined when additional analyses are done in cohorts of women only on HAART and when HAART adherence and effectiveness are considered.
As both HIV and HPV are sexually transmitted infections, the increase in cervical lesions found in HIV-positive women may be due to shared behavioral risk factors such as high number of sexual partners or high risk sexual partners. However, biological mechanisms have also been implicated. The increased risk of cervical lesion development and progression seen in women with HIV is postulated to be due to both an impaired immune system and an increased potential for unregulated cell growth. For example, cervical molecular profiles of HIV-positive women show alterations in oncogenic and immunogenic protein expression43–45. Additionally, there is evidence linking the HIV-1 transactivator protein (Tat) with increased expression of HPV oncogenes and proliferation of HPV-infected cells46. Together these data indicate an altered immune response to HPV infection and an altered natural history of cervical cancer in HIV-positive women. In alignment with the hypothesis that impaired immune function reduces the ability to clear HPV infection, thus increasing the probability of developing cervical precancer and cancer, most studies found an inverse association between level of immunosuppression, as indicated by CD4 counts, and the incidence and progression of cervical lesions7,10,19,21,26,27,33,37.
Our review is limited, however, by the small number of studies reporting rates for cervical lesion incidence and progression, making between-study comparisons difficult. Where possible, rates were approximated based on median follow-up time. This review identified gaps in the literature on incidence and progression of cervical precancer in HIV-positive women. Most studies had a median age for study participants in the low 30s; few studies reported on older populations. Given the increased life expectancy of HIV-positive women due to HAART, assessment of this growing demographic of women 30 years and older is needed. Additionally, HIV viral load was not systematically extracted in this analysis due to the general lack of consistent data throughout the studies. Also, we did not find studies meeting our criteria that addressed the effects of HAART use on progression risk from low-grade lesions. A recent large study, which was not included in our literature review, reported that HAART use reduced the rate of cervical lesion progression from LSIL (rate ratio 0.52 for HAART users versus ART non-users)41. HAART use has also been associated with a reduction in the combined rate for incidence and progression of cervical neoplasia (rate of any progression from normal or LSIL baseline), though not always reaching statistical significance28,41,47–49. Additionally, evidence shows that HAART use increases the rate of regression of precancerous lesions32,50,51.
The compiled research highlights the high risk of cervical precancer development and progression in HIV-positive women. This risk appears to be greater in women with reduced immune function but more evidence is needed to determine how HAART use modifies risk of incidence and progression. Our findings reinforce the importance of screening for cervical lesions in HIV-positive populations, particularly those with lower CD4 counts that are at a higher risk for both incident and progressive cervical disease outcomes. Given the healthcare resources focused on HIV care, integrating cervical screening with HIV treatment programs is essential to reduce untimely death of women from this preventable cancer.
Acknowledgments
Funding:
This manuscript was supported by CFAR development award AI50410.
Footnotes
Disclosure of interests:
The authors declare no competing interests.
Contribution to Authorship:
SD performed the literature search, extracted data, and prepared the manuscript. AR helped with manuscript preparation, contributed subject matter expertise, edited and reviewed the manuscript. CF provided subject matter expertise, edited and reviewed the manuscript. JT checked the data extraction, edited and reviewed the manuscript. JS provided subject matter expertise, edited and reviewed the manuscript.
Details of ethics approval:
Ethics approval was not obtained as this manuscript is a systematic summary of published data.
References
- 1.UNAIDS, WHO. AIDS Epidemic Update. 2009 Dec; http://data.unaids.org/pub/report/2009/jc1700_epi_update_2009_en.pdf.
- 2.Scheurer ME, Tortolero-Luna G, KA-S Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15(5):727–746. doi: 10.1111/j.1525-1438.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 3.De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008;17(6):545–54. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 4.Firnhaber C, Zungu K, Levin S, et al. Diverse and high prevalence of human papillomavirus associated with a significant high rate of cervical dysplasia in human immunodeficiency virus-infected women in Johannesburg, South Africa. Acta Cytol. 2009;53(1):10–7. doi: 10.1159/000325079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahdieh L, Munoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol. 2000;151(12):1148–57. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 6.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC., Jr Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337(19):1343–9. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 7.Delmas MC, Larsen C, van Benthem B, et al. Cervical squamous intraepithelial lesions in HIV-infected women: prevalence, incidence and regression. European Study Group on Natural History of HIV Infection in Women. AIDS. 2000;14(12):1775–84. doi: 10.1097/00002030-200008180-00013. [DOI] [PubMed] [Google Scholar]
- 8.Maiman M, Fruchter RG, Sedlis A, et al. Prevalence, risk factors, and accuracy of cytologic screening for cervical intraepithelial neoplasia in women with the human immunodeficiency virus. Gynecol Oncol. 1998;68(3):233–9. doi: 10.1006/gyno.1998.4938. [DOI] [PubMed] [Google Scholar]
- 9.Olaitan A, Mocroft A, McCarthy K, Phillips A, Reid W, Johnson M. Cervical abnormality and sexually transmitted disease screening in human immunodeficiency virus-positive women. Obstet Gynecol. 1997;89(1):71–5. doi: 10.1016/s0029-7844(96)00377-8. [DOI] [PubMed] [Google Scholar]
- 10.Six C, Heard I, Bergeron C, et al. Comparative prevalence, incidence and short-term prognosis of cervical squamous intraepithelial lesions amongst HIV-positive and HIV-negative women. AIDS. 1998;12(9):1047–56. [PubMed] [Google Scholar]
- 11.Wright TC, Jr, Ellerbrock TV, Chiasson MA, Van Devanter N, Sun XW. Cervical intraepithelial neoplasia in women infected with human immunodeficiency virus: prevalence, risk factors, and validity of Papanicolaou smears. New York Cervical Disease Study. Obstet Gynecol. 1994;84(4):591–7. [PubMed] [Google Scholar]
- 12.Massad LS, Riester KA, Anastos KM, et al. Prevalence and predictors of squamous cell abnormalities in Papanicolaou smears from women infected with HIV-1. Women’s Interagency HIV Study Group. J Acquir Immune Defic Syndr. 1999;21(1):33–41. doi: 10.1097/00126334-199905010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Firnhaber C, Van Le H, Pettifor A, et al. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. MMWR. 1992;41(RR17) [PubMed] [Google Scholar]
- 15.United States Department of State. Pink Ribbon Red Ribbon Overview and Press Release. 2011 www.state.gov/r/pa//prs/ps/2011/09/172244.htm.
- 16.La Ruche G, Ramon R, Mensah-Ado I, et al. Squamous intraepithelial lesions of the cervix, invasive cervical carcinoma, and immunosuppression induced by human immunodeficiency virus in Africa. Dyscer-CI Group. Cancer. 1998;82(12):2401–8. [PubMed] [Google Scholar]
- 17.Branca M, Garbuglia AR, Benedetto A, et al. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow-up. Int J STD AIDS. 2003;14(6):417–25. doi: 10.1258/095646203765371321. [DOI] [PubMed] [Google Scholar]
- 18.Heard I, Bergeron C, Jeannel D, Henrion R, Kazatchkine MD. Papanicolaou smears in human immunodeficiency virus-seropositive women during follow-up. Obstet Gynecol. 1995;86(5):749–53. doi: 10.1016/0029-7844(95)00282-V. [DOI] [PubMed] [Google Scholar]
- 19.Lehtovirta P, Finne P, Nieminen P, et al. Prevalence and risk factors of squamous intraepithelial lesions of the cervix among HIV-infected women - a long-term follow-up study in a low-prevalence population. Int J STD AIDS. 2006;17(12):831–4. doi: 10.1258/095646206779307649. [DOI] [PubMed] [Google Scholar]
- 20.Petry KU, Bohmer G, Iftner T, Flemming P, Stoll M, Schmidt RE. Human papillomavirus testing in primary screening for cervical cancer of human immunodeficiency virus-infected women, 1990–1998. Gynecol Oncol. 1999;75(3):427–31. doi: 10.1006/gyno.1999.5639. [DOI] [PubMed] [Google Scholar]
- 21.Sirera G, Videla S, Lopez-Blazquez R, et al. Evolution of cervical cytologic changes among HIV-infected women with normal cytology in the HAART era. AIDS Res Hum Retroviruses. 2007;23(8):965–71. doi: 10.1089/aid.2006.0293. [DOI] [PubMed] [Google Scholar]
- 22.Soncini E, Zoncada A, Condemi V, Antoni AD, Bocchialini E, Soregotti P. Reduction of the risk of cervical intraepithelial neoplasia in HIV-infected women treated with highly active antiretroviral therapy. Acta Biomed. 2007;78(1):36–40. [PubMed] [Google Scholar]
- 23.Kitchener H, Nelson L, Adams J, et al. Colposcopy is not necessary to assess the risk to the cervix in HIV-positive women: an international cohort study of cervical pathology in HIV-1 positive women. Int J Cancer. 2007;121(11):2484–91. doi: 10.1002/ijc.22947. [DOI] [PubMed] [Google Scholar]
- 24.Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283(8):1031–7. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 25.Massad LS, Seaberg EC, Wright RL, et al. Squamous cervical lesions in women with human immunodeficiency virus: long-term follow-up. Obstet Gynecol. 2008;111(6):1388–93. doi: 10.1097/AOG.0b013e3181744619. [DOI] [PubMed] [Google Scholar]
- 26.Schuman P, Ohmit SE, Klein RS, et al. Group HIVERS. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188(1):128–36. doi: 10.1086/375783. [DOI] [PubMed] [Google Scholar]
- 27.Hawes SE, Critchlow CW, Sow PS, et al. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV-1) and HIV-2. J Natl Cancer Inst. 2006;98(2):100–9. doi: 10.1093/jnci/djj010. [DOI] [PubMed] [Google Scholar]
- 28.Omar T, Schwartz S, Hanrahan C, et al. Progression and regression of premalignant cervical lesions in HIV-infected women from Soweto: a prospective cohort. AIDS. 2011;25(1):87–94. doi: 10.1097/QAD.0b013e328340fd99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalermchockcharoenkit A, Chayachinda C, Thamkhantho M, Komoltri C. Prevalence and cumulative incidence of abnormal cervical cytology among HIV-infected Thai women: a 5.5-year retrospective cohort study. BMC Infect Dis. 2011;11:8. doi: 10.1186/1471-2334-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branca M, Garbuglia AR, Benedetto A, et al. Group DCS. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow-up. Int J STD AIDS. 2003;14(6):417–25. doi: 10.1258/095646203765371321. [DOI] [PubMed] [Google Scholar]
- 31.Sirera G, Videla S, Lopez-Blazquez R, et al. Highly active antiretroviral therapy and incidence of cervical squamous intraepithelial lesions among HIV-infected women with normal cytology and CD4 counts above 350 cells/mm3. J Antimicrob Chemother. 2008;61(1):191–4. doi: 10.1093/jac/dkm412. [DOI] [PubMed] [Google Scholar]
- 32.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681–90. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duerr A, Paramsothy P, Jamieson DJ, et al. Effect of HIV infection on atypical squamous cells of undetermined significance. Clin Infect Dis. 2006;42(6):855–61. doi: 10.1086/500404. [DOI] [PubMed] [Google Scholar]
- 34.Kirby TO, Allen ME, Alvarez RD, Hoesley CJ, Huh WK. High-risk human papillomavirus and cervical intraepithelial neoplasia at time of atypical squamous cells of undetermined significance cytologic results in a population with human immunodeficiency virus. J Low Genit Tract Dis. 2004;8(4):298–303. doi: 10.1097/00128360-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Massad LS, Evans CT, Minkoff H, et al. Natural history of grade 1 cervical intraepithelial neoplasia in women with human immunodeficiency virus. Obstet Gynecol. 2004;104(5 Pt 1):1077–85. doi: 10.1097/01.AOG.0000143256.63961.c0. [DOI] [PubMed] [Google Scholar]
- 36.Robinson WR, Andersen J, Darragh TM, Kendall MA, Clark R, Maiman M. Isotretinoin for low-grade cervical dysplasia in human immunodeficiency virus-infected women. Obstet Gynecol. 2002;99(5 Pt 1):777–84. doi: 10.1016/s0029-7844(02)01949-x. [DOI] [PubMed] [Google Scholar]
- 37.Nappi L, Carriero C, Bettocchi S, Herrero J, Vimercati A, Putignano G. Cervical squamous intraepithelial lesions of low-grade in HIV-infected women: recurrence, persistence, and progression, in treated and untreated women. Eur J Obstet Gynecol Reprod Biol. 2005;121(2):226–32. doi: 10.1016/j.ejogrb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Del Mistro A, Bertorelle R, Franzetti M, et al. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis. 2004;38(5):737–42. doi: 10.1086/381681. [DOI] [PubMed] [Google Scholar]
- 39.La Ruche G, Leroy V, Mensah-Ado I, et al. Short-term follow up of cervical squamous intraepithelial lesions associated with HIV and human papillomavirus infections in Africa. Int J STD AIDS. 1999;10(6):363–8. doi: 10.1258/0956462991914276. [DOI] [PubMed] [Google Scholar]
- 40.Calore EE, Manzione CR, Nadal SR, Cavalieri MJ, Calore NM, Santos RP. Ki-67 expression in anal intraepithelial neoplasia in AIDS. Sao Paulo Med J. 2001;119(3):119–21. doi: 10.1590/S1516-31802001000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firnhaber C, Westreich D, Schulze D, et al. Highly active antiretroviral therapy and cervical dysplasia in HIV-positive women in South Africa. J Int AIDS Soc. 2012;15(2):1–6. doi: 10.7448/IAS.15.2.17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adler DH, Kakinami L, Modisenyane T, et al. Increased regression and decreased incidence of HPV-related cervical lesions among HIV-infected women on HAART. AIDS. 2012;26(13):1645–52. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clifford GM, Franceschi S. Cancer risk in HIV-infected persons: influence of CD4(+) count. Future Oncol. 2009;5(5):669–78. doi: 10.2217/fon.09.28. [DOI] [PubMed] [Google Scholar]
- 44.Nicol AF, Fernandes AT, da Bonecini-Almeida MG. Immune response in cervical dysplasia induced by human papillomavirus: the influence of human immunodeficiency virus-1 co-infection -- review. Mem Inst Oswaldo Cruz. 2005;100(1):1–12. doi: 10.1590/s0074-02762005000100001. [DOI] [PubMed] [Google Scholar]
- 45.Nicol AF, Pires AR, de Souza SR, et al. Cell-cycle and suppressor proteins expression in uterine cervix in HIV/HPV co-infection: comparative study by tissue micro-array (TMA) BMC Cancer. 2008;8:289. doi: 10.1186/1471-2407-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim RH, Yochim JM, Kang MK, Shin KH, Christensen R, Park NH. HIV-1 Tat enhances replicative potential of human oral keratinocytes harboring HPV-16 genome. Int J Oncol. 2008;33(4):777–82. [PubMed] [Google Scholar]
- 47.Minkoff H, Ahdieh L, Massad LS, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15(16):2157–64. doi: 10.1097/00002030-200111090-00011. [DOI] [PubMed] [Google Scholar]
- 48.Paramsothy P, Jamieson DJ, Heilig CM, et al. The effect of highly active antiretroviral therapy on human papillomavirus clearance and cervical cytology. Obstet Gynecol. 2009;113(1):26–31. doi: 10.1097/AOG.0b013e31819225cb. [DOI] [PubMed] [Google Scholar]
- 49.Lillo F, Ferrari D, Veglia F, et al. Human papillomavirus infection and associated cervical disease in human immunodeficiency virus-infected women: Effect of highly active antiretroviral therapy. The Journal of Infectious Diseases. 2001;184:547–51. doi: 10.1086/322856. [DOI] [PubMed] [Google Scholar]
- 50.Heard I, Tassie JM, Kazatchkine MD, Orth G. Highly active antiretroviral therapy enhances regression of cervical intraepithelial neoplasia in HIV-seropositive women. AIDS. 2002;16(13):1799–802. doi: 10.1097/00002030-200209060-00013. [DOI] [PubMed] [Google Scholar]
- 51.Adler DH, Kakinami L, Modisenyane T, et al. Increased regression and decreased incidence of HPV-related cervical lesions among HIV-infected women on HAART. AIDS. 2012;26(13):1645–1652. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]